Enhancing Native Plant Establishment in Mine Tailings under Drought Stress Conditions through the Application of Organo-Mineral Amendments and Microbial Inoculants
Abstract
1. Introduction
2. Results
2.1. Influence of Irrigation Regimes and Microbial Inoculants on Soil Chemical Properties
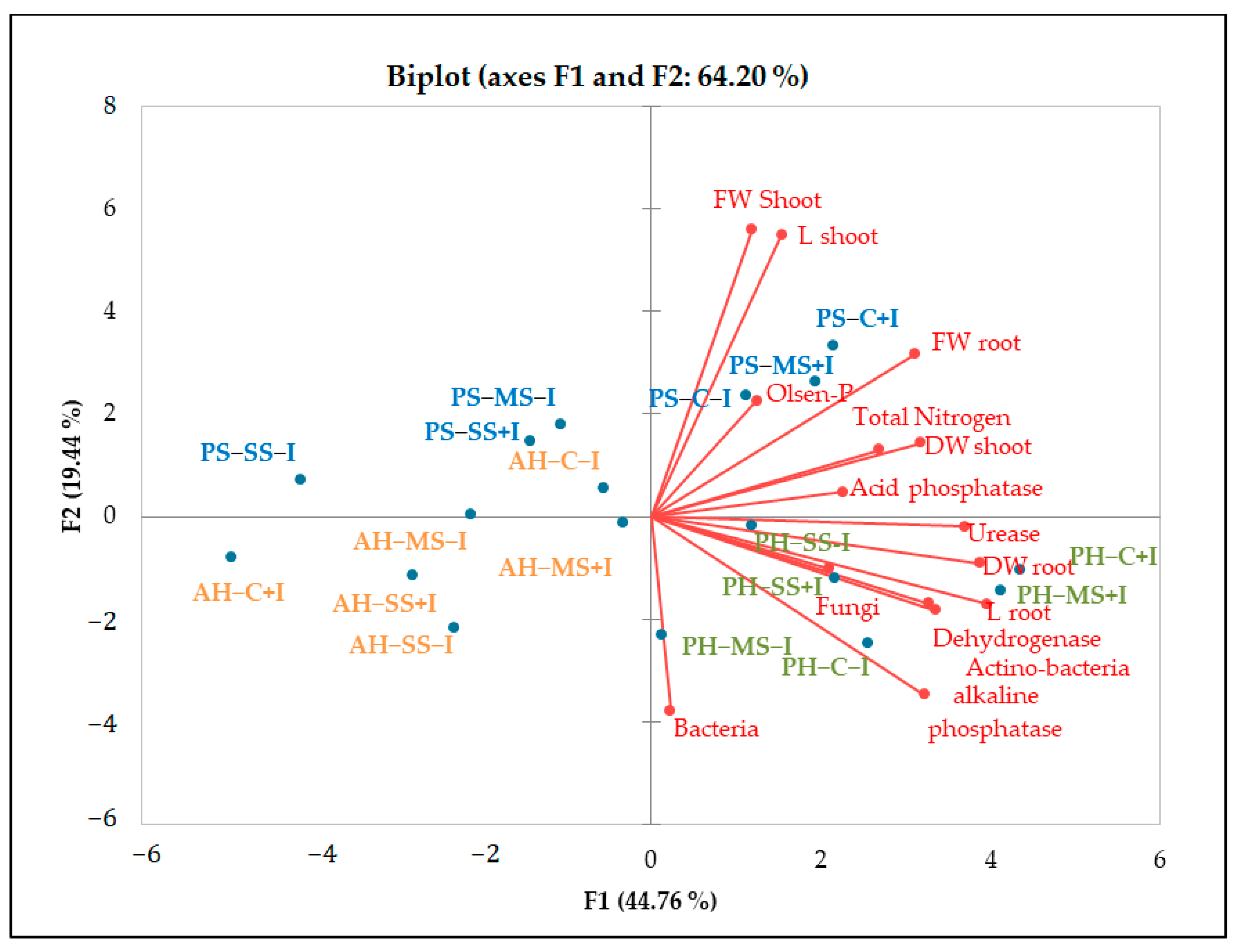
2.2. Influence of Irrigation Regimes and Microbial Inoculants on Soil Microbial Activity and Structure
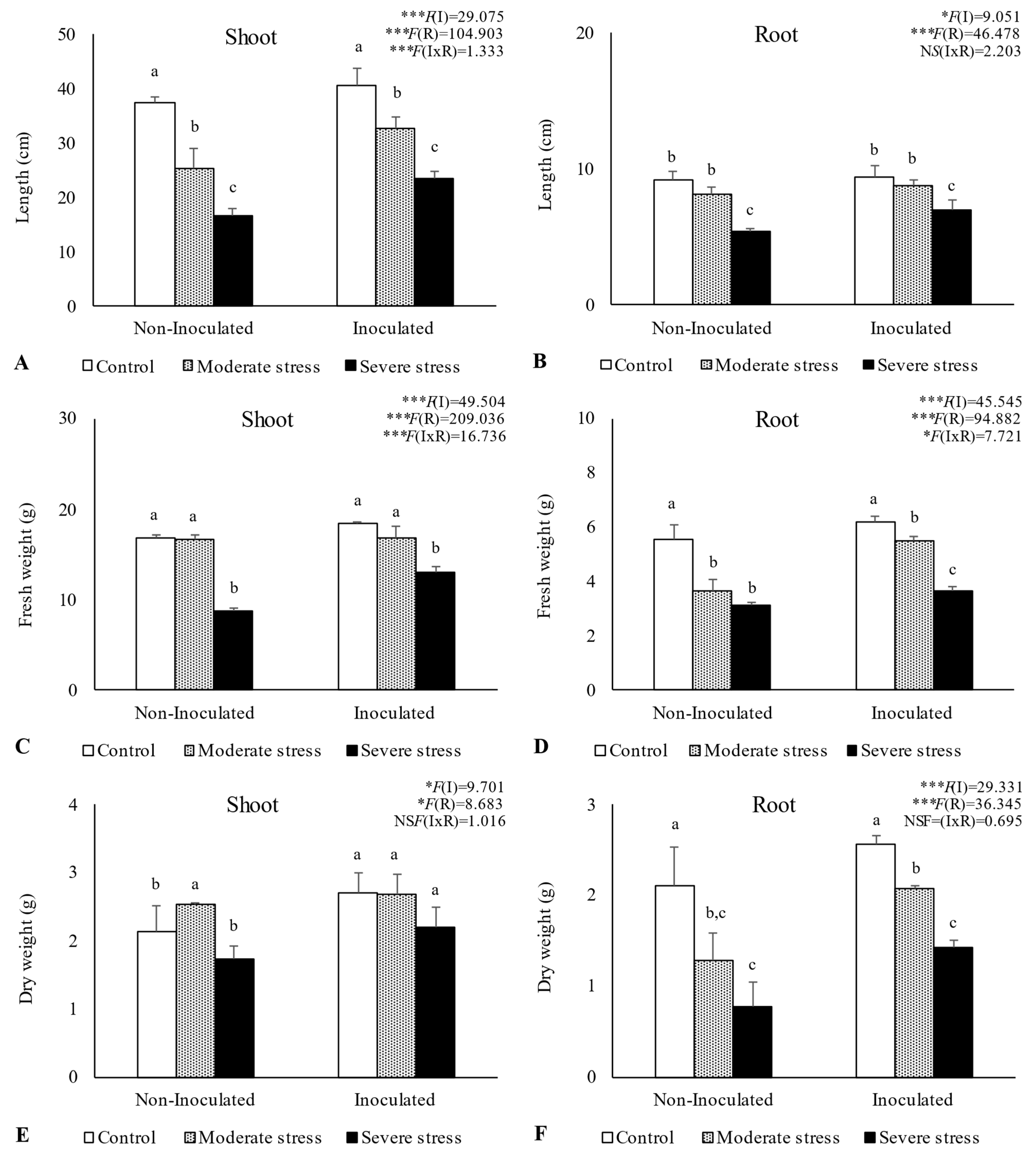
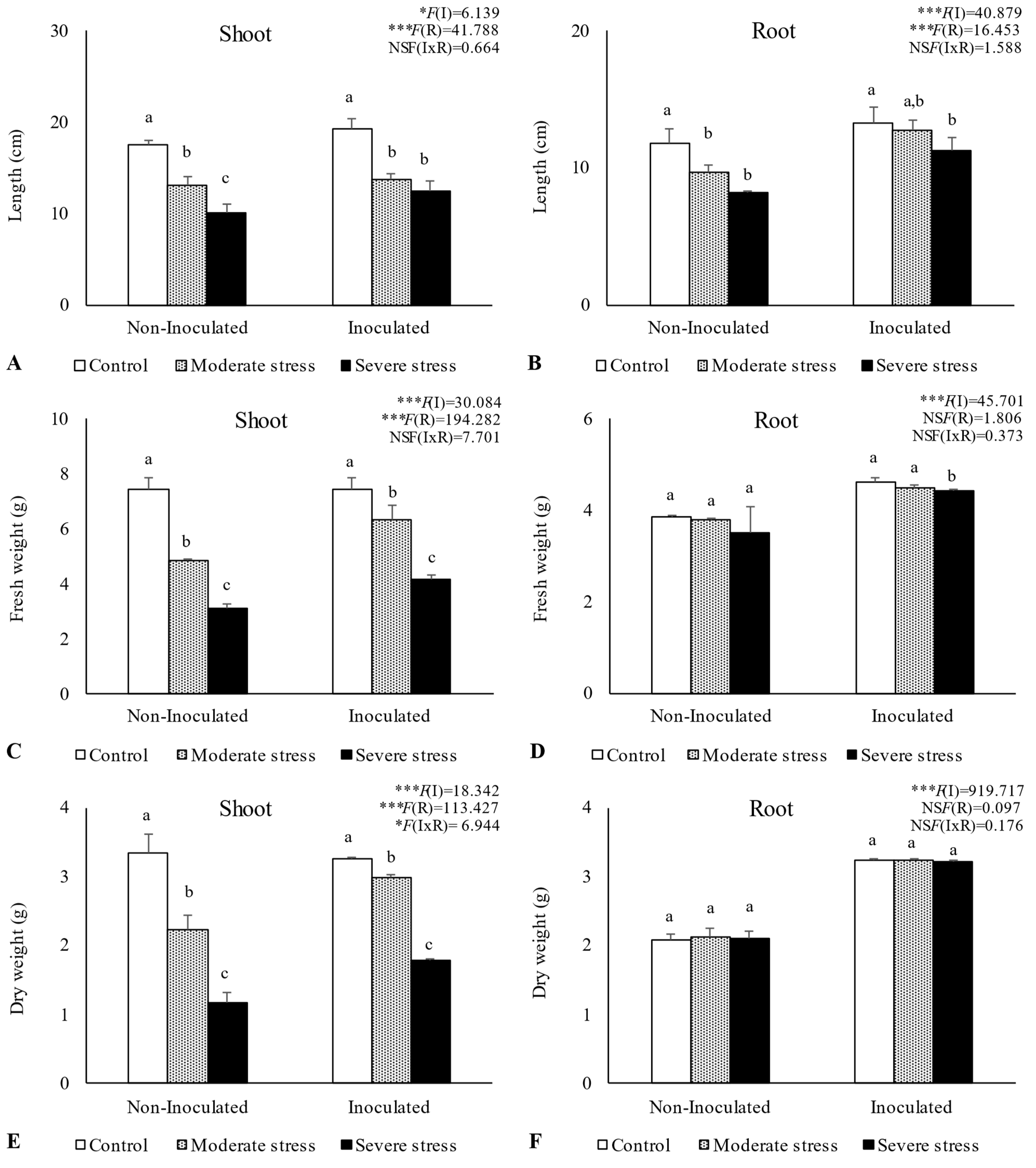
2.3. Influence of Irrigation Regimes and Microbial Inoculants on Plant Growth
3. Discussion
3.1. Improvement of Mine Tailings’ Chemical and Microbiological Properties by the Combined Use of Organo-Mineral Amendments and Bacterial Inoculants
3.2. Plant Growth in Amended Mine Tailings under Water Stress Conditions
4. Materials and Methods
4.1. Plant Species
4.2. PGPR Consortium, Seed Sterilization, and Seedling Growth
4.3. Sampling and Characterization of Mine Tailings, Topsoil, and Organo-Mineral Amendments
4.4. Greenhouse Pot Experiment
4.4.1. Soil Physicochemical Analysis
4.4.2. Soil Enzymatic Activity
4.4.3. Enumeration of Culturable Microorganisms
4.4.4. Plant Biometric Parameters
4.4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franzaring, J.; Ancora, S.; Paoli, L.; Fongoh, A.; Büttner, P.; Fangmeier, A.; Schlosser, S.; Monaci, F. Phytotoxicity of polymetallic mine wastes from southern Tuscany and Saxony. Ecotoxicol. Environ. Saf. 2018, 162, 505–513. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Ozdemiroglu, S.; Fonti, V.; Beolchini, F. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015, 86, 24–36. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of heavy metals: Understanding of principles and practices. In Plant-Metal Interactions; Springer: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Zhang, Q.; Li, J.; Zhang, F.; Liu, Z. Effects of peat on plant growth and lead and zinc phytostabilization from lead-zinc mine tailing in southern China: Screening plant species resisting and accumulating metals. Ecotoxicol. Environ. Saf. 2019, 176, 42–49. [Google Scholar] [CrossRef]
- Testiati, E.; Parinet, J.; Massiani, C.; Laffont-Schwob, I.; Rabier, J.; Pfeifer, H.-R.; Lenoble, V.; Masotti, V.; Prudent, P. Trace metal and metalloid contamination levels in soils and in two native plant species of a former industrial site: Evaluation of the phytostabilization potential. J. Hazard. Mater. 2013, 248, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Dary, M.; Chamber-Pérez, M.; Palomares, A.; Pajuelo, E. “In Situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pardo, T.; Martínez-Fernández, D.; Bernal, M.P. The use of a halophytic plant species and organic amendments for the remediation of a trace elements-contaminated soil under semi-arid conditions. J. Hazard. Mater. 2012, 223–224, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef]
