Abstract
Prefoldins (PFDs) are ubiquitous co-chaperone proteins that originated in archaea during evolution and are present in all eukaryotes, including yeast, mammals, and plants. Typically, prefoldin subunits form hexameric PFD complex (PFDc) that, together with class II chaperonins, mediate the folding of nascent proteins, such as actin and tubulin. In addition to functioning as a co-chaperone in cytoplasm, prefoldin subunits are also localized in the nucleus, which is essential for transcription and post-transcription regulation. However, the specific and critical roles of prefoldins in plants have not been well summarized. In this review, we present an overview of plant prefoldin and its related proteins, summarize the structure of prefoldin/prefoldin-like complex (PFD/PFDLc), and analyze the versatile landscape by prefoldin subunits, from cytoplasm to nucleus regulation. We also focus the specific role of prefoldin-mediated phytohormone response and global plant development. Finally, we overview the emerging prefoldin-like (PFDL) subunits in plants and the novel roles in related processes, and discuss the next direction in further studies.
1. Introduction
Molecular chaperones are a class of proteins that are ubiquitous from prokaryotes to eukaryotes. From the defined concept, chaperone proteins can bind noncovalently to nascent peptide chains or unfolded proteins, helping them to fold, assemble, transport, and degrade, but do not exist in their final conformation. Chaperonins are divided into two types: the group I chaperonins are present in the mitochondria, chloroplasts, and the bacterial cytoplasm; the group II components are found in the cytoplasm of archaea and eukaryotic cells [1,2]. One of these auxiliary co-chaperones is prefoldin (PFD), found in archaea and eukaryotes, whose best-known function is to assist in the folding of unnatural proteins (especially cytoskeletal proteins) by delivering them to group II chaperonins [3,4]. There are six prefoldin subunits (PFD1–PFD6) that make up the prefoldin complex (PFDc) in eukaryotes [5,6,7,8,9]. The discovery of prefoldins is more than 20 years old and was initially named as Gims (genes involved in microtubule biogenesis) in a screen for mutants that are synthetically lethal with a mutated yeast γ-tubulin strain [10]. It was later renamed as “prefoldin” because it presented homologs in archaea and eukaryotes and was characterized as a co-chaperone protein in the cytoplasm [4]. In contrast to eukaryotic prefoldin, the archaeal prefoldin was soon systematically identified in Methanobacterium thermoautotrophicum [11]. While prefoldin chaperones in yeast and mammals have been intensively studied in recent decades, systematic research on the prefoldin in plants began only as late as 2009, despite the early report of bioinformatic analyses [12,13]. In recent studies, prefoldin subunits or complexes have been identified in the regulation of gene expression, transcription, transcript splicing, and protein homeostasis and have further been linked to human disease and plant growth [3,7,9,14,15,16,17,18], indicating the versatile capacity of prefoldins in multiple organisms.
In addition to the typical PFDc, there is another prefoldin-like (PFDL) complex (PFDLc) present in all eukaryotes, but not in archaea. As that PFDc, human PFDLc is constituted by prefoldin subunits with additional variant prefoldin partners to form the heterohexamer [7,16,19,20]. Unlike typical PFDc, the PFDLc has the additional ability to participate in RNA polymerases assembly [21,22], to stabilize phosphatidylinositol-3 kinase-associated protein kinases [23], and to inhibit certain cytoplasmic enzymes [24], and may also provide a platform for the stabilization and maturation of intracellular multiprotein complexes [25]. A very recent study demonstrated the existence of PFDLc in Arabidopsis thaliana (Arabidopsis) [26], indicating the conserved process in species evolution.
In fact, prefoldins and prefoldin-like subunits or complexes play fundamental roles in cell life. As fixed-growth organisms, plants have attracted much attention to harmonize the external variable environment with intracellular events for better cell survival. Although the study of plant prefoldin lags behind by a decade compared with yeast and mammals, the conserved and specific roles of plant prefoldins have been gradually aroused in recent years, implying their great potential in the plant kingdom. In this review, we first present the structure of PFDc and PFDLc. We then analyze the dynamic landscapes of plant prefoldins related processes, from cytoplasm to nucleus, and from phytohormone to global development. Finally, we put an unconventional PFDL subunit URI (unconventional prefoldin RPB5 interactor) as a paradigm, to review its conservation and specificities.
2. Structure of the Prefoldin Complex and Prefoldin-Like Complex
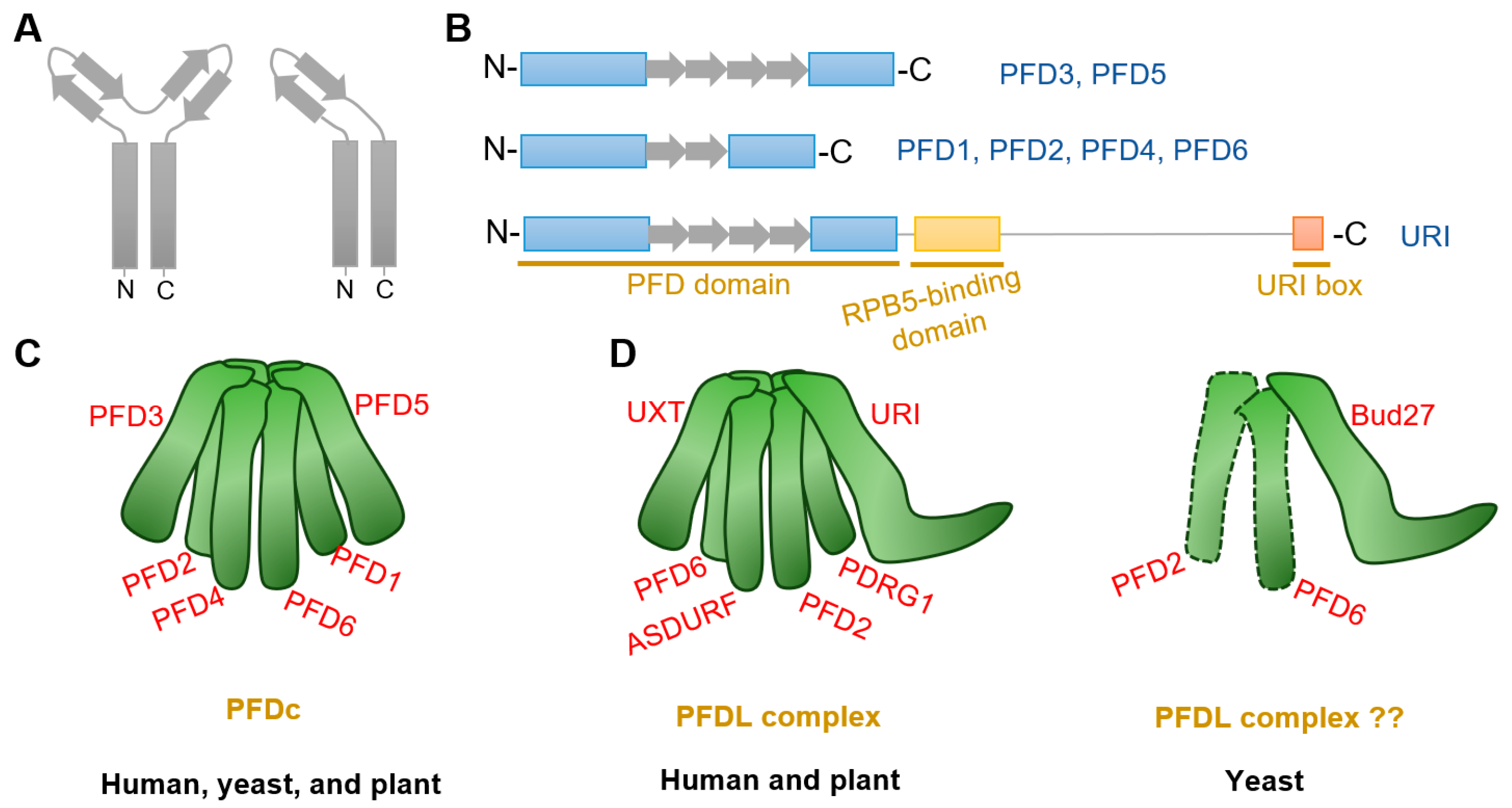
Prefoldin subunits are small proteins consisting of two coiled-coil structural domains and four or two β-strands that form α-type and β-type hairpin structures, respectively (Figure 1A,B). Eukaryotes PFDc consists two different α-types (PFD3 and PFD5) and four different β-types (PFD1, PFD2, PFD4, and PFD6) to form a heterohexameric complex (Table 1) [8,15], whereas in archaea, each type is replaced by either two or four copies [5,11,27]. Although yeast PFDc is still customarily called as “Gim complex”, all eukaryotes’ PFDc forms a similar jellyfish-like conformation with a rigid β barrel backbone in the center, from which six flexible coiled tentacles extend (Figure 1C). These six tentacles extend in the same direction and able to bind a variety of nascent client proteins based on substrate affinities formed by hydrophobic cavities generated by intrinsic residues in the distal region [5,6]. Unlike other chaperones, PFDc does not hydrolyze ATP when it physical interacts with its client proteins, but undergoes a stranding period until it is transferred to the group II chaperonin, CCT (chaperonins containing T-complex polypeptide-1, also named as TRiC (T-complex polypeptide-1 ring complex)), for further folding [6]. In addition, PFDc also protects unnatural proteins when they are captured by CCT and in the releasing stage [5]. Finally, when PFDc comes into contact with CCT, immature and conformationally unstable proteins are naturally transferred to CCT due to its high affinity and PFDc is automatically released [28].
