Difference in Germination Traits between Congeneric Native and Exotic Species May Affect Invasion
Abstract
1. Introduction
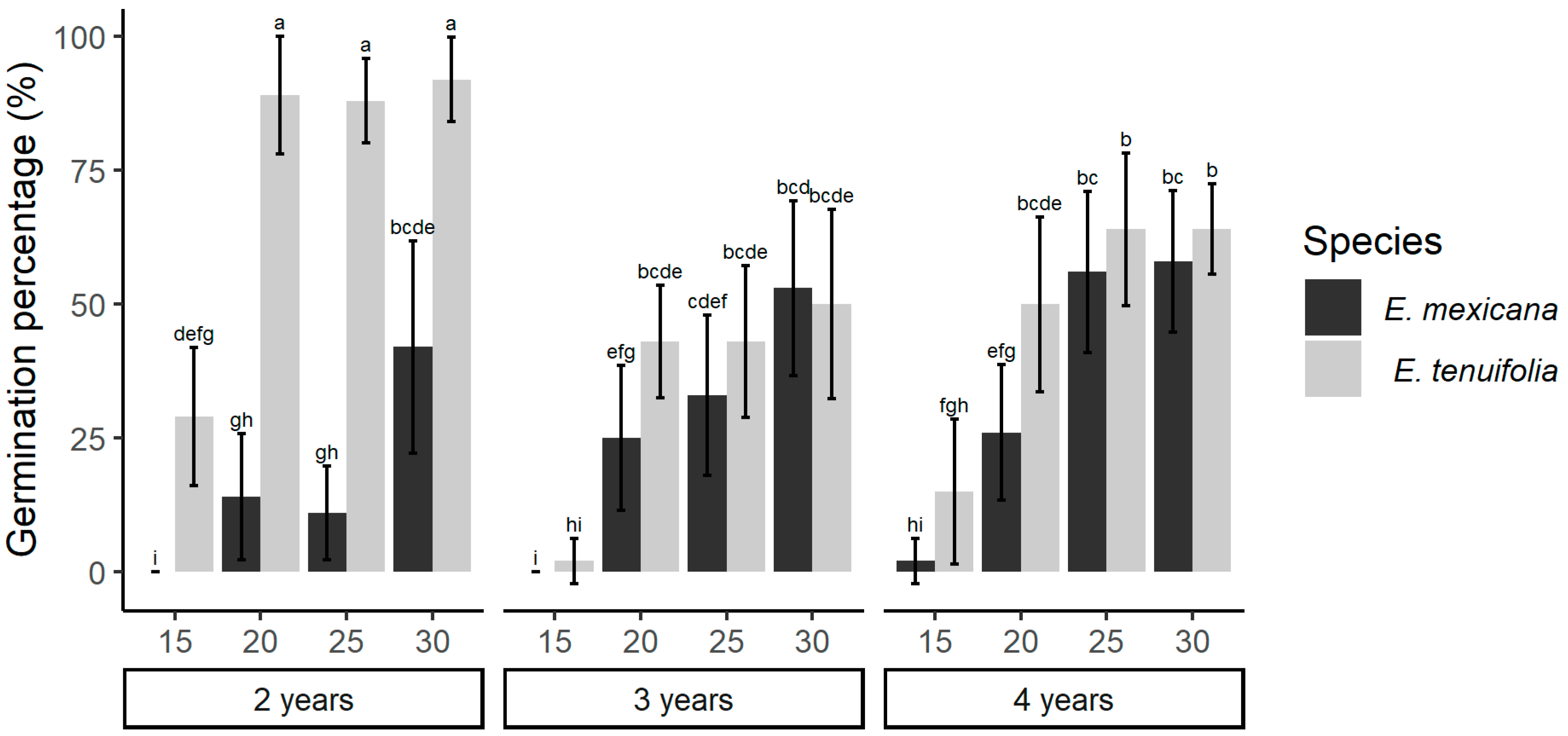
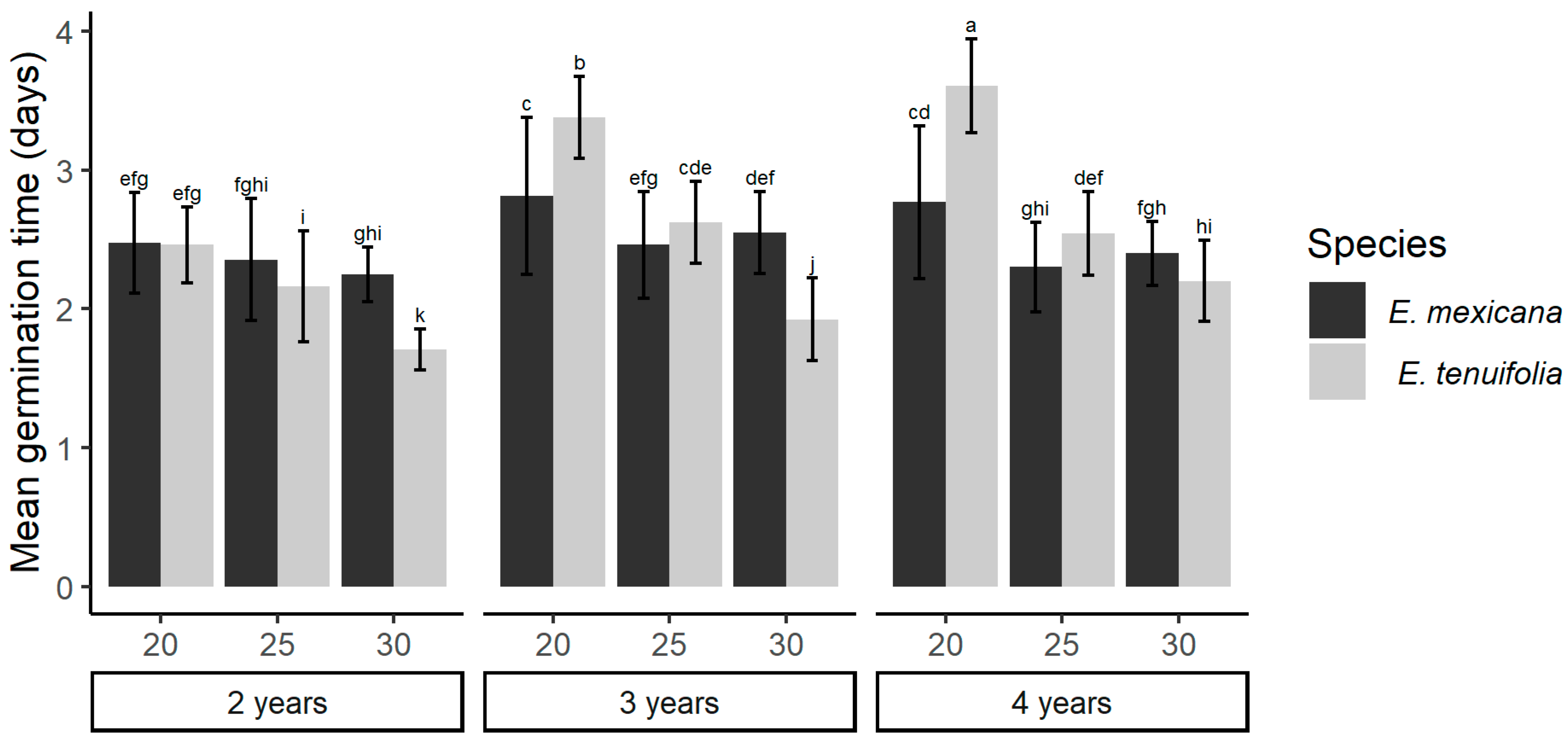
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rejmánek, M.; Richardson, D.M.; Higgins, S.I.; Pitcairn, M.J.; Grotkopp, E. Ecology of invasive plants: State of the art. In Invasive Alien Species: A New Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Pyšek, P.; Richardson, D.M. The biogeography of naturalization in alien plants. J. Biogeogr. 2006, 33, 2040–2050. [Google Scholar] [CrossRef]
- Küster, E.C.; Kühn, I.; Bruelheide, H.; Klotz, S. Trait interactions help explain plant invasion success in the German flora. J. Ecol. 2008, 96, 860–868. [Google Scholar] [CrossRef]
- Grman, E.; Suding, K.N. Within-Year Soil Legacies Contribute to Strong Priority Effects of Exotics on Native California Grassland Communities. Restor. Ecol. 2010, 18, 664–670. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Pergl, J.; Randall, R.; Chytrý, M.; Kühn, I.; Tichý, L.; Danihelka, J.; Jun, J.C.; Sádlo, J. The global invasion success of Central European plants is related to distribution characteristics in their native range and species traits. Divers. Distrib. 2009, 15, 891–903. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Hierro, J.L. Community Assembly Theory as a Framework for Biological Invasions. Trends Ecol. Evol. 2018, 33, 313–325. Available online: https://www.sciencedirect.com/science/article/pii/S0169534718300533 (accessed on 30 July 2023). [CrossRef]
