Trade-Offs and Partitioning Strategy of Carbon Source-Sink During Fruit Development of Camellia oleifera
Abstract
1. Introduction
2. Results
2.1. The Disparity of NSCs in Camellia oleifera Organs
2.2. The Dynamics of NSCs in C. oleifera Organs at the Critical Stage of Fruit Development
2.3. The Correlation of NSCs in C. oleifera Organs at the Critical Stage of Fruit Development
2.4. The Allometric Partitioning Characteristics of NSCs in C. oleifera Organs at the Critical Stage of Fruit Development
3. Discussion
3.1. The Disparity of NSCs in Four C. oleifera Organs
3.2. The Dynamics of NSCs Within C. oleifera Organs at Critical Stage of Fruit Development
3.3. The NSCs Partitioning Characteristics of Four C. oleifera Organs in Critical Stage with the Allometric Analysis
4. Materials and Methods
4.1. Study Site Description
4.2. Field Sampling
4.3. Nonstructural Carbohydrate Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCs | Non-structural carbohydrates |
| C | Carbon |
References
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Palo Alto, CA, USA, 2014; Volume 65, pp. 667–687. [Google Scholar]
- Hartmann, H.; Bahn, M.; Carbone, M.; Richardson, A.D. Plant carbon allocation in a changing world–challenges and progress: Introduction to a Virtual Issue on carbon allocation Introduction to a virtual issue on carbon allocation. New Phytol. 2020, 227, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Kong, L.; Hu, S.; Deng, M.; Yang, G.; Wei, Q.; Yu, F. Emerging Insights into the Roles of the Rhizome-Culm System in Bamboo Shoot Development through Analysis of Non-Structural Carbohydrate Changes. Plants 2024, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Shi, T.; Zhou, S.; Gao, S.; Zhao, Y.; Shi, G. Non-Structural Carbohydrates Accumulation in Seedlings Improved Flowering Quality of Tree Peony under Forcing Culture Conditions, with Roots Playing a Crucial Role. Plants 2024, 13, 2837. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, Y.; Jiang, Y.; Chen, M.; Zhang, H.; Wu, P.; Ma, X. Dynamics of Non-Structural Carbohydrates Release in Chinese Fir Topsoil and Canopy Litter at Different Altitudes. Plants 2023, 12, 729. [Google Scholar] [CrossRef]
- Lu, L.-L.; Liu, H.; Wang, J.; Zhao, K.-P.; Miao, Y.; Li, H.-C.; Hao, G.-Y.; Han, S.-J. Seasonal patterns of nonstructural carbohydrate storage and mobilization in two tree species with distinct life-history traits. Tree Physiol. 2024, 44, tpae042. [Google Scholar] [CrossRef]
- Signori-Mueller, C.; Oliveira, R.S.; Barros, F.d.V.; Tavares, J.V.; Gilpin, M.; Carvalho Diniz, F.; Marca Zevallos, M.J.; Salas Yupayccana, C.A.; Acosta, M.; Bacca, J.; et al. Non-structural carbohydrates mediate seasonal water stress across Amazon forests. Nat. Commun. 2021, 12, 2310. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Davidson, A.M.; Orozco, J.; Cooper, K.B.; Guzman-Delgado, P. The impact of non-structural carbohydrates (NSC) concentration on yield in Prunus dulcis, Pistacia vera, and Juglans regia. Sci. Rep. 2022, 12, 4360. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees–from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Zhang, G.; Maillard, P.; Mao, Z.; Brancheriau, L.; Engel, J.; Gerard, B.; Fortunel, C.; Maeght, J.-L.; Martinez-Vilalta, J.; Ramel, M.; et al. Non-structural carbohydrates and morphological traits of leaves, stems and roots from tree species in different climates. BMC Res. Notes. 2022, 15, 251. [Google Scholar] [CrossRef]