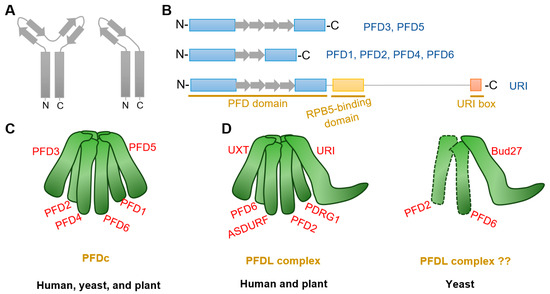
Figure 1.
Structure of PFDc and PFDLc. (A,B) Structure of α-type (including PFD3 and PFD5) and β-type (including PFD1, PFD2, PFD4, and PFD6) prefoldins. Rectangles and arrows represent coiled-coil domain and β strands, respectively. α-type prefoldins contain four β strands to form two hairpins, whereas β-type prefoldins construct only one hairpin. In addition to the typical prefoldin subunits, another PFDL named URI constitutes with external RPB5-binding domain and a URI box. (C) Model of canonical PFDc in human (Homo sapiens), yeast (Saccharomyces cerevisiae), and plant (Arabidopsis thaliana). Note that all species showed similar PFDc conformation, although yeast is customarily named “Gim” (see Table 1). (D) Model of PFDLc in human, yeast, and plant. Note that human and plant PFDLc display similar confirmation, while there is no direct evidence for the existence of the yeast PFDL complex.
Plant PFDc also contains six different subunits as that in eukaryotes (Table 1) [29]. Interestingly, each subunit in Arabidopsis showing more similarity to the orthologs of the corresponding subunit in human than to other subunits in the same organisms [12]. Arabidopsis PFDc showed high similarity confirmation with that in human [29]. Furthermore, analysis of prefoldin gene families from 14 plant species showed that all subunit genes have their close orthologues [30]. Moreover, both plant and human subunits can complement the defective phenotypes by deletion of yeast prefoldins [10,13]. All these findings strength the conservative properties of plant prefoldins.

Table 1.
The canonical and prefoldin-like subunit in different organism and research advances in plants.
Table 1.
The canonical and prefoldin-like subunit in different organism and research advances in plants.
| Type | Name in Arabidopsis | AGI * Number | Name in Human | Name in Yeast | Functions in Plant |
|---|---|---|---|---|---|
| prefoldin subunit | |||||
| α-type | PFD3 | AT5G49510 | PFD3; VBP1 | Gim2 | cytoskeleton [13]; chromatin remodeling [31]; salt stress [13]; PINs trafficking [32]; GA-dependent interaction [33] |
| PFD5 | AT5G23290 | PFD5; MM1 | Gim5 | cytoskeleton [13]; chromatin remodeling [31]; salt stress [13]; PINs trafficking [32]; GA-dependent interaction [33] | |
| β-type | PFD1 | AT2G07340 | PFD1 | Gim6 | cytoskeleton [29]; chromatin remodeling [31] |
| PFD2 | AT3G22480 | PFD2 | Gim4 | cytoskeleton [29]; chromatin remodeling [31] | |
| PFD4 | AT1G08780 | PFD4 | Gim3 | cytoskeleton [18]; chromatin remodeling [31]; splicing [14]; cold acclimation [18] | |
| PFD6 | AT1G29990 | PFD6 | Gim1 | cytoskeleton [34]; chromatin remodeling [31]; PINs trafficking [35] | |
| prefoldin-like subunit | |||||
| α-type | URI | AT1G03760 | URI; RMP | Bud27 | cytoskeleton; PINs trafficking [32] |
| UXT | AT1G26660 | UXT; STAP1 | - | ? | |
| β-type | PDRG1 | AT3G15351 | PDRG1 | - | ? |
| ASDURF | AT1G49245 | ASDURF | - | ? | |
* AGI: Arabidopsis Gene Identifier. The short line (-) represent it do not exist in this organism, and the question mark (?) represent the function is unknown.
Another special PFDLc exists in the eukaryote kingdom. Similarly, human PFDLc is constituted of α2β4 elements. Two α-types are replaced by two variants called UXT (ubiquitously expressed transcript) and URI (unconventional prefoldin RNA polymerase binding subunit 5 (RPB5) interactor), while four β-types are composed of the typical PFD2, PFD6, an additional PDRG1 (p53 and DNA damage-regulated gene 1), and another β-type PFDL called ASDURF (asparagine synthetase domain-containing 1 upstream reading frame) (Table 1; Figure 1D) [15,16,19,25,36]. Yet, it contains only the URI homologue Bud27, as the unique PFDL protein exist in yeast (Table 1) [37,38]. Although Bud27 may interact with PFD6 by yeast two-hybrid assay and affinity purification in Saccharomyces cerevisiae [21,39,40], there is currently no direct and valid evidence for the exact subunits that constitute the yeast PFDL complex (Figure 1D).
Very recent studies in Arabidopsis indicates the existence of PFDL subunits in plants [26,32]. Arabidopsis URI ortholog was firstly identified by forward genetic screening with altered auxin distribution [32]. Further analysis demonstrated that URI was the PFDL subunits and showed conserved roles in Arabidopsis [26,32]. Moreover, putative ortholog analysis identified PDRG1, UXT, and ASDURF genes (Table 1), and AP-MS assay demonstrated URI, PFD2, PFD6, PDRG1, and ASDURF as interactors of UXT [26]. The interactions between URI and PFD2/PFD6 were also verified by yeast-two-hybrid assay and co-immunoprecipitate assay [32]. All these findings confirm the presence of PFDLc in the plant kingdom, which is similar to that in humans (Figure 1D).
3. Dynamic Functions of Prefoldin Proteins in Plants
3.1. Proteins Folding by PFDc and Its Associate Partner CCT
3.1.1. Prefoldin Subunits in Cytoskeleton Organization
Newly unfolded proteins easily aggregate to disturb the proteostasis [1,41,42]. Nascent actin and tubulin always spontaneously self-organize, so the PFDc protects them until they are correctly folded. Therefore, PFDc plays the fundamental role in cytoskeleton organization. Plant cytoskeleton networks are involved in massive developmental processes, including pollen tube growth, root hair elongation, trichome morphogenesis, response to light signals, salt, pathogen attacks, and other biotic or abiotic stresses [43,44,45,46,47]. In fact, defects in one of these subunits in Arabidopsis are sufficient to cause abnormal cytoskeleton (Table 1) and links to developmental defects, with the aggravated phenotype by multiple mutants [13,14,18,34]. All prefoldin subunit mutants except pfd1 exhibited abnormal microtubule arrangement in root elongation zones or hypocotyl cells compared with the wild type; all prefoldin single or multiple mutants are more sensitive to the microtubule depolymerizing drug oryzalin; and all prefoldin single mutants are sufficient to decrease the α-tubulin level [13,18,29,34]. Notably, prefoldin subunits are involved in microtubule-associated processes only as part of the PFDc, since the sextuple mutant does not increase sensitivity to oryzalin or decrease the α-tubulin level [29], suggesting that the prefoldin subunits act as the PFDc that promote microtubule organization and mediate the associated phenotypes in plants. However, there is a lack of direct experimental evidence as to whether the prefoldin subunits or the PFDc are required for actin organization in plant cells.
The critical function of prefoldins for cytoskeleton-related phenotypes is conserved in various species. For example, reductions in functional prefoldins reduce levels of α-tubulin and microtubule growth rates, leading to abnormal distal tip cell migration in Caenorhabditis elegans [48]. Mutations in Drosophila allele result in abnormal spindle and centrosome deletions and disruption of neuroblast polarity due to tubulin instability [49,50]. These landscapes reveal the intrinsic and conserved role of prefoldins in animals and plants. Nevertheless, from another point of view, sessile plants’ adaptation to perpetual environmental challenges associated with cytoskeleton organization is a specific mechanism for the evolution of green plants. Further studies could also focus on the coordination between PFDc and environmental stress stimuli.
