Morphophysiological Characterisation of Guayule (Parthenium argentatum A. Gray) in Response to Increasing NaCl Concentrations: Phytomanagement and Phytodesalinisation in Arid and Semiarid Areas
Abstract
1. Introduction
2. Results
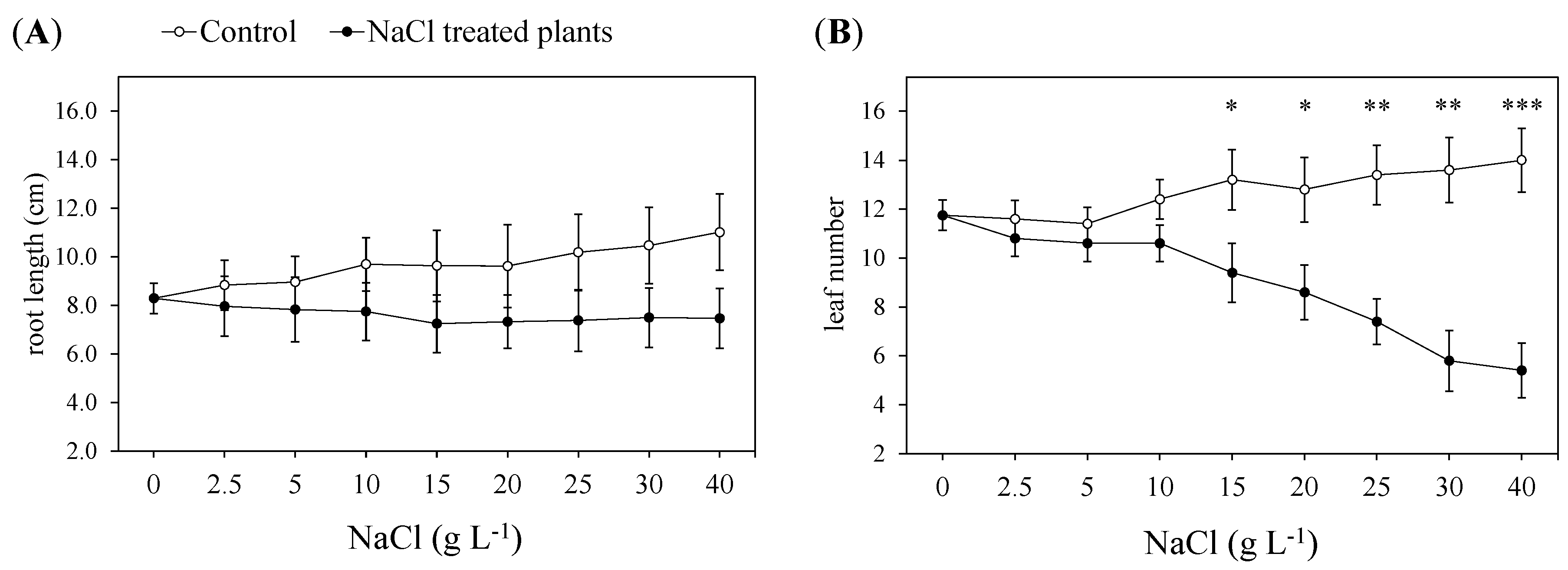
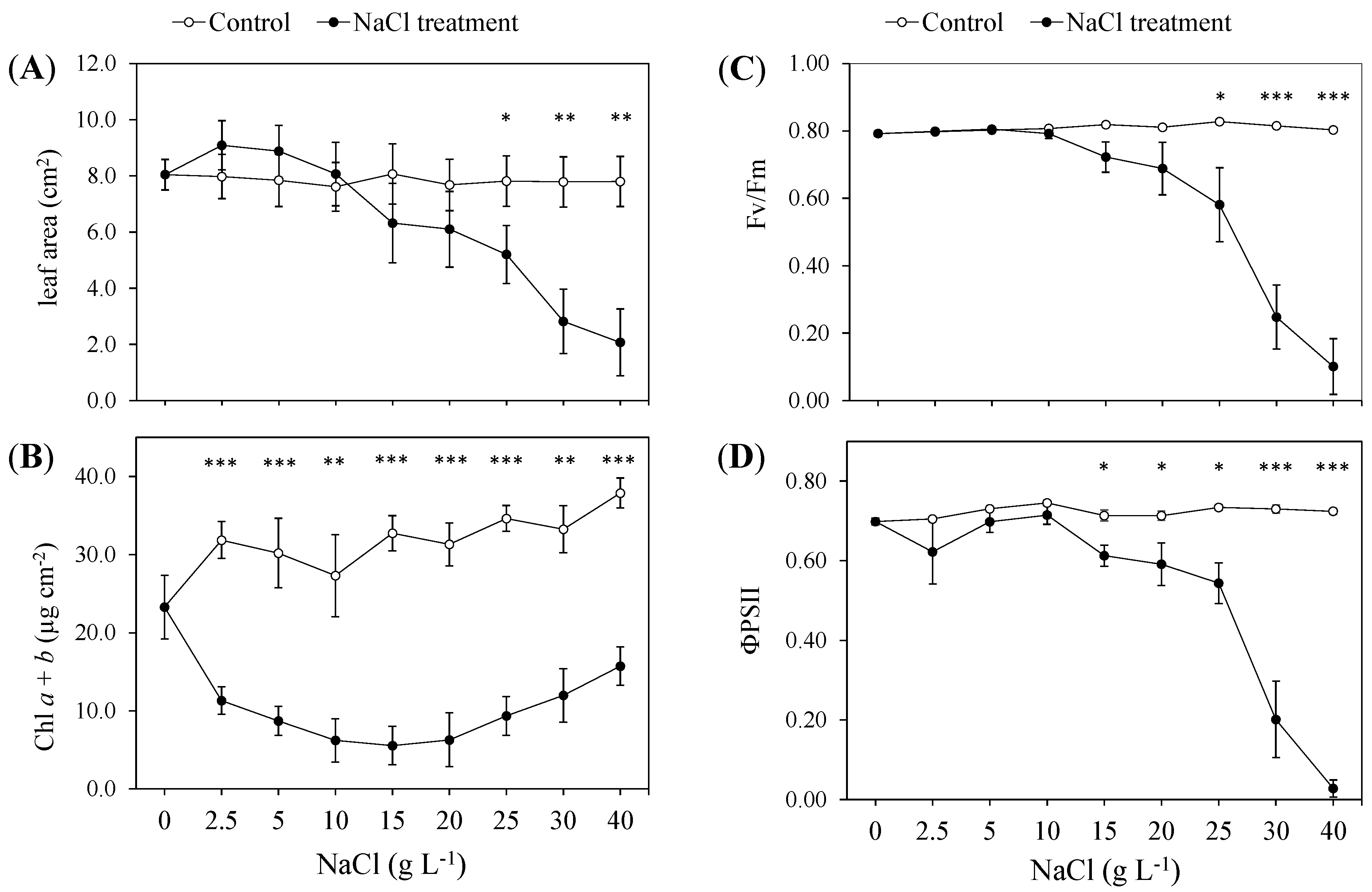
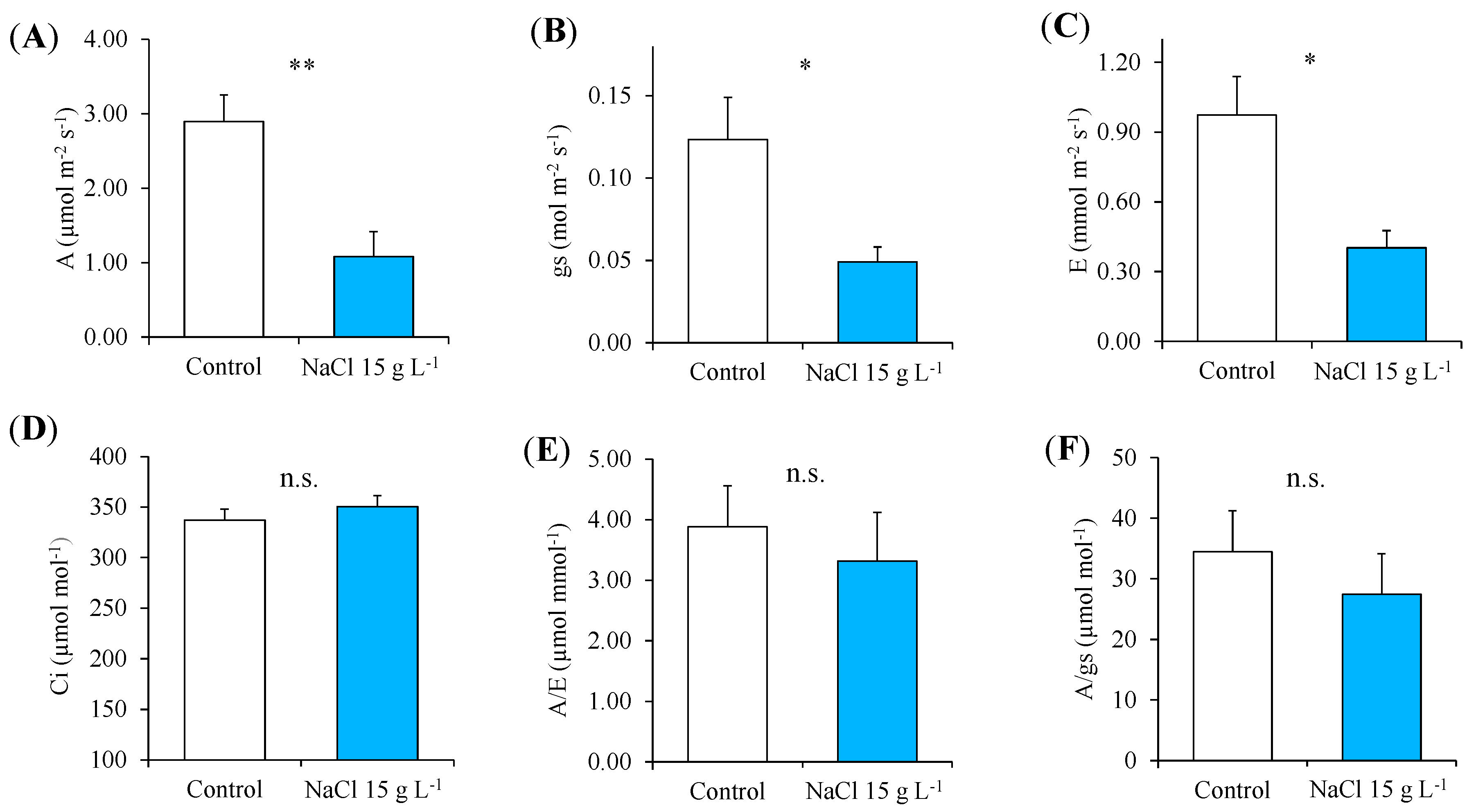
2.1. Nondestructive Determinations: Monitoring of the Morphological, Chlorophyll Fluorescence and Gas-Exchange Parameters during the Test
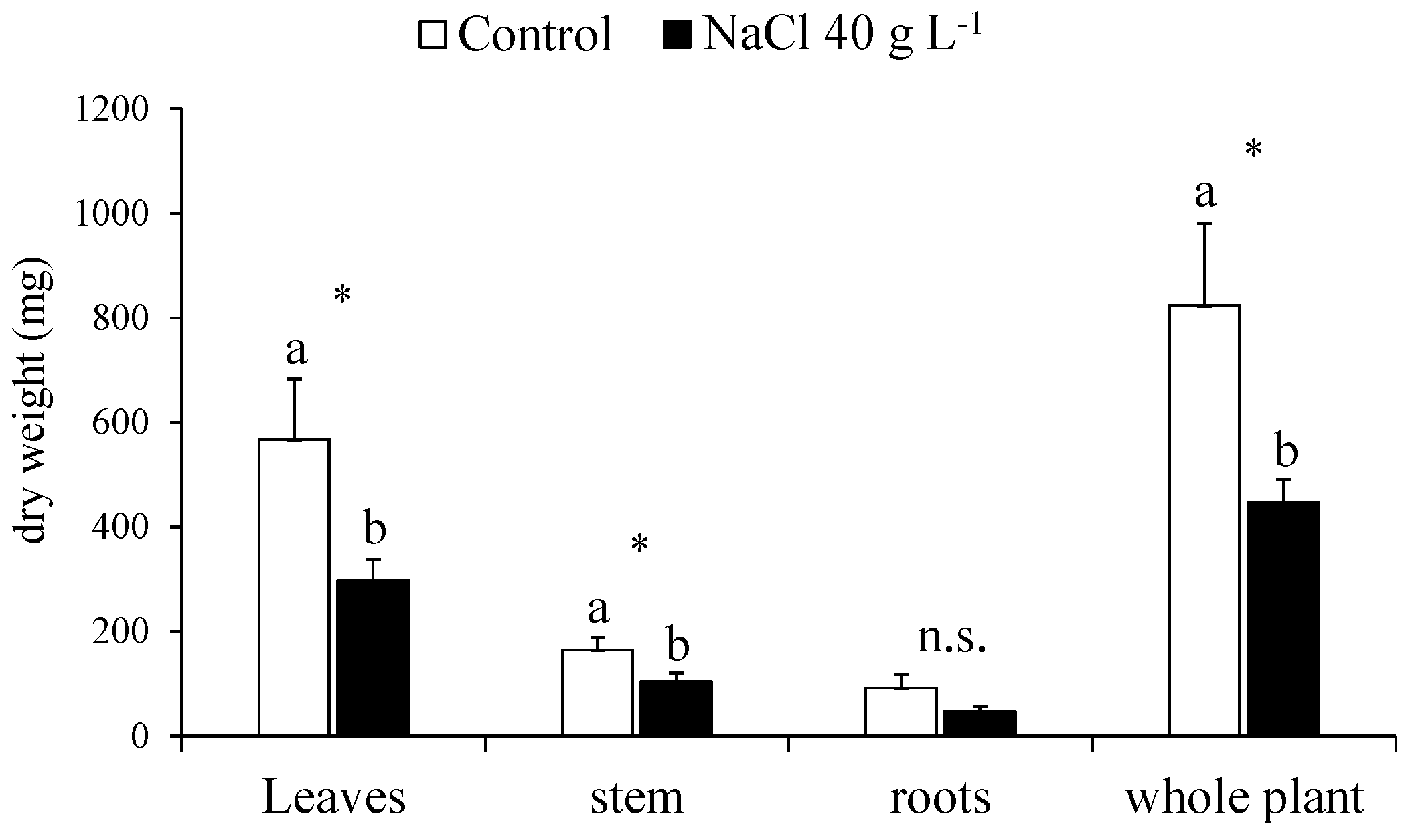
2.2. Destructive Determinations: Growth, Biomass Partitioning and Water Content
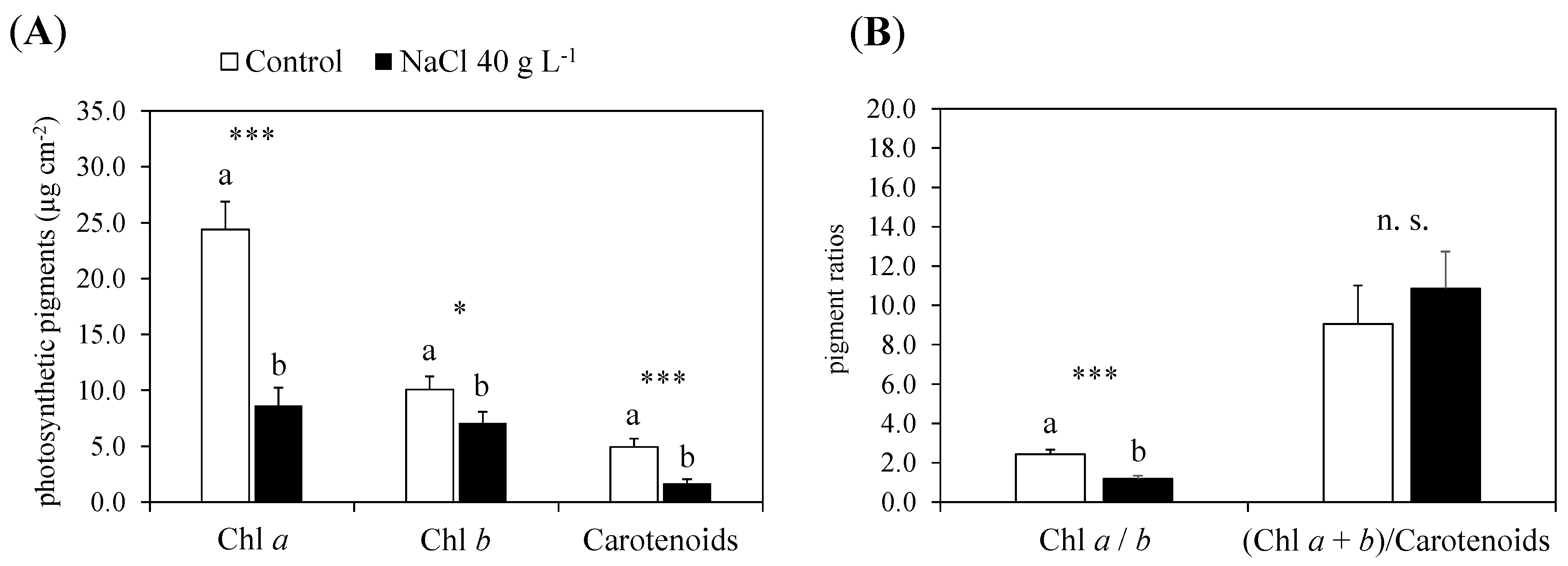
2.2.1. Photosynthetic Pigments and Nutrients
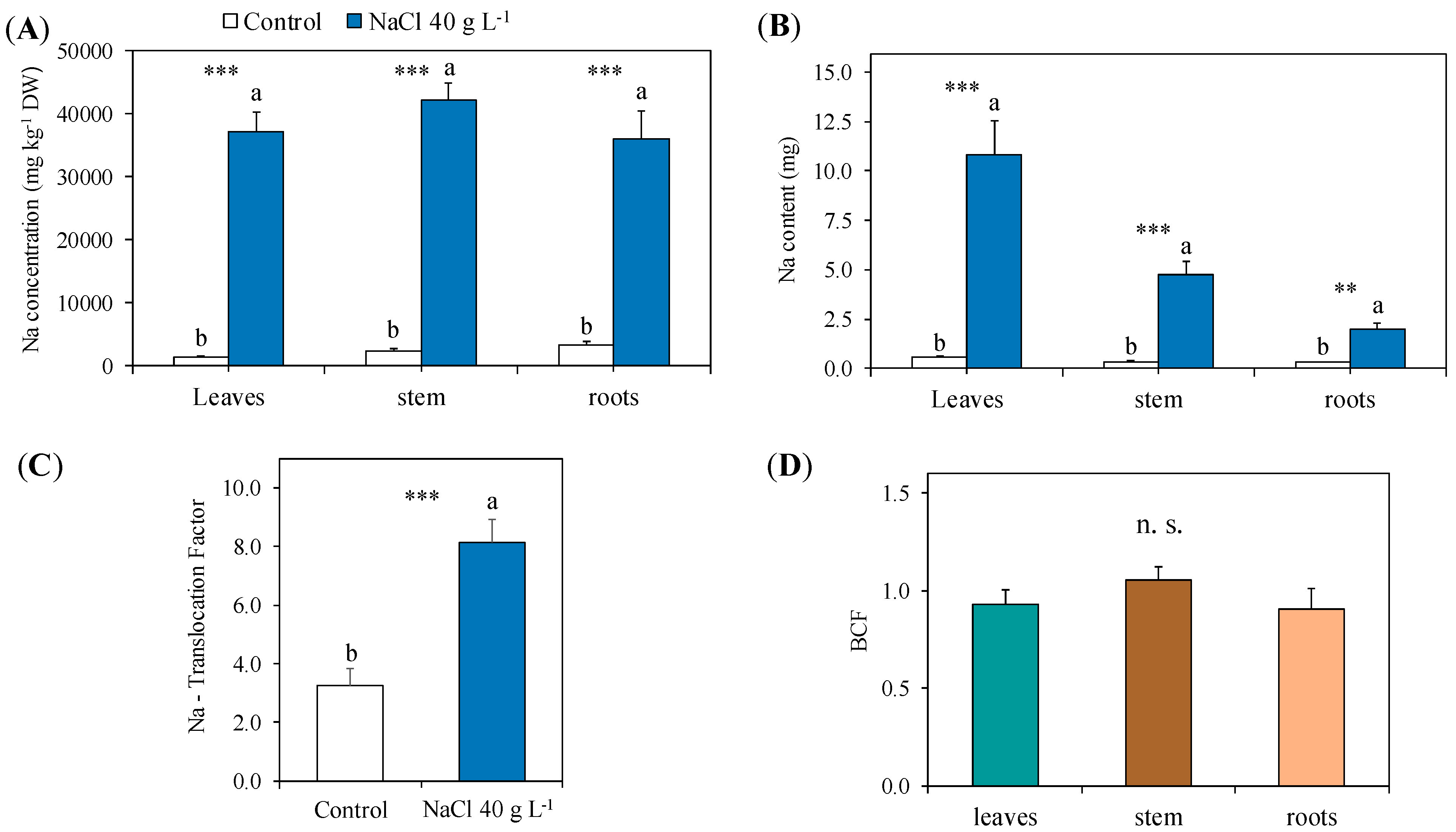
2.2.2. Sodium Concentration and Content: Distribution, Translocation, Accumulation and Relation with the Major Cations
3. Discussion
3.1. Morphophysiological Response to Increasing NaCl Concentrations
3.2. Biomass Partitioning, the Distribution of Nutrients and Sodium
4. Materials and Methods
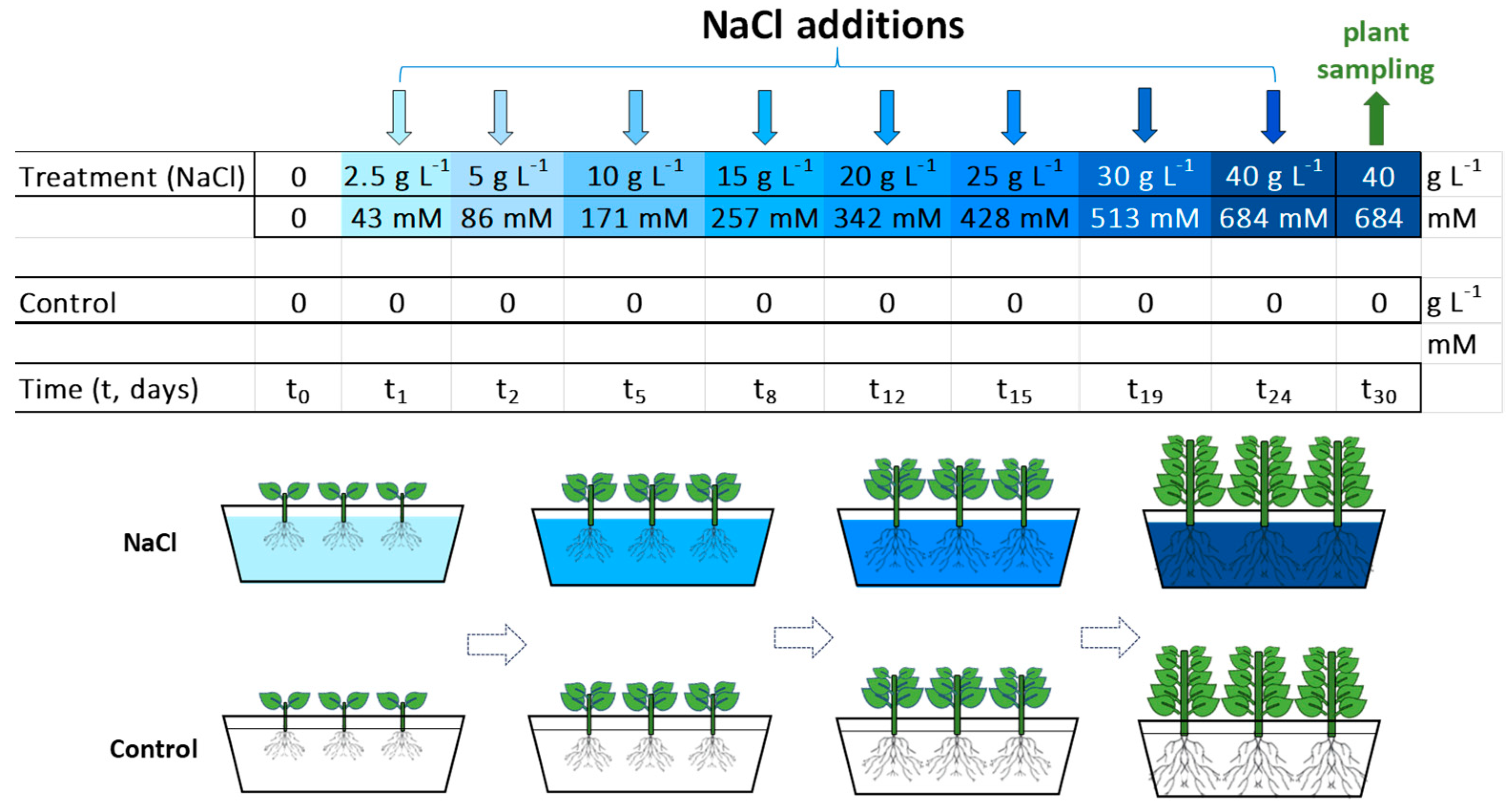
4.1. Plant Material, Preculture, Growth Conditions and Treatments
4.2. Monitoring through Nondestructive Measurements
4.2.1. Biometric Parameters and Total Chlorophyll
4.2.2. Chlorophyll Fluorescence
4.2.3. Gas-Exchange Parameters
4.3. Analysis through Destructive Measurements
4.3.1. Growth, Hydration Status, Biomass Partitioning and Analysis
4.3.2. Extraction of Photosynthetic Pigments