- Visser, A.; Kroes, J.; van Vliet, M.T.; Blenkinsop, S.; Fowler, H.J.; Broers, H.P. Climate change impacts on the leaching of a heavy metal contamination in a small lowland catchment. J. Contam. Hydrol. 2012, 127, 47–64. [Google Scholar] [CrossRef]
- Jha, S.; Srivastava, R. Impact of drought on vegetation carbon storage in arid and semi-arid regions. Remote Sens. Appl. Soci. Environ. 2018, 11, 22–29. [Google Scholar] [CrossRef]
- Parraga-Aguado, I.; González-Alcaraz, M.N.; Álvarez-Rogel, J.; Conesa, H.M. Assessment of the employment of halophyte plant species for the phytomanagement of mine tailings in semiarid areas. Ecol. Eng. 2014, 71, 598–604. [Google Scholar] [CrossRef]
- Boularbah, A.; Schwartz, C.; Bitton, G.; Morel, J.L. Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 2006, 63, 811–817. [Google Scholar] [CrossRef]
- Boularbah, A.; Schwartz, C.; Bitton, G.; Morel, J.L. Heavy metal contamination from mining sites in South Morocco: 1. Use of a biotest to assess metal toxicity of tailings and soils. Chemosphere 2006, 63, 802–810. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments–an emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environ. Exp. Biol. 2010, 68, 1–13. [Google Scholar] [CrossRef]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ. Earth Sci. 2012, 65, 621–630. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals: Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Meeinkuirt, W.; Pokethitiyook, P.; Kruatrachue, M.; Tanhan, P.; Chaiyarat, R. Phytostabilization of a Pb-contaminated mine tailing by various tree species in potand field trial experiments. Int. J. Phytoremediation 2012, 14, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Wasilkowski, D.; Nowak, A.; Płaza, G.; Mrozik, A. Effects of pulp and Na bentonite amendments on the mobility of trace elements, soil enzymes activity and microbial parameters under ex situ aided phytostabilization. PLoS ONE 2017, 12, e0169688. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, C.; Wang, D.; Arthur, E.; Zhang, Z.; Guo, Z.; Peng, X.; Mooney, S.J. Mooney. Effect of long-term organic amendments on the full-range soil water retention characteristics of a Vertisol. Soil Tillage Res. 2020, 202, 104663. [Google Scholar] [CrossRef]
- Kang, S.-W.; Ahn, K.-H. The Influence of Organic Matter Origin on the Chlorine Bulk Decay Coefficient in Reclaimed Water. Water 2022, 14, 765. [Google Scholar] [CrossRef]
- Taban, M.; Naeini, S.A.R.M. Movahedi Naeini. Effect of aquasorb and organic compost amendments on soil water retention and evaporation with different evaporation potentials and soil textures. Commun. Soil Sci. Plant Anal. 2006, 37, 2031–2055. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Zainab, N.; Din, B.U.; Javed, M.T.; Afridi, M.S.; Mukhtar, T.; Kamran, M.A.; Chaudhary, H.J. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol. Biochem. 2020, 152, 90–99. [Google Scholar] [CrossRef]