3.1.2. CCT Chaperonin, the Protein Folding Partner with PFDc
CCT chaperonin is the typical PFDc-anchored target protein. CCT complex is a conserved multi-subunit complex consisting of two back-to-back rings, each containing eight related but distinct paralogous subunits (named CCT1 to CCT8), forming a complex of approximately 1000 kDa. Eukaryotic CCT complex mediates nascent client peptides folding by encapsulating them in its enclosed central cavity [51,52,53,54,55]. Actin and tubulin captured by PFDc absolutely require CCT for refolding so the cytoskeleton is much more dependent on this biomacromolecules. In human, dysfunction of the CCT chaperonin leads to severe disease symptoms such as neurological-related disorders and cancer, largely due to their dysregulation in protein proteostasis and aggregation [51,54,55,56,57]. With similar construction and function, the Arabidopsis CCT complex is also assembled from eight CCT subunits, except that CCT6 has two coding regions that are 96% identical [12,58]. Plant CCT deficient cells exhibited depletion of cortical microtubules, accompanied by reduced tubulin level due to protein degradation [58], which is similar to the disorganized arrangement of tubulin in the PFD gene depletion mutants [13,18,29,34]. Moreover, prefoldin subunits also involved in the pathogenesis of human neurodegenerative diseases and tumors [7,9,59], which reveals the tightly correlation with CCT. Thus, PFD-CCT could be the conserved module in controlling the protein folding for proteostasis in organisms, both in mammals and plants.
There is a specific role of CCT subunits in determining the maintenance of plant stem cells. A recent study found that intrinsic enrichment levels of different CCT subunits in root stem cells ensured protein folding activity and proteomic integrity, thereby conferring proteotoxic stress [60]. For example, cct7-2 and cct8-2 exhibit a shorter root and disturbed stem cell niche, especially abnormal cell division and QC identity [60] and defective cell-to-cell trafficking [61]. Interestingly, the co-chaperone PFDc is also highly expressed in stem cells [60], implying that the PFD-CCT module may co-regulate stem cell identity and root growth and development. Because one subunit of the CCT subunits is sufficient to cause lethality in Arabidopsis [61], new biotechnological approaches, such as RNAi or bioinformatics analysis, could be considered to dissect the mechanism of PFD-CCT module in plant plasticity development, and of course, this is a permanent topic need to be concerned.
In addition to the cytoskeleton, there is approximately 10% other cellular substrates bind to the CCT complex for folding [55,62]. These proteins are involved in many important cellular processes, including cell cycle, cell division, DNA maintenance, metabolism, RNA processing, etc. [55]. In recent years, the catalytic subunit of protein phosphatase 4 (PP4c) has emerged as a candidate novel substrate for plant CCT; another protein, Tap46, appears to stabilize PP4c and prevent its degradation when bound to the CCT complex [58]. This finding implying the possibility linking CCT to protein phosphatase status, which may be associated with plant metabolism and growth.
3.2. Prefoldins in Nucleus
The previous section focuses on the function of prefoldin as a co-chaperone with CCT chaperonin for protein folding, especially for tubulin organization; clearly, this process occurs in the cytoplasm. It should be emphasized that plant prefoldins are also localized into nucleus [14,18,33]. Although prefoldins cannot act directly as transcription factors due to their lack of ability to bind DNA, they are also required in animals and plants to mediate nuclear gene expression in various aspects of the regulatory hierarchy. Here, we provide examples to fully understand their functions in various aspects.
3.2.1. Transcription Regulation
Lots of studies put forward the critical role of prefoldins involved in transcript gene expression. Excess PFD1 in human lung tumors triggers its aggregation in the nucleus and binds to the transcription start site of the Cyclin A gene, repressing its expression [63]. The yeast prefoldin subunits bind to chromatin and affect RNA polymerase II activity, which regulate the transcription elongation [64]. Other in-depth studies have found that human PFD5 can act as a bridge protein to recruit other transcription co-repressors and functions as a tumor suppressor [65,66,67]. In addition, PFD6 directly binds to the FOXO (forkhead box O) transcription factor and enhances its transcriptional activity, thereby modulating the longevity response of Caenorhabditis elegans [68]. Therefore, nuclear prefoldins appears to regulate gene expression either by directly affecting the activity of core transcription factors or indirectly by recruiting other factors to regulate downstream cellular events.
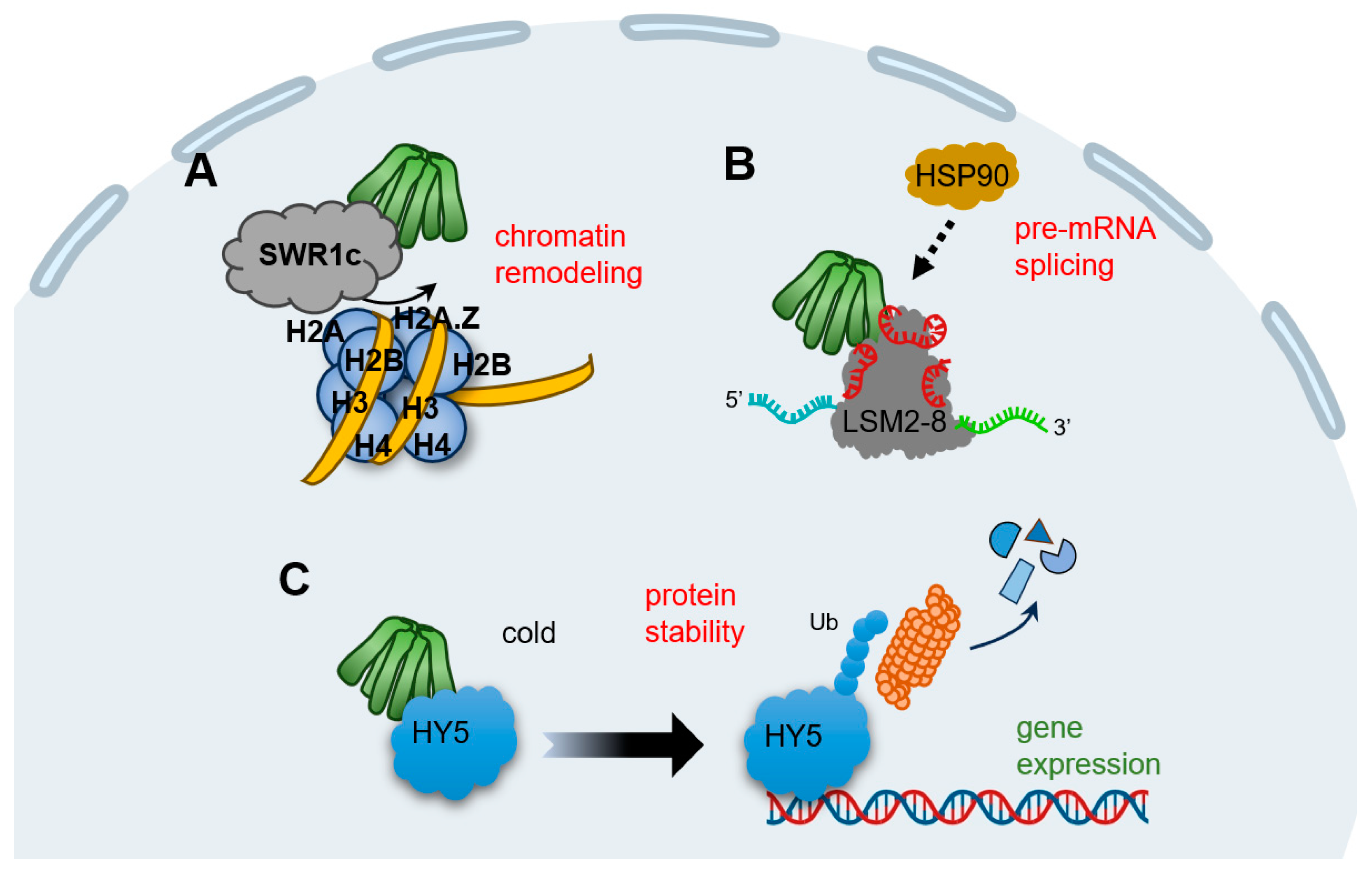
Transcriptomic studies and ChIP-seq experiments in the model plant Arabidopsis demonstrate that prefoldins affect multiple groups of gene profiles [14,29,31]. This mechanism is much dependent on the requirement of prefoldins in chromatin remodeling (Figure 2A). SWR1c (SWI2/SNF2-related 1 chromatin remodeling complex) is the highly conserved complex to promote the exchange of H2A/H2B dimers with H2A.Z/H2B, which affects nucleosome stability and chromatin structure [69,70,71,72]. Notably, all prefoldin subunits interact with at least one of the components of SWR1c, indicating PFDc associate with chromatin remodeling to mediate gene expression (Table 1) [31]. More interestingly, the contribution of prefoldins to H2A.Z deposition corresponds to environment responsive genes [31], further strengthening the notion that prefoldins in the environmental adaptation. In next studies, it is necessary to dissect how prefoldins regulate SWR1c activity, and more importantly, to occupy the specific location in a set of gene loci responding to the environmental challenge.