4.3.3. Chemical Organ Analysis: Distribution of Sodium and Nutrients
4.3.4. Sodium Translocation, Accumulation and Concentration Factors
4.4. Regression Models for the Leaf Area and Total Chlorophyll Determinations through Nondestructive Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- FAO. Global Symposium on Salt-Affected Soils: Outcome Document; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global Warming and 21st century Drying. Clim. Dyn. 2014, 43, 2607–2627. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.-T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Koganti, T.; Santos, F.A.M.; Triantafilis, J. Mapping Soil Salinity and a Fresh-Water Intrusion in Three-Dimensions Using a Quasi-3d Joint-Inversion of DUALEM-421S and EM34 Data. Sci. Total Environ. 2017, 577, 395–404. [Google Scholar] [CrossRef] [PubMed]
- USDA-ARS. Research Databases. Bibliography on Salt Tolerance; Brown, G.E., Jr., Ed.; U.S. Salinity Laboratory, Agricultural Research Service: Riverside, CA, USA, 2008. Available online: http://www.ars.usda.gov/Services/docs.htm?docid=8908 (accessed on 27 September 2023).
- Cabral, R.L.; Ferreira, T.O.; Nóbrega, G.N.; Barcellos, D.; Roiloa, S.R.; Zandavalli, R.B.; Otero, X.L. How Do Plants and Climatic Conditions Control Soil Properties in Hypersaline Tidal Flats? Appl. Sci. 2020, 10, 7624. [Google Scholar] [CrossRef]
