Understanding the Relations between Soil Biochemical Properties and N2O Emissions in a Long-Term Integrated Crop–Livestock System
Abstract
1. Introduction
2. Results
2.1. Soil C and N
2.2. Microbial Biomass C and N
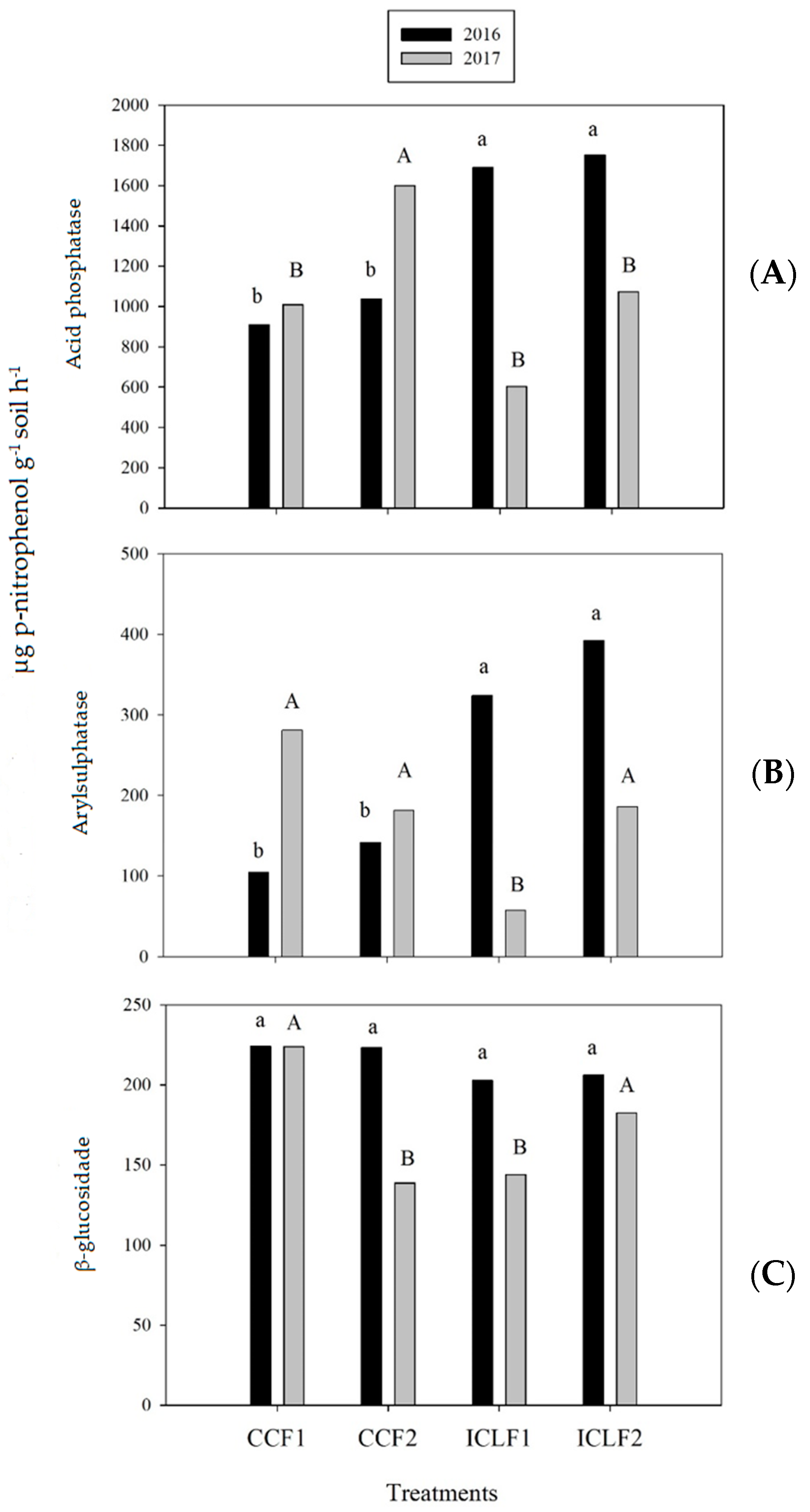
2.3. Cumulative N2O Emissions and Soil Enzymes
3. Discussion
3.1. Effect of Management Systems on Soil C and N