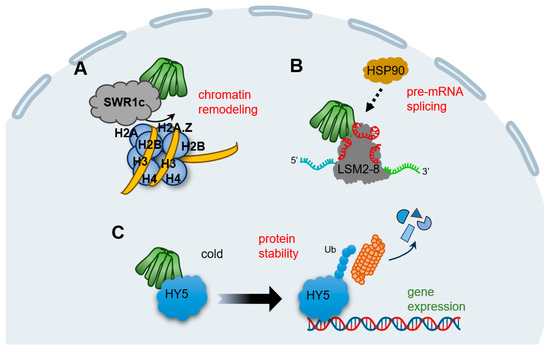
Figure 2.
Schematic representation of the known plant PFDc in nucleus. (A) Prefoldins associate with SWR1c to favor H2A/H2B into H2A.Z/H2B to affect chromatin structure. (B) Prefoldins interact and mediate the activity of LSM2-8 spliceosome and then direct the pre-mRNA splicing. PFD4 may be a subunit to recruit HSP90. (C) In cold stimulation, HY5 promotes the expression of cold-inducible transcripts, while PFD4 interact with HY5, triggering its ubiquitination and degradation, to attenuate this process and ensure the proper products of downstream transcripts.
3.2.2. For Pre-mRNA Splicing
Pre-mRNA splicing is a fundamental process to expand proteome of eukaryotic organisms in post-transcriptional level [73,74]. In Arabidopsis, the key component of LSM2-8 (Sm-like 2–8) spliceosome complex is encoded by LSM genes, and PFDs are tightly co-expressed with LSMs [14]. Besides, all six prefoldins interact with LSM8, a specialized subunit required for LSM2-8 complex formation; this interaction maintained the adequate level of LSM8, and therefore, mediated the activity of the LSM2-8 spliceosome in response to U6 snRNA levels and pre-mRNA splicing patterns (Figure 2B) [14]. Interestingly, PFD4 acts as a co-chaperone associated with HSP90 that regulate LSM8 activity and mediate their interaction (Table 1) [14]. Although it is unclear whether PFD4 interacts directly with HSP90 or requires another co-chaperone, there is clearly an essential role for prefoldins involved in pre-mRNA splicing events in plant, which may open the door to a role for molecular chaperones in pre-mRNA splicing regulation. In line with this, human prefoldins act locally in the genome to regulate co-transcriptional pre-mRNA splicing [17], implying that prefoldins mediate the transcriptional splicing across different organisms.
3.2.3. Protein Stability Regulation
Typical prefoldins are involved in gene expression not only by regulating the transcription or post-transcription processes, but also by mediating proteins stability. For instance, human PFD5 promotes c-Myc degradation by recruiting the proteasomes and a novel ubiquitin E3 ligase [75]. Another typical example is that prefoldins mediate the expression of viral genes by facilitating the interaction of HIV integrase with the VHL (von Hippel–Lindau) ubiquitin ligase, triggering its polyubiquitination and degradation [76]. In the case of plants, we enumerate a vital example of prefoldin-mediated low temperature acclimatization. HY5 (ELONGATED HYPOCOTYL 5), a member of bZIP (basic leucine zipper) transcription factors that integrates various processes including light, hormones, nutrients, and stress conditions [77,78,79,80,81], could be regulated by prefoldins at the post-translational level [18]. When plants perceive the cold stimulation, nuclear HY5 promotes the expression of cold-inducible transcripts, including anthocyanin biosynthesis genes, whereas prefoldins accumulate into nucleus and the interaction of PFD4-HY5 triggers HY5 ubiquitination and degradation, which in turn reduce its protein level and ensures the proper products of anthocyanins under cold stress (Table 1) (Figure 2C) [18]. Overall, this feedback loop ensures the balance transcripts in response to cold conditions. Remarkably, this nucleocytoplasmic shuttling of prefoldins is DELLA-dependent, a transcriptional regulator found in plants but not in other animals [82]. Therefore, although prefoldins link gene expression by modifying proteins stability in both humans and plants, distinct regulators exert the specific mechanism in different organism.
3.3. Special Correlation of Prefoldins to Plant Hormones
Plant hormones have been extensively studied in recent decades. These low-concentration molecules produced by plant metabolism are widely involved in plant growth, development, and stress response to the environment. Among them, the gibberellic acid (GA) and auxin are two important regulators of plant growth related to prefoldins. Here, we focus on the mechanism of prefoldin to GA signaling pathway and auxin transporters trafficking in plant cells.
3.3.1. GA Signaling
The two most critical components of GA signaling include the GA receptor GID1 (gibberellin insensitive dwarf 1) and DELLA growth inhibitors. The current model of GA signaling suggests that GID1 senses GA signal and binds to DELLA to form the GA-GID1-DELLA trimeric complex, which subsequently triggers the polyubiquitinylation of DELLA and its degradation via the 26S proteasome pathway [82,83,84]. Therefore, when the GA level is high, DELLA is degraded and switches on GA signaling to promote plant growth; conversely, DELLA inhibits plant growth. Furthermore, DELLA integrates other hormonal signals and environmental cues to regulate plant growth [82,84,85]. The current study reveals a novel mechanism by which PFDc integrates GA-DELLA signaling and microtubules-mediated cell wall expansion. According to the yeast two-hybrid screen, PFD3 and PFD5 were captured as DELLA-interaction proteins (Table 1); this interaction triggers the accumulation of prefoldins in the nucleus and regulates their equilibrium level between the cytoplasm and the nucleus [33]. Notably, the localization of the entire PFDc rather than individual subunits into the nucleus depends on the presence of DELLA, although only PFD3/PFD5 interact with DELLA [33]. Thus, in the presence of GA, PFDc loses its ability to interact with DELLA because of its degradation and stays in the cytoplasm and functions as the cytoskeleton folding co-chaperone, whereas in the absence of GA, PFDc shuttles into the nucleus and severely compromises microtubules heterodimer. Therefore, PFDc is linked to GA signaling and regulates the balance of cell wall expansion through DELLA-dependent subcellular localization. Furthermore, the interaction between prefoldins and DELLA appears to be conserved, as PdPFD2.2 interacts with a putative DELLA in Populus deltoides, and the localization of PdPFD2.2 is regulated by this interaction [86]. Moreover, there are five GA responsive elements upstream of the PdPFD2.2 gene locus, suggesting the possibility in responsive to GA [86]. Interestingly, overexpression PdPFD2.2 increased biofuel conversion, which provides potential for bioenergy utilizing in the crop Populus [86,87].
3.3.2. Auxin Signaling and Transport
A number of clues suggest a correlation between prefoldins and auxin signaling. Firstly, functional network analysis of maize prefoldins mapped a number of candidate interactors, including ABP1 (auxin-binding protein 1); in addition, 13 prefoldin genes in maize showed different responses to IAA treatment, with 3 genes up-regulated, 2 unchanged, and others down-regulated; furthermore, there are multiple ARF (auxin response factor) binding sites, auxin-inducible elements, and other hormone- or stress-inducible elements upstream in prefoldin genes locus [30]. All these findings implying that prefoldins may associate with auxin signaling. However, the delicate molecular mechanisms between prefoldin and auxin signaling pathways are still puzzling, so revealing their relationship is important and interesting for further work.
A unique property of auxin is the targeted cell-to-cell transport to establish the auxin gradients and maxima. Auxin transporters, including PINs (PIN-FORMED), exert the critical role in this process [88,89,90]. In particular, PINs turn out to circulate continuously between the PM (plasma membrane) and endosomes, which has been termed as PINs trafficking; this trafficking mechanism include the recycling pathway and the degradation pathway. In the former, PM-localized PINs are continuously endocytosed, and subsequently, returned to the PM with the secretion of the GNOM protein [88,90,91,92]; in the latter, PINs are degraded via endosome-mediated vacuolar targeting in a manner that is dependent on ubiquitin modification [92,93]. An effective way to monitor the cycling of PINs is BFA (brefeldin A) treatment, a fungal toxin that triggers the aggregation of PINs to form the so-called “BFA compartments” [91,94]. Interestingly, prefoldin mutants reduce the incidence of PIN2 in the PM [35]; this event may be related to defects in the transport of PIN2 from the PM to the endosome or back to the PM, as BFA treatment reveal extensive BFA compartment in pfd3 or pfd5 mutants [32]. Therefore, prefoldin subunits can link the distribution of auxin by regulating polarity localization of auxin transporters (e.g., PINs), at least in their mode of transport (Table 1).