- Barbafieri, M.; Bretzel, F.; Scartazza, A.; Di Baccio, D.; Rosellini, I.; Grifoni, M.; Pini, R.; Clementi, A.; Franchi, E. Response to Hypersalinity of Four Halophytes Growing in Hydroponic Floating Systems: Prospects in the Phytomanagement of High Saline Wastewaters and Extreme Environments. Plants 2023, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Vera-Gargallo, B.; Hernández, M.; Dumont, M.G.; Ventosa, A. Thrive or survive: Prokaryotic life in hypersaline soils. Environ. Microbiome 2023, 18, 17. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Nat. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Jlassi, A.; Zorrig, W.; Khouni, A.E.; Lakhdar, A.; Smaoui, A.; Abdelly, C.; Rabhi, M. Phytodesalination of a moderately-salt-affected soil by Sulla carnosa. Int. J. Phytoremediation 2013, 15, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Kalanaki, M.; Ritzema, H.; Bamshad, R.; Jones, E.; Fazilatnia, M. Application of biodesalinization for reclamation of salt-affected soil under composted cow manure and deficit irrigation with saline water. PAWE 2020, 18, 469–479. [Google Scholar] [CrossRef]
- Sarath, N.G.; Sruthi, P.; Shackira, A.M.; Puthur, J.T. Halophytes as effective tool for phytodesalination and land reclamation (Chapter 16). In Frontiers in Plant-Soil Interaction Molecular Insights into Plant Adaptation; Academic Press Elsevier Inc.: Amsterdam, NV, USA, 2021; pp. 459–494. [Google Scholar] [CrossRef]
- Ray, D.T.; Dierig, D.A.; Thompson, A.E.; Coffelt, T.A. Registration of six guayule germplasms with high yielding ability. Crop Sci. 1999, 39, 300. [Google Scholar] [CrossRef]