3.2. Effect of Management Systems on Biomass C and N
3.3. Effect of Management Systems on Cumulative N2O Emissions and Soil Enzymes
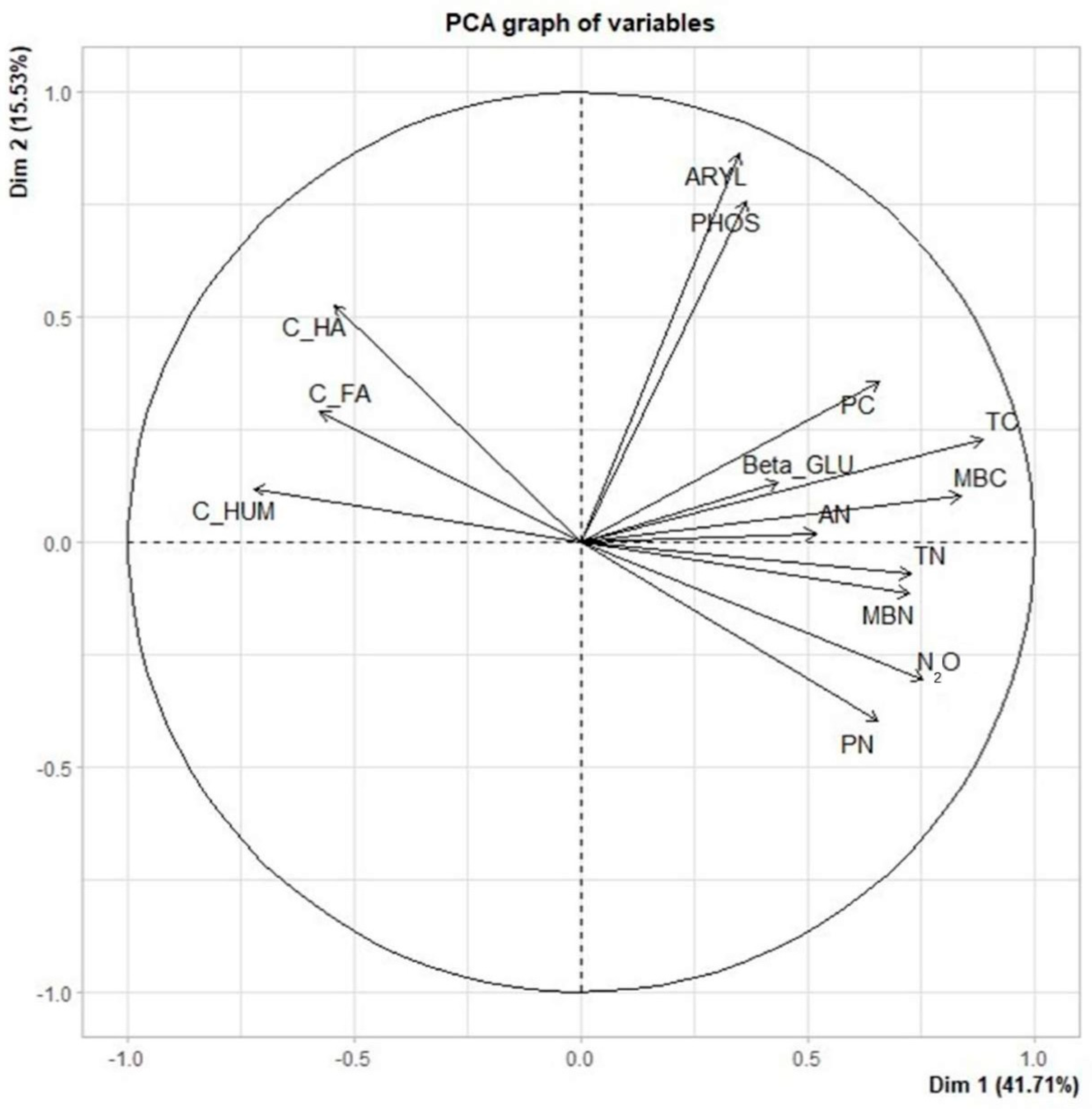
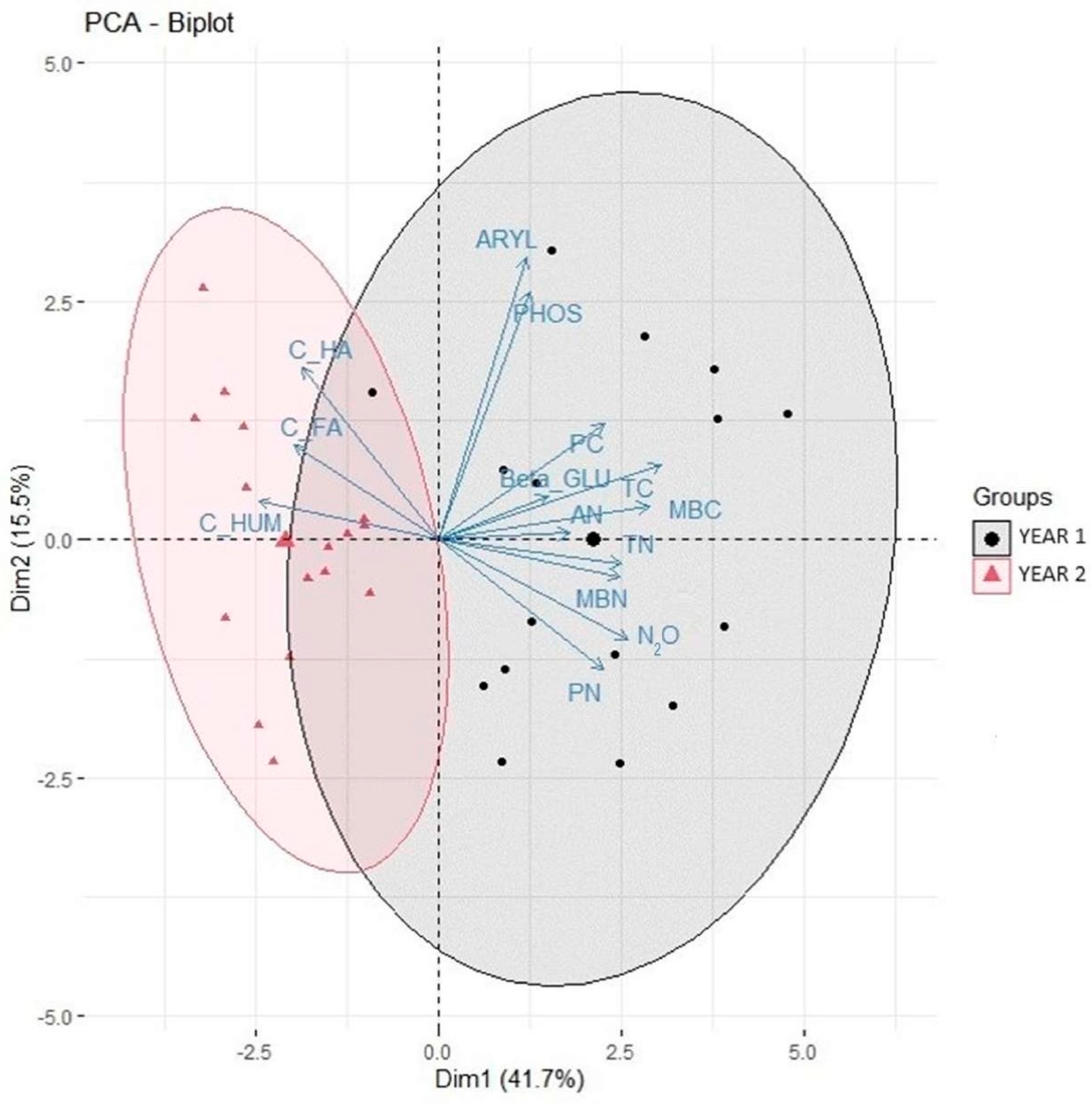
3.4. Relationship between Soil Properties, Cumulative N2O Emissions, and Soil Enzymes
4. Materials and Methods
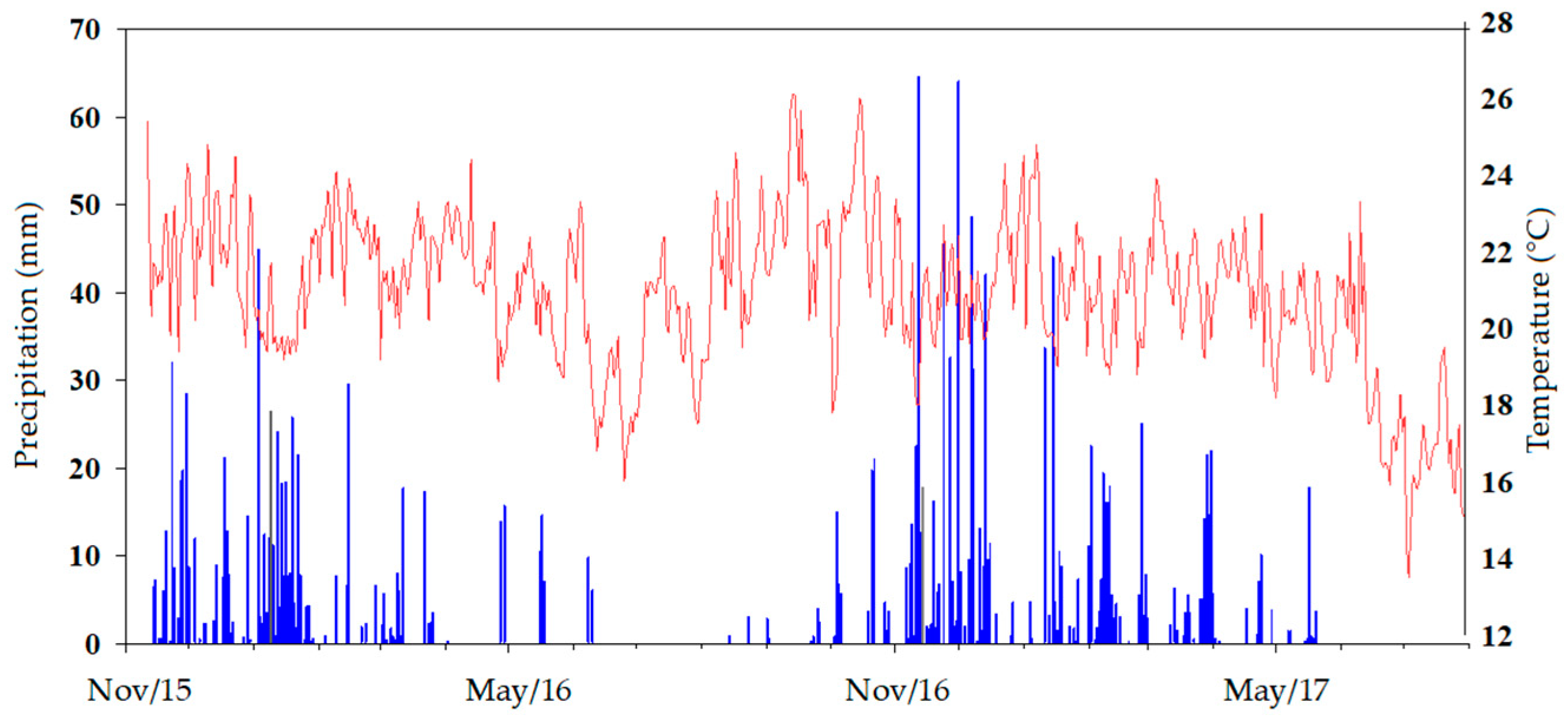
4.1. Site Description and Experimental Design
4.2. Soil Sampling and Analysis
4.2.1. Soil Total C and N
4.2.2. Soil Particulate C and N Fractions
4.2.3. Soil Microbial Biomass C and N
4.2.4. Chemical Fractionation of Soil Organic Matter
4.2.5. Soil Available N
4.2.6. Soil Enzymes
4.2.7. Nitrous Oxide Sampling and Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 659–740. [Google Scholar]
- Tian, H.; Yang, J.; Xu, R.; Lu, C.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Chang. Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef]
- Ciais, P.C.; Sabine, G.; Bala, L.; Bopp, V.; Brovkin, J.; Canadell, A.; Chhabra, R.; DeFries, J.; Galloway, M.; Heimann, C.; et al. Carbon and Other Biogeochemical Cycles. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/WG1AR5_Chapter06_FINAL.pdf (accessed on 2 December 2023).