3.3.3. Are Prefoldins Required for the Coordination between GA and Auxin?
Multiple studies have revealed the tightly relationship between GA and auxin. For example, auxin is required to promote GA signaling response [95]. In turn, GA modulates the transport of PINs to regulate the auxin gradient, as evidenced by the fact that high GA levels required for PINs to circulate back to the PM and inhibit their vacuolar targeting, whereas low GA levels promote their vacuolar transport [96,97]. Besides, some ARFs may be associated with DELLA, and thus, massive downstream components could be regulated [98,99]. Therefore, one concerned question aims that the coordination between prefoldins and phytohormone crosstalk. Notably, prefoldins do action in GA-facilitated PINs transport [35], indicating that prefoldins are required in the crosstalk between auxin and GA pathways and further regulate hormone-mediated plant development. Further studies could focus on the functions of prefoldins in other hormones, which is a special manner to distinguish those animals.
3.4. Global Regulation for Plant Growth in a PFDc-Dependent or -Independent Manner
To dissect the different processes regulated by PFDc or individual subunits under conditions of plant development or environmental stress, a recent study generated a sextuple mutant by crossing that were defective in the activity for each subunit [29]. The antagonistic regulation between GA and abscisic acid (ABA) signal is thought to be a major determinant in the control of seed germination [100,101]. An earlier study indicates that PFD4 maybe one candidate ABI3 (abscisic acid insensitive 3)-interacting partners [102], but it lacks validation later. Recent transcriptomic data also enrich germination-related terms in the 6×pfd mutant, and all prefoldin genes are transcriptionally active in imbibed seeds [29]. Therefore, prefoldin subunits may be involved in seed germination through an ABA-dependent signaling pathway. However, this process may be dependent on the entire PFDc rather than individual subunits, as all pfd single mutants exhibited a similar delay in germination compared with the 6×pfd mutant [29].
The growth of hypocotyl and root are also much dependent on the canonical complex, as the 6×pfd mutant did not show any exacerbated phenotype. Consistent with this, the flowering time is regulated in a PFDc-dependent manner. However, the growth of another organ, the rosette leaf, may be regulated in at least two ways because of the smaller size of the rosette leaf in the 6×pfd compared with the single pfd mutant [29]. Therefore, there are at least two ways to regulate plant growth in different scenarios.
Prefoldins are also involved in abiotic stress adaptation. Earlier studies showed that the yeast pfd mutants are hypersensitivities to salt stress [10,13,103]. With parallel evolution, all prefoldin subunits in Arabidopsis are required for root growth under salt stress (100 mM NaCl). Interestingly, this response may be Na+-specific because neither ionic nor osmotic stress requires each subunit [29]. It is possible that the PFDc is involved in salt stress response in a manner associated with cytoskeletal reorientation, as salt stress induces depolymerization and reorganization of cortical microtubules [104,105,106,107]. Another abiotic stress response, cold acclimation, is also dependent on the entire PFDc, as any stacking effect is lacking in the 6×pfd mutant [29].
The individual prefoldin subunit is much exert to regulate gene expression. Transcriptomic data showed that more differentially expressed genes (DEGs) were found in 6×pfd compared with pfd4 single mutant (a total of 1186 DEGs in 6×pfd, while only 198 DEGs in pfd4) [14,29], highlighting the relevance of the individual subunit of the prefoldin, or the alternative complex, rather than the entire hexamer, in mediating gene expression. Interestingly, the DEGs in the 6×pfd can be divided into three gene clusters and each of which is associated with a number of key transcription factors [29]. Thus, single prefoldin subunit or alternative recombinants are required to regulate gene expression for plant development.
4. The Unconventional Prefoldin RPB5 Interactor, a Representative PFDL Subunit
In addition to the classic prefoldin subunits, there are also PFDL members exist in eukaryotes kingdom. Human and plant PFDL subunits include URI, UXT, PDRG1, and ASDURF [26,36], while Bud27 (URI homologue) is the exclusive PFDL in yeast [38]. Here, we put plant URI as the paradigm and summarize the conservations and specificities compared with that in animals.
The URI and its orthologues across species contain three conserved regions known as the PFD domain, the RPB5-binding domain, and the URI box (Figure 1B) [32,37,38,108]. The PFD domain is highly conserved and thought to be required for interaction with PFDL modules, therefore forming the PFDL complex [19]. The mutation in the PFD domain of Arabidopsis URI eliminate its close interaction with PFD6 also strength this notion [32]. In fact, this interaction is associated with the cytoskeleton arrangement and auxin transport, at least by PINs trafficking (Table 1) [32].
Downstream of the PFD domain is a large extend fragment and so called as the intrinsically disordered region [26]. The disordered structure is important for various effector functions, because the flexibility region provide a platform to interact with other partners or undergo protein modifications [109,110]. In Chlamydomonas reinhardtii, some intrinsically disordered proteins are related to unfolded protein binding or chaperone function [111], indicating the tightly association between molecular chaperones and disordered regions. Nevertheless, this unique region endowed URI more functions beyond as a co-chaperone. For example, the RPB5-binding domain in this region is essential for RNA polymerase-related events. Both the human URI and its yeast ortholog Bud27 bind and regulate the stability of RPB5, a common subunit of three eukaryotic RNA polymerases, affecting RNA polymerase assembly or activity [19,21,22,112]. Yeast Bud27 also regulates RNA polymerase-dependent transcription and ribosome biogenesis processes [113,114]. In Arabidopsis, URI has an extensive interactome than UXT, another PFDL subunit that lack the extended region downstream in PFD domain, and more importantly, most of the URI-associate partners are related to RNA-binding or metabolic processing [26]. In conclusion, the RPB5-binding domain confers a variety of functions not found in typical prefoldins. It is interesting to explore how plant URI facilitates RNA polymerase related processes, such as ribosomal biogenesis, transcription, or translation.
As for the last “URI box”, which encodes a short α-helix peptide at the C-terminus (Figure 1B), little is known about the exact function. A few animal studies imply that it may be required for transcription or translation [39,115]. However, the URI box in plants has not been accurately annotated.
In human, URI also functions as a signaling protein by phosphorylation transformation. For example, URI phosphorylation prevents its interaction with PP1γ (protein phosphatase 1γ) [24], and the released URI could regulate O-linked N-acetylglucosamine transferase activity [116], both of which regulate cell survival. Particularly, there are 13 phosphorylated Ser/Thr residues in Arabidopsis URI, and these phosphorylation residues are required to maintain its stability [26]. Interestingly, all of them are in the disordered region, which is in line with two phosphorylated sites in human [19,26]. Further work could focus on the mechanism of plant URI phosphorylation and the linkage of this phosphorylation transformation to cell life.
5. Conclusions and Future Directions
Generic prefoldin chaperones are not only involved in cytoskeleton folding together with their associated partners (e.g., CCT), but are also required for protein stability and gene expression regulation at the transcription or post-transcription level. The role of plant prefoldins have some specificities compared to the progress in animals. In this review, we summarize the conserved roles of prefoldin in the structure of the prefoldin complex, the associated protein CCT, and cytoskeleton folding processes. In addition, we focus on the specific roles of prefoldin in plant development and give some paradigms for a comprehensive understanding. These roles involve coordination with hormonal signals, such as GA and auxin, PFDc-dependent or independent responses to abiotic stress, and regulation of environment adaptation by nuclear prefoldins. In addition to the classical prefoldin subunits, other PFDL members have gained attention in recent years. The URI prefoldin-like subunit appears to be required for a wider range of cellular processes according to the extend region. Certain aspects of prefoldin-related modes of action require further research.
For the prefoldin subunits, some of the following issues can be addressed: (i) what transcription factors can be recruited and how do they coordinate gene expression with prefoldins; (ii) since prefoldin is required for the GA and auxin pathways, how does it mediate upstream environmental signals and regulate intracellular biosynthesis or transport; (iii) why are there PFDc-dependent and -independent ways during plant growth or abiotic stresses, and what is the intrinsic mechanism of substituting subunits during these processes; and (iv) the molecular mechanism by which prefoldins work together with their co-chaperone proteins to promote protein stability remains undermined.
For prefoldin-like subunits, some questions remain to be answered: (i) how do the URI contribute to RNA polymerase-related processes, such as ribosome synthesis, transcription initiation/elongation, or translation in plants, and what are the specific roles of the URI in plants; (ii) does URI manipulate those cellular events by its individual subunit or PFDL complex; and (iii) what kind of proteins could be recruited and regulate URI phosphorylation and how do they work. All these questions need to be seriously considered.
Author Contributions
Writing—original draft preparation, Y.Y. and G.Z.; writing—review and editing, Y.Y., M.S., Q.S. and Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by project grants from the Natural Science Foundation of Shandong of China (ZR2023QC138; ZR2020QC034); the Shandong Province Higher Educational Science and Technology Program (2023KJ270); and the Talent Introduction Project of Dezhou University of China (2022xjrc410; 2022xjrc427).
Data Availability Statement
Data from this study are available in this article.