- Foster, M.A.; Coffelt, T.A. Guayule agronomics: Establishment, irrigated production, and weed control. Ind. Crops Prod. 2005, 22, 27–40. [Google Scholar] [CrossRef]
- Monti, A. The Future of Guayule in Europe. In Proceedings of the AAIC (Association for the Advancement of Industrial Crops) Congress, Montpellier, France, 21–24 April 2014. [Google Scholar]
- Zanetti, F. Guayule Rubber a Strategic Raw Material for Europe with a Long-Term History within the AAIC (Association for the Advancement of Industrial Crops). In Proceedings of the AAIC Congress, Montpellier, France, 14–19 July 2019. [Google Scholar]
- Cornish, K.; Brichta, J.L.; Yu, P.; Wood, D.F.; Mcglothlin, M.W.; Martin, J.A. Guayule Latex Provides a Solution for the Critical Demands of the Non-Allergenic Medical Products Market. Agro Food Ind. Hi Technol. 2001, 12, 27–32. [Google Scholar]
- Rousset, A.; Amor, A.; Punvichai, T.; Perino, S.; Palu, S.; Dorget, M.; Pioch, D.; Chemat, F. Guayule (Parthenium argentatum A. Gray), a Renewable Resource for Natural Polyisoprene and Resin: Composition, Processes and Applications. Molecules 2021, 26, 664. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Meloni, P.; Idda, I.; Mariani, A.; Pilo, M.; Nurchi, V.; Lachowicz, J.; Rivera, E.; Orona-Espino, A.; Sanna, G. Assessment, Validation and Application to Real Samples of an RP-HPLC Method for the Determination of Guayulins A, B, C and D in Guayule Shrub. Separations 2018, 5, 23. [Google Scholar] [CrossRef]