- Davidson, A.M.; Le, S.T.; Cooper, K.B.; Lange, E.; Zwieniecki, M.A. No time to rest: Seasonal dynamics of non-structural carbohydrates in twigs of three Mediterranean tree species suggest year-round activity. Sci. Rep. 2021, 11, 5181. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Lin, T.; Zhang, B.; Lai, Y.; Chen, X.; Xiao, Y.; Xie, Y.; Zhu, J.; Yang, Y.; Wang, J. Regulating carbon and water balance as a strategy to cope with warming and drought climate in Cunninghamia lanceolata in southern China. Front. Plant Sci. 2022, 13, 1048930. [Google Scholar] [CrossRef]
- Furze, M.E.; Wainwright, D.K.; Huggett, B.A.; Knipfer, T.; McElrone, A.J.; Brodersen, C.R. Ecologically driven selection of nonstructural carbohydrate storage in oak trees. New Phytol. 2021, 232, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zheng, H.; Huang, Z.; Wang, J.; Zhu, J. Non-Structural Carbohydrate Dynamics in Leaves and Branches of Pinus massoniana (Lamb.) Following 3-Year Rainfall Exclusion. Forests 2018, 9, 315. [Google Scholar] [CrossRef]
- Tixier, A.; Gambetta, G.A.; Godfrey, J.; Orozco, J.; Zwieniecki, M.A. Non-structural Carbohydrates in Dormant Woody Perennials; The Tale of Winter Survival and Spring Arrival. Front. For. Glob. Change 2019, 2, 18. [Google Scholar] [CrossRef]
- Tixier, A.; Orozco, J.; Roxas, A.A.; Earles, J.M.; Zwieniecki, M.A. Diurnal Variation in Nonstructural Carbohydrate Storage in Trees: Remobilization and Vertical Mixing. Plant Physiol. 2018, 178, 1602–1613. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Wang, X. Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees-Struct. Funct. 2014, 28, 77–89. [Google Scholar] [CrossRef]
- Huang, X.; Guo, W.; Yang, L.; Zou, Z.; Zhang, X.; Addo-Danso, S.D.; Zhou, L.; Li, S. Effects of Drought Stress on Non-Structural Carbohydrates in Different Organs of Cunninghamia lanceolata. Plants 2023, 12, 2477. [Google Scholar] [CrossRef]
- Lacointe, A. Carbon allocation among tree organs: A review of basic processes and representation in functional-structural tree models. Ann. For. Sci. 2000, 57, 521–533. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Lang, X.; Huang, X. Non-structural carbohydrates regulated by season and species in the subtropical monsoon broad-leaved evergreen forest of Yunnan Province, China. Sci. Rep. 2018, 8, 1083. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, S.; Yang, Z.; Watkins, C.B.; Wang, K. The physiology, molecular biology and biochemistry in ripening and stored fruit. Front. Plant Sci. 2023, 14, 1296816. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, G.C.; Orr, R.; Bennett, D.; Bally, I.S.E. The roles of non-structural carbohydrates in fruiting: A review focusing on mango (Mangifera indica). Funct. Plant Biol. 2024, 51, FP23195. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Granell, A. Fruit development and ripening Preface. J. Exp. Bot. 2014, 65, 4489–4490. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, J.; Gao, X.; Wang, L. The Accumulation and Conversion of Non-Structural Carbohydrate in Branches and Leaves at Different Phenological Stages Determine the Fruit Yield of Xanthoceras sorbifolium Bunge. J. Plant Growth Regul. 2024, 43, 829–839. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Q.; Chen, L.; Liu, D.; Yang, H.; Xu, C.; Hong, J.; Li, J.; Ding, Y.; Sakr, S.; et al. Sink Strength Promoting Remobilization of Non-Structural Carbohydrates by Activating Sugar Signaling in Rice Stem during Grain Filling. Int. J. Mol. Sci. 2022, 23, 4864. [Google Scholar] [CrossRef]
- Mesa, K.; Serra, S.; Masia, A.; Gagliardi, F.; Bucci, D.; Musacchi, S. Seasonal trends of starch and soluble carbohydrates in fruits and leaves of ‘Abbe Fetel’ pear trees and their relationship to fruit quality parameters. Sci. Hortic. 2016, 211, 60–69. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Bonghi, C.; Drincovich, M.F.; Falchi, R.; Lara, M.V.; Moscatello, S.; Vizzotto, G.; Famiani, F. Non-structural Carbohydrate Metabolism in the Flesh of Stone Fruits of the Genus Prunus (Rosaceae)—A Review. Front. Plant Sci. 2020, 11, 549921. [Google Scholar] [CrossRef]
- Tixier, A.; Guzman-Delgado, P.; Sperling, O.; Roxas, A.A.; Laca, E.; Zwieniecki, M.A. Comparison of phenological traits, growth patterns, and seasonal dynamics of non-structural carbohydrate in Mediterranean tree crop species. Sci. Rep. 2020, 10, 347. [Google Scholar] [CrossRef]
- Zhao, W.; Xiao, C.; Li, M.; Xu, L.; Li, X.; He, N. Spatial variation and allocation of sulfur among major plant organs in China. Sci. Total Environ. 2022, 844, 157155. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Ann. Bot. 2006, 97, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Koven, C.D.; Kueppers, L.M. Allometric relationships and trade-offs in 11 common Mediterranean-climate grasses. Ecol. Appl. 2024, 34, e2976. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Yu, J.; Yan, W.; Zhang, J.; Yang, D.; Yao, G.; Liu, Z.; Wu, Y.; Hou, X. Integrative iTRAQ-based proteomic and transcriptomic analysis reveals the accumulation patterns of key metabolites associated with oil quality during seed ripening of Camellia oleifera. Hortic. Res. 2021, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Meng, S.; Jia, Q.; Liu, Q.; Zhou, G.; Wang, H.; Yu, J. Aboveground Biomass Allocation and Additive Allometric Models for Natural Larix gmelinii in the Western Daxing’anling Mountains, Northeastern China. Forests 2019, 10, 150. [Google Scholar] [CrossRef]
- Milla, R.; Westgeest, A.J.; Maestre-Villanueva, J.; Nunez-Castillo, S.; Gomez-Fernandez, A.; Vasseur, F.; Violle, C.; Balarynova, J.; Smykal, P. Evolutionary pathways to lower biomass allocation to the seed coat in crops: Insights from allometric scaling. New Phytol. 2024, 243, 466–476. [Google Scholar] [CrossRef]