Acknowledgments
The authors thank Robert Wang and Alan Ahlquist for their help with reviewing the English language.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Horwich, A.L.; Fenton, W.A.; Chapman, E.; Farr, G.W. Two families of chaperonin: Physiology and mechanism. Annu. Rev. Cell Dev. Biol. 2007, 23, 115–145. [Google Scholar] [CrossRef] [PubMed]
- Millán-Zambrano, G.; Chávez, S. Nuclear functions of prefoldin. Open Biol. 2014, 4, 140085. [Google Scholar] [CrossRef] [PubMed]
- Vainberg, I.E.; Lewis, S.A.; Rommelaere, H.; Ampe, C.; Vandekerckhove, J.; Klein, H.L.; Cowan, N.J. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell 1998, 93, 863–873. [Google Scholar] [CrossRef]
- Siegert, R.; Leroux, M.R.; Scheufler, C.; Hartl, F.U.; Moarefi, I. Structure of the molecular chaperone prefoldin: Unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell 2000, 103, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Sahlan, M.; Zako, T.; Yohda, M. Prefoldin, a jellyfish-like molecular chaperone: Functional cooperation with a group II chaperonin and beyond. Biophys. Rev. 2018, 10, 339–345. [Google Scholar] [CrossRef]
- Liang, J.; Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Yi, P.; Han, Y.; Luo, X.; Wang, H.; Tang, L.; et al. The functions and mechanisms of prefoldin complex and prefoldin-subunits. Cell Biosci. 2020, 10, 87. [Google Scholar] [CrossRef]
- Arranz, R.; Martín-Benito, J.; Valpuesta, J.M. Structure and Function of the Cochaperone Prefoldin. Adv. Exp. Med. Biol. 2018, 1106, 119–131. [Google Scholar]
- Tahmaz, I.; Shahmoradi Ghahe, S.; Topf, U. Prefoldin Function in Cellular Protein Homeostasis and Human Diseases. Front. Cell Dev. Biol. 2021, 9, 816214. [Google Scholar] [CrossRef]
- Geissler, S.; Siegers, K.; Schiebel, E. A novel protein complex promoting formation of functional α- and γ-tubulin. Embo J. 1998, 17, 952–966. [Google Scholar] [CrossRef]
- Leroux, M.R.; Fändrich, M.; Klunker, D.; Siegers, K.; Lupas, A.N.; Brown, J.R.; Schiebel, E.; Dobson, C.M.; Hartl, F.U. MtGimC, a novel archaeal chaperone related to the eukaryotic chaperonin cofactor GimC/prefoldin. Embo J. 1999, 18, 6730–6743. [Google Scholar] [CrossRef]
- Hill, J.E.; Hemmingsen, S.M. Arabidopsis thaliana type I and II chaperonins. Cell Stress Chaperones 2001, 6, 190–200. [Google Scholar] [CrossRef]
- Rodríguez-Milla, M.A.; Salinas, J. Prefoldins 3 and 5 play an essential role in Arabidopsis tolerance to salt stress. Mol. Plant 2009, 2, 526–534. [Google Scholar] [CrossRef]
- Esteve-Bruna, D.; Carrasco-López, C.; Blanco-Touriñán, N.; Iserte, J.; Calleja-Cabrera, J.; Perea-Resa, C.; Úrbez, C.; Carrasco, P.; Yanovsky, M.J.; Blázquez, M.A.; et al. Prefoldins contribute to maintaining the levels of the spliceosome LSM2-8 complex through Hsp90 in Arabidopsis. Nucleic Acids Res. 2020, 48, 6280–6293. [Google Scholar] [CrossRef]
- Herranz-Montoya, I.; Park, S.; Djouder, N. A comprehensive analysis of prefoldins and their implication in cancer. iScience 2021, 24, 103273. [Google Scholar] [CrossRef] [PubMed]
- Payán-Bravo, L.; Peñate, X.; Chávez, S. Functional Contributions of Prefoldin to Gene Expression. Adv. Exp. Med. Biol. 2018, 1106, 1–10. [Google Scholar]
- Payán-Bravo, L.; Fontalva, S.; Peñate, X.; Cases, I.; Guerrero-Martínez, J.A.; Pareja-Sánchez, Y.; Odriozola-Gil, Y.; Lara, E.; Jimeno-González, S.; Suñé, C.; et al. Human prefoldin modulates co-transcriptional pre-mRNA splicing. Nucleic Acids Res. 2021, 49, 6267–6280. [Google Scholar] [CrossRef]
- Perea-Resa, C.; Rodríguez-Milla, M.A.; Iniesto, E.; Rubio, V.; Salinas, J. Prefoldins Negatively Regulate Cold Acclimation in Arabidopsis thaliana by Promoting Nuclear Proteasome-Mediated HY5 Degradation. Mol. Plant 2017, 10, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Mita, P.; Savas, J.N.; Ha, S.; Djouder, N.; Yates, J.R., 3rd; Logan, S.K. Analysis of URI nuclear interaction with RPB5 and components of the R2TP/prefoldin-like complex. PLoS ONE 2013, 8, e63879. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Hernández, H.; Pal, M.; Rodríguez, C.F.; Prodromou, C.; Pearl, L.H.; Llorca, O. Advances on the Structure of the R2TP/Prefoldin-like Complex. Adv. Exp. Med. Biol. 2018, 1106, 73–83. [Google Scholar]
- Mirón-García, M.C.; Garrido-Godino, A.I.; García-Molinero, V.; Hernández-Torres, F.; Rodríguez-Navarro, S.; Navarro, F. The prefoldin bud27 mediates the assembly of the eukaryotic RNA polymerases in an rpb5-dependent manner. PLoS Genet. 2013, 9, e1003297. [Google Scholar] [CrossRef]
- Vernekar, D.V.; Bhargava, P. Yeast Bud27 modulates the biogenesis of Rpc128 and Rpc160 subunits and the assembly of RNA polymerase III. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2015, 1849, 1340–1353. [Google Scholar] [CrossRef]
- Horejsí, Z.; Takai, H.; Adelman, C.A.; Collis, S.J.; Flynn, H.; Maslen, S.; Skehel, J.M.; de Lange, T.; Boulton, S.J. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol. Cell 2010, 39, 839–850. [Google Scholar] [CrossRef]
- Djouder, N.; Metzler, S.C.; Schmidt, A.; Wirbelauer, C.; Gstaiger, M.; Aebersold, R.; Hess, D.; Krek, W. S6K1-mediated disassembly of mitochondrial URI/PP1γ complexes activates a negative feedback program that counters S6K1 survival signaling. Mol. Cell 2007, 28, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lynham, J.; Houry, W.A. The Multiple Functions of the PAQosome: An R2TP- and URI1 Prefoldin-Based Chaperone Complex. Adv. Exp. Med. Biol. 2018, 1106, 37–72. [Google Scholar] [PubMed]
- Gómez-Mínguez, Y.; Palacios-Abella, A.; Costigliolo-Rojas, C.; Barber, M.; Hernández-Villa, L.; Úrbez, C.; Alabadí, D. The prefoldin-like protein AtURI exhibits characteristics of instrinsically disordered proteins. FEBS Lett. 2023. early view. [Google Scholar] [CrossRef]
- Lim, S.; Glover, D.J.; Clark, D.S. Prefoldins in Archaea. Adv. Exp. Med. Biol. 2018, 1106, 11–23. [Google Scholar] [PubMed]
- Zako, T.; Iizuka, R.; Okochi, M.; Nomura, T.; Ueno, T.; Tadakuma, H.; Yohda, M.; Funatsu, T. Facilitated release of substrate protein from prefoldin by chaperonin. FEBS Lett. 2005, 579, 3718–3724. [Google Scholar] [CrossRef]
- Blanco-Touriñán, N.; Esteve-Bruna, D.; Serrano-Mislata, A.; Esquinas-Ariza, R.M.; Resentini, F.; Forment, J.; Carrasco-López, C.; Novella-Rausell, C.; Palacios-Abella, A.; Carrasco, P.; et al. A genetic approach reveals different modes of action of prefoldins. Plant Physiol. 2021, 187, 1534–1550. [Google Scholar] [CrossRef]
- Cao, J. Analysis of the Prefoldin Gene Family in 14 Plant Species. Front. Plant Sci. 2016, 7, 317. [Google Scholar] [CrossRef]
- Marí-Carmona, C.; Forment, J.; Blázquez, M.A.; Alabadí, D. A role for prefoldins in H2A.Z deposition in Arabidopsis. bioRxiv 2021. [CrossRef]
- Yang, Y.; Liu, F.; Liu, L.; Zhu, M.; Yuan, J.; Mai, Y.X.; Zou, J.J.; Le, J.; Wang, Y.; Palme, K.; et al. The unconventional prefoldin RPB5 interactor mediates the gravitropic response by modulating cytoskeleton organization and auxin transport in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1916–1934. [Google Scholar] [CrossRef] [PubMed]
- Locascio, A.; Blázquez, M.A.; Alabadí, D. Dynamic regulation of cortical microtubule organization through prefoldin-DELLA interaction. Curr. Biol. 2013, 23, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Deng, Z.; Paredez, A.R.; DeBolt, S.; Wang, Z.Y.; Somerville, C. Prefoldin 6 is required for normal microtubule dynamics and organization in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 18064–18069. [Google Scholar] [CrossRef] [PubMed]