- Cheng, F.; Dehghanizadeh, M.; Audu, M.A.; Jarvis, J.M.; Holguin, F.O.; Brewer, C.E. Characterization and evaluation of guayule processing residues as potential feedstock for biofuel and chemical production. Ind. Crops Prod. 2020, 150, 112311. [Google Scholar] [CrossRef]
- Piluzza, G.; Campesi, G.; Molinu, M.G.; Re, G.A.; Sulas, L. Bioactive Compounds from Leaves and Twigs of Guayule Grown in a Mediterranean Environment. Plants 2020, 9, 442. [Google Scholar] [CrossRef]
- Sundar, D.; Chaitanya, K.V.; Ramachandra Reddy, A. Stress-induced proteins in Parthenium argentatum leaves. Biol. Plant. 2003, 46, 313–316. [Google Scholar] [CrossRef]
- Placido, D.F.; Heinitz, C.; McMahan, C.M.; Bañuelos, G.S. Guayule is an industrial crop that can be grown for its natural rubber production and phytoremediation capability in the Western San Joaquin Valley, California. Curr. Plant Biol. 2021, 28, 100223. [Google Scholar] [CrossRef]
- Miyamoto, S.; Bucks, D.A. Water quantity and quality requirements of guayule: Current assessment. Agric. Water Manag. 1985, 10, 205–219. [Google Scholar] [CrossRef]
- Hunsaker, D.J.; Elshikha, D.M.; Bronson, K.F. High guayule rubber production with subsurface irrigation in the US desert Southwest. Agric. Water Manag. 2019, 220, 1–12. [Google Scholar] [CrossRef]
- Miyamoto, S.; Piela, K.; Davis, J.; Fenn, L.B. Salt effects on emergence and seedling mortality of guayule. Agron. J. 1984, 76, 295–300. [Google Scholar] [CrossRef]