- Xu, Z.; Du, W.; Zhou, G.; Qin, L.; Meng, S.; Yu, J.; Sun, Z.; SiQing, B.; Liu, Q. Aboveground biomass allocation and additive allometric models of fifteen tree species in northeast China based on improved investigation methods. For. Ecol. Manag. 2022, 505, 119918. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, M.; Liu, Z.; Li, P.; Xie, B.; Peng, C. Dynamic allometric scaling of tree biomass and size. Nat. Plants 2021, 7, 42–49. [Google Scholar] [CrossRef]
- Yan, Z.; Li, P.; Chen, Y.; Han, W.; Fang, J. Nutrient allocation strategies of woody plants: An approach from the scaling of nitrogen and phosphorus between twig stems and leaves. Sci. Rep. 2016, 6, 20099. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.; He, N.; Xia, F.; Wang, Q.; Wang, R.; Xu, Z.; Jia, Y. Invariant allometric scaling of nitrogen and phosphorus in leaves, stems, and fine roots of woody plants along an altitudinal gradient. J. Plant Res. 2016, 129, 647–657. [Google Scholar] [CrossRef]
- Jiang, P.; Yang, C.; Zhang, X.; Tong, B.; Xie, X.; Li, X.; Fan, S. The Growth and Non-Structural Carbohydrate Response Patterns of Siberian Elm (Ulmus pumila) under Salt Stress with Different Intensities and Durations. Forests 2024, 15, 1004. [Google Scholar] [CrossRef]
- Franceschini, T.; Schneider, R. Influence of shade tolerance and development stage on the allometry of ten temperate tree species. Oecologia 2014, 176, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jing, H.; Wu, J. Non-structural carbohydrate (NSC) content and C:N:P stoichiometry of Pinus yunnanensis seedling needles in response to shade treatment. Ind. Crops Prod. 2024, 210, 118138. [Google Scholar] [CrossRef]
- Song, Q.; Ji, K.; Mo, W.; Wang, L.; Chen, L.; Gao, L.; Gong, W.; Yuan, D. Dynamics of sugars, endogenous hormones, and oil content during the development of Camellia oleifera fruit. Botany 2021, 99, 515–529. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Wang, Z.; Niu, A.; Xue, Y.; Zhou, D.; Zhou, G.; Liu, J. Research progress and management strategies of fungal diseases in Camellia oleifera. Front. Microbiol. 2023, 14, 1215024. [Google Scholar] [CrossRef]
- Liu, S.; Chen, T.; Ye, D.; Chen, Q.; Ni, J.; Rao, M. Prediction of distributional patterns of four major Camellia oilseed species in China under climate and land use changes. Ecol. Indic. 2023, 155, 110996. [Google Scholar] [CrossRef]
- Qin, P.; Shen, J.; Wei, J.; Chen, Y. A critical review of the bioactive ingredients and biological functions of camellia oleifera oil. Curr. Res. Food Sci. 2024, 8, 100753. [Google Scholar] [CrossRef]
- Yan, J.; He, J.; Li, J.; Ren, S.; Wang, Y.; Zhou, J.; Tan, X. Analysis of Camellia oleifera transcriptome reveals key pathways and hub genes involved during different photoperiods. BMC Plant Biol. 2022, 22, 435. [Google Scholar] [CrossRef]
- Yang, D.; Wang, R.; Lai, H.; He, Y.; Chen, Y.; Xun, C.; Zhang, Y.; He, Z. Comparative Transcriptomic and Lipidomic Analysis of Fatty Acid Accumulation in Three Camellia oleifera Varieties During Seed Maturing. J. Agric. Food Chem. 2024, 72, 18257–18270. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Zhou, J.; Gu, Y.; Tan, X. Comparative study on fruit development and oil synthesis in two cultivars of Camellia oleifera. BMC Plant Biol. 2021, 21, 348. [Google Scholar] [CrossRef]