- Salanenka, Y.; Verstraeten, I.; Löfke, C.; Tabata, K.; Naramoto, S.; Glanc, M.; Friml, J. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, P.; Poitras, C.; Faubert, D.; Bouchard, A.; Blanchette, M.; Gauthier, M.S.; Coulombe, B. Upstream ORF-Encoded ASDURF Is a Novel Prefoldin-like Subunit of the PAQosome. J. Proteome Res. 2020, 19, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gstaiger, M.; Luke, B.; Hess, D.; Oakeley, E.J.; Wirbelauer, C.; Blondel, M.; Vigneron, M.; Peter, M.; Krek, W. Control of nutrient-sensitive transcription programs by the unconventional prefoldin URI. Science 2003, 302, 1208–1212. [Google Scholar] [CrossRef]
- Martínez-Fernández, V.; Garrido-Godino, A.I.; Cuevas-Bermudez, A.; Navarro, F. The Yeast Prefoldin Bud27. Adv. Exp. Med. Biol. 2018, 1106, 109–118. [Google Scholar]
- Deplazes, A.; Möckli, N.; Luke, B.; Auerbach, D.; Peter, M. Yeast Uri1p promotes translation initiation and may provide a link to cotranslational quality control. EMBO J. 2009, 28, 1429–1441. [Google Scholar] [CrossRef]
- Ito, T.; Chiba, T.; Ozawa, R.; Yoshida, M.; Hattori, M.; Sakaki, Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 2001, 98, 4569–4574. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Hartl, F.U. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Bhandari, D.D.; Brandizzi, F. Plant endomembranes and cytoskeleton: Moving targets in immunity. Curr. Opin. Plant Biol. 2020, 58, 8–16. [Google Scholar] [CrossRef]
- Kost, B.; Mathur, J.; Chua, N.H. Cytoskeleton in plant development. Curr. Opin. Plant Biol. 1999, 2, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.J.; Huang, R.D. Cytoskeleton and plant salt stress tolerance. Plant Signal Behav. 2011, 6, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lian, N.; Zhang, Y.; Man, Y.; Chen, L.; Yang, H.; Lin, J.; Jing, Y. The Cytoskeleton in Plant Immunity: Dynamics, Regulation, and Function. Int. J. Mol. Sci. 2022, 23, 15553. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, T. Understanding the functions and mechanisms of plant cytoskeleton in response to environmental signals. Curr. Opin. Plant Biol. 2019, 52, 86–96. [Google Scholar] [CrossRef]
- Lundin, V.F.; Srayko, M.; Hyman, A.A.; Leroux, M.R. Efficient chaperone-mediated tubulin biogenesis is essential for cell division and cell migration in C. elegans. Dev. Biol. 2008, 313, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rai, M.; Wang, C.; Gonzalez, C.; Wang, H. Prefoldin and Pins synergistically regulate asymmetric division and suppress dedifferentiation. Sci. Rep. 2016, 6, 23735. [Google Scholar] [CrossRef] [PubMed]
- Delgehyr, N.; Wieland, U.; Rangone, H.; Pinson, X.; Mao, G.; Dzhindzhev, N.S.; McLean, D.; Riparbelli, M.G.; Llamazares, S.; Callaini, G.; et al. Drosophila Mgr, a Prefoldin subunit cooperating with von Hippel Lindau to regulate tubulin stability. Proc. Natl. Acad. Sci. USA 2012, 109, 5729–5734. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Willardson, B.M. Mechanistic insights into protein folding by the eukaryotic chaperonin complex CCT. Biochem. Soc. Trans. 2022, 50, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.; Dalton, K.; Frydman, J. The Mechanism and Function of Group II Chaperonins. J. Mol. Biol. 2015, 427, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Joachimiak, L.A.; Walzthoeni, T.; Liu, C.W.; Aebersold, R.; Frydman, J. The structural basis of substrate recognition by the eukaryotic chaperonin TRiC/CCT. Cell 2014, 159, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liu, C.; Han, W.; Cong, Y. TRiC/CCT Chaperonin: Structure and Function. Subcell. Biochem. 2019, 93, 625–654. [Google Scholar] [PubMed]
- Wang, D.Y.; Kamuda, K.; Montoya, G.; Mesa, P. The TRiC/CCT Chaperonin and Its Role in Uncontrolled Proliferation. Adv. Exp. Med. Biol. 2020, 1243, 21–40. [Google Scholar] [PubMed]
- Ghozlan, H.; Cox, A.; Nierenberg, D.; King, S.; Khaled, A.R. The TRiCky Business of Protein Folding in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 906530. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J. The Molecular Chaperone CCT/TRiC: An Essential Component of Proteostasis and a Potential Modulator of Protein Aggregation. Front. Genet. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Yoon, J.T.; Choi, I.; Kim, S.; Lee, H.S.; Pai, H.S. Functional characterization of chaperonin containing T-complex polypeptide-1 and its conserved and novel substrates in Arabidopsis. J. Exp. Bot. 2019, 70, 2741–2757. [Google Scholar] [CrossRef]
- Mo, S.J.; Zhao, H.C.; Tian, Y.Z.; Zhao, H.L. The Role of Prefoldin and Its Subunits in Tumors and Their Application Prospects in Nanomedicine. Cancer Manag. Res. 2020, 12, 8847–8856. [Google Scholar] [CrossRef]
- Llamas, E.; Torres-Montilla, S.; Lee, H.J.; Barja, M.V.; Schlimgen, E.; Dunken, N.; Wagle, P.; Werr, W.; Zuccaro, A.; Rodríguez-Concepción, M.; et al. The intrinsic chaperone network of Arabidopsis stem cells confers protection against proteotoxic stress. Aging Cell 2021, 20, e13446. [Google Scholar] [CrossRef]
- Xu, X.M.; Wang, J.; Xuan, Z.; Goldshmidt, A.; Borrill, P.G.; Hariharan, N.; Kim, J.Y.; Jackson, D. Chaperonins facilitate KNOTTED1 cell-to-cell trafficking and stem cell function. Science 2011, 333, 1141–1144. [Google Scholar] [CrossRef]
- Yam, A.Y.; Xia, Y.; Lin, H.T.; Burlingame, A.; Gerstein, M.; Frydman, J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 2008, 15, 1255–1262. [Google Scholar] [CrossRef]
- Wang, D.; Shi, W.; Tang, Y.; Liu, Y.; He, K.; Hu, Y.; Li, J.; Yang, Y.; Song, J. Prefoldin 1 promotes EMT and lung cancer progression by suppressing cyclin A expression. Oncogene 2017, 36, 885–898. [Google Scholar] [CrossRef]
- Millán-Zambrano, G.; Rodríguez-Gil, A.; Peñate, X.; de Miguel-Jiménez, L.; Morillo-Huesca, M.; Krogan, N.; Chávez, S. The prefoldin complex regulates chromatin dynamics during transcription elongation. PLoS Genet. 2013, 9, e1003776. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Taira, T.; Maeda, Y.; Tanaka, S.; Nishihara, H.; Iguchi-Ariga, S.M.; Nagashima, K.; Ariga, H. MM-1, a c-Myc-binding protein, is a candidate for a tumor suppressor in leukemia/lymphoma and tongue cancer. J. Biol. Chem. 2001, 276, 45137–45144. [Google Scholar] [CrossRef]
- Mori, K.; Maeda, Y.; Kitaura, H.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. MM-1, a novel c-Myc-associating protein that represses transcriptional activity of c-Myc. J. Biol. Chem. 1998, 273, 29794–29800. [Google Scholar] [CrossRef] [PubMed]
- Satou, A.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. A novel transrepression pathway of c-Myc. Recruitment of a transcriptional corepressor complex to c-Myc by MM-1, a c-Myc-binding protein. J. Biol. Chem. 2001, 276, 46562–46567. [Google Scholar] [CrossRef]
- Son, H.G.; Seo, K.; Seo, M.; Park, S.; Ham, S.; An, S.W.A.; Choi, E.S.; Lee, Y.; Baek, H.; Kim, E.; et al. Prefoldin 6 mediates longevity response from heat shock factor 1 to FOXO in C. elegans. Genes. Dev. 2018, 32, 1562–1575. [Google Scholar] [CrossRef]
- Mizuguchi, G.; Shen, X.; Landry, J.; Wu, W.H.; Sen, S.; Wu, C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 2004, 303, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Fakher, B.; Jakada, B.H.; Cao, S.; Qin, Y. SWR1 Chromatin Remodeling Complex: A Key Transcriptional Regulator in Plants. Cells 2019, 8, 1621. [Google Scholar] [CrossRef]
- Iyer, V.R. The specificity of H2A.Z occupancy in the yeast genome and its relationship to transcription. Curr. Genet. 2020, 66, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Scacchetti, A.; Becker, P.B. Variation on a theme: Evolutionary strategies for H2A.Z exchange by SWR1-type remodelers. Curr. Opin. Cell Biol. 2021, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef]