- Miyamoto, S.; Davis, J.; Madrid, L. Salt Tolerance of Guayule; Texas A&M University: College Station, TX, USA, 1990; p. 18. [Google Scholar]
- Poscher, E. Salinity Effects on Guayule Leaf Anatomy and Physiology. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 28 April 2005. Available online: https://repository.arizona.edu/handle/10150/194362?show=full (accessed on 10 November 2023).
- Bañuelos, G.S.; Placido, D.F.; Zhu, H.; Centofanti, T.; Zambrano, M.C.; Heinitz, C.; Lone, T.A.; McMahan, C.M. Guayule as an alternative crop for natural rubber production grown in B- and Se-laden soil in Central California. Ind. Crops Prod. 2022, 189, 115799. [Google Scholar] [CrossRef]
- Shao, T.; Chen, Y.; Gao, X.; Zhou, Z.; Long, X.; Rengel, Z. Salt stress affects the biomass of industrial crop Jerusalem artichoke by affecting sugar transport and metabolism. Heliyon 2023, 9, e14107. [Google Scholar] [CrossRef]
- Ren, F.; Yang, G.; Li, W.; He, X.; Gao, Y.; Tian, L.; Li, F.; Wang, Z.; Liu, S. Yield-compatible salinity level for growing cotton (Gossypium hirsutum L.) under mulched drip irrigation using saline water. Agric. Water Manag. 2021, 250, 106859. [Google Scholar] [CrossRef]
- Evancho, B.; Dial, H. Plant Guide for guayule (Parthenium argentatum); USDA-Natural Resources Conservation Service, Tucson Plant Materials Center: Tucson, AZ, USA, 2020. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_PAAR5.pdf (accessed on 4 September 2023).
