Unveiling the Biological Function of Phyllostachys edulis FBA6 (PeFBA6) through the Identification of the Fructose-1,6-Bisphosphate Aldolase Gene
Abstract
1. Introduction
2. Results
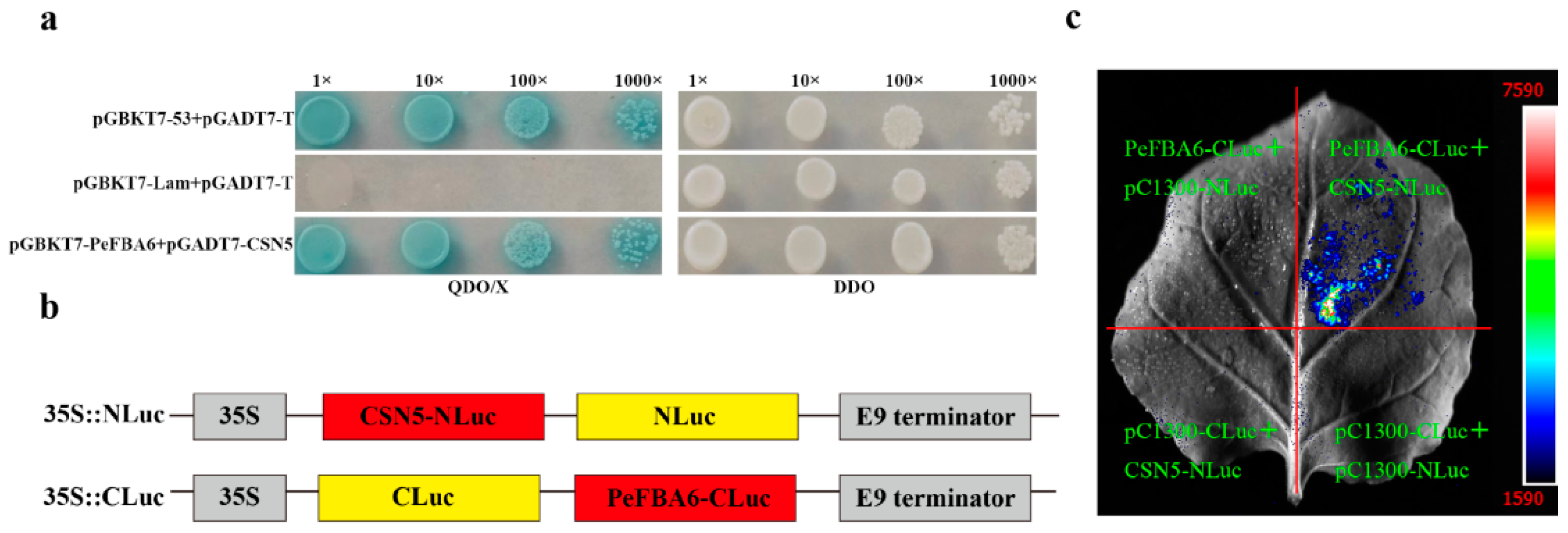
2.1. Verification of the Interaction between CSN5 and PeFBA6
2.2. Determination of Cytoplasmic FBA and Chloroplast FBA Enzyme Activities
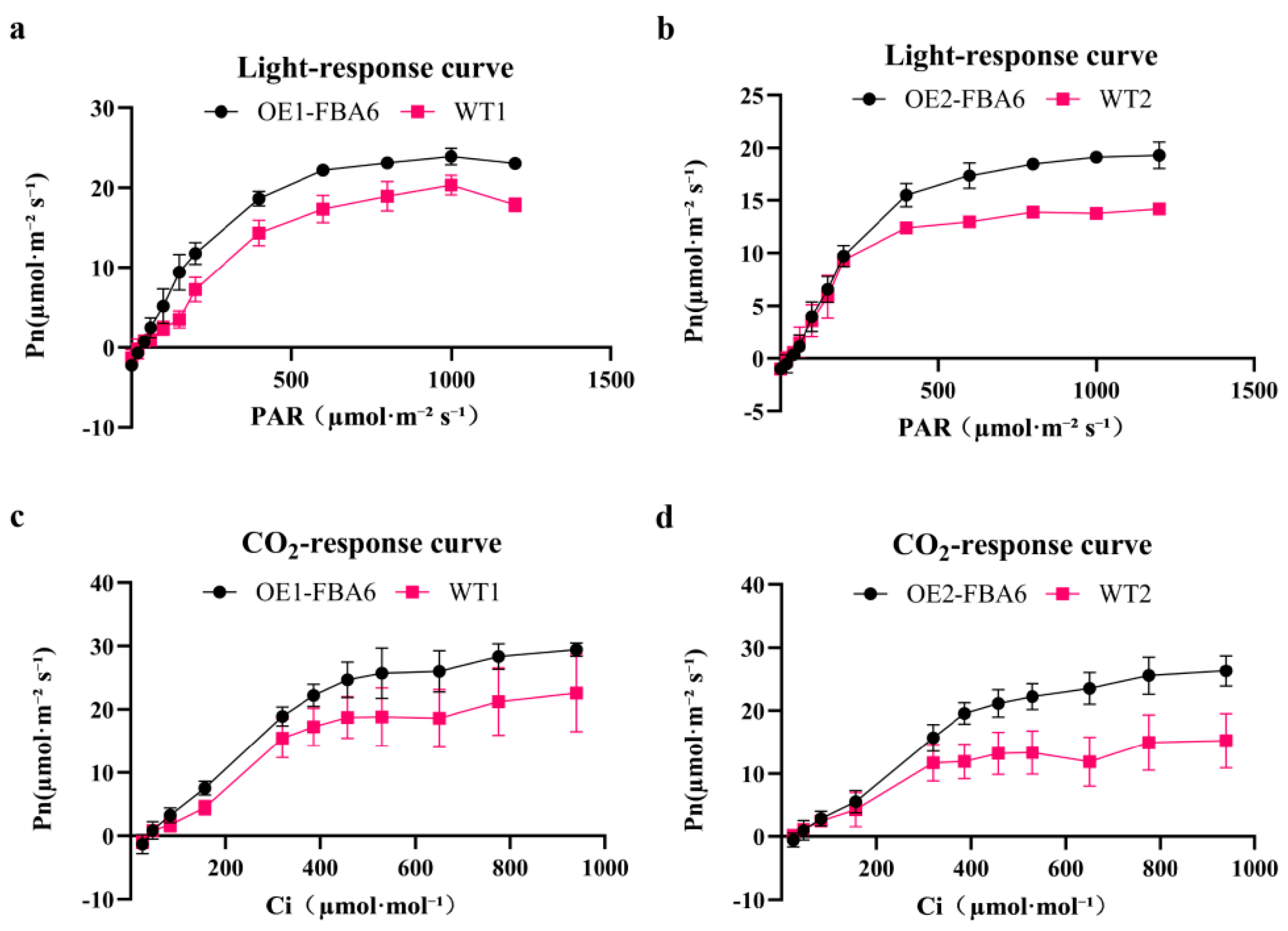
2.3. Response Curves of WT and Overexpressed Rice
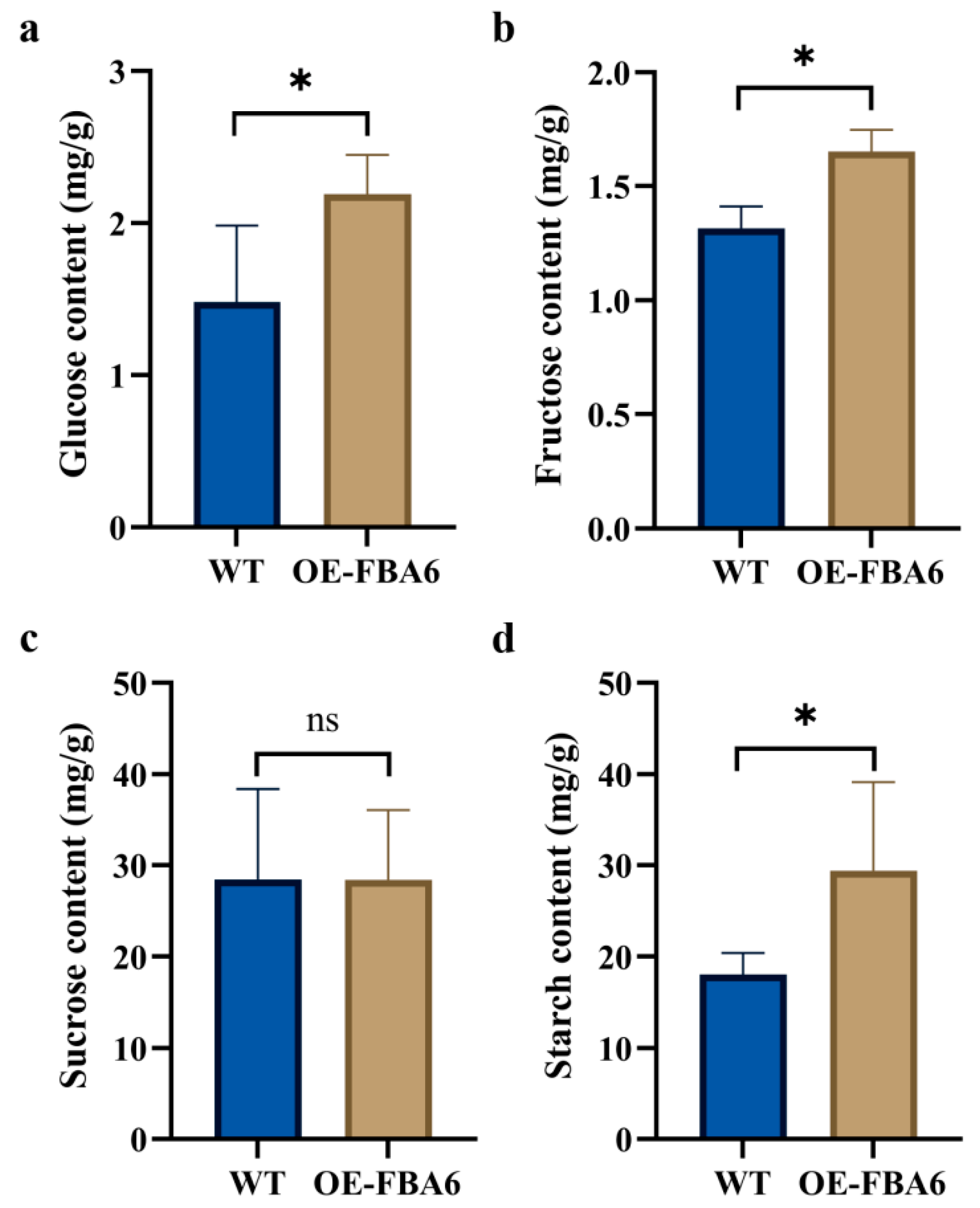
2.4. Glucose, Fructose, Sucrose, and Starch Contents
3. Discussion
4. Materials and Methods
4.1. Construction of a Rice Genetic Transformation Vector
4.2. Screening of Putative Interaction Proteins of PeFBA6
4.3. Yeast Two-Hybrid (Y2H) Assay
4.4. Imaging of Firefly Luciferase Complementation
4.5. Measurement of the Rice Light Response Curve and the CO2 Response Curve
4.6. Determination of Glucose, Fructose, and Sucrose Contents
4.7. Determination of Starch Content
4.8. FBA Enzyme Activity Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef] [PubMed]
- Plaxton, W.C. The organization and regulation of plant glycolysis. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef]
- Ronimus, R.S.; Morgan, H.W. Distribution and phylogenies of enzymes of the Embden-Meyerhof-Parnas pathway from archaea and hyperthermophilic bacteria support a gluconeogenic origin of metabolism. Archaea 2003, 1, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Hers, H.G.; Hue, L. Gluconeogenesis and related aspects of glycolysis. Annu. Rev. Biochem. 1983, 52, 617–653. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Plaxton, W.C. Multifaceted functions of post-translational enzyme modifications in the control of plant glycolysis. Curr. Opin. Plant Biol. 2020, 55, 28–37. [Google Scholar] [CrossRef]
- Schulze-Niemand, E.; Naumann, M. The COP9 signalosome: A versatile regulatory hub of Cullin-RING ligases. Trends Biochem. Sci. 2023, 48, 82–95. [Google Scholar] [CrossRef]