- da Silva, F.A.M.; de Oliveira, A.D.; de Carvalho, A.M.; Marchão, R.L.; Luiz, A.J.B.; Ribeiro, F.P.; Müller, A.G. Effects of agricultural management and of climate change on N2O emissions in an area of the Brazilian Cerrado: Measurements and simulations using the STICS soil-crop model. Agric. Ecosyst. Environ. 2024, 363, 108842. [Google Scholar] [CrossRef]
- Ponti, S.M.C.; Videla, C.C.; Monterubbianesi, M.G.; Andrade, F.H.; Rizzalli, R.H. Crop intensification with sustainable practices did not increase N2O emissions. Agric. Ecosyst. Environ. 2020, 292, 106828. [Google Scholar] [CrossRef]
- Ramzan, S.; Rasool, T.; Bhat, R.A.; Ahmad, P.; Ashraf, I.; Rashid, N.; Shafiq, M.U.; Mir, I.A. Agricultural soils a trigger to nitrous oxide: A persuasive greenhouse gas and its management. Environ. Monit. Assess. 2020, 192, 1–21. [Google Scholar] [CrossRef]
- MCTI. Ministério da Ciência e Tecnologia. Coordenação Geral de Mudanças Globais do Clima. Quarta Comunicação Nacional do Brasil à Convenção-Quadro das Nações Unidas Sobre Mudança do Clima; Brasília, D.F., Ed.; Ministério da Ciência e Tecnologia: Brasília, Brazil, 2021. [Google Scholar]
- de Figueiredo, C.C.; de Oliveira, A.D.; dos Santos, I.L.; Ferreira, E.A.B.; Malaquias, J.V.; de Sá, M.A.C.; de Carvalho, A.M.; dos Santos, J.D.D.G. Relationships between soil organic matter pools and nitrous oxide emissions of agroecosystems in the Brazilian Cerrado. Sci. Total Environ. 2018, 618, 1572–1582. [Google Scholar] [CrossRef]
- Ammann, C.; Neftel, A.; Jocher, M.; Fuhrer, J.; Leifeld, J. Effect of management and weather variations on the greenhouse gas budget of two grasslands during a 10-year experiment. Agric. Ecosyst. Environ. 2020, 292, 106814. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management strategies to mitigate N2O emissions in agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef]
- Brazil Ministry of the Environment. Guidelines for a National Strategy for Climate Neutrality. 2022. Available online: http://www.mma.gov.br (accessed on 4 January 2024).
- Amadori, C.; Dieckow, J.; Zanatta, J.A.; de Moraes, A.; Zaman, M.; Bayer, C. Nitrous oxide and methane emissions from soil under integrated farming systems in southern Brazil. Sci. Total Environ. 2022, 828, 154555. [Google Scholar] [CrossRef]
- Pereira, G.S.; Angnes, G.; Franchini, J.C.; Damian, J.M.; Cerri, C.E.P.; Rocha, C.H.; da Silva, R.V.; dos Santos, E.L.; Tavares Filho, J. Soil nitrous oxide emissions after the introduction of integrated cropping systems in subtropical conditions. Agric. Ecosyst. Environ. 2022, 323, 107684. [Google Scholar] [CrossRef]
- Sato, J.H.; de Figueiredo, C.C.; Marchão, R.L.; de Oliveira, A.D.; Vilela, L.; Delvico, F.M.; Alves, B.J.R.; de Carvalho, A.M. Understanding the relations between soil organic matter fractions and N2O emissions in a long-term integrated crop-livestock system. Eur. J. Soil Sci. 2019, 70, 1183–1196. [Google Scholar] [CrossRef]
- Thomas, B.W.; Hao, X.; Larney, F.J.; Goyer, C.; Chantigny, M.H.; Charles, A. Non-legume cover crops can increase non-growing season nitrous oxide emissions. Soil Sci. Soc. Am. J. 2017, 81, 189–199. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Bareche, J.; Pareja-Sánchez, E.; Justes, É.; Cantero-Martínez, C. No-tillage reduces long-term yield-scaled soil nitrous oxide emissions in rainfed Mediterranean agroecosystems: A field and modelling approach. Agric. Ecosyst. Environ. 2018, 262, 36–47. [Google Scholar] [CrossRef]