- He, Y.; Chen, R.; Yang, Y.; Liang, G.; Zhang, H.; Deng, X.; Xi, R. Sugar Metabolism and Transcriptome Analysis Reveal Key Sugar Transporters during Camellia oleifera Fruit Development. Int. J. Mol. Sci. 2022, 23, 822. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, N.; Yu, G.; Wang, Q.; Sun, J. Leaf non-structural carbohydrates regulated by plant functional groups and climate: Evidences from a tropical to cold-temperate forest transect. Ecol. Indic. 2016, 62, 22–31. [Google Scholar] [CrossRef]
- Wiley, E.; Hoch, G.; Landhausser, S.M. Dying piece by piece: Carbohydrate dynamics in aspen (Populus tremuloides) seedlings under severe carbon stress. J. Exp. Bot. 2017, 68, 5221–5232. [Google Scholar] [CrossRef]
- Bao, Q.-X.; Mu, X.-R.; Tong, C.; Li, C.; Tao, W.-Z.; Zhao, S.-T.; Liu, Y.-X.; Wang, W.-N.; Wei, Y.-T.; Yu, F.-H.; et al. Sugar status in preexisting leaves determines systemic stomatal development within newly developing leaves. Proc. Natl. Acad. Sci. USA 2023, 120, e2302854120. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Q.; Liu, X.; Xie, F.; Li, T.; Zhang, Q.; Dang, H. Variations in carbon source-sink relationships in subalpine fir across elevational gradients. Plant Biol. 2019, 21, 64–70. [Google Scholar] [CrossRef]
- Silvestro, R.; Mencuccini, M.; Garcia-Valdes, R.; Antonucci, S.; Arzac, A.; Biondi, F.; Butto, V.; Camarero, J.J.; Campelo, F.; Cochard, H.; et al. Partial asynchrony of coniferous forest carbon sources and sinks at the intra-annual time scale. Nat. Commun. 2024, 15, 6169. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Li, Q.; Hu, Y.; Yang, J.; Song, Z. Effects of NSC in different organs and at different growth stages on the yield of oil peony Fengdan with different ages. Front. Plant Sci. 2023, 14, 1108668. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef]
- Hoch, G.; Popp, M.; Körner, C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos. 2002, 98, 361–374. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-Sink Relationships in Crop Plants and Their Influence on Yield Development and Nutritional Quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Vitasse, Y.; Hoch, G. Coordination between growth, phenology and carbon storage in three coexisting deciduous tree species in a temperate forest. Tree Physiol. 2016, 36, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Aubrey, D.P.; Wang, X.; Sun, H. Seasonal non-structural carbohydrate dynamics differ between twig bark and xylem tissues. Trees-Struct. Funct. 2022, 36, 1231–1245. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Zhu, G.; Jin, C.; Yan, J.; Suo, J.; Yu, W.; Hu, Y.; Wu, J. Shortage of storage carbohydrates mainly determines seed abscission in Torreya grandis ‘Merrillii’. Hort. Plant J. 2025, 11, 619–632. [Google Scholar] [CrossRef]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 25908. [Google Scholar] [CrossRef]