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W.; Peters, W.S. The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol. 2002, 129, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Healey, P.L.; Mehta, I.J.; Westerling, K.E. Leaf trichomes of some Parthenium species. Am. J. Bot. 1986, 73, 1093–1099. [Google Scholar] [CrossRef]
- James, R.A.; Rivelli, A.R.; Munns, R.; von Caemmerer, S. Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Funct. Plant Biol. 2002, 29, 1393–4032002. [Google Scholar] [CrossRef]
- Pak, V.A.; Nabipour, M.; Meskarbashee, M. Effect of salt stress on chlorophyll content, fluorescence, Na+ and K+ ions content in rape plants (Brassica napus L.). Asian J. Agric. Res. 2009, 3, 28–37. [Google Scholar] [CrossRef]
- Placido, D.F.; Dierig, D.A.; Cruz, V.M.V.; Ponciano, G.; Dong, C.; Dong, N.; Huynh, T.; Williams, T.; Cahoon, R.E.; Wall, G.W.; et al. Downregulation of an allene oxide synthase gene improves photosynthetic rate and alters phytohormone homeostasis in field-grown guayule. Ind. Crops Prod. 2020, 153, 112341. [Google Scholar] [CrossRef]
- Martinez-Alonso, A.; Garcia-Ibañez, P.; Bárzana, G.; Carvajal, M. Leaf Gas Exchange and Growth Responses of Tomato Plants to External Flavonoids Application as Biostimulators under Normal and Salt-Stressed Conditions. Agronomy 2022, 12, 3230. [Google Scholar] [CrossRef]
- Anderson, J.; Chow, W.S.; De Las Rivas, J. Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: The grana enigma. Photosynth. Res. 2008, 98, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Fei, P.; Song, J.; Li, D.; Ge, C.; Chen, W. Responses of rice leaf thickness, SPAD readings and chlorophyll a/b ratios to different nitrogen supply rates in paddy field. Field Crop. Res. 2009, 114, 426–432. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askem, A.P. A Modern Tool for Classical Plant Growth Analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Moore, B.D.; Franceschi, V.R.; Cheng, S.-H.; Wu, J.; Ku, M.S.B. Photosynthetic characteristics of the C3-C4 intermediate Parthenium hysterophorus. Plant Physiol. 1987, 85, 984–989. [Google Scholar] [CrossRef]
- Gilliland, M.G.; van Staden, J.; Mitchell, J.J. CO2 fixation and rubber deposition in the bark and leaves of guayule during winter. J. Plant Physiol. 1985, 121, 369–379. [Google Scholar] [CrossRef]
- Tejera, N.A.; Soussi, M.; Lluch, C. Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Environ. Exp. Bot. 2006, 58, 17–24. [Google Scholar] [CrossRef]
- Schachtman, D.; Liu, W. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999, 4, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Nevo, R.; Charuvi, D.; Tsabari, O.; Reich, Z. Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J. 2012, 70, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.B.; Aung, B.; Amyot, L.; Lalin, I.; Lachaal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- Hu, Y.; Schnidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant. Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Bloss, H.-E. Growth and nutrition of guayule (Parthenium argentatum) in a saline soil as influenced by vesicular-arbuscular mycorrhiza and phosphorus fertilization. New Phytol. 1988, 108, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Amiriani, M.R. Effect of salinity stress on growth, mineral composition, proline content, antioxidant enzymes of soybean. Am. J. Plant Physiol. 2010, 5, 350–360. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Wang, X.; Huang, X.; Ma, C.; Liang, H.; Liu, B. Study on the Relationship of Ions (Na, K, Ca) Absorption and Distribution to Photosynthetic Response of Salix matsudana Koidz Under Salt Stress. Front. Plant Sci. 2022, 13, 860111. [Google Scholar] [CrossRef] [PubMed]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; López-Angulo, J.; Escudero, A.; Palacio, S.; Abedi, M.; Akhani, H. Functional structure of plant communities along salinity gradients in Iranian salt marshes. Plant-Environ. Interact. 2022, 3, 16–27. [Google Scholar] [CrossRef]
- Di Baccio, D.; Castagna, A.; Tognetti, R.; Ranieri, A.; Sebastiani, L. Early responses to cadmium of two poplar clones that differ in stress tolerance. J. Plant Physiol. 2014, 171, 1693–1705. [Google Scholar] [CrossRef]
- Bonanno, G.; Vymazal, J.; Cirelli, G.L. Translocation, accumulation and bioindication of trace elements in wetland plants. Sci. Total. Environ. 2018, 631, 252–261. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Grasso, R.; Gallardo, M.; Thompson, R.B. Influence of time of day on measurement with chlorophyll meters and canopy reflectance sensors of different crop N status. Precis. Agric. 2019, 20, 1087–1106. [Google Scholar] [CrossRef]
- Scartazza, A.; Fambrini, M.; Mariotti, L.; Picciarelli, P.; Pugliesi, C. Energy conversion processes and related gene expression in a sunflower mutant with altered salicylic acid metabolism. Plant Physiol. Biochem. 2020, 148, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, L.; Guglielminetti, L.; Mariotti, L.; Curadi, M.; Picciarelli, P.; Scartazza, A.; Sarrocco, S.; Vannacci, G. Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. Micro-Tom growth. Plant Soil 2016, 400, 351–366. [Google Scholar] [CrossRef]