- Qin, N.X.; Xu, D.Q.; Li, J.G.; Deng, X.W. COP9 signalosome: Discovery, conservation, activity, and function. J. Integr. Plant Biol. 2020, 62, 90–103. [Google Scholar] [CrossRef]
- Krassikova, L.; Zhang, B.X.; Nagarajan, D.; Queiroz, A.L.; Kacal, M.; Samakidis, E.; Vakifahmetoglu-Norberg, H.; Norberg, E. The deubiquitinase JOSD2 is a positive regulator of glucose metabolism. Cell Death Differ. 2021, 28, 1091–1109. [Google Scholar] [CrossRef]
- Fushinobu, S.; Nishimasu, H.; Hattori, D.; Song, H.J.; Wakagi, T. Structural basis for the bifunctionality of fructose-1,6-bisphosphate aldolase/phosphatase. Nature 2011, 478, 538–541. [Google Scholar] [CrossRef]
- Haake, V.; Zrenner, R.; Sonnewald, U.; Stitt, M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998, 14, 147–157. [Google Scholar] [CrossRef]
- Raines, C.A. The Calvin cycle revisited. Photosynth. Res. 2003, 75, 1–10. [Google Scholar] [CrossRef]
- Guo, L.H.; Cheng, W.; Wang, D.B. Discussion on several problems in the teaching of “Calvin Cycle”. Plant Physiol. J. 2006, 42, 296–298. (In Chinese) [Google Scholar]
- Woodrow, I.E.; Berry, J.A. Enzymatic regulation of photosynthetic CO2, fixation in C3 plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 533–594. [Google Scholar] [CrossRef]
- D R Geiger, a.; Servaites, J.C. Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 235–256. [Google Scholar] [CrossRef]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012, 63, 3001–3009. [Google Scholar] [CrossRef]
- Portis, A.R., Jr.; Parry, M.A. Discoveries in rubisco (Ribulose 1,5-bisphosphate carboxylase/oxygenase): A historical perspective. Photosynth. Res. 2007, 94, 121–143. [Google Scholar] [CrossRef]
- Spreitzer, R.J. Genetic dissection of rubisco structure and function. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 411–434. [Google Scholar] [CrossRef]
- Krapp, A.; Chaves, M.M.; David, M.M.; Rodriques, M.L.; Pereira, J.S.; Stitt, M. Decreased ribulose-1,5-bisphosphate carboxylase/oxygenase in transgenic tobacco transformed with ‘antisense’ rbcS.VIII. Impact on photosynthesis and growth in tobacco growing under extreme high irradiance and high temperature. Plant Cell Environ. 1994, 17, 945–953. [Google Scholar] [CrossRef]
- Hartman, F.C.; Harpel, M.R. Structure, function, regulation, and assembly of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Annu. Rev. Biochem. 1994, 63, 197–234. [Google Scholar] [CrossRef] [PubMed]
- Quick, W.P.; Neuhaus, H.E. The regulation and control of photosynthetic carbon assimilation. In A Molecular Approach to Primary Metabolism in Higher Plants, 1st ed.; Foyer, C., Quick, W.P., Eds.; CRC Press: London, UK, 1997. [Google Scholar]
- Hudson, G.S.; Evans, J.R.; von Caemmerer, S.; Arvidsson, Y.B.C.; Andrews, T.J. Reduction of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Content by Antisense RNA Reduces Photosynthesis in Transgenic Tobacco Plants 1. Plant Physiol. 1992, 98, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Lunn, J.; Usadel, B. Arabidopsis and primary photosynthetic metabolism—More than the icing on the cake. Plant J. 2010, 61, 1067–1091. [Google Scholar] [CrossRef]
- Raines, C.A. Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ. 2006, 29, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Schulze, D. Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ. 1994, 17, 465–487. [Google Scholar] [CrossRef]
- Jacquot, J.P.; Lopez-Jaramillo, J.; Chueca, A.; Cherfils, J.; Lemaire, S.; Chedozeau, B.; Miginiac-Maslow, M.; Decottignies, P.; Wolosiuk, R.; Lopez-Gorge, J. High-level expression of recombinant pea chloroplast fructose-1,6-bisphosphatase and mutagenesis of its regulatory site. Eur. J. Biochem. 1995, 229, 675–681. [Google Scholar] [PubMed]
- Henkes, S.; Sonnewald, U.; Badur, R.; Flachmann, R.; Stitt, M. A small decrease of plastid transketolase activity in antisense tobacco transformants has dramatic effects on photosynthesis and phenylpropanoid metabolism. Plant Cell 2001, 13, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.P.; Willingham, N.M.; Lloyd, J.C.; Raines, C.A. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 1997, 204, 27–36. [Google Scholar] [CrossRef]
- Haake, V.; Geiger, M.; Walch-Liu, P.; Engels, C.O.; Zrenner, R.; Stitt, M. Changes in aldolase activity in wild-type potato plants are important for acclimation to growth irradiance and carbon dioxide concentration, because plastid aldolase exerts control over the ambient rate of photosynthesis across a range of growth conditions. Plant J. 1999, 17, 479–489. [Google Scholar] [CrossRef]
- Wei, Q.; Guo, L.; Jiao, C.; Fei, Z.J.; Chen, M.; Cao, J.J.; Ding, Y.L.; Yuan, Q.S. Characterization of the developmental dynamics of the elongation of a bamboo internode during the fast growth stage. Tree Physiol. 2019, 39, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Ramakrishnan, M.; Fei, Z.J.; Vinod, K.K.; Ding, Y.L.; Jiao, C.; Gao, Z.P.; Zha, R.F.; Wang, C.Y.; et al. Rapid growth of Moso bamboo (Phyllostachys edulis): Cellular roadmaps, transcriptome dynamics, and environmental factors. Plant Cell 2022, 34, 3577–3610. [Google Scholar] [CrossRef]
- Song, X.Z.; Peng, C.H.; Zhou, G.M.; Gu, H.H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 25908. [Google Scholar] [CrossRef]
- Reitsma, J.M.; Liu, X.; Reichermeier, K.M.; Moradian, A.; Sweredoski, M.J.; Hess, S.; Deshaies, R.J. Composition and regulation of the cellular repertoire of SCF ubiquitin ligases. Cell 2017, 171, 1326–1339.e14. [Google Scholar] [CrossRef]
- Mohamed, W.I.; Schenk, A.D.; Kempf, G.; Cavadini, S.; Basters, A.; Potenza, A.; Abdul Rahman, W.; Rabl, J.; Reichermeier, K.; Thomä, N.H. The CRL4(DCAF1) cullin-RING ubiquitin ligase is activated following a switch in oligomerization state. EMBO J. 2021, 40, e108008. [Google Scholar] [CrossRef]
- Vasanthaiah, H.K.; Ravishankar, K.V.; Shivashankara, K.S.; Anand, L.; Narayanaswamy, P.; Mukunda, G.; Prasad, T.G. Cloning and characterization of differentially expressed genes of internal breakdown in mango fruit (Mangifera indica). J. Plant Physiol. 2006, 163, 671–679. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Kang, S.Y.; Kim, D.S.; Kim, J.B.; Seo, Y.W. Wheat F-box protein recruits proteins and regulates their abundance during wheat spike development. Mol. Biol. Rep. 2012, 39, 9681–9696. [Google Scholar] [CrossRef]
- Lenk, S.E.; Susan, P.P.; Hickson, I.; Jasionowski, T.; Dunn, W.A., Jr. Ubiquitinated aldolase B accumulates during starvation-induced lysosomal proteolysis. J. Cell. Physiol. 1999, 178, 17–27. [Google Scholar] [CrossRef]
- Wei, N.; Deng, X.W. The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 2003, 19, 261–286. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Xu, S.X.; Xu, M.; He, L.; Zhang, J.F.; Luo, Z.P.; Xie, X.D.; Wu, M.Z.; Yang, J. Identifying loci controlling total starch content of leaf in Nicotiana tabacum through genome-wide association study. Funct. Integr. Genom. 2022, 22, 537–552. [Google Scholar] [CrossRef]
- Flechner, A.; Gross, W.; Martin, W.F.; Schnarrenberger, C. Chloroplast class I and class II aldolases are bifunctional for fructose-1,6-biphosphate and sedoheptulose-1,7-biphosphate cleavage in the Calvin cycle. FEBS Lett. 1999, 447, 200–202. [Google Scholar] [CrossRef]
- Yan, X.H.; Yin, J.H.; Duan, S.H.; Zhou, B.; Hu, W.H.; Liu, S. Photosynthesis light response curves of four rice varieties and model fitting. Chin. J. Ecol. 2013, 32, 604–610. (In Chinese) [Google Scholar]
- Saradadevi, K.; Raghavendra, A.S. Dark respiration protects photosynthesis against photoinhibition in mesophyll protoplasts of pea (Pisum sativum). Plant Physiol. 1992, 99, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.P.; Yang, X.L.; Feng, G.P. Comparative study on light-response models of electron transport rates of plants. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2018, 39, 97–104. (In Chinese) [Google Scholar]
- Zhang, Y.M.; Zhou, G.S. Advances in leaf maximum carboxylation rate and its response to environmental factors. Acta Ecol. Sin. 2012, 32, 5907–5917. (In Chinese) [Google Scholar] [CrossRef]
- Makowka, A.; Nichelmann, L.; Schulze, D.; Spengler, K.; Wittmann, C.; Forchhammer, K.; Gutekunst, K. Glycolytic shunts replenish the calvin-benson-bassham cycle as anaplerotic reactions in cyanobacteria. Mol. Plant 2020, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.P.; Kang, H.J. Study on biological significance of coefficients inmodified model of photosynthesis-irradiance. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2012, 33, 51–57. (In Chinese) [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, H.; Zhu, C.; Yang, K.; Lin, Z.; Wang, J.; Gao, Z. Unveiling the Biological Function of Phyllostachys edulis FBA6 (PeFBA6) through the Identification of the Fructose-1,6-Bisphosphate Aldolase Gene. Plants 2024, 13, 968. https://doi.org/10.3390/plants13070968
Li T, Li H, Zhu C, Yang K, Lin Z, Wang J, Gao Z. Unveiling the Biological Function of Phyllostachys edulis FBA6 (PeFBA6) through the Identification of the Fructose-1,6-Bisphosphate Aldolase Gene. Plants. 2024; 13(7):968. https://doi.org/10.3390/plants13070968
Chicago/Turabian StyleLi, Tiankuo, Hui Li, Chenglei Zhu, Kebin Yang, Zeming Lin, Jiangfei Wang, and Zhimin Gao. 2024. "Unveiling the Biological Function of Phyllostachys edulis FBA6 (PeFBA6) through the Identification of the Fructose-1,6-Bisphosphate Aldolase Gene" Plants 13, no. 7: 968. https://doi.org/10.3390/plants13070968
APA StyleLi, T., Li, H., Zhu, C., Yang, K., Lin, Z., Wang, J., & Gao, Z. (2024). Unveiling the Biological Function of Phyllostachys edulis FBA6 (PeFBA6) through the Identification of the Fructose-1,6-Bisphosphate Aldolase Gene. Plants, 13(7), 968. https://doi.org/10.3390/plants13070968