- Benidire, L.; Pereira, S.; Aboudrar, W.; Hafidi, M.; Castro, P.; Boularbah, A. Remediation of metal-contaminated mine tailings by the application of organic and mineral amendments. J. Soils Sediments 2022, 22, 482–495. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V.; Gupta, P.; Chandra, A. Potential use of Solanum lycopersicum and plant growth promoting rhizobacterial (PGPR) strains for the phytoremediation of endosulfan stressed soil. Chemosphere 2021, 279, 130589. [Google Scholar] [CrossRef]
- Madline, A.; Benidire, L.; Boularbah, A. Alleviation of salinity and metal stress using plant growth-promoting rhizobacteria isolated from semiarid Moroccan copper-mine soils. Environ. Sci. Pollut. Res. 2021, 28, 67185–67202. [Google Scholar] [CrossRef]
- Sandhya, V.S.K.Z.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2020, 62, 21–30. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Severi, J.; Butterly, C.R.; Baldock, J.A. Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol. 2016, 211, 864–873. [Google Scholar] [CrossRef]
- Gispert, M.; Emran, M.; Pardini, G.; Doni, S.; Ceccanti, B. The impact of land management and abandonment on soil enzymatic activity, glomalin content and aggregate stability. Geoderma 2013, 202–203, 51–61. [Google Scholar] [CrossRef]
- Yadav, B.K.; Akhtar, M.; Panwar, J. Rhizospheric plant-microbe interactions: Key factors to soil fertility and plant nutrition. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 127–145. [Google Scholar]
- Bowsher, A.W.; Ali, R.; Harding, S.A.; Tsai, C.J.; Donovan, L.A. Evolutionary divergences in root exudate composition among ecologically-contrasting Helianthus species. PLoS ONE 2016, 11, e0148280. [Google Scholar] [CrossRef]
- Gamage SS, W.; Masakorala, K.; Brown, M.T.; Gamage, S.M.K.W. Comparative phytoremediation potentials of Impatiens balsamina L. and Crotalaria retusa L. for soil contaminated with used lubricating oil. Environ. Adv. 2021, 5, 100095. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.-J.; Nam, I.-H. Effect of treating acid sulfate soils with phosphate solubilizing bacteria on germination and growth of tomato (Lycopersicon esculentum L.). Int. J. Environ. Res. Public Health 2021, 18, 8919. [Google Scholar] [CrossRef]
- Altaf, M. Functional diversity of nitrogen-fixing plant growth-promoting Rhizobacteria: The story so far. In Soil Nitrogen Ecology; Springer: Cham, Switzerland, 2021; pp. 327–348. [Google Scholar]
- Zeng, Q.; Ding, X.; Wang, J.; Han, X.; Iqbal, H.; Bilal, M. Insight into soil nitrogen and phosphorus availability and agricultural sustainability by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2022, 29, 45089–45106. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 2020, 262, 127803. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere 2021, 263, 128345. [Google Scholar] [CrossRef]