- Scartazza, A.; Picciarelli, P.; Mariotti, L.; Curadi, M.; Barsanti, L.; Gualtieri, P. The role of Euglena gracilis paramylon in modulating xylem hormone levels, photosynthesis and water-use efficiency in Solanum lycopersicum L. Physiol. Plant. 2017, 161, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Di Baccio, D.; Tognetti, R.; Minnocci, A.; Sebastiani, L. Responses of the Populus × euramericana clone I-214 to excess zinc: Carbon assimilation, structural modifications, metal distribution and cellular localization. Environ. Exp. Bot. 2009, 67, 153–163. [Google Scholar] [CrossRef]
- Pepe, A.; Di Baccio, D.; Magnani, E.; Giovannetti, M.; Sbrana, C. Zinc and Iron Biofortification and Accumulation of Health-Promoting Compounds in Mycorrhizal Cichorium intybus L. J. Plant. Nutr. Soil Sci. 2022, 22, 4703–4716. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 1; Klute, A., Ed.; ASA-SSSA: Madison, WI, USA, 1992; pp. 403–430. [Google Scholar] [CrossRef]
| Growth Parameter | NaCl Treatment | Statistics | ||
|---|---|---|---|---|
| leaf | Ctr. | Tr. | F | p |
| FW/DW | 4.28 ± 0.43 a | 1.28 ± 0.11 b | 45.07 | <0.001 |
| RWC (%) | 78.75 ± 4.96 a | 28.94 ± 11.03 b | 20.89 | 0.001 |
| LMA (g m−2) | 56.43 ± 1.29 b | 145.23 ± 12.95 a | 46.57 | 0.002 |
| LAR (m2 kg−1) | 12.03 ± 0.37 a | 4.13 ± 0.50 b | 164.28 | <0.001 |
| LMR (g g−1) | 0.67 ± 0.02 | 0.66 ± 0.05 | 0.01 | 0.922 |
| NAR (g m−2 d−1) | 3.39 ± 0.58 | 3.32 ± 1.64 | 0.002 | 0.969 |
| stem | Ctr. | Tr. | ||
| FW/DW | 3.07 ± 0.14 a | 2.46 ± 0.18 b | 7.13 | 0.028 |
| SL (cm) | 4.88 ± 0.23 | 4.66 ± 0.45 | 0.19 | 0.672 |
| SLMR (cm g−1) | 31.59 ± 2.21 | 40.23 ± 6.23 | 2.06 | 0.194 |
| SMR (g g−1) | 0.23 ± 0.03 | 0.24 ± 0.04 | 0.046 | 0.835 |
| stem/leaf | 0.35 ± 0.05 | 0.40 ± 0.12 | 0.16 | 0.697 |
| root | Control | Tr. | ||
| FW/DW | 9.35 ± 0.77 | 7.54 ± 0.78 | 0.88 | 0.377 |
| RL (cm) | 9.49 ± 1.32 | 9.00 ± 1.33 | 0.068 | 0.801 |
| RLMR (cm g−1) | 132.94 ± 16.92 | 157.68 ± 23.11 | 0.69 | 0.433 |
| RMR (g g−1) | 0.11 ± 0.02 | 0.10 ± 0.02 | 0.049 | 0.830 |
| Whole plant | Ctr. | Tr. | ||
| LA (cm2) | 87.65 ± 25.40 a | 16.02 ± 2.61 b | 7.87 | 0.049 |
| FW/DW | 4.49 ± 0.46 a | 2.14 ± 0.11 b | 24.28 | 0.001 |
| RGR (d−1) | 4.00 × 10−2 ± 5.75 × 10−3 a | 2.44 × 10−2 ± 3.11 × 10−3 b | 5.66 | 0.044 |
| shoot/root | 10.02 ± 2.94 | 10.65 ± 3.22 | 0.021 | 0.889 |
| Element | Plant Organ | NaCl Treatment | Statistics | ||
|---|---|---|---|---|---|
| Ctr. | Tr. | F | p | ||
| K (mg g−1) | leaves | 36.51 ± 4.69 | 30.22 ± 2.89 | 1.31 | 0.297 |
| stem | 16.46 ± 2.24 | 12.06 ± 2.19 | 1.98 | 0.209 | |
| roots | 31.82 ± 5.03 a | 10.21 ± 1.31 b | 17.26 | 0.006 | |
| P (mg g−1) | leaves | 1.84 ± 0.57 | 1.61 ± 0.06 | 0.16 | 0.705 |
| stem | 4.10 ± 1.17 | 5.77 ± 0.61 | 1.61 | 0.251 | |
| roots | 7.47 ± 0.91 | 9.67 ± 2.71 | 0.59 | 0.471 | |
| Ca (mg g−1) | leaves | 17.32 ± 0.73 | 18.20 ± 2.35 | 0.13 | 0.734 |
| stem | 11.12 ± 1.30 a | 5.39 ± 1.84 b | 6.47 | 0.044 | |
| roots | 7.81 ± 2.39 | 9.13 ± 1.59 | 0.21 | 0.662 | |
| Mg (mg g−1) | leaves | 4.05 ± 0.16 | 4.85 ± 0.68 | 1.30 | 0.297 |
| stem | 0.49 ± 0.08 b | 1.14 ± 0.04 a | 49.05 | <0.001 | |
| roots | 1.44 ± 0.23 | 1.30 ± 0.08 | 0.33 | 0.588 | |
| NaCl Treatment | Statistics | |||
|---|---|---|---|---|
| K/Na | Ctr. | Tr. | F | p |
| leaves | 34.02 ± 11.01 a | 0.84 ± 0.13 b | 9.09 | 0.024 |
| stem | 8.31 ± 2.37 a | 0.29 ± 0.05 b | 11.40 | 0.015 |
| roots | 10.75 ± 3.72 a | 0.29 ± 0.03 b | 7.90 | 0.031 |
| Ca/Na | Ctr. | Tr. | ||
| leaves | 15.09 ± 3.64 a | 0.51 ± 0.10 b | 16.01 | 0.007 |
| stem | 5.36 ± 1.21 a | 0.13 ± 0.04 b | 18.55 | 0.005 |
| roots | 2.09 ± 0.49 a | 0.26 ± 0.05 b | 13.71 | 0.010 |
| Mg/Na | Ctr. | Tr. | ||
| leaves | 3.44 ± 0.69 a | 0.14 ± 0.03 b | 22.97 | 0.003 |
| stem | 0.26 ± 0.10 a | 0.027 ± 0.002 b | 5.42 | 0.050 |
| roots | 0.42 ± 0.04 a | 0.04 ± 0.01 b | 96.12 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Baccio, D.; Lorenzi, A.; Scartazza, A.; Rosellini, I.; Franchi, E.; Barbafieri, M. Morphophysiological Characterisation of Guayule (Parthenium argentatum A. Gray) in Response to Increasing NaCl Concentrations: Phytomanagement and Phytodesalinisation in Arid and Semiarid Areas. Plants 2024, 13, 378. https://doi.org/10.3390/plants13030378
Di Baccio D, Lorenzi A, Scartazza A, Rosellini I, Franchi E, Barbafieri M. Morphophysiological Characterisation of Guayule (Parthenium argentatum A. Gray) in Response to Increasing NaCl Concentrations: Phytomanagement and Phytodesalinisation in Arid and Semiarid Areas. Plants. 2024; 13(3):378. https://doi.org/10.3390/plants13030378
Chicago/Turabian StyleDi Baccio, Daniela, Aurora Lorenzi, Andrea Scartazza, Irene Rosellini, Elisabetta Franchi, and Meri Barbafieri. 2024. "Morphophysiological Characterisation of Guayule (Parthenium argentatum A. Gray) in Response to Increasing NaCl Concentrations: Phytomanagement and Phytodesalinisation in Arid and Semiarid Areas" Plants 13, no. 3: 378. https://doi.org/10.3390/plants13030378
APA StyleDi Baccio, D., Lorenzi, A., Scartazza, A., Rosellini, I., Franchi, E., & Barbafieri, M. (2024). Morphophysiological Characterisation of Guayule (Parthenium argentatum A. Gray) in Response to Increasing NaCl Concentrations: Phytomanagement and Phytodesalinisation in Arid and Semiarid Areas. Plants, 13(3), 378. https://doi.org/10.3390/plants13030378