- Pyšek, P.; Manceur, A.M.; Alba, C.; McGregor, K.F.; Pergl, J.; Štajerová, K.; Chytrý, M.; Danihelka, J.; Kartesz, J.; Klimešová, J.; et al. Naturalization of central European plants in North America: Species traits, habitats, propagule pressure, residence time. Ecology 2015, 96, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Gioria, M.; Carta, A.; Baskin, C.C.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; van Kleunen, M.; Weigelt, P.; Winter, M.; et al. Persistent soil seed banks promote naturalisation and invasiveness in flowering plants. Ecol. Lett. 2021, 24, 1655–1667. [Google Scholar] [CrossRef]
- Grubb, P.J. The Maintenance of Species-Richness in Plant Communities: The Importance of the Regeneration Niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Koch, M.; Huthmann, M.; Bernhardt, K.-G. Cardamine amara L. (Brassicaceae) in dynamic habitats: Genetic composition and diversity of seed bank and established populations. Basic Appl. Ecol. 2003, 4, 339–348. Available online: https://www.sciencedirect.com/science/article/pii/S1439179104701280 (accessed on 30 July 2023). [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Donohue, K.; Rubio De Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Donohue, K. Seeds and seasons: Interpreting germination timing in the field. Seed Sci. Res. 2005, 15, 175–187. [Google Scholar] [CrossRef]
- Donohue, K. Setting the Stage: Phenotypic Plasticity as Habitat Selection. Int. J. Plant Sci. 2003, 164, S79–S92. [Google Scholar] [CrossRef]
- Van Couwenberghe, R.; Gégout, J.C.; Lacombe, E.; Collet, C. Light and competition gradients fail to explain the coexistence of shade-tolerant Fagus sylvatica and shade-intermediate Quercus petraea seedlings. Ann. Bot. 2013, 112, 1421–1430. [Google Scholar] [CrossRef]
- Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Effects of germination season on life history traits and on transgenerational plasticity in seed dormancy in a cold desert annual. Sci. Rep. 2016, 6, 25076. [Google Scholar] [CrossRef]
- Leverett, L.D. Germination phenology determines the propensity for facilitation and competition. Ecology 2017, 98, 2437–2446. [Google Scholar] [CrossRef]
- Gentili, R.; Ambrosini, R.; Augustinus, B.A.; Caronni, S.; Cardarelli, E.; Montagnani, C.; Müller-Schärer, H.; Schaffner, U.; Citterio, S. High Phenotypic Plasticity in a Prominent Plant Invader along Altitudinal and Temperature Gradients. Plants 2021, 10, 2144. [Google Scholar] [CrossRef]
- Eyster, H.N.; Wolkovich, E.M. Comparisons in the native and introduced ranges reveal little evidence of climatic adaptation in germination traits. Clim. Chang. Ecol. 2021, 2, 100023. Available online: https://www.sciencedirect.com/science/article/pii/S266690052100023X (accessed on 30 July 2023). [CrossRef]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Cleland, E.E. Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biol. Invasions 2013, 15, 2253–2264. [Google Scholar] [CrossRef]
- Huebner, C.D. Chapter 18—Effects of global climate change on regeneration of invasive plant species from seeds. In Plant Regeneration from Seeds; Baskin, C.C., Baskin, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 243–257. Available online: https://www.sciencedirect.com/science/article/pii/B9780128237311000068 (accessed on 30 July 2023).