- Dovis, V.L.; Machado, E.C.; Ribeiro, R.V.; Magalhaes Filho, J.R.; Marchiori, P.E.R.; Sales, C.R.G. Roots are important sources of carbohydrates during flowering and fruiting in ‘Valencia’ sweet orange trees with varying fruit load. Sci. Hortic. 2014, 174, 87–95. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Long, R.W.; Dudley, T.L.; D’Antonio, C.M.; Grady, K.C.; Bush, S.E.; Hultine, K.R. Spenders versus savers: Climate-induced carbon allocation trade-offs in a recently introduced woody plant. Funct. Ecol. 2021, 35, 1640–1654. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Tsogtsaikhan, T.; Yang, X.; Gao, R.; Liu, J.; Tang, W.; Liu, G.; Ye, X.; Huang, Z. Biomass allocation between reproductive and vegetative organs of Artemisia along a large environmental gradient. BMC Plant Biol. 2025, 25, 27. [Google Scholar] [CrossRef]
- Hoch, G. Fruit-bearing branchlets are carbon autonomous in mature broad-leaved temperate forest trees. Plant Cell Environ. 2005, 28, 651–659. [Google Scholar] [CrossRef]
- Blume, H.-P.; Brümmer, G.W.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. (Eds.) Scheffer/Schachtschabel Soil Science; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhou, C.; Yao, X.; Lin, P.; Wang, K.; Chang, J.; Mo, R. Constituents changes associated with seeds development of Camellia oleifera Abel (in Chinese). Chin. J. Oil Crop Sci. 2013, 35, 680–685. [Google Scholar]
- Tang, J.; Feng, J.; Yang, Z.; Chen, S.; Chen, H.; Bai, Y. Changes of endogenous hormones in fruit and their effects on the fruit development of Camellia oleifera (in Chinese). J. For. Environ. 2015, 35, 331–336. [Google Scholar]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]




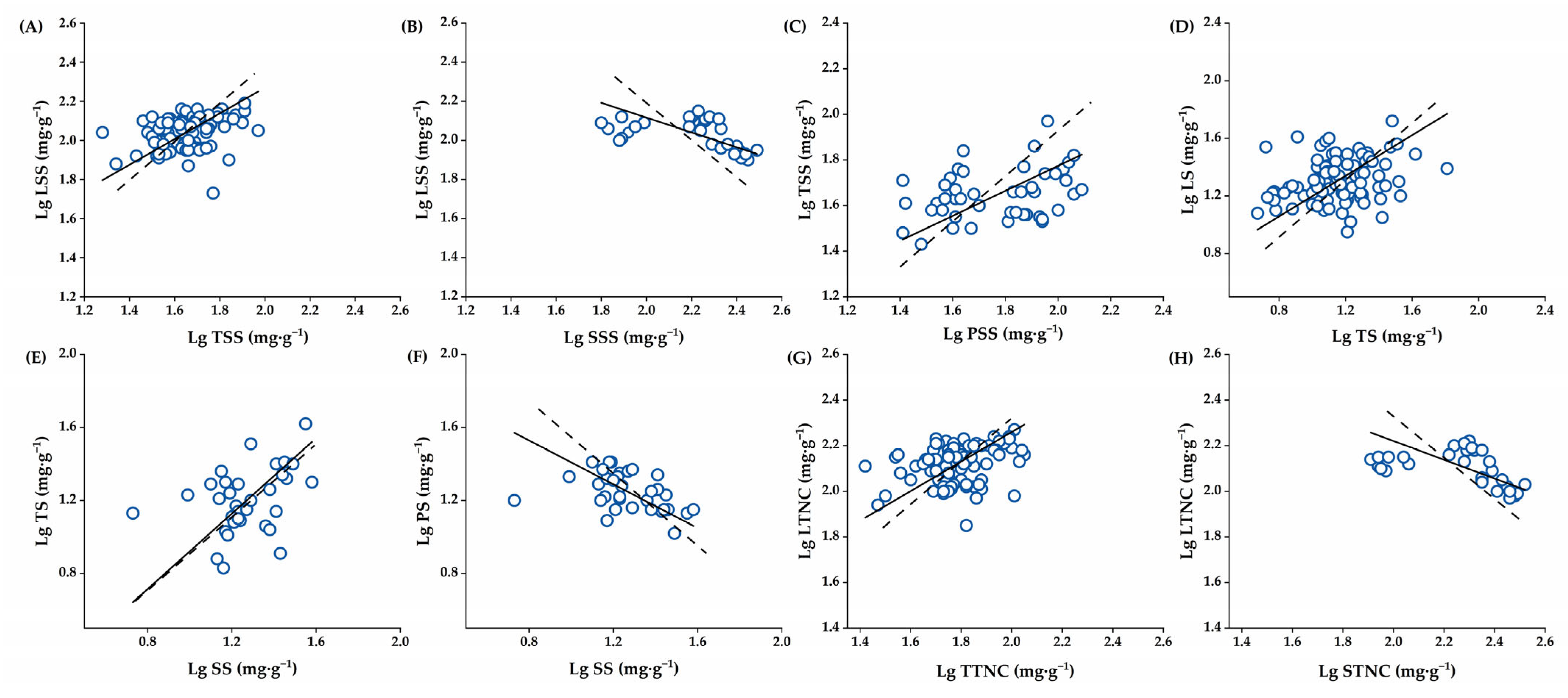

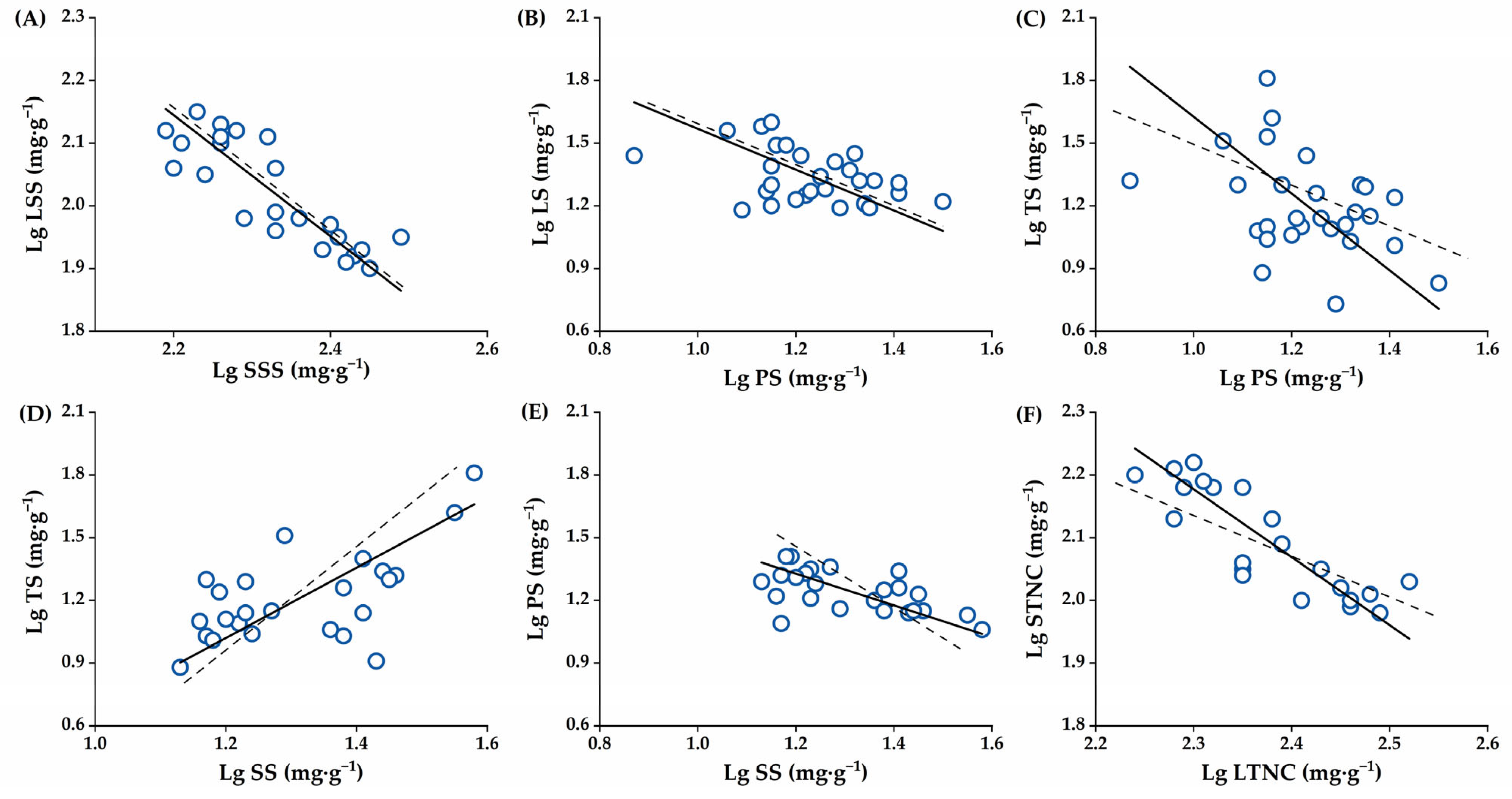
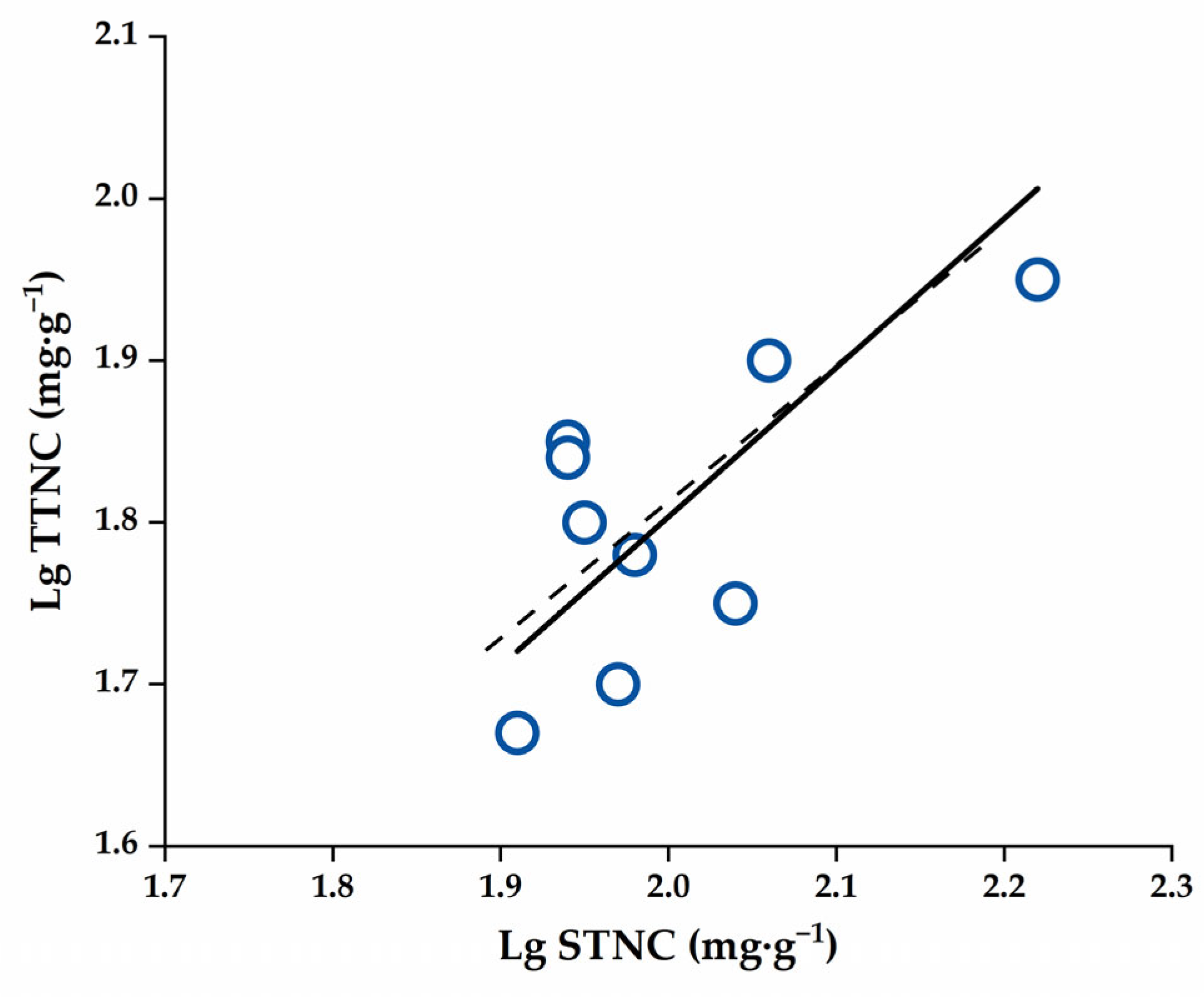

| Index | Organ (Y–X) | N | R2 | p | αSMA | 95% CI | βSMA | 95% CI | P1.0 |
|---|---|---|---|---|---|---|---|---|---|
| Soluble Sugar (mg·g−1) | SsLeaf-Twig | 87 | 0.089 | ** | 0.657 | (0.534, 0.806) | 0.955 | (0.731, 1.180) | ** |
| SsLeaf-Peel | 47 | 0.005 | ns | −0.433 | (−0.582, −0.322) | 2.807 | (2.574, 3.040) | ** | |
| SsLeaf-Seed | 33 | 0.229 | ** | −0.379 | (−0.520, −0.276) | 2.874 | (2.603, 3.146) | ** | |
| SsTwig-Peel | 47 | 0.116 | * | 0.548 | (0.415, 0.725) | 0.678 | (0.401, 0.956) | ** | |
| SsTwig-Seed | 33 | 0.034 | ns | −0.445 | (−0.633, −0.313) | 2.629 | (2.272, 2.987) | ** | |
| SsPeel-Seed | 33 | 0.087 | ns | −0.788 | (−1.111, −0.559) | 3.608 | (2.993, 4.223) | ns | |
| Starch (mg·g−1) | SLeaf-Twig | 87 | 0.056 | * | 0.705 | (0.572, 0.867) | 0.495 | (0.319, 0.670) | ** |
| SLeaf-Peel | 47 | 0.012 | ns | −0.685 | (−0.920, −0.511) | 2.113 | (1.870, 2.355) | * | |
| SLeaf-Seed | 33 | 0.052 | ns | 0.717 | (0.506, 1.017) | 0.405 | (0.076, 0.734) | ns | |
| STwig-Peel | 47 | 0.063 | ns | 1.091 | (0.819, 1.453) | −0.105 | (−0.480, 0.270) | ns | |
| STwig-Seed | 33 | 0.132 | * | 1.033 | (0.739, 1.444) | −0.113 | (−0.566, 0.341) | ns | |
| SPeel-Seed | 33 | 0.170 | * | −0.600 | (−0.833, −0.432) | 2.009 | (1.752, 2.266) | ** | |
| Total NSCs (mg·g−1) | TLeaf-Twig | 87 | 0.086 | ** | 0.648 | (0.528, 0.795) | 0.965 | (0.726, 1.204) | ** |
| TLeaf-Peel | 47 | 0.02 | ns | −0.441 | (−0.591, −0.329) | 2.942 | (2.695, 3.190) | ** | |
| TLeaf-Seed | 33 | 0.184 | * | −0.411 | (−0.568, −0.297) | 3.041 | (2.732, 3.351) | ** | |
| TTwig-Peel | 47 | 0.044 | ns | 0.571 | (0.428, 0.763) | 0.704 | (0.387, 1.020) | ** | |
| TTwig-Seed | 33 | 0.021 | ns | −0.462 | (−0.659, −0.324) | 2.829 | (2.448, 3.211) | ** | |
| TPeel-Seed | 33 | 0.098 | ns | −0.749 | (−1.054, −0.533) | 3.658 | (3.064, 4.251) | ns |
| Month | Index | Organ (Y–X) | N | R2 | p | αSMA | 95% CI | βSMA | 95% CI | P1.0 |
|---|---|---|---|---|---|---|---|---|---|---|
| 4–6 | Soluble Sugar (mg·g−1) | SsLeaf-Twig | 24 | 0.567 | ** | 0.394 | (0.296, 0.525) | 1.421 | (1.231, 1.611) | ** |
| SsLeaf-Peel | 10 | 0.473 | * | 0.552 | (0.315, 0.969) | 1.169 | (0.643, 1.694) | * | ||
| SsTwig-Peel | 10 | 0.074 | ns | 1.060 | (0.516, 2.179) | −0.018 | (−1.294, 1.258) | ns | ||
| Starch (mg·g−1) | SLeaf-Twig | 24 | 0.311 | ** | 0.719 | (0.502, 1.029) | 0.553 | (0.261, 0.844) | ns | |
| SLeaf-Peel | 10 | 0.009 | ns | 1.127 | (0.537, 2.366) | 0.212 | (−0.688, 1.111) | ns | ||
| STwig-Peel | 10 | 0.345 | ns | 1.184 | (0.637, 2.201) | −0.041 | (−0.714, 0.631) | ns | ||
| Total NSCs(mg·g−1) | TLeaf-Twig | 24 | 0.547 | ** | 0.425 | (0.317, 0.570) | 1.400 | (1.177, 1.623) | ** | |
| TLeaf-Peel | 10 | 0.302 | ns | 0.608 | (0.322, 1.150) | 1.098 | (0.394, 1.802) | ns | ||
| TTwig-Peel | 10 | 0.133 | ns | 1.121 | (0.556, 2.259) | −0.115 | (−1.492, 1.263) | ns |
| Month | Index | Organ (Y–X) | N | R2 | p | αSMA | 95% CI | βSMA | 95% CI | P1.0 |
|---|---|---|---|---|---|---|---|---|---|---|
| 7–9 | Soluble Sugar (mg·g−1) | SsLeaf-Twig | 47 | 0.005 | ns | 0.864 | (0.643, 1.161) | 0.589 | (0.159, 1.019) | ns |
| SsLeaf-Peel | 28 | 0.022 | ns | −0.632 | (−0.932, −0.428) | 3.162 | (2.706, 3.618) | * | ||
| SsLeaf-Seed | 24 | 0.720 | ** | −0.966 | (−1.218, −0.766) | 4.269 | (3.743, 4.794) | ns | ||
| SsTwig-Peel | 28 | 0.065 | ns | 0.700 | (0.478, 1.024) | 0.404 | (−0.091, 0.898) | ns | ||
| SsTwig-Seed | 24 | 0.098 | ns | −1.087 | (−1.636. −0.722) | 4.165 | (3.102, 5.227) | ns | ||
| SsPeel-Seed | 24 | 0.042 | ns | 1.838 | (1.207, 2.798) | −2.449 | (−4.300, −0.598) | ** | ||
| Starch (mg·g−1) | SLeaf-Twig | 47 | 0.005 | ns | 0.637 | (0.474, 0.855) | 0.571 | (0.340, 0.802) | ** | |
| SLeaf-Peel | 28 | 0.172 | * | −0.977 | (−1.399, −0.682) | 2.545 | (2.101, 2.990) | ns | ||
| SLeaf-Seed | 24 | 0.090 | ns | 0.854 | (0.567, 1.287) | 0.206 | (−0.271, 0.683) | ns | ||
| STwig-Peel | 28 | 0.155 | * | −1.838 | (−2.642, −1.279) | 3.465 | (2.619, 4.311) | ** | ||
| STwig-Seed | 24 | 0.370 | ** | 1.683 | (1.193, 2.375) | −1.000 | (−1.780, −0.220) | ** | ||
| SPeel-Seed | 24 | 0.341 | ** | −0.758 | (−1.078, −0.534) | 2.239 | (1.879, 2.598) | ns | ||
| Total NSCs (mg·g−1) | TLeaf-Twig | 47 | 0.003 | ns | 0.896 | (0.667, 1.205) | 0.495 | (0.011, 0.978) | ns | |
| TLeaf-Peel | 28 | 0.067 | ns | −0.695 | (−1.016, −0.475) | 3.433 | (2.914, 3.951) | ns | ||
| TLeaf-Seed | 24 | 0.742 | ** | −1.084 | (−1.355, −0.868) | 4.671 | (4.095, 5.248) | ns | ||
