Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi
Abstract
1. Introduction
2. Results
2.1. Toxicity of Thiamethoxam against Rhopalosiphum padi
2.2. Impact of Sublethal Concentrations of Thiamethoxam on Parental Rhopalosiphum padi (F0)
2.3. Developmental Duration and Adult Longevity of Rhopalosiphum padi Progeny Generation (F1)
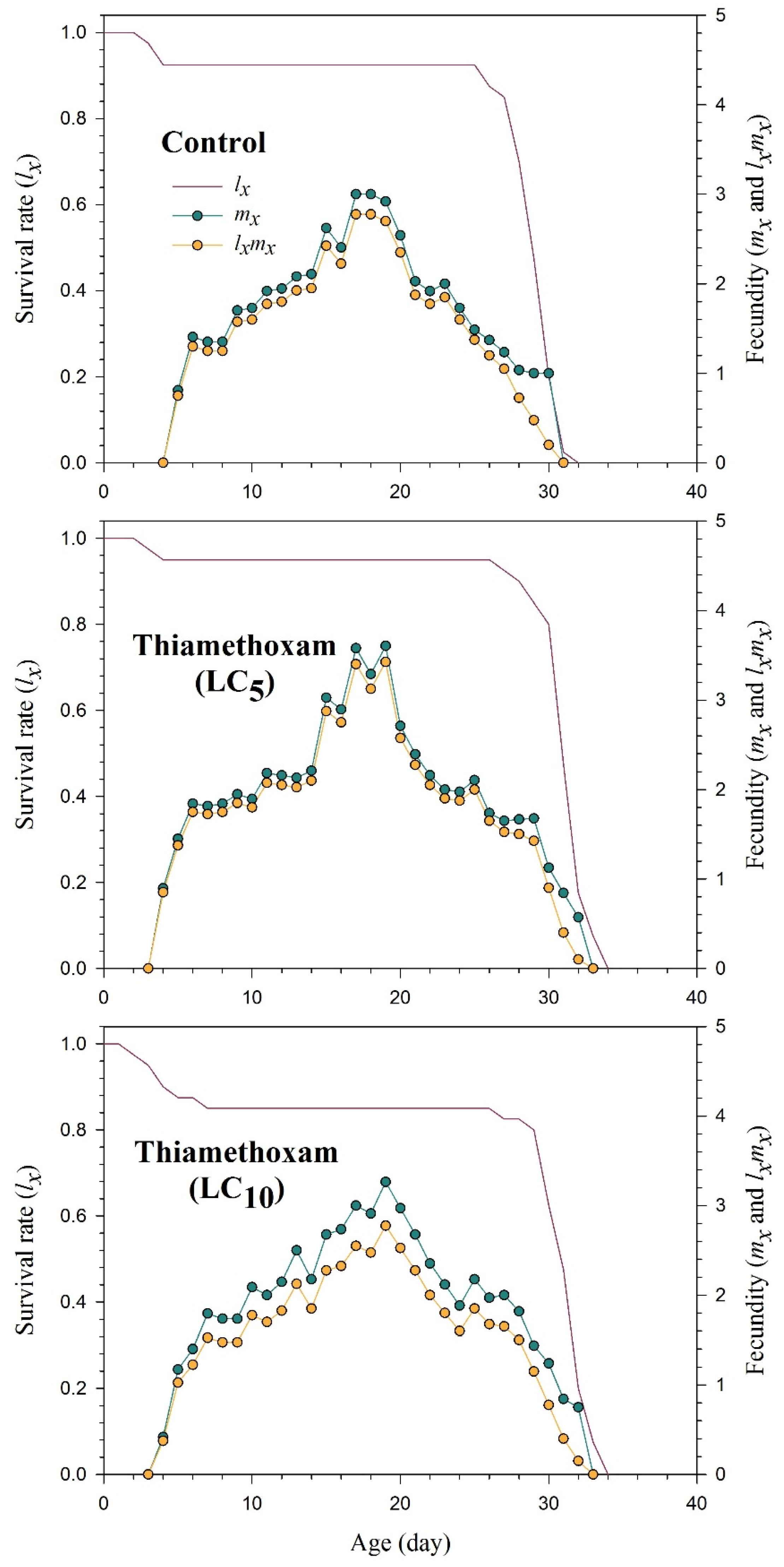
2.4. Demographic Traits and Fecundity of Rhopalosiphum padi Progeny Generation (F1)
2.5. Population Projection
2.6. Sublethal Effects of Thiamethoxam on Feeding Behavior of F0 and F1 Rhopalosiphum padi
3. Material and Methods
3.1. Toxicity Bioassays
3.2. Sublethal Effects of Thiamethoxam on Rhopalosiphum padi (F0)
3.3. Intergenerational Impact of Thiamethoxam on Rhopalosiphum padi (F1)
3.4. Electro Penetrography of Rhopalosiphum padi Feeding Behavior
3.5. Data Analysis
3.6. Life Table Data Analysis
3.7. Population Projection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs, 2 Volume Set; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests; Cabi: Wallingford, UK, 2017. [Google Scholar]
- Hullé, M.; Chaubet, B.; Turpeau, E.; Simon, J. Encyclop’Aphid: A website on aphids and their natural enemies. Entomol. Gen. 2020, 40, 97–101. [Google Scholar] [CrossRef]
- Yan, S.; Yin, M.-Z.; Shen, J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: Mechanisms, current status and challenges. Entomol. Gen. 2023, 43, 21–30. [Google Scholar] [CrossRef]
- Pan, M.-z.; Zhang, Y.; Cao, H.-h.; Wang, X.-x.; Liu, T.-x. Research progresses, application, and prospects in aphid biological control on main crops in China. J. Plant Prot. 2023, 49, 146–172. [Google Scholar]
- Bellefeuille, Y.; Fournier, M.; Lucas, E. Biological control of the foxglove aphid using a banker plant with Eupeodes americanus (Diptera: Syrphidae) in experimental and commercial greenhouses. Biol. Control 2021, 155, 104541. [Google Scholar] [CrossRef]
- Umina, P.A.; Reidy-Crofts, J.; Babineau, M.; Maino, J.L.; Edwards, O.R. Susceptibility of the bird cherry-oat aphid, Rhopalosiphum padi (Hemiptera: Aphididae), to four insecticides. Austral Entomol. 2020, 59, 838–844. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, S.; Liu, Y.; Ma, L.; Li, X.; Zhang, Y.; Fan, Y.; Song, D.; Gao, X. Slow resistance evolution to neonicotinoids in field populations of wheat aphids revealed by insecticide resistance monitoring in China. Pest. Manag. Sci. 2022, 78, 1428–1437. [Google Scholar] [CrossRef]
- Atta, B.; Rizwan, M.; Sabir, A.M.; Gogi, M.D.; Farooq, M.A.; Jamal, A. Lethal and sublethal effects of clothianidin, imidacloprid and sulfoxaflor on the wheat aphid, Schizaphis graminum (Hemiptera: Aphididae) and its coccinellid predator, Coccinella septempunctata. Int. J. Trop. Insect Sci. 2021, 41, 345–358. [Google Scholar] [CrossRef]
- Jie, M.; Gao, Y.; Kuang, D.; Shi, Y.; Wang, H.; Jing, W. Relationship between imidacloprid residues and control effect on cotton aphids in arid region. Environ. Geochem. Health 2021, 43, 1941–1952. [Google Scholar] [CrossRef]
- Desneux, N.; Fauvergue, X.; Dechaume-Moncharmont, F.-X.; Kerhoas, L.; Ballanger, Y.; Kaiser, L. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 2005, 98, 9–17. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Gao, H.; Wang, C.; Li, M.; Zhang, Y.; Li, X.; Liu, E.; Zhu, X. Field evolved resistance to pyrethroids, neonicotinoids, organophosphates and macrolides in Rhopalosiphum padi (Linnaeus) and Sitobion avenae (Fabricius) from China. Chemosphere 2021, 269, 128747. [Google Scholar] [CrossRef]
- Ding, W.; Guo, L.; Xue, Y.; Wang, M.; Li, C.; Zhang, R.; Zhang, S.; Xia, X. Life Parameters and Physiological Reactions of Cotton Aphids Aphis gossypii (Hemiptera: Aphididae) to Sublethal Concentrations of Afidopyropen. Agronomy 2024, 14, 258. [Google Scholar] [CrossRef]
- Zhang, A.; Zhou, W.; Wu, D.; Han, L.; Zhao, K. Effects of multigenerational imidacloprid and thiamethoxam stress on metabolism and physiology of Aphis glycines Matsumura (Hemiptera: Aphididae). PLoS ONE 2022, 17, e0271069. [Google Scholar] [CrossRef]
- Khurshid, A.; Inayat, R.; Basit, A.; Mobarak, S.H.; Gui, S.H.; Liu, T.X. Effects of Thiamethoxam on Physiological and Molecular Responses to Potato Plant (Solanum tuberosum), Green Peach Aphid (Myzus persicae) and Parasitoid (Aphidius gifuensis). Pest. Manag. Sci. 2024. [Google Scholar] [CrossRef]
- Tian, F.; Qiao, C.; Wang, C.; Pang, T.; Guo, L.; Li, J.; Pang, R.; Liu, H.; Xie, H. Comparison of the effectiveness of thiamethoxam and its main metabolite clothianidin after foliar spraying and root irrigation to control Myzus persicae on peach. Sci. Rep. 2022, 12, 16883. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Zhan, E.L.; Zhang, S.G.; Wang, Y.; Song, P.P.; Jones, A.K.; Han, Z.J.; Zhao, C.Q. Broflanilide prolongs the development of fall armyworm Spodoptera frugiperda by regulating biosynthesis of juvenile hormone. Entomol. Gen. 2022, 42, 761–769. [Google Scholar] [CrossRef]
- Margus, A.; Tikka, S.; Karvanen, J.; Lindström, L. Transgenerational sublethal pyrethroid exposure gives rise to insecticide resistance in a pest insect. Sci. Total Environ. 2024, 908, 168114. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Zeng, Q.-H.; Yang, H.; Zhang, C.; Ding, B.; Yang, H.-Z.; Yang, M.-F. Sublethal and transgenerational effects of broflanilide on Myzus persicae (Sulzer)(Hemiptera: Aphididae). Crop. Prot. 2023, 174, 106421. [Google Scholar] [CrossRef]
- Fouad, E.A.; El-Sherif, S.A.; Mokbel, E.-S.M. Flupyradifurone induces transgenerational hormesis effects in the cowpea aphid, Aphis craccivora. Ecotoxicology 2022, 31, 909–918. [Google Scholar] [CrossRef]
- Deevey Jr, E.S. Life tables for natural populations of animals. Q. Rev. Biol. 1947, 22, 283–314. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead)(Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer)(Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Özgökçe, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Chi, H.; Kara, H.; Özgökçe, M.S.; Atlihan, R.; Güncan, A.; Rişvanlı, M.R. Innovative application of set theory, Cartesian product, and multinomial theorem in demographic research. Entomol. Gen. 2022, 42, 863–874. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C.; et al. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–735. [Google Scholar] [CrossRef]
- Milenovic, M.; Wosula, E.N.; Rapisarda, C.; Legg, J.P. Impact of host plant species and whitefly species on feeding behavior of Bemisia tabaci. Front. Plant Sci. 2019, 10, 1. [Google Scholar] [CrossRef]
- Shang, J.; Dong, W.; Fang, H.; Wang, C.; Yang, H.; Chen, Z.; Guo, X.; Wang, H.; Liang, P.; Shi, X. Effects of dimpropyridaz on feeding behavior, locomotivity and biological parameters of Aphis gossypii. Pestic. Biochem. Phys. 2023, 197, 105694. [Google Scholar] [CrossRef]
- Sauge, M.H.; Lacroze, J.P.; Poëssel, J.L.; Pascal, T.; Kervella, J. Induced resistance by Myzus persicae in the peach cultivar ‘Rubira’. Entomol. Exp. Appl. 2002, 102, 29–37. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MS Chart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2024. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 5 January 2024).
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. An Introduction to the Bootstrap; Chapman and 913 Hall. Inc.: New York, NY, USA, 1993; Volume 914. [Google Scholar]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Akköprü, E.P.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University in Taiwan. 2024. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 5 January 2024).
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Huang, H.-W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, L.; Wang, H.; Qiao, K.; Wang, D.; Wang, K. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest. Manag. Sci. 2011, 67, 1528–1533. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef]
- Gul, H.; ul Haq, I.; Ullah, F.; Khan, S.; Yaseen, A.; Shah, S.H.; Tariq, K.; Güncan, A.; Desneux, N.; Liu, X. Impact of sublethal concentrations of flonicamid on key demographic parameters and feeding behavior of Schizaphis graminum. Ecotoxicology 2023, 32, 756–767. [Google Scholar] [CrossRef]
- Gul, H.; Güncan, A.; Ullah, F.; Ning, X.; Desneux, N.; Liu, X. Sublethal concentrations of thiamethoxam induce transgenerational hormesis in cotton aphid, Aphis gossypii Glover. CABI Agric. Biosci. 2023, 4, 50. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Liang, P.; Li, J.; Gao, X. A sublethal concentration of afidopyropen suppresses the population growth of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). J. Integr. Agric. 2022, 21, 2055–2064. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, Y.-T.; Guo, T.-X.; Zhang, P.; Li, X.-a.; Kong, F.-B.; Zhang, B.-Z. Sublethal effects of imidacloprid on the fitness of two species of wheat aphids, Schizaphis graminum (R.) and Rhopalosiphum padi (L.). PLoS ONE 2023, 18, e0294877. [Google Scholar] [CrossRef]
- Cui, L.; Yuan, H.; Wang, Q.; Wang, Q.; Rui, C. Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae). Sci. Rep. 2018, 8, 8915. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Luo, C.; Lv, H.; Zhang, L.; Desneux, N.; You, H.; Li, J.; Ullah, F.; Ma, K. Impact of sublethal and low lethal concentrations of flonicamid on key biological traits and population growth associated genes in melon aphid, Aphis gossypii Glover. Crop Prot. 2022, 152, 105863. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, K.; Chi, H.; Hou, Y.; Gao, X. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer)(Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef]
- Gong, Y.; Cheng, S.; Desneux, N.; Gao, X.; Xiu, X.; Wang, F.; Hou, M. Transgenerational hormesis effects of nitenpyram on fitness and insecticide tolerance/resistance of Nilaparvata lugens. J. Pest. Sci. 2023, 96, 161–180. [Google Scholar] [CrossRef]
- Ayyanath, M.-M.; Cutler, G.C.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef]
- Sial, M.U.; Zhao, Z.; Zhang, L.; Zhang, Y.; Mao, L.; Jiang, H. Evaluation of insecticides induced hormesis on the demographic parameters of Myzus persicae and expression changes of metabolic resistance detoxification genes. Sci. Rep. 2018, 8, 16601. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and insects: Effects and interactions in agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef]
- Rix, R.R.; Ayyanath, M.M.; Christopher Cutler, G. Sublethal concentrations of imidacloprid increase reproduction, alter expression of detoxification genes, and prime Myzus persicae for subsequent stress. J. Pest. Sci. 2016, 89, 581–589. [Google Scholar] [CrossRef]
- Lee, S.T.; Davis, J.A. The impact of thiamethoxam on the feeding and behavior of 2 soybean herbivore feeding guilds. J. Econ. Entomol. 2023, 116, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Sun, L.; Yang, D.; Yan, X.; Yuan, H. Effects of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, on the feeding behaviour of Sitobion avenae. Pest. Manag. Sci. 2012, 68, 1484–1491. [Google Scholar] [CrossRef]
- Miao, J.; Du, Z.B.; Wu, Y.Q.; Gong, Z.J.; Jiang, Y.L.; Duan, Y.; Li, T.; Lei, C.L. Sub-lethal effects of four neonicotinoid seed treatments on the demography and feeding behaviour of the wheat aphid Sitobion avenae. Pest. Manag. Sci. 2014, 70, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Noor, M.; Backus, E.A.; Hussain, A.; Ali, A.; Peng, W.; Zhang, H. The toxicity of flonicamid to cotton leafhopper, Amrasca biguttula (Ishida), is by disruption of ingestion: An electropenetrography study. Pest. Manag. Sci. 2017, 73, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; He, Y.; Wu, J.; Tang, Y.; Gu, J.; Ding, W.; Zhang, Y. Sublethal effects of cyantraniliprole and imidacloprid on feeding behavior and life table parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 1595–1602. [Google Scholar] [CrossRef]
- Koo, H.N.; Lee, S.W.; Yun, S.H.; Kim, H.K.; Kim, G.H. Feeding response of the cotton aphid, Aphis gossypii, to sublethal rates of flonicamid and imidacloprid. Entomol. Exp. Appl. 2015, 154, 110–119. [Google Scholar] [CrossRef]

| Parameters | Control | Thiamethoxam (LC5) | Thiamethoxam (LC10) |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Adult longevity (days) | 25.05 ± 0.12 a | 19.00 ± 0.29 b | 14.00 ± 0.32 c |
| Fecundity (nymphs/female) | 48.00 ± 0.70 a | 41.00 ± 0.84 b | 32.00 ± 0.63 c |
| Reproductive days (days) | 22.73 ± 0.25 a | 18.70 ± 0.33 b | 13.58 ± 0.27 c |
| Stage | Control | Thiamethoxam (LC5) | Thiamethoxam (LC10) | |||
|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| First-instar nymph | 40 | 1.45 ± 0.09 a | 40 | 1.20 ± 0.06 b | 40 | 1.33 ± 0.07 ab |
| Second-instar nymph | 38 | 1.24 ± 0.07 a | 40 | 1.03 ± 0.02 b | 39 | 1.03 ± 0.03 b |
| Third-instar nymph | 38 | 1.32 ± 0.08 a | 38 | 1.11 ± 0.05 b | 37 | 1.24 ± 0.08 ab |
| Fourth-instar nymph | 37 | 1.41 ± 0.08 a | 38 | 1.11 ± 0.05 b | 35 | 1.20 ± 0.07 ab |
| Pre-adult | 37 | 5.38 ± 0.08 a | 38 | 4.42 ± 0.09 c | 35 | 4.80 ± 0.12 b |
| Adult (Female) | 37 | 24.00 ± 0.20 b | 38 | 27.00 ± 0.20 a | 35 | 26.00 ± 0.73 a |
| Total longevity (Female) | 37 | 29.38 ± 0.23 b | 38 | 31.42 ± 0.24 a | 35 | 30.80 ± 0.74 ab |
| Parameters a | Control | Thiamethoxam (LC5) | Thiamethoxam (LC10) |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| R0 (offspring/individual) | 42.55 ± 2.01 b | 55.10 ± 2.10 a | 48.13 ± 3.18 ab |
| r (day−1) | 0.2810 ± 0.0055 b | 0.3311 ± 0.0065 a | 0.2988 ± 0.0083 b |
| λ (day−1) | 1.3244 ± 0.0073 b | 1.3926 ± 0.0090 a | 1.3482 ± 0.0112 b |
| T (days) | 13.35 ± 0.16 a | 12.11 ± 0.18 b | 12.96 ± 0.22 a |
| F (nymphs/female) | 46.00 ± 0.65 b | 58.00 ± 0.67 a | 55.00 ± 1.58 a |
| RPd(days) | 22.76 ± 0.26 b | 26.08 ± 0.19 a | 24.77 ± 0.71 a |
| APRP (days) | 0.14 ± 0.07 a | 0.08 ± 0.04 a | 0.20 ± 0.09 a |
| TPRP (days) | 5.51 ± 0.11 a | 4.50 ± 0.10 c | 5.00 ± 0.16 b |
| Treatments | Np | C | G | E1 | E2 |
|---|---|---|---|---|---|
| Control | 972.6 ± 279.36 b | 4112.7 ± 660.94 b | 1374.1 ± 603.65 a | 476.6 ± 177.69 a | 14,261 ± 1091.98 a |
| LC5 | 3081.5 ± 331.49 a | 5381.4 ± 448.28 b | 1368.1 ± 392.78 a | 1005.3 ± 148.62 a | 10,241 ± 687.65 b |
| LC10 | 2257.5 ± 619.97 ab | 8188.7 ± 967.94 a | 1258.7 ± 730.75 a | 959.4 ± 201.42 a | 7112 ± 487.52 c |
| Treatments | Np | C | G | E1 | E2 |
|---|---|---|---|---|---|
| Control | 1358.7 ± 373.08 a | 5269.5 ± 829.03 a | 2932.7 ± 1226.44 a | 342.33 ± 87.66 a | 11,287 ± 1708.69 b |
| LC5 | 1240.3 ± 261.34 a | 2315.5 ± 389.59 b | 699.7 ± 335.34 a | 684.3 ± 125.59 a | 15,711 ± 583.21 a |
| LC10 | 2061.4 ± 403.84 a | 2496.4 ± 353.60 b | 998.2 ± 494.17 a | 670.38 ± 123.40 a | 15,326 ± 713.29 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, H.; Haq, I.u.; Güncan, A.; Abbas, A.; Khan, S.; Yaseen, A.; Ullah, F.; Desneux, N.; Liu, X. Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi. Plants 2024, 13, 865. https://doi.org/10.3390/plants13060865
Gul H, Haq Iu, Güncan A, Abbas A, Khan S, Yaseen A, Ullah F, Desneux N, Liu X. Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi. Plants. 2024; 13(6):865. https://doi.org/10.3390/plants13060865
Chicago/Turabian StyleGul, Hina, Ihsan ul Haq, Ali Güncan, Arzlan Abbas, Shanza Khan, Aqsa Yaseen, Farman Ullah, Nicolas Desneux, and Xiaoxia Liu. 2024. "Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi" Plants 13, no. 6: 865. https://doi.org/10.3390/plants13060865
APA StyleGul, H., Haq, I. u., Güncan, A., Abbas, A., Khan, S., Yaseen, A., Ullah, F., Desneux, N., & Liu, X. (2024). Thiamethoxam-Induced Intergenerational Sublethal Effects on the Life History and Feeding Behavior of Rhopalosiphum padi. Plants, 13(6), 865. https://doi.org/10.3390/plants13060865