- Gioria, M.; Pyšek, P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol. Invasions 2017, 19, 1055–1080. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Ackerman, J.D. Spatial distribution and performance of native and invasive Ardisia (Myrsinaceae) species in Puerto Rico: The anatomy of an invasion. Biol. Invasions 2011, 13, 1543–1558. [Google Scholar] [CrossRef]
- Beckmann, M.; Bruelheide, H.; Erfmeier, A. Germination responses of three grassland species differ between native and invasive origins. Ecol. Res. 2011, 26, 763–771. [Google Scholar] [CrossRef]
- Xu, X.; Wolfe, L.; Diez, J.; Zheng, Y.; Guo, H.; Hu, S. Differential germination strategies of native and introduced populations of the invasive species Plantago virginica. NeoBiota 2019, 43, 101–118. [Google Scholar] [CrossRef]
- Bouteiller, X.P.; Moret, F.; Ségura, R.; Klisz, M.; Martinik, A.; Monty, A.; Pino, J.; van Loo, M.; Wojda, T.; Porté, A.J.; et al. The seeds of invasion: Enhanced germination in invasive European populations of black locust (Robinia pseudoacacia L.) compared to native American populations. Plant Biol. 2021, 23, 1006–1017. [Google Scholar] [CrossRef]
- Forbis, T.A. Germination phenology of some Great Basin native annual forb species. Plant Species Biol. 2010, 25, 221–230. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Pucheta, E.; García-Muro, V.J.; Rolhauser, A.G.; Quevedo-Robledo, L. Invasive potential of the winter grass Schismus barbatus during the winter season of a predominantly summer-rainfall desert in Central-Northern Monte. J. Arid. Environ. 2011, 75, 390–393. [Google Scholar] [CrossRef]
- Winn, A.A.; Miller, T.E. Effect of density on magnitude of directional selection on seed mass and emergence time in Plantago wrightiana Dcne. (Plantaginaceae). Oecologia 1995, 103, 365–370. [Google Scholar] [CrossRef]
- Brändle, M.; Stadler, J.; Klotz, S.; Brandl, R. Distributional range size of weedy plant species is correlated to germination patterns. Ecology 2003, 84, 136–144. [Google Scholar] [CrossRef]
- Venable, D.L. Bet hedging in a guild of desert annuals. Ecology 2007, 88, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Flora of North America Editorial Committee. Flora of North America North of Mexico, Eragrostis Mexicana. Available online: http://floranorthamerica.org/Eragrostis_mexicana (accessed on 30 July 2023).
- Calderón, G.; Rzedowski, J. Flora fanerogámica del Valle de México; Instituto de Ecología: Coyoacán, Mexico, 2001. [Google Scholar]
- Jung, M.J.; Veldkamp, J.F.; Kuoh, C.S. Notes on Eragrostis wolf (Poaceae) for the flora of Taiwan. Taiwania 2008, 53, 96–102. [Google Scholar]
- POWO. Botanic Gardens, Kew. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. E. tenuifolia. 2023. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:1064189-2#other-data (accessed on 11 December 2023).
- Ackerman, B.A.; Manrique, F.E.; Jaramillo, L.V.; Guerrero, S.P.; Miranda, S.J.A.; INúñez, T.I. Las Gramíneas de México, Tomo II. México, D.F.: Secretaría de Agricultura y Recursos Hidráulicos; Comisión Técnico Consultiva de Coeficientes de Agostadero: Tlalpan, Mexico, 1987.
- Njuguna, J.G.M.; Gordon, D.T.; Louie, R. Wild grass hosts of maize streak virus and its Cicadulina leafhopper vectors in Kenya. In Proceedings of the Eastern and Southern Africa Regional Maize Conference, Arusha, Tanzania, 3–7 June 1996; CIMMYT: El Batan, Mexico, 1997. [Google Scholar]
- Sanchez-Muñoz, A.d.J. Invasive Lehmann Lovegrass (Eragrostis lehmanniana) in Chihuahua, Mexico: Consequences of Invasion. Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2009. [Google Scholar]
- Bittencourt, H.v.H.; Bonome, L.T.d.S.; Pagnoncelli, F.d.B.; Lana, M.A.; Trezzi, M.M. Seed germination and emergence of Eragrostis tenuifolia (A. Rich.) Hochst. ex Steud. in response to environmental factors. J. Plant. Prot. Res. 2016, 56, 32–38. [Google Scholar] [CrossRef]
- Goulart, I.C.G.R.; Merotto Junior, A.; Perez, N.B.; Kalsing, A. Controle de capim-annoni-2 (Eragrostis plana) com herbicidas pré-emergentes em associação com diferentes métodos de manejo do campo nativo. Planta Daninha 2009, 27, 181–190. [Google Scholar] [CrossRef][Green Version]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Csontos, P. Seed banks: Ecological definitions and sampling considerations. Community Ecol. 2007, 8, 75–85. [Google Scholar] [CrossRef]