- Abalos, D.; Recous, S.; Butterbach-Bahl, K.; Notaris, C.; Rittl, T.F.; Topp, C.F.E.; Søren, P.O.; Hansen, S.; Bleken, M.A.; Rees, R.M.; et al. A review and meta-analysis of mitigation measures for nitrous oxide emissions from crop residues. Sci. Total Environ. 2022, 828, 154388. [Google Scholar] [CrossRef]
- Coser, T.R.; Ramos, M.L.G.; de Figueiredo, C.C.D.; de Carvalho, A.M.; Cavalcante, E.; Moreira, M.K.R.; Araújo, P.S.M.; de Oliveira, S.A. Soil microbiological properties and available nitrogen for corn in monoculture and intercropped with forage. Pesqui. Agropecu. Bras. 2016, 51, 1660–1667. [Google Scholar] [CrossRef]
- Huang, J.; Hu, B.; Qi, K.; Chen, W.; Pang, X.; Bao, W.; Tian, G. Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol. 2016, 72, 35–41. [Google Scholar] [CrossRef]
- de Carvalho, A.M.; de Oliveira, W.R.D.; Ramos, M.L.G.; Coser, T.R.; de Oliveira, A.D.; Pulrolnik, K.; Souza, K.W.; Vilela, L.; Marchão, R.L. Soil N2O fluxes in integrated production systems, continuous pasture and Cerrado. Nutr. Cycl. Agroecosyst. 2017, 108, 69–83. [Google Scholar] [CrossRef]
- Lopes, A.A.C.; de Sousa, D.M.G.; Chaer, G.M.; dos Reis Júnior, F.B.; Goedert, W.J.; Mendes, I.C. Interpretation of microbial soil indicators as a function of crop yield and organic carbon. Soil Sci. Soc. Am. J. 2013, 77, 461–472. [Google Scholar] [CrossRef]
- Borase, D.N.; Nath, C.P.; Hazra, K.K.; Senthilkumar, M.; Singh, S.S.; Praharaj, C.S.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Poggere, G.; Corrêa, R.S.; Hungria, M.; Mendes, I.C. Soil enzymatic activity in Brazilian biomes under native vegetation and contrasting cropping and management. Appl. Soil Ecol. 2019, 190, 105014. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil. 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Maiga, A.; Alhameid, A.; Singh, S.; Polat, A.; Singh, J.; Kumar, S.; Osborne, S. Responses of soil organic carbon, aggregate stability, carbon and nitrogen fractions to 15 and 24 years of no-till diversified crop rotations. Soil Res. 2019, 57, 149–157. [Google Scholar] [CrossRef]
- de Carvalho, A.M.; dos Santos, D.C.R.; Ramos, M.L.G.; Marchão, R.L.; Vilela, L.; de Sousa, T.R.; Malaquias, J.V.; Gonçalves, A.D.M.A.; Coser, T.T.; de Oliveira, A.D. Nitrous oxide emissions from a long-term integrated crop–livestock system with two levels of P and K fertilization. Land 2022, 11, 1535. [Google Scholar] [CrossRef]
- Soares, D.S.; Ramos, M.L.G.; Marchão, R.L.; Maciel, G.A.; de Oliveira, A.D.; Malaquias, J.V.; de Carvalho, A.M. How diversity of crop residues in long-term no-tillage systems affect chemical and microbiological soil properties. Soil Tillage Res. 2019, 194, 104316. [Google Scholar] [CrossRef]
- Costa, A.A.; Dias, B.D.O.; Fraga, V.D.S.; Santana, C.C.; Sampaio, T.F.; Silva, N.D. Physical fractionation of organic carbon in areas under different land uses in the Cerrado. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 534–540. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Resck, D.V.S.; Carneiro, M.A.C.; Ramos, M.L.G.; Sá, J.C.M. Stratification Ratio of Organic Matter Pools Influenced by Management Systems in a Weathered Oxisol from a Tropical Agro-Ecoregion in Brazil. Soil Res. 2013, 51, 133–141. [Google Scholar] [CrossRef]