- Shenasa, H.; Bentley, D.L. Pre-mRNA splicing and its cotranscriptional connections. Trends Genet. 2023, 39, 672–685. [Google Scholar] [CrossRef]
- Kimura, Y.; Nagao, A.; Fujioka, Y.; Satou, A.; Taira, T.; Iguchi-Ariga, S.M.; Ariga, H. MM-1 facilitates degradation of c-Myc by recruiting proteasome and a novel ubiquitin E3 ligase. Int. J. Oncol. 2007, 31, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Mousnier, A.; Kubat, N.; Massias-Simon, A.; Ségéral, E.; Rain, J.C.; Benarous, R.; Emiliani, S.; Dargemont, C. von Hippel–Lindau binding protein 1-mediated degradation of integrase affects HIV-1 gene expression at a postintegration step. Proc. Natl. Acad. Sci. USA 2007, 104, 13615–13620. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J.F. The Multifaceted Roles of HY5 in Plant Growth and Development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef]
- Xiao, Y.; Chu, L.; Zhang, Y.; Bian, Y.; Xiao, J.; Xu, D. HY5: A Pivotal Regulator of Light-Dependent Development in Higher Plants. Front. Plant Sci. 2021, 12, 800989. [Google Scholar] [CrossRef]
- Cluis, C.P.; Mouchel, C.F.; Hardtke, C.S. The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J. 2004, 38, 332–347. [Google Scholar] [CrossRef]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2-COP1-HY5-BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Fu, X. GA action: Turning on de-DELLA repressing signaling. Curr. Opin. Plant Biol. 2007, 10, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Huang, X.Z.; Xiao, S.L.; Fu, X.D. Evolutionarily conserved DELLA-mediated gibberellin signaling in plants. J. Integr. Plant Biol. 2008, 50, 825–834. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Li, M.; Ding, J.; Pu, Y.; Bryan, A.C.; Rottmann, W.; Winkeler, K.A.; Collins, C.M.; Singan, V.; et al. Overexpression of a Prefoldin β subunit gene reduces biomass recalcitrance in the bioenergy crop Populus. Plant Biotechnol. J. 2020, 18, 859–871. [Google Scholar] [CrossRef]
- Macaya-Sanz, D.; Chen, J.G.; Kalluri, U.C.; Muchero, W.; Tschaplinski, T.J.; Gunter, L.E.; Simon, S.J.; Biswal, A.K.; Bryan, A.C.; Payyavula, R.; et al. Agronomic performance of Populus deltoides trees engineered for biofuel production. Biotechnol. Biofuels 2017, 10, 253. [Google Scholar] [CrossRef]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef]
- Michniewicz, M.; Brewer, P.B.; Friml, J. Polar auxin transport and asymmetric auxin distribution. Arabidopsis Book 2007, 5, e0108. [Google Scholar]
- Sauer, M.; Kleine-Vehn, J. PIN-FORMED and PIN-LIKES auxin transport facilitators. Development 2019, 146, dev168088. [Google Scholar] [CrossRef]
- Geldner, N.; Anders, N.; Wolters, H.; Keicher, J.; Kornberger, W.; Muller, P.; Delbarre, A.; Ueda, T.; Nakano, A.; Jürgens, G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003, 112, 219–230. [Google Scholar] [CrossRef]
- Zhou, J.J.; Luo, J. The PIN-FORMED Auxin Efflux Carriers in Plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Zhang, Y.; Li, Y.; Pei, Y.; Zhang, M. Regulation of PIN-FORMED Protein Degradation. Int. J. Mol. Sci. 2023, 24, 843. [Google Scholar] [CrossRef]
- Friml, J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur. J. Cell Biol. 2010, 89, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Harberd, N.P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Löfke, C.; Zwiewka, M.; Heilmann, I.; Van Montagu, M.C.; Teichmann, T.; Friml, J. Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism. Proc. Natl. Acad. Sci. USA 2013, 110, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Willige, B.C.; Isono, E.; Richter, R.; Zourelidou, M.; Schwechheimer, C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell 2011, 23, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Ben-Targem, M.; Ripper, D.; Bayer, M.; Ragni, L. Auxin and gibberellin signaling cross-talk promotes hypocotyl xylem expansion and cambium homeostasis. J. Exp. Bot. 2021, 72, 3647–3660. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Su, H.; Cao, H.; Wei, H.; Fu, X.; Jiang, X.; Song, Q.; He, X.; Xu, C.; Luo, K. AUXIN RESPONSE FACTOR7 integrates gibberellin and auxin signaling via interactions between DELLA and AUX/IAA proteins to regulate cambial activity in poplar. Plant Cell 2022, 34, 2688–2707. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.; Jones, H.D.; Holdsworth, M.J. Interactions of the developmental regulator ABI3 with proteins identified from developing Arabidopsis seeds. Plant J. 2000, 21, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Warringer, J.; Ericson, E.; Fernandez, L.; Nerman, O.; Blomberg, A. High-resolution yeast phenomics resolves different physiological features in the saline response. Proc. Natl. Acad. Sci. USA 2003, 100, 15724–15729. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Yuan, M. Salt tolerance requires cortical microtubule reorganization in Arabidopsis. Plant Cell Physiol. 2007, 48, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Yuan, M.; Ge, Y.; Liu, Y.; Fan, J.; Ruan, Y.; Cui, Z.; Tong, S.; Zhang, S. The microfilament cytoskeleton plays a vital role in salt and osmotic stress tolerance in Arabidopsis. Plant Biol. 2010, 12, 70–78. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Li, C.; Zhu, L.; Fu, Y.; Guo, Y. Imaging of Cortical Microtubules in Plants Under Salt Stress. Methods Mol. Biol. 2023, 2604, 257–261. [Google Scholar]
- Zhou, S.; Chen, Q.; Li, X.; Li, Y. MAP65-1 is required for the depolymerization and reorganization of cortical microtubules in the response to salt stress in Arabidopsis. Plant Sci. 2017, 264, 112–121. [Google Scholar] [CrossRef]
- Thomas, P.A.; Mita, P.; Ha, S.; Logan, S.K. Role of the Unconventional Prefoldin Proteins URI and UXT in Transcription Regulation. Adv. Exp. Med. Biol. 2018, 1106, 85–94. [Google Scholar]
- Trivedi, R.; Nagarajaram, H.A. Intrinsically Disordered Proteins: An Overview. Int. J. Mol. Sci. 2022, 23, 14050. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Launay, H.; Schramm, A.; Lebrun, R.; Gontero, B. Exploring intrinsically disordered proteins in Chlamydomonas reinhardtii. Sci. Rep. 2018, 8, 6805. [Google Scholar] [CrossRef]
- Martínez-Fernández, V.; Navarro, F. Rpb5, a subunit shared by eukaryotic RNA polymerases, cooperates with prefoldin-like Bud27/URI. AIMS Genet. 2018, 5, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, V.; Cuevas-Bermúdez, A.; Gutiérrez-Santiago, F.; Garrido-Godino, A.I.; Rodríguez-Galán, O.; Jordán-Pla, A.; Lois, S.; Triviño, J.C.; de la Cruz, J.; Navarro, F. Prefoldin-like Bud27 influences the transcription of ribosomal components and ribosome biogenesis in Saccharomyces cerevisiae. RNA 2020, 26, 1360–1379. [Google Scholar] [CrossRef] [PubMed]
- Mirón-García, M.C.; Garrido-Godino, A.I.; Martínez-Fernández, V.; Fernández-Pevida, A.; Cuevas-Bermúdez, A.; Martín-Expósito, M.; Chávez, S.; de la Cruz, J.; Navarro, F. The yeast prefoldin-like URI-orthologue Bud27 associates with the RSC nucleosome remodeler and modulates transcription. Nucleic Acids Res. 2014, 42, 9666–9676. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Gu, J.X.; Zhu, C.Q.; Sun, F.Y.; Dorjsuren, D.; Lin, Y.; Murakami, S. Interaction with general transcription factor IIF (TFIIF) is required for the suppression of activated transcription by RPB5-mediating protein (RMP). Cell Res. 2003, 13, 111–120. [Google Scholar] [CrossRef]
- Burén, S.; Gomes, A.L.; Teijeiro, A.; Fawal, M.A.; Yilmaz, M.; Tummala, K.S.; Perez, M.; Rodriguez-Justo, M.; Campos-Olivas, R.; Megías, D.; et al. Regulation of OGT by URI in Response to Glucose Confers c-MYC-Dependent Survival Mechanisms. Cancer Cell 2016, 30, 290–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).