- Iglesias-Fernández, R.; del Carmen Rodríguez-Gacio, M.; Matilla, A.J. Progress in research on dry afterripening. Seed Sci. Res. 2011, 21, 69–80. Available online: https://www.cambridge.org/core/article/progress-in-research-on-dry-afterripening/625592962A4C54D0D21520C13BA6A9E6 (accessed on 30 July 2023). [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef]
- Chrobock, T.; Kempel, A.; Fischer, M.; van Kleunen, M. Introduction bias: Cultivated alien plant species germinate faster and more abundantly than native species in Switzerland. Basic Appl. Ecol. 2011, 12, 244–250. [Google Scholar] [CrossRef]
- Goergen, E.; Daehler, C.C. Reproductive Ecology of a Native Hawaiian Grass (Heteropogon contortus; Poaceae) versus Its Invasive Alien Competitor (Pennisetum setaceum; Poaceae). Int. J. Plant Sci. 2001, 162, 317–326. [Google Scholar] [CrossRef]
- Callaway, J.C.; Josselyn, M.N. The introduction and spread of smooth cordgrass (Spartina alterniflora) in South San Francisco Bay. Estuaries 1992, 15, 218–226. [Google Scholar] [CrossRef]
- Díaz-Segura, O.; Golubov, J.; Mandujano, M.C.; Zavala-Hurtado, J.A. Reproductive characteristics that favor invasiveness in Leonotis nepetifolia (L.) R. Br. Plant Species Biol. 2020, 35, 270–282. [Google Scholar] [CrossRef]
- Navarrete-Sauza, E.; Rojas-Arechiga, M. Germination of the exotic Calotropis procera (Aiton) W.T. (Apocynaceae) in Mexico. Bot. Sci. 2023, 101, 854–864. [Google Scholar] [CrossRef]
- Hsu, H.M.; Kao, W.Y. Vegetative and reproductive growth of an invasive weed Bidens pilosa L. var. radiata and its noninvasive congener Bidens bipinnata in Taiwan. Taiwania 2014, 59, 119–126. [Google Scholar]
- Dyer, A.R.; Fenech, A.; Rice, K.J. Accelerated seedling emergence in interspecific competitive neighbourhoods. Ecol. Lett. 2000, 3, 523–529. [Google Scholar] [CrossRef]
- Porceddu, M.; Pritchard, H.W.; Mattana, E.; Bacchetta, G. Differential Interpretation of Mountain Temperatures by Endospermic Seeds of Three Endemic Species Impacts the Timing of In Situ Germination. Plants 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cardina, J. Germination patterns and implications for invasiveness in three Taraxacum (Asteraceae) species. Weed Res. 2012, 52, 112–121. [Google Scholar] [CrossRef]
- Rzedowski, J.; Calderon de Rzedowski, G. Sinopsis numérica de la flora fanerogámica del Valle de México. Acta Bot. Mex. 1989, 8, 15–30. [Google Scholar] [CrossRef]
- Villaseñor, J.L.; Espinosa-Garcia, F.J. The alien flowering plants of Mexico. Divers Distrib. 2004, 10, 113–123. [Google Scholar] [CrossRef]
- Naturalista. Naturalista. 2023. Available online: https://www.naturalista.mx/ (accessed on 30 July 2023).
- Royal Botanical Garden. Royal Botanical Garden. 2023. Available online: https://powo.science.kew.org/ (accessed on 30 July 2023).
- Van Kleunen, M.; Johnson, S.D. South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. J. Ecol. 2007, 95, 674–681. [Google Scholar] [CrossRef]
- Vibrans. Haike. Malezas de México. Ficha Eragrostis tenuifolia (A. Rich.) Hochst. ex Steud. 2010. Available online: http://www.conabio.gob.mx/malezasdemexico/poaceae/eragrostis-tenuifolia/fichas/ficha.htm (accessed on 30 July 2023).
- Lozano-Isla, F.; Benites-Alfaro, O.E.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application “GerminaQuant for R”. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Ranal, M.A.; de Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomé-Díaz, J.; Golubov, J.; Eguiarte, L.E.; Búrquez, A. Difference in Germination Traits between Congeneric Native and Exotic Species May Affect Invasion. Plants 2024, 13, 478. https://doi.org/10.3390/plants13040478
Salomé-Díaz J, Golubov J, Eguiarte LE, Búrquez A. Difference in Germination Traits between Congeneric Native and Exotic Species May Affect Invasion. Plants. 2024; 13(4):478. https://doi.org/10.3390/plants13040478
Chicago/Turabian StyleSalomé-Díaz, Julieta, Jordan Golubov, Luis E. Eguiarte, and Alberto Búrquez. 2024. "Difference in Germination Traits between Congeneric Native and Exotic Species May Affect Invasion" Plants 13, no. 4: 478. https://doi.org/10.3390/plants13040478
APA StyleSalomé-Díaz, J., Golubov, J., Eguiarte, L. E., & Búrquez, A. (2024). Difference in Germination Traits between Congeneric Native and Exotic Species May Affect Invasion. Plants, 13(4), 478. https://doi.org/10.3390/plants13040478