- Fernandes, F.C.S.; Libardi, P.L.; Carvalho, L.A.D. Internal drainage and nitrate leaching in a corn-black oat-corn succession with two split nitrogen applications. Sci. Agric. 2006, 63, 483–492. [Google Scholar] [CrossRef]
- Turner, S.; Meyer-Stüve, S.; Schippers, A.; Guggenberger, G.; Schaarschmidt, F.; Wild, B.; Richter, A.; Dohrmann, R.; Mikutta, R. Microbial utilization of mineral-associated nitrogen in soils. Soil Biol. Biochem. 2017, 104, 185–196. [Google Scholar] [CrossRef]
- Jilling, A.; Kane, D.; Williams, A.; Yannarell, A.C.; Davis, A.; Jordan, N.R.; Koide, R.T.; Mortesen, D.A.; Smith, R.G.; Snapp, S.S.; et al. Rapid and distinct response of particulate and mineral-associated organic nitrogen to conservation tillage and cover crops. Geoderma 2020, 359, 114001. [Google Scholar] [CrossRef]
- Villarino, S.H.; Talab, E.; Contisciani, L.; Videla, C.; Di Geronimo, P.; Mastrángelo, M.E.; Georgiou, K.; Jackson, R.J.; Piñeiro, G. A large nitrogen supply from the stable mineral-associated soil organic matter fraction. Biol. Fertil. Soils 2023, 59, 833–841. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Gutknecht, J.L.M.; Stuart Grandy, A. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 2021, 158, 108265. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Sci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Baptistella, J.L.C.; de Andrade, S.A.L.; Favarin, J.L.; Mazzafera, P. Urochloa in Tropical Agroecosystems. Front. Sustain. Food Syst. 2020, 4, 119. [Google Scholar] [CrossRef]
- Soussana, J.F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Contosta, A.R.; Frey, S.; Schimel, J.; Schnecker, J.; Smith, R.G.; Tiemann, L.; Grandy, A.S. Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 2018, 139, 103–122. [Google Scholar] [CrossRef]
- Meneghin, M.F.S.; Ramos, M.L.G.; Oliveira, S.A.D.; Ribeiro Junior, W.Q.; Amabile, R.F. Avaliação da disponibilidade de nitrogênio no solo para o trigo em Latossolo vermelho do Distrito Federal. Rev. Bras. Cienc. Solo 2008, 32, 1941–1948. [Google Scholar] [CrossRef][Green Version]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of permanganate-oxidizable carbon and mineralizable carbon for assessment of organic matter stabilization and mineralization. Soil Sci. Soc. Am. J. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- Reis, M.M.; Santos, L.D.; Pegoraro, R.F.; Colen, F.; Rocha, L.M.; Ferreira, G.A.D.P. Nutrition of Tithonia diversifolia and attributes of the soil fertilized with bio fertilizer in irrigated system. Rev. Bras. Eng. Agric. Ambient. 2016, 20, 1008–1013. [Google Scholar] [CrossRef]
- Čapek, P.; Choma, M.; Tahovská, K.; Kaňa, J.; Kopáček, J.; Šantrůčková, H. Coupling the resource stoichiometry and microbial biomass turnover to predict nutrient mineralization and immobilization in soil. Geoderma 2021, 385, 114884. [Google Scholar] [CrossRef]
- Santos, J.V.; Bento, L.R.; Bresolin, J.D.; Mitsuyuki, M.C.; Oliveira, P.P.A.; Pezzopane, J.R.M.; Bernardi, A.C.C.; Mendes, I.C.; Martin-Neto, L. The long-term effects of intensive grazing and silvopastoral systems on soil physicochemical properties, enzymatic activity, and microbial biomass. Catena 2022, 219, 106619. [Google Scholar] [CrossRef]
- Xu, S.; Geng, W.; Sayer, E.J.; Zhou, G.; Zhou, P.; Liu, C. Soil microbial biomass and community responses to experimental precipitation change: A meta-analysