Stability Analysis and Multi-Trait Selection of Flowering Phenology Parameters in Olive Cultivars Under Multi-Environment Trials
Abstract
1. Introduction
2. Results
2.1. ANOVA and BLUP-Based Mean Performance of Flowering Traits Across Four Environments
2.2. BLUP-Based Variance Components and Genetic Parameters
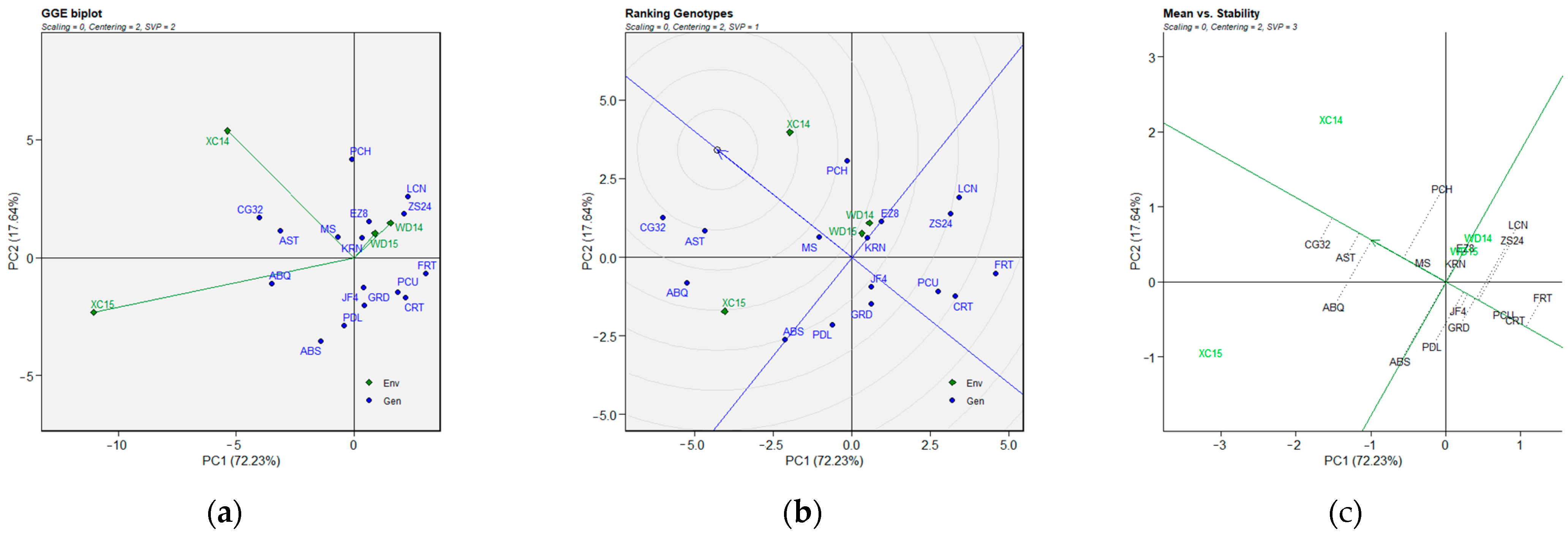
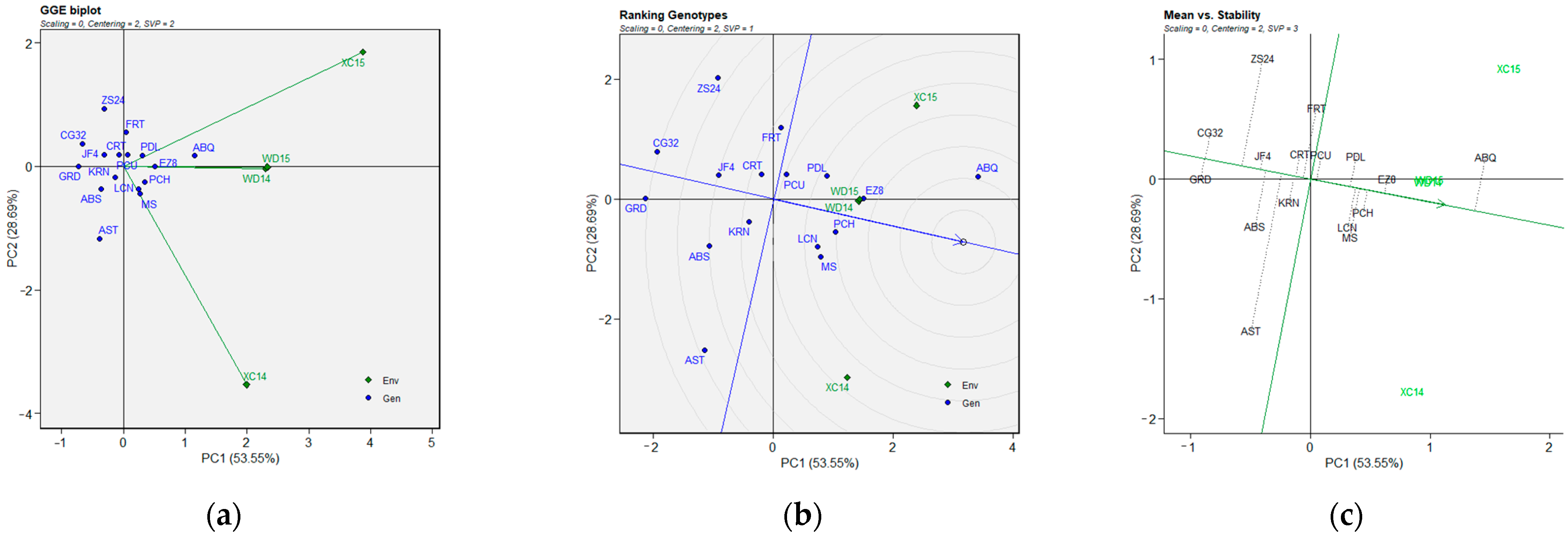
2.3. BLUP-Based GGE Biplot Analysis
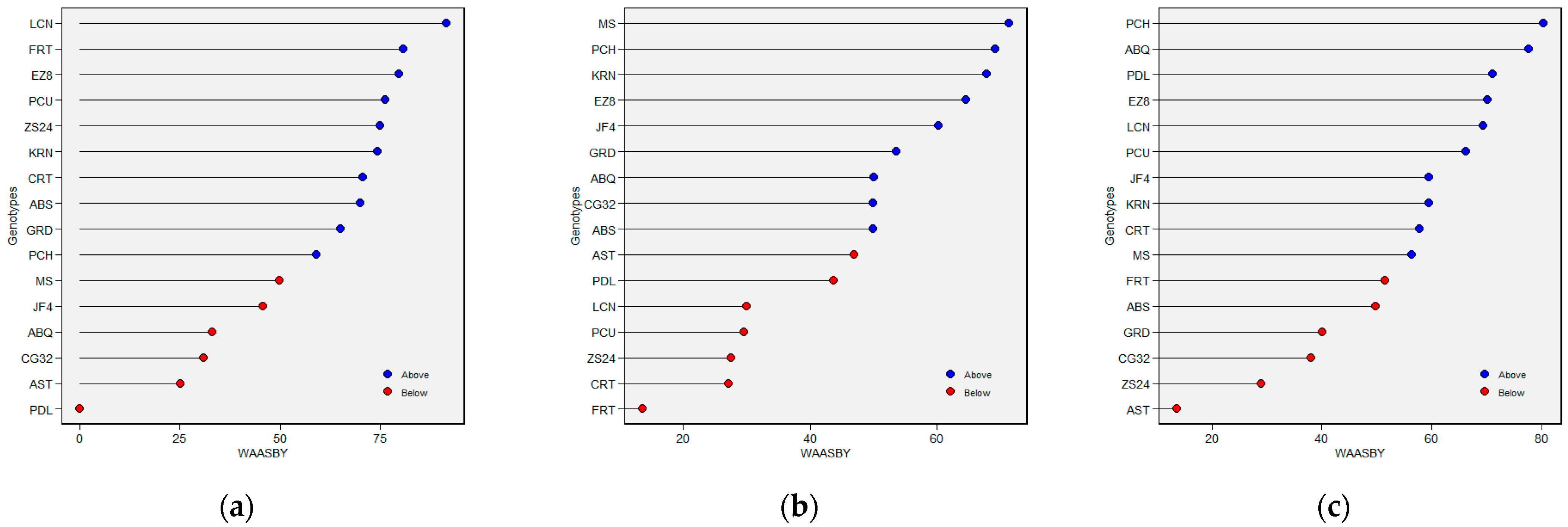
2.4. Selection of Olive Genotypes Based on MPS Index
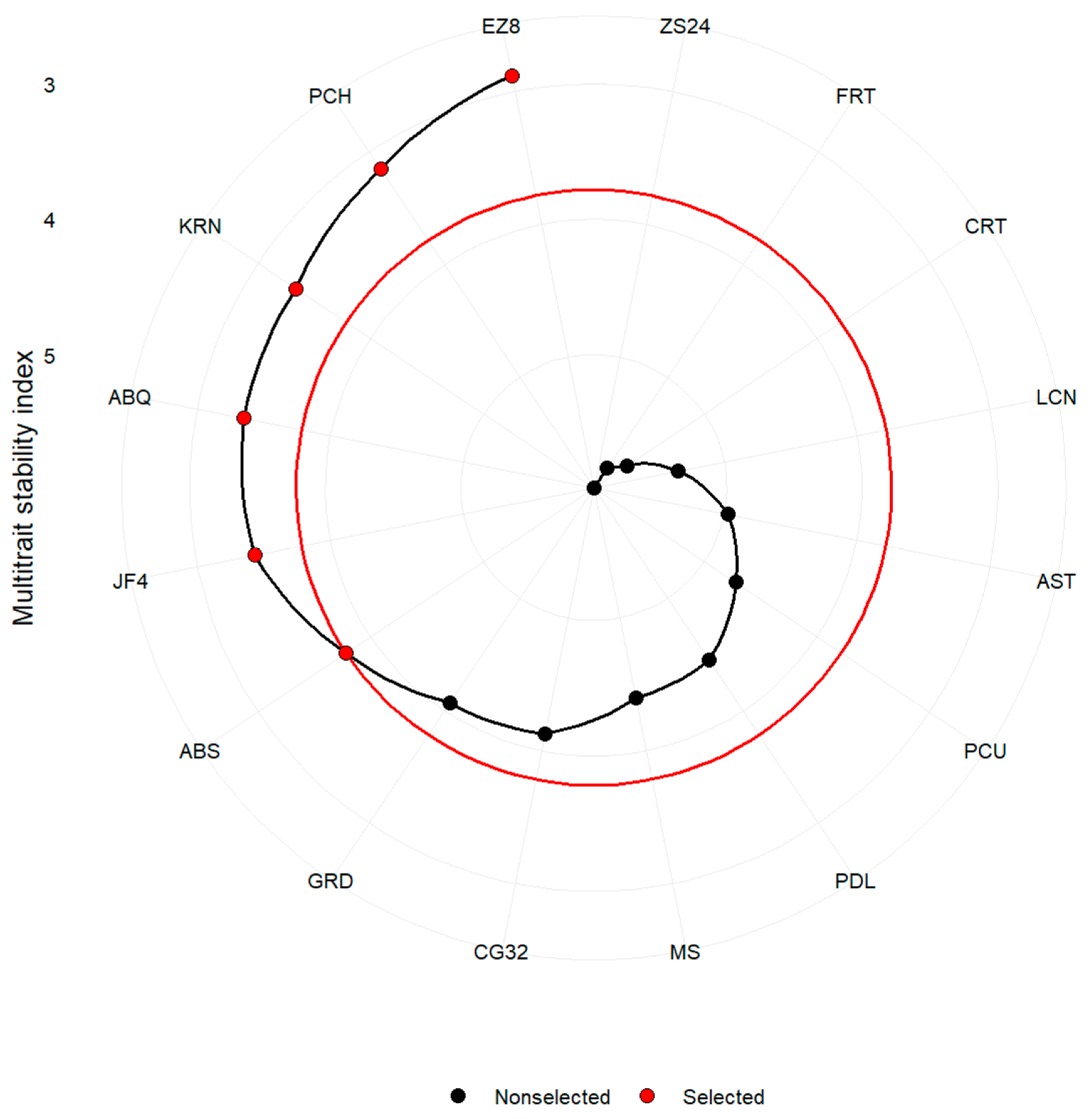
2.5. Multi-Trait Selection for Performance and Stability Based on MTMPS Index
3. Discussion
3.1. Effects of GEI and Genetic Parameters on Olive Flowering Phenology
3.2. Selection of Genotypes via MPS
3.3. Selection of Genotypes via Multi-Trait Index MTMPS
4. Materials and Methods
4.1. Plant Materials
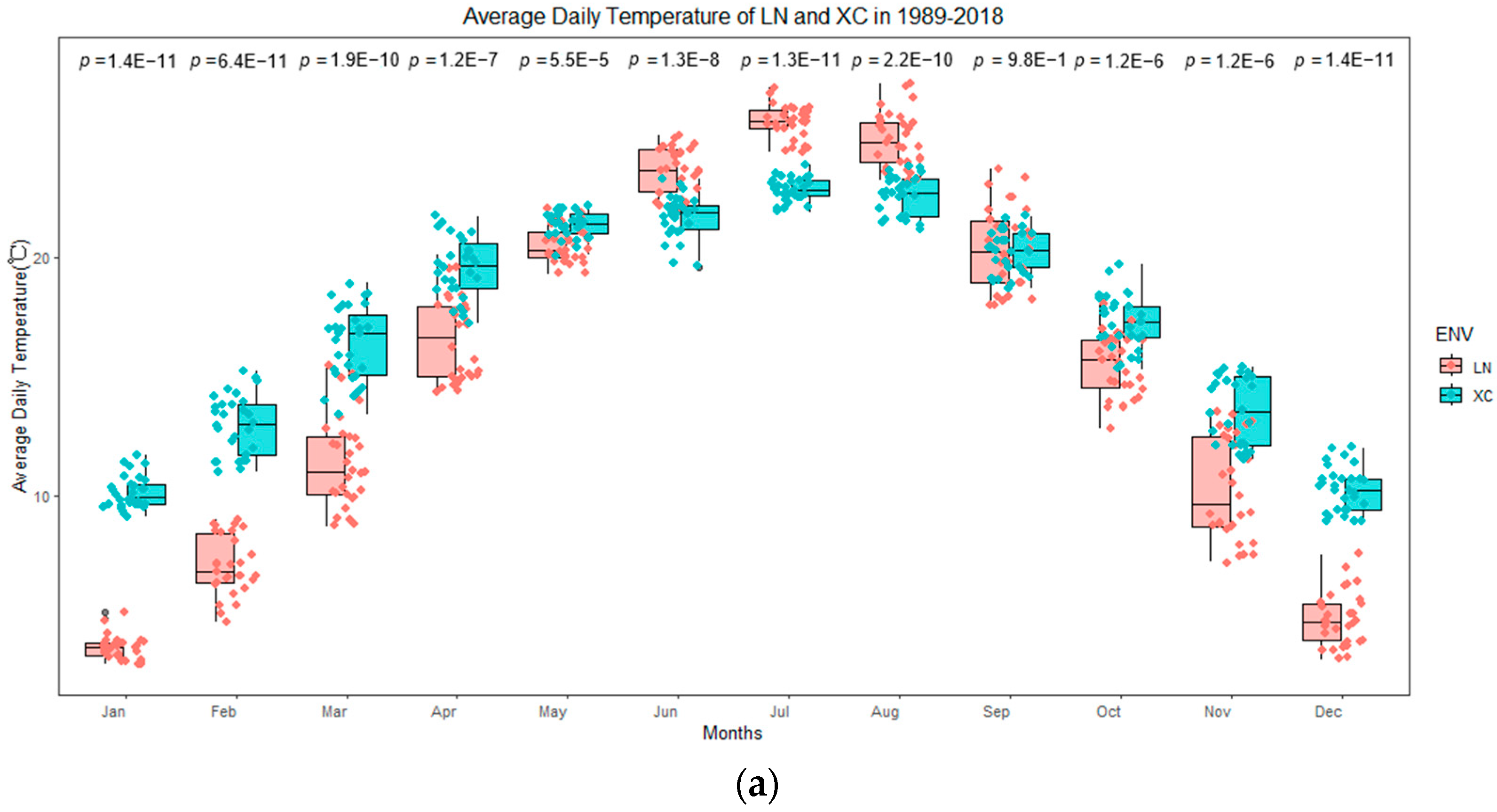
4.2. Experimental Sites and Design
4.3. Flowering Observations
- The length of the flowering period (FP): The number of days from when Stage 61 appeared to have begun to when Stage 68 (where the majority of petals have fallen) appeared to be the most common stage.
- The length of the full-bloom period (FBP): The number of days from when Stage 61 appeared to be the most common stage to when Stage 65 (full bloom, with at least 50% of flowers open) appeared to be the most common stage.
- The full-bloom date (FBD): The average Julian date of the start and end of the FBP, expressed as the DOY (day of the year).
4.4. Statistical Analysis
4.4.1. Variance Component Analysis and Genetic Parameters
4.4.2. GGE Biplot Analysis of BLUP
4.4.3. Mean Performance and Stability (MPS) of Single Traits
4.4.4. Mean Performance and Stability of Multiple Traits (MTMPS)
4.4.5. Selection Differentials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FBD | full-bloom date |
| FP | flowering-period length |
| FBP | full-bloom period length |
| BLUP | best linear unbiased prediction |
| MPS | mean performance and stability |
| MTMPS | multi-trait mean performance and stability |
| METs | multi-environment trials |
| REML | restricted maximum likelihood |
| LMM | linear mixed model |
| LRT | likelihood ratio test |
| WAAS | weighted average of the absolute scores |
| SVD | singular value decomposition |
| IPCAs | interaction principal component axes |
| SD | selection differential |
| GEI | genotype–environment interaction |
| ANOVA | analysis of variance |
| GGE | genotype + genotype by environment interaction |
References
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Hamze, L.M.; Trentacoste, E.R.; Searles, P.S.; Rousseaux, M.C. Spring Reproductive and Vegetative Phenology of Olive (Olea Europaea L.) Cultivars at Different Air Temperatures along a Latitudinal-Altitudinal Gradient in Argentina. Sci. Hortic. 2022, 304, 111327. [Google Scholar] [CrossRef]
- Brugnara, E.C.; Sabião, R.R. Olive Reproductive Phenology in a Warm and Humid Region of Santa Catarina, Brazil. Agrocienc. Urug. 2022, 26, e898. [Google Scholar] [CrossRef]
- Medina-Alonso, M.G.; Navas, J.F.; Cabezas, J.M.; Weiland, C.M.; Ríos-Mesa, D.; Lorite, I.J.; León, L.; La Rosa, R.D. Differences on Flowering Phenology under Mediterranean and Subtropical Environments for Two Representative Olive Cultivars. Environ. Exp. Bot. 2020, 180, 104239. [Google Scholar] [CrossRef]
- Conde-Innamorato, P.; Arias-Sibillotte, M.; Villamil, J.J.; Bruzzone, J.; Bernaschina, Y.; Ferrari, V.; Zoppolo, R.; Villamil, J.; Leoni, C. It Is Feasible to Produce Olive Oil in Temperate Humid Climate Regions. Front. Plant Sci. 2019, 10, 1544. [Google Scholar] [CrossRef]
- Lorite, I.J.; Gabaldón-Leal, C.; Ruiz-Ramos, M.; Belaj, A.; De La Rosa, R.; León, L.; Santos, C. Evaluation of Olive Response and Adaptation Strategies to Climate Change under Semi-Arid Conditions. Agric. Water Manag. 2018, 204, 247–261. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G. Environmental Stresses and Sustainable Olive Growing. Acta Hort. 2011, 924, 19–30. [Google Scholar] [CrossRef]
- Elloumi, O.; Ghrab, M.; Chatti, A.; Chaari, A.; Ben Mimoun, M. Phenological Performance of Olive Tree in a Warm Production Area of Central Tunisia. Sci. Hortic. 2020, 259, 108759. [Google Scholar] [CrossRef]
- Ben-Ari, G.; Biton, I.; Many, Y.; Namdar, D.; Samach, A. Elevated Temperatures Negatively Affect Olive Productive Cycle and Oil Quality. Agronomy 2021, 11, 1492. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Sánchez-Lucas, R.; Benlloch, M.; Ricardo, F.-E. An Approach to Global Warming Effects on Flowering and Fruit Set of Olive Trees Growing under Field Conditions. Sci. Hortic. 2018, 240, 405–410. [Google Scholar] [CrossRef]
- Medina-Alonso, M.G.; Cabezas, J.M.; Ríos-Mesa, D.; Lorite, I.J.; León, L.; De La Rosa, R. Flowering Phenology of Olive Cultivars in Two Climate Zones with Contrasting Temperatures (Subtropical and Mediterranean). Agriculture 2023, 13, 1312. [Google Scholar] [CrossRef]
- Didevarasl, A.; Costa Saura, J.M.; Spano, D.; Deiana, P.; Snyder, R.L.; Mulas, M.; Nieddu, G.; Zelasco, S.; Santona, M.; Trabucco, A. Modeling Phenological Phases across Olive Cultivars in the Mediterranean. Plants 2023, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- Houssam-eddine, B.; Osama, K.; Meryem, E.; Halima, H.; Eike, L.; Mehdi Ben, M.; Mohamed, G.; Ahmed El, B.; Hakim, O.; Jamal, C.; et al. Phenological Response of Olive Cultivars to Climate Variation in Morocco. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Garcia, R.; Cabrita, M.J.; Dias, A.B. Olive Growing Systems, Varieties and Olive Oil Production Systems. In The Olive Landscapes of the Mediterranean: Key Challenges and Opportunities for their Sustainability in the Early XXIst Century; Muñoz-Rojas, J., García-Ruiz, R., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 303–314. ISBN 978-3-031-57956-1. [Google Scholar]
- Lavee, S.; Rallo, L.; Rapoport, H.F.; Troncoso, A. The Floral Biology of the Olive: Effect of Flower Number, Type and Distribution on Fruitset. Sci. Hortic. 1996, 66, 149–158. [Google Scholar] [CrossRef]
- Biton, I.; Many, Y.; Mazen, A.; Ben-Ari, G. Compatibility between “Arbequina” and “Souri” Olive Cultivars May Increase Souri Fruit Set. Agronomy 2020, 10, 910. [Google Scholar] [CrossRef]
- Didevarasl, A.; Costa-Saura, J.M.; Spano, D.; Deiana, P.; Snyder, R.L.; Rechid, D.; Bülow, K.; Mulas, M.; Nieddu, G.; Trabucco, A. The Phenological Phases of Early and Mid-Late Budbreak Olive Cultivars in a Changing Future Climate over the Euro-Mediterranean Region. Eur. J. Agron. 2025, 168, 127658. [Google Scholar] [CrossRef]
- Garcia-Mozo, H.; Orlandi, F.; Galan, C.; Fornaciari, M.; Romano, B.; Ruiz, L.; Diaz De La Guardia, C.; Trigo, M.M.; Chuine, I. Olive Flowering Phenology Variation between Different Cultivars in Spain and Italy: Modeling Analysis. Theor. Appl. Climatol. 2009, 95, 385–395. [Google Scholar] [CrossRef]
- De Melo-Abreu, J. Modelling Olive Flowering Date Using Chilling for Dormancy Release and Thermal Time. Agric. For. Meteorol. 2004, 125, 117–127. [Google Scholar] [CrossRef]
- Alcalá, A.R.; Barranco, D. Prediction of Flowering Time in Olive for the Cordoba Olive Collection. HortSci 1992, 27, 1205–1207. [Google Scholar] [CrossRef]
- Abou-Saaid, O.; El Yaacoubi, A.; Moukhli, A.; El Bakkali, A.; Oulbi, S.; Delalande, M.; Farrera, I.; Kelner, J.-J.; Lochon-Menseau, S.; El Modafar, C.; et al. Statistical Approach to Assess Chill and Heat Requirements of Olive Tree Based on Flowering Date and Temperatures Data: Towards Selection of Adapted Cultivars to Global Warming. Agronomy 2022, 12, 2975. [Google Scholar] [CrossRef]
- Belaj, A.; De La Rosa, R.; León, L.; Gabaldón-Leal, C.; Santos, C.; Porras, R.; De La Cruz-Blanco, M.; Lorite, I.J. Phenological Diversity in a World Olive Germplasm Bank: Potential Use for Breeding Programs and Climate Change Studies. Span. J. Agric. res. 2020, 18, e0701. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Martos-García, I.; Benlloch, M.; Fernández-Escobar, R. High Temperatures Have a Different Effect on Vegetative and Reproductive Processes of ‘Picual’ and ‘Arbequina’ Olive in Spain. Sci. Hortic. 2024, 337, 113560. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, P.; Xie, J.; Zhao, G.; Wei, Z. Advances in the Pollination Biology of Olive (Olea Europaea L.). Acta Ecol. Sin. 2013, 33, 64–71. [Google Scholar] [CrossRef]
- Baldoni, L.; Belaj, A. Olive. In Oil Crops; Springer: New York, NY, USA, 2009; pp. 397–421. [Google Scholar] [CrossRef]
- Navas-Lopez, J.F.; León, L.; Rapoport, H.F.; Moreno-Alías, I.; Lorite, I.J.; De La Rosa, R. Genotype, Environment and Their Interaction Effects on Olive Tree Flowering Phenology and Flower Quality. Euphytica 2019, 215, 184. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; De La Rosa, R.; Rapoport, H.F. Floral Quality Components of a New Olive Cultivar and Its Parents. Sci. Hortic. 2013, 154, 17–19. [Google Scholar] [CrossRef]
- Aqbouch, L.; Zunino, L.; Abou-Saaid, O.; Sarah, G.; Mournet, P.; El Bakkali, A.; Zaher, H.; Costes, E.; Cubry, P.; Khadari, B. Characterization of an Olive Population Relevant for Genome-Wide Association Studies on Flowering Time. Acta Hortic. 2024, 1412, 129–136. [Google Scholar] [CrossRef]
- Aqbouch, L.; Abou-Saaid, O.; Sarah, G.; Zunino, L.; Segura, V.; Mournet, P.; Bonal, F.; Zaher, H.; El Bakkali, A.; Cubry, P.; et al. Genome-Wide Association Analysis of Flowering Date in a Collection of Cultivated Olive Tree. Hortic. Res. 2025, 12, uhae265. [Google Scholar] [CrossRef]
- Lamoumni, O.; Bakkali, A.E.; Abou-Saaid, O.; Cubry, P.; Leon, L.; Delalande, M.; Mournet, P.; Droc, G.; Sarah, G.; Zaher, H.; et al. Unraveling the Genetic Basis of Full Flowering Date in Olive Tree through QTL Mapping Approach: Towards Climate-Adaptive Breeding. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Mancuso, S. Phenology Modeling and Forecasting in Olive (Olea Europaea L.) Using Artificial Neural Networks. Adv. Hortic. Sci. 2002, 16, 3–4. [Google Scholar] [CrossRef]
- Rapoport, H.F. The Reproductive Biology of the Olive Tree and Its Relationship to Extreme Environmental Conditions. Acta Hortic. 2014, 1057, 41–50. [Google Scholar] [CrossRef]
- Orlandi, F.; Garcia-Mozo, H.; Galán, C.; Romano, B.; De La Guardia, C.D.; Ruiz, L.; Del Mar Trigo, M.; Dominguez-Vilches, E.; Fornaciari, M. Olive Flowering Trends in a Large Mediterranean Area (Italy and Spain). Int. J. Biometeorol. 2010, 54, 151–163. [Google Scholar] [CrossRef]
- Bonofiglio, T.; Orlandi, F.; Sgromo, C.; Romano, B.; Fornaciari, M. Influence of Temperature and Rainfall on Timing of Olive (Olea Europaea) Flowering in Southern Italy. New Zealand J. Crop Hortic. Sci. 2008, 36, 59–69. [Google Scholar] [CrossRef]
- Osborne, C.P.; Chuine, I.; Viner, D.; Woodward, F.I. Olive Phenology as a Sensitive Indicator of Future Climatic Warming in the Mediterranean. Plant Cell Environ. 2000, 23, 701–710. [Google Scholar] [CrossRef]
- Sanz-Cortés, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological Growth Stages of Olive Trees (Olea Europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Navas-López, J.F.; León, L.; Rapoport, H.F.; Moreno-Alías, I.; Medina, M.G.; Santos, C.; Porras, R.; Lorite, I.J.; De La Rosa, R. Flowering Phenology and Flower Quality of Cultivars ‘Arbequina’, ‘Koroneiki’ and ‘Picual’ in Different Environments of Southern Spain. Acta Hortic. 2018, 1229, 257–262. [Google Scholar] [CrossRef]
- Sampaio Filho, J.S.; Olivoto, T.; Campos, M.D.S.; Oliveira, E.J.D. Multi-Trait Selection in Multi-Environments for Performance and Stability in Cassava Genotypes. Front. Plant Sci. 2023, 14, 1282221. [Google Scholar] [CrossRef] [PubMed]
- Isik, F.; Holland, J.; Maltecca, C.; Isik, F.; Holland, J.; Maltecca, C. Multi Environmental Trials. In Genetic Data Analysis for Plant and Animal Breeding; Springer: Cham, Switzerland, 2017; pp. 227–262. [Google Scholar] [CrossRef]
- Yue, H.; Olivoto, T.; Bu, J.; Li, J.; Wei, J.; Xie, J.; Chen, S.; Peng, H.; Nardino, M.; Jiang, X. Multi-Trait Selection for Mean Performance and Stability of Maize Hybrids in Mega-Environments Delineated Using Envirotyping Techniques. Front. Plant Sci. 2022, 13, 1030521. [Google Scholar] [CrossRef]
- De Leon, N.; Jannink, J.; Edwards, J.W.; Kaeppler, S.M. Introduction to a Special Issue on Genotype by Environment Interaction. Crop Science 2016, 56, 2081–2089. [Google Scholar] [CrossRef]
- Yan, W. Analysis and Handling of G × E in a Practical Breeding Program. Crop Science 2016, 56, 2106–2118. [Google Scholar] [CrossRef]
- Sellami, M.H.; Di Mola, I.; Ottaiano, L.; Cozzolino, E.; De Vita, P.; Mori, M. Assessing Temporal Variability in Durum Wheat Performance and Stability through Multi-Trait Mean Performance Selection in Mediterranean Climate. Front. Agron. 2024, 6, 1466040. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Khalili, M.; Poczai, P.; Olivoto, T. Stability Indices to Deciphering the Genotype-by-Environment Interaction (GEI) Effect: An Applicable Review for Use in Plant Breeding Programs. Plants 2022, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Olivoto, T.; Lúcio, A.D.C.; Da Silva, J.A.G.; Sari, B.G.; Diel, M.I. Mean Performance and Stability in Multi-environment Trials II: Selection Based on Multiple Traits. Agron. J. 2019, 111, 2961–2969. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists. In GGE Biplot Analysis; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Garcia-Mozo, H.; Oteros, J.; Galan, C. Phenological Changes in Olive (Ola Europaea L.) Reproductive Cycle in Southern Spain Due to Climate Change. Ann Agric Environ Med. 2015, 22, 421–428. [Google Scholar] [CrossRef]
- Aybar, V.E.; De Melo-Abreu, J.P.; Searles, P.S.; Matias, A.C.; Del Rio, C.; Caballero, J.M.; Rousseaux, M.C. Evaluation of Olive Flowering at Low Latitude Sites in Argentina Using a Chilling Requirement Model. Span. J. Agric. Res. 2015, 13, e0901. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Sanjani, S.; Nikkhah-Chamanabad, H.; Mehrvar, M.R.; Asadi, A.; Amini, A. Identification of Salt-Tolerant Barley Genotypes Using Multiple-Traits Index and Yield Performance at the Early Growth and Maturity Stages. Bull Natl Res Cent 2021, 45, 117. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot Analysis of Multi-Environment Trial Data: Principles and Applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; Da Silva, J.A.G.; Marchioro, V.S.; De Souza, V.Q.; Jost, E. Mean Performance and Stability in Multi-environment Trials I: Combining Features of AMMI and BLUP Techniques. Agron. J. 2019, 111, 2949–2960. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M.; Meira, D.; Meier, C.; Follmann, D.N.; De Souza, V.Q.; Konflanz, V.A.; Baretta, D. Multi-trait Selection for Mean Performance and Stability in Maize. Agron. J. 2021, 113, 3968–3974. [Google Scholar] [CrossRef]
- Koundinya, A.V.V.; Ajeesh, B.R.; Vivek, H.; Sheela, M.N.; Mohan, C.; Asha, K.I. Genetic Parameters, Stability and Selection of Cassava Genotypes between Rainy and Water Stress Conditions Using AMMI, WAAS, BLUP and MTSI. Sci. Hortic. 2021, 281, 109949. [Google Scholar] [CrossRef]
- Nataraj, V.; Bhartiya, A.; Singh, C.P.; Devi, H.N.; Deshmukh, M.P.; Verghese, P.; Singh, K.; Mehtre, S.P.; Kumari, V.; Maranna, S.; et al. WAASB-based Stability Analysis and Simultaneous Selection for Grain Yield and Early Maturity in Soybean. Agronomy J. 2021, 113, 3089–3099. [Google Scholar] [CrossRef]
- Olivoto, T.; Diel, M.I.; Schmidt, D.; Lúcio, A.D. MGIDI: A Powerful Tool to Analyze Plant Multivariate Data. Plant Methods 2022, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Deng, M.; Yu, N. Study on the Regions of China Adaptable to Olive Growing. Olivae 1998, 70, 19–31. [Google Scholar]
- He, S.; Gu, Y.; Liu, L. Olive Introduction and Breeding in China. I. The Theory and Practice of Olive Introduction in China. Riv. Ortoflorofruttic. Italiana 1981, 65, 413–432. [Google Scholar]
- Wang, J.; Zhang, D.; Farooqi, T.J.A.; Ma, L.; Deng, Y.; Jia, Z. The Olive (Olea Europaea L.) Industry in China: Its Status, Opportunities and Challenges. Agroforest Syst 2019, 93, 395–417. [Google Scholar] [CrossRef]
- Su, C.; Sun, J.; Zhu, W.; Peng, L. History, Distribution, and Potential of the Olive Industry in China: A Review. Sustainability 2018, 10, 1426. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; Rapoport, H.F.; Martins, P.C. Morphological Limitations in Floral Development among Olive Tree Cultivars. Acta Hortic. 2012, 23–27. [Google Scholar] [CrossRef]
- Reale, L.; Sgromo, C.; Bonofiglio, T.; Orlandi, F.; Fornaciari, M.; Ferranti, F.; Romano, B. Reproductive Biology of Olive (Olea Europaea L.) DOP Umbria Cultivars. Sex Plant Reprod. 2006, 19, 151–161. [Google Scholar] [CrossRef]
- Marco, F.; Fabio, O.; Emma, T. Long Term Analysis on Olive Flowering and Climatic Relationships in Central Italy. Eur. J. Agron. 2025, 162, 127435. [Google Scholar] [CrossRef]
- Rojo, J.; Pérez-badia, R. Effects of Topography and Crown-Exposure on Olive Tree Phenology. Trees 2014, 28, 449–459. [Google Scholar] [CrossRef]
- Mousavi, S.; De La Rosa, R.; Moukhli, A.; El Riachy, M.; Mariotti, R.; Torres, M.; Pierantozzi, P.; Stanzione, V.; Mastio, V.; Zaher, H.; et al. Plasticity of Fruit and Oil Traits in Olive among Different Environments. Sci. Rep. 2019, 9, 16968. [Google Scholar] [CrossRef] [PubMed]
- Olivoto, T.; Lúcio, A.D. Metan: An R Package for Multi-environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.0; R Foundation for Statistical Computing: Vienna, Austria, 2024.
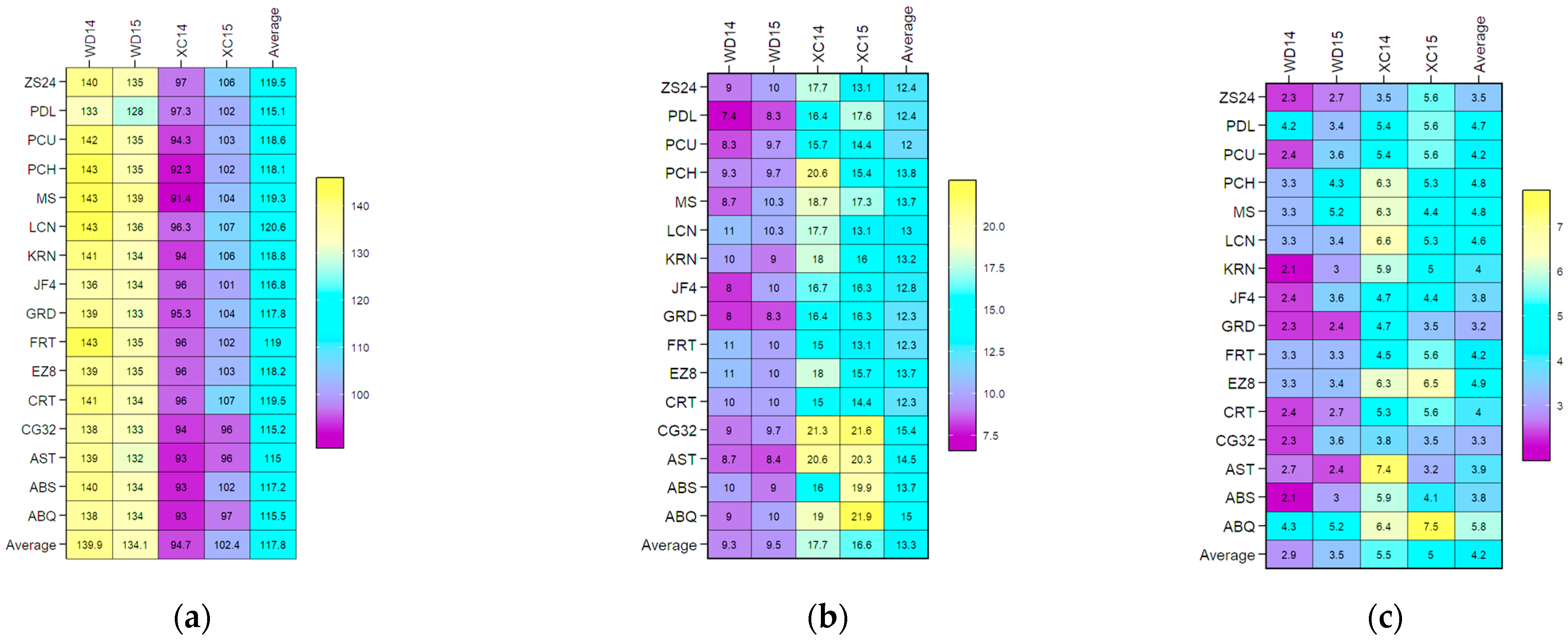






| Source of Variation | df | FBD | FP | FBP |
|---|---|---|---|---|
| ENV | 3 | 2.45 × 104 *** | 969.924 *** | 76.102 *** |
| REP(/ENV) | 8 | 3.80 × 10−1 *** | 0.193 | 0.922 *** |
| GEN | 15 | 3.72 × 101 *** | 13.065 *** | 6.175 *** |
| GEN:ENV (GEI) | 45 | 1.57 × 101 *** | 10.379 *** | 2.262 *** |
| Residuals | 120 | 8.02 × 10−2 | 0.170 | 0.244 |
| CV (%) | 0.241 | 3.11 | 11.70 |
| Parameters | FBD | FP | FBP |
|---|---|---|---|
| PV | 7.08 | 3.80 | 1.24 |
| heritability | 0.253 | 0.0589 | 0.262 |
| GEIr2 | 0.736 | 0.896 | 0.541 |
| 0.578 | 0.206 | 0.634 | |
| AS | 0.760 | 0.453 | 0.796 |
| rge | 0.985 | 0.952 | 0.734 |
| CVg | 1.14 | 3.56 | 13.5 |
| CVr | 0.241 | 3.11 | 11.7 |
| CV ratio | 4.72 | 1.15 | 1.16 |
| Mean Performance | WAASBY | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Factor | Xo | Xs | SD | SD% | Sense | Goal | Factor | Xo | Xs | SD | SD% |
| FP | FA1 | 13.28 | 13.69 | 0.413 | 3.11 | Increase | 100 | FA1 | 47.2 | 60.4 | 13.1 | 27.8 |
| FBP | FA1 | 4.22 | 4.53 | 0.304 | 7.19 | Increase | 100 | FA1 | 55.6 | 66.2 | 10.5 | 18.9 |
| FBD | FA2 | 117.7 | 117.4 | −0.299 | −0.25 | Decrease | 100 | FA2 | 58.3 | 66.1 | 7.80 | 13.4 |
| Cultivar Name | Origin Country | Genotype Code | Cultivar Name | Origin Country | Genotype Code |
|---|---|---|---|---|---|
| Arbequina | Spain | ABQ | Leccino | Italy | LCN |
| Arbosana | Spain | ABS | Pendolino | Italy | PDL |
| Gordal | Spain | GRD | Koroneiki | Greece | KRN |
| Manzanilla de Sevilla | Spain | MS | Picholine | France | PCH |
| Picual | Spain | PCU | Chenggu32 | Seedling selection | CG32 |
| Ascolano Tenera | Italy | AST | Ezhi8 | Seedling selection | EZ8 |
| Coratina | Italy | CRT | Jiufeng4 | Seedling selection | JF4 |
| Frantoio | Italy | FRT | Zhongshan24 | Seedling selection | ZS24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jia, D.; Zhou, Z.; Du, J.; Xiao, Q.; Cao, M. Stability Analysis and Multi-Trait Selection of Flowering Phenology Parameters in Olive Cultivars Under Multi-Environment Trials. Plants 2025, 14, 1906. https://doi.org/10.3390/plants14131906
Li J, Jia D, Zhou Z, Du J, Xiao Q, Cao M. Stability Analysis and Multi-Trait Selection of Flowering Phenology Parameters in Olive Cultivars Under Multi-Environment Trials. Plants. 2025; 14(13):1906. https://doi.org/10.3390/plants14131906
Chicago/Turabian StyleLi, Jinhua, Dongxu Jia, Zhenyuan Zhou, Jincheng Du, Qiangang Xiao, and Mingrong Cao. 2025. "Stability Analysis and Multi-Trait Selection of Flowering Phenology Parameters in Olive Cultivars Under Multi-Environment Trials" Plants 14, no. 13: 1906. https://doi.org/10.3390/plants14131906
APA StyleLi, J., Jia, D., Zhou, Z., Du, J., Xiao, Q., & Cao, M. (2025). Stability Analysis and Multi-Trait Selection of Flowering Phenology Parameters in Olive Cultivars Under Multi-Environment Trials. Plants, 14(13), 1906. https://doi.org/10.3390/plants14131906