- Madejón, P.; Navarro-Fernández, C.M.; Madejón, E.; López-García, Á.; Marañón, T. Plant response to mycorrhizal inoculation and amendments on a contaminated soil. Sci. Total Environ. 2021, 789, 147943. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; Laghmari, G.; Lemjereb, M.; Hnadi, H.; Amrani, K.; El Ghachtouli, N. Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatal. Agric. Biotechnol. 2021, 31, 101914. [Google Scholar] [CrossRef]
- Dey, G.; Banerjee, P.; Maity, J.P.; Sharma, R.K.; Gnanachandrasamy, G.; Huang, Y.H.; Chen, C.Y. Heavy metals distribution and ecological risk assessment including arsenic resistant PGPR in tidal mangrove ecosystem. Mar. Pollut. Bull. 2022, 181, 113905. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil. Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Pohlon, E.; Fandino, A.O.; Marxsen, J. Bacterial community composition and extracellular enzyme activity in temperate streambed sediment during drying and rewetting. PLoS ONE 2013, 8, e83365. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Klimkowicz-Pawlas, A.; Gałązka, A.; Grządziel, J.; Stuczyński, T. Impact of water stress on microbial community and activity in sandy and loamy soils. Agronomy 2020, 10, 1429. [Google Scholar] [CrossRef]
- Schreckinger, J.; Mutz, M.; Mendoza-Lera, C.; Frossard, A. Attributes of drying define the structure and functioning of microbial communities in temperate riverbed sediment. Front. Microbiol. 2021, 12, 676615. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Soil enzyme activity in a Mediterranean forest after six years of drought. Soil. Sci. Soc. Am. J. 2010, 74, 838–851. [Google Scholar] [CrossRef]
- Gao, W.; Reed, S.C.; Munson, S.M.; Rui, Y.; Fan, W.; Zheng, Z.; Hao, Y. Responses of soil extracellular enzyme activities and bacterial community composition to seasonal stages of drought in a semiarid grassland. Geoderma 2021, 401, 115327. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Rathore, A.P.; Sharma, S. Effect of halotolerant plant growth promoting rhizobacteria inoculation on soil microbial community structure and nutrients. Appl. Soil Ecol. 2022, 150, 103461. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Chen, F.; Li, C.; Wu, L. Effects of the successive planting of Eucalyptus urophylla on soil bacterial and fungal community structure, diversity, microbial biomass, and enzyme activity. Land Degrad. Dev. 2019, 30, 636–646. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef]
- Piromyou, P.; Noisangiam, R.; Uchiyama, H.; Tittabutr, P.; Boonkerd, N.; Teaumroong, N. Indigenous microbial community structure in rhizosphere of Chinese kale as affected by plant growth-promoting rhizobacteria inoculation. Pedosphere 2013, 23, 577–592. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; Ali, Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Shi, G.; Xia, S.; Ye, J.; Huang, Y.; Liu, C.; Zhang, Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ. Exp. Bot. 2015, 111, 127–134. [Google Scholar] [CrossRef]
- Wang, J.Q.; Li, H.; Liu, Q.; Xiang, D. Effects of drought stress on root development and physiological characteristics of sweet potato at seedling stage. J. Appl. Ecol. 2019, 30, 3155–3163. [Google Scholar] [CrossRef]
- Xiao, X.X. The physiological and biochemical response of longan (Dimocarpus longan Lour.) to aluminum stress and rectification of aluminum toxicity. Fujian J. Agric. Sci. 2002, 17, 182–185. [Google Scholar]
- Orrego, F.; Ortíz-Calderón, C.; Lutts, S.; Ginocchio, R. Effect of single and combined Cu, NaCl and water stresses on three Atriplex species with phytostabilization potential. S. Afr. J. Bot. 2020, 131, 161–168. [Google Scholar] [CrossRef]
- Nedjimi, B.; Beladel, B.; Guit, B. Biodiversity of halophytic vegetation in chott Zehrez lake of Djelfa (Algeria). Am. J. Plant Sci. 2012, 3, 1513–1660. [Google Scholar] [CrossRef]
- Geng, M.; Xu, M.; Xiao, H.; Wang, H.; He, L.; Zhao, Z.; Yu, M. Protective role of mucilage against Al toxicity to root apex of pea (Pisum sativum). Acta Physiol. Plant. 2012, 34, 1261–1266. [Google Scholar] [CrossRef]
- Liao, M.; Xie, X.M. Cadmium release in contaminated soils due to organic acids. Pedosphere 2004, 14, 223–228. [Google Scholar]
- Bali, A.S.; Sidhu GP, S.; Kumar, V. Root exudates ameliorate cadmium tolerance in plants: A review. Environ. Chem. Lett. 2020, 18, 1243–1275. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sharp, R.E. Complexity and coordination of root growth at low water potentials: Recent advances from transcriptomic and proteomic analyses. Plant Cell Environ. 2010, 33, 590–603. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Abdelkrim, S.; Jebara, S.H.; Saadani, O.; Abid, G.; Taamalli, W.; Zemni, H.; Jebara, M. In Situ effects of Lathyrus sativus-PGPR to remediate and restore quality and fertility of Pb and Cd polluted soils. Ecotoxicol. Environ. Saf. 2020, 192, 110260. [Google Scholar] [CrossRef]
- Morcillo, R.J.; Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Ahmed, B.; Shahid, M.; Syed, A.; Rajput, V.D.; Elgorban, A.M.; Minkina, T.; Lee, J. Drought tolerant Enterobacter sp./Leclercia adecarboxylata secretes indole-3-acetic acid and other biomolecules and enhances the biological attributes of Vigna radiata (L.) R. Wilczek in water deficit conditions. Biology 2021, 10, 1149. [Google Scholar] [CrossRef]
- Hou, X.; Teng, W.; Hu, Y.; Yang, Z.; Li, C.; Scullion, J.; Zheng, R. Potential phytoremediation of soil cadmium and zinc by diverse ornamental and energy grasses. BioResources 2020, 15, 616–640. [Google Scholar] [CrossRef]
- Benidire, L.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Assessment of plant growth-promoting bacterial populations in the rhizosphere of metallophytes from the Kettara mine, Marrakech. Environ. Sci. Pollut. Res. 2016, 23, 21751–21765. [Google Scholar] [CrossRef] [PubMed]
- El Hamiani, O.; El Khalil, H.; Sirguey, C.; Ouhammou, A.; Bitton, G.; Schwartz, C.; Boularbah, A. Metal concentrations in plants from mining areas in South Morocco: Health risks assessment of consumption of edible and aromatic plants. CLEAN–Soil. Air Water 2015, 43, 399–407. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Amer Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Aubert, G. Methodes D’analyses Des Sols, 2nd ed.; Centre régional de Documentation Pédagogique, C.R.D.P.: Marseille, France, 1978; p. 360. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil. Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 1995; 569p. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]



| pH | EC (mS cm−1) | P Olsen (mg kg−1) | TN (%) | OC (%) | |||
|---|---|---|---|---|---|---|---|
| Pre-plant | 7.55 ± 0.20 | 4.71 ± 0.04 | 42.84 ± 0.61 | 0.32 ± 0.01 | 3.11 ± 0.05 | ||
| Atriplex halimus | Non inoculated | Control | 6.72 ± 0.04 a,** | 4.35 ± 0.02 b,** | 40.86 ± 3.63 b,NS | 0.51 ± 0.02 a,** | 4.70 ± 0.02 a,** |
| Moderate stress | 6.62 ± 0.05 b,** | 4.34 ± 0.01 b,** | 40.17 ± 5.35 b,NS | 0.42 ± 0.03 b,** | 4.68 ± 0.11 a,** | ||
| Severe stress | 6.75 ± 0.03 a,** | 4.42 ± 0.02 a,** | 52.68 ± 3.06 a,** | 0.38 ± 0.04 b,NS | 3.68 ± 0.09 b,** | ||
| Inoculated | Control | 6.57 ± 0.02 a,** | 4.21 ± 0.04 b,** | 51.30 ± 5.31 a,** | 0.54 ± 0.01 a,** | 4.71 ± 0.04 a,** | |
| Moderate stress | 6.54 ± 0.05 a,** | 4.32 ± 0.05 a,** | 55.30 ± 5.85 a,** | 0.53 ± 0.04 a,** | 4.37 ± 0.58 a,** | ||
| Severe stress | 6.51 ± 0.02 a,** | 3.90 ± 0.04 c,** | 55.31 ± 6.09 a,** | 0.38 ± 0.43 b,** | 4.68 ± 0.11 a,** | ||
| Two-Way ANOVA | *** F(I) = 72.532 | *** F(I) = 180.549 | ** F(I) = 15.793 | ** F(I) = 7.506 | NS F(I) = 0.015 | ||
| * F(R) = 5.277 | *** F(R) = 36.093 | * F(R) = 4.152 | *** F(R) = 25.434 | * F(R) = 10.111 | |||
| * F(IxR) = 6.004 | * ** F(IxR) = 78.615 | NS F(IxR) = 2.370 | * F(IxR) = 3.945 | NS F(IxR) = 1.393 | |||
| Penisetum setaceum | Non inoculated | Control | 6.66 ± 0.05 a,** | 4.21 ± 0.04 b,** | 47.97 ± 1.35 a,NS | 0.42 ± 0.02 a,** | 3.64 ± 0.02 a,** |
| Moderate stress | 6.72 ± 0.03 a,** | 4.32 ± 0.05 a,** | 48.63 ± 0.15 a | 0.45 ± 0.07 a,** | 3.60 ± 0.03 a,** | ||
| Severe stress | 6.72 ± 0.04 a,** | 3.90 ± 0.04 c,** | 45.31 ± 2.89 a,NS | 0.34 ± 0.04 b,** | 3.71 ± 0.04 a,** | ||
| Inoculated | Control | 7.25 ± 0.01 a,** | 4.32 ± 0.02 b,** | 60.86 ± 4.38 a,** | 0.50 ± 0.07 bc,** | 4.20 ± 0.02 b,** | |
| Moderate stress | 6.51 ± 0.05 b,** | 4.34 ± 0.04 b,** | 63.51 ± 1.35 a,** | 0.54 ± 0.02 b,** | 4.20 ± 0.02 b,** | ||
| Severe stress | 6.46 ± 0.30 b,** | 4.42 ± 0.02 a,** | 41.68 ± 3.50 b,NS | 0.56 ± 0.03 ab,** | 4.36 ± 0.03 a,** | ||
| Two-Way ANOVA | NS F(I) = 0.379 | *** F(I) = 180.549 | *** F(I) = 41.102 | *** F(I) = 85.32 | *** F(I) = 611.163 | ||
| *** F(R) = 14.706 | ** F(R) = 36.093 | *** F(R) = 39.541 | * F(R) = 4.316 | * F(R) = 10.866 | |||
| *** F(IxR) = 19.82 | *** F(IxR) = 78.61 | *** F(IxR) = 21.87 | ** F(IxR) = 11.15 | NSF(IxR) = 0.89 | |||
| Peganum harmala | Non inoculated | Control | 7.12 ± 0.02 a,** | 3.67 ± 0.02 c,** | 37.42 ± 2.50 a,NS | 0.42 ± 0.04 a,** | 3.81 ± 0.01 a,** |
| Moderate stress | 6.44 ± 0.02 b,** | 4.35 ± 0.04 b,** | 40.29 ± 1.87 a,NS | 0.43 ± 0.01 a,** | 3.77 ± 0.02 a,** | ||
| Severe stress | 6.35 ± 0.03 c,** | 4.46 ± 0.02 a,** | 38.21 ± 1.56 a,NS | 0.45 ± 0.03 a,** | 3.65 ± 0.04 b,** | ||
| Inoculated | Control | 7.10 ± 0.02 a,** | 4.23 ± 0.01 a,** | 54.15 ± 1.78 c,** | 0.53 ± 0.07 a,** | 4.67 ± 0.05 a,** | |
| Moderate stress | 6.31 ± 0.02 b,** | 4.23 ± 0.03 a,** | 57.42 ± 0.94 b,** | 0.53 ± 0.08 a,** | 4.67 ± 0.01 a,** | ||
| Severe stress | 6.32 ± 0.01 b,** | 3.87 ± 0.07 a,** | 60.29 ± 1.87 a,** | 0.51 ± 0.02 a,** | 4.43 ± 0.02 b,** | ||
| Two-Way ANOVA | *** F(I) = 119.102 | * F(I) = 6.258 | *** F(I) = 472.278 | *** F(I) = 152.581 | *** F(I) = 1293.901 | ||
| *** F(R) = 2258.193 | *** F(R) = 110.180 | * F(R) = 6.516 | NSF(R) = 0.070 | *** F(R) = 31.168 | |||
| NSF(IxR) = 2.261 | *** F(IxR) = 309.705 | * F(IxR) = 4.021 | * F(IxR) = 4.395 | NSF(IxR) = 2.806 | |||
| Bacteria (×107 CFU g−1 dry soil) | Fungi (×104 CFU g−1 dry soil) | Actinobacteria (×103 CFU g−1 dry soil) | |||
|---|---|---|---|---|---|
| Atriplex halimus | Non inoculated | Control | 10.79 ± 0.01 a | 2.33 ± 0.51 b | 28.72 ± 0.01 a |
| Moderate stress | 10.95 ± 0.01 a | 5.00 ± 0.60 a | 24.05 ± 0.02 b | ||
| Severe stress | 9.053 ± 0.02 b | 4.53 ± 0.11 a | 20.08 ± 0.04 c | ||
| Inoculated | Control | 11.32 ± 0.01 a | 5.40 ± 0.30 a | 34.72 ± 0.01 a | |
| Moderate stress | 11.34 ± 0.03 a | 4.93 ± 0.57 a | 33.33 ± 0.03 a | ||
| Severe stress | 10.33 ± 0.02 b | 3.20 ± 0.40 b | 22.72 ± 0.02 b | ||
| Two-Way ANOVA | *** F(I) = 105.585 | * F(I) = 7.440 | *** F(I) = 33.136 | ||
| *** F(R) = 182.036 | *** F(R) = 12.964 | *** F(R) = 34.682 | |||
| *** F(IxR) = 16.730 | *** F(IxR) = 41.226 | NSF(IxR) = 3.409 | |||
| Penisetum setaceum | Non inoculated | Control | 11.48 ± 0.48 a | 3.47 ± 0.23 a | 22.05 ± 0.20 a |
| Moderate stress | 10.68 ± 0.40 b | 2.40 ± 0.01 b | 25.34 ± 0.41 b | ||
| Severe stress | 9.413 ± 0.12 c | 1.80 ± 0.20 c | 14.72 ± 0.20 c | ||
| Inoculated | Control | 12.63 ± 0.18 a | 4.13 ± 0.46 a | 25.32 ± 0.23 a | |
| Moderate stress | 12.29 ± 0.13 b | 4.73 ± 0.23 a | 27.36 ± 0.23 a | ||
| Severe stress | 10.67 ± 0.23 c | 2.67 ± 0.42 b | 18.08 ± 0.11 b | ||
| Two-Way ANOVA | *** F(I) = 113.483 | *** F(I) = 78.233 | * F(I) = 6.036 | ||
| *** F(R) = 105.897 | *** F(R) = 44.860 | *** F(R) = 25.857 | |||
| NSF(IxR) = 1.303 | *** F(IxR) = 13.000 | NSF(IxR) = 0.143 | |||
| Pegau harmala | Non inoculated | Control | 99.10 ± 0.32 a | 4.80 ± 0.69 a | 31.33 ± 0.11 a |
| Moderate stress | 94.51 ± 0.61 b | 3.73 ± 0.23 b | 32.02 ± 0.20 a | ||
| Severe stress | 88.57 ± 0.14 c | 2.40 ± 0.40 c | 14.73 ± 0.23 b | ||
| Inoculated | Control | 106.21 ± 0.72 a | 5.27 ± 0.30 a | 46.75 ± 0.30 a | |
| Moderate stress | 105.03 ± 0.11 b | 4.47 ± 0.11 b | 40.08 ± 0.34 b | ||
| Severe stress | 103.24 ± 0.30 c | 3.67 ± 0.11 c | 32.03 ± 0.20 c | ||
| Two-Way ANOVA | *** F(I) = 212.253 | *** F(I) = 22.443 | *** F(I) = 137.815 | ||
| *** F(R) = 29.521 | *** F(R) = 44.328 | *** F(R) = 69.148 | |||
| *** F(IxR) = 9.23 | *** F(IxR) = 1.836 | * F(IxR) = 6.037 | |||
| Parameters | Mine Tailings | Topsoil | Marble Sludge | Sheep Manure | Amended Mine Tailings |
|---|---|---|---|---|---|
| pH H2O | 2.36 ± 0.01 | 8.02 ± 0.07 | 10.33 ± 0.24 | 8.36 ± 0.11 | 7.55 ± 0.2 |
| pH KCl | 2.19 ± 0.12 | 7.88 ± 0.13 | 8.89 ± 0.11 | 7.92 ± 0.21 | 6.53 ± 0.16 |
| EC (mS cm−1) | 7.86 | 2.44 | 0.198 | 11.47 | 4.71 ± 0.04 |
| Organic Carbon (%) | Nd * | 1.13 ± 0.08 | 1.2 ± 0.05 | 40 ± 0.11 | 3.11 ± 0.05 |
| Total N (%) | Nd * | 0.17 ± 0.06 | 0.14 ± 0.16 | 1.28 ± 0.05 | 0.32 ± 0.01 |
| Olsen-P (mg kg−1) | Nd * | 43.42 ± 0.04 | 20 ± 1.23 | 1047 ± 11.24 | 42.84 ± 0.61 |
| CaCO3 (%) | - | 16.89 ± 0.14 | 57.42 ± 0.25 | - | - |
| Texture | Loam | Sandy Loam | - | - | Sandy Loam |
| Clay (%) | 7.47 | 5.58 | - | - | 6 |
| Silt (%) | 37.37 | 39.08 | - | - | 41 |
| Sand (%) | 50.47 | 52.89 | - | - | 53 |
| Moisture content (%) | 41.92 | 40.72 | 40.62 | 40.49 | 39.11 |
| Total Cd (mg kg−1) | 1.07 ± 0.04 | 0.08 ± 0.03 | 0.05 ± 0.01 | 0.12 ± 0.01 | 0.01 ± 0.001 |
| Total Pb (mg kg−1) | 178.48 ± 7.52 | 31.01 ± 7.37 | 1.2 ± 0.23 | 17.25 ± 0.41 | 45.47 ± 5.07 |
| Total Zn (mg kg−1) | 275.93 ± 10.66 | 102.47 ± 10.67 | 7.5 ± 0.31 | 73.82 ± 0.33 | 118.09 ± 7.53 |
| Total Cu (mg kg−1) | 1084.19 ± 64.2 | 424.08 ± 44.37 | 8.53 ± 0.22 | 17.03 ± 0.37 | 414.36 ± 43.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atika, M.; Leila, B.; Pereira, S.I.A.; Castro, P.M.L.; Ali, B. Enhancing Native Plant Establishment in Mine Tailings under Drought Stress Conditions through the Application of Organo-Mineral Amendments and Microbial Inoculants. Plants 2024, 13, 863. https://doi.org/10.3390/plants13060863
Atika M, Leila B, Pereira SIA, Castro PML, Ali B. Enhancing Native Plant Establishment in Mine Tailings under Drought Stress Conditions through the Application of Organo-Mineral Amendments and Microbial Inoculants. Plants. 2024; 13(6):863. https://doi.org/10.3390/plants13060863
Chicago/Turabian StyleAtika, Madline, Benidire Leila, Sofia I. A. Pereira, Paula M. L. Castro, and Boularbah Ali. 2024. "Enhancing Native Plant Establishment in Mine Tailings under Drought Stress Conditions through the Application of Organo-Mineral Amendments and Microbial Inoculants" Plants 13, no. 6: 863. https://doi.org/10.3390/plants13060863
APA StyleAtika, M., Leila, B., Pereira, S. I. A., Castro, P. M. L., & Ali, B. (2024). Enhancing Native Plant Establishment in Mine Tailings under Drought Stress Conditions through the Application of Organo-Mineral Amendments and Microbial Inoculants. Plants, 13(6), 863. https://doi.org/10.3390/plants13060863