| TTwig-Peel | 28 | 0.000 | ns | 0.795 | (0.537, 1.178) | 0.288 | (−0.326, 0.903) | ns | ||
| TTwig-Seed | 24 | 0.068 | ns | −1.259 | (−1.906, −0.831) | 4.770 | (3.497, 6.043) | ns | ||
| TPeel-Seed | 24 | 0.015 | ns | 1.780 | (1.163, 2.724) | −2.286 | (−4.136, −0.436) | ** |
| Month | Index | Organ (Y–X) | N | R2 | p | αSMA | 95% CI | βSMA | 95% CI | P1.0 |
|---|---|---|---|---|---|---|---|---|---|---|
| 10–11 | Soluble Sugar (mg·g−1) | SsLeaf-Twig | 16 | 0.073 | ns | 0.590 | (0.348, 0.999) | 1.086 | (0.544, 1.628) | * |
| SsLeaf-Peel | 9 | 0.052 | ns | 0.449 | (0.204, 0.985) | 1.175 | (0.403, 1.946) | * | ||
| SsLeaf-Seed | 9 | 0.010 | ns | 0.338 | (0.152, 0.753) | 1.409 | (0.829, 1.990) | ** | ||
| SsTwig-Peel | 9 | 0.246 | ns | 1.080 | (0.529, 2.205) | −0.461 | (−2.116, 1.195) | ns | ||
| SsTwig-Seed | 9 | 0.243 | ns | 0.814 | (0.398, 1.663) | 0.103 | (−1.117, 1.324) | ns | ||
| SsPeel-Seed | 9 | 0.095 | ns | 0.754 | (0.348, 1.630) | 0.522 | (−0.714, 1.759) | ns | ||
| Starch (mg·g−1) | SLeaf-Twig | 16 | 0.059 | ns | 1.628 | (0.958, 2.768) | −0.744 | (−1.864, 0.377) | ns | |
| SLeaf-Peel | 9 | 0.081 | ns | −1.159 | (−2.520, −0.533) | 2.730 | (1.479, 3.982) | ns | ||
| SLeaf-Seed | 9 | 0.000 | ns | −0.703 | (−1.571, −0.315) | 2.087 | (1.347, 2.826) | ns | ||
| STwig-Peel | 9 | 0.006 | ns | 0.877 | (0.393, 1.957) | 0.124 | (−0.862, 1.110) | ns | ||
| STwig-Seed | 9 | 0.138 | ns | 0.532 | (0.249, 1.133) | 0.611 | (0.094, 1.128) | ns | ||
| SPeel-Seed | 9 | 0.108 | ns | −0.607 | (−1.306, −0.282) | 1.949 | (1.349, 2.548) | ns | ||
| Total NSCs (mg·g−1) | TLeaf-Twig | 16 | 0.116 | ns | 0.433 | (0.258, 0.725) | 1.355 | (0.934, 1.775) | ** | |
| TLeaf-Peel | 9 | 0.206 | ns | 0.337 | (0.163, 0.700) | 1.438 | (0.886, 1.990) | ** | ||
| TLeaf-Seed | 9 | 0.215 | ns | 0.252 | (0.122, 0.522) | 1.626 | (1.225, 2.026) | ** | ||
| TTwig-Peel | 9 | 0.122 | ns | 1.230 | (0.574, 2.635) | −0.721 | (−2.838, 1.395) | ns | ||
| TTwig-Seed | 9 | 0.451 | * | 0.921 | (0.494, 1.714) | −0.038 | (−1.259, 1.184) | ns | ||
| TPeel-Seed | 9 | 0.081 | ns | 0.748 | (0.344, 1.626) | 0.556 | (−0.728, 1.840) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xie, Y.; Zhang, Y.; Fang, X.; Wang, J. Trade-Offs and Partitioning Strategy of Carbon Source-Sink During Fruit Development of Camellia oleifera. Plants 2025, 14, 1920. https://doi.org/10.3390/plants14131920
Li Y, Xie Y, Zhang Y, Fang X, Wang J. Trade-Offs and Partitioning Strategy of Carbon Source-Sink During Fruit Development of Camellia oleifera. Plants. 2025; 14(13):1920. https://doi.org/10.3390/plants14131920
Chicago/Turabian StyleLi, Yueling, Yiqing Xie, Yue Zhang, Xuan Fang, and Jian Wang. 2025. "Trade-Offs and Partitioning Strategy of Carbon Source-Sink During Fruit Development of Camellia oleifera" Plants 14, no. 13: 1920. https://doi.org/10.3390/plants14131920
APA StyleLi, Y., Xie, Y., Zhang, Y., Fang, X., & Wang, J. (2025). Trade-Offs and Partitioning Strategy of Carbon Source-Sink During Fruit Development of Camellia oleifera. Plants, 14(13), 1920. https://doi.org/10.3390/plants14131920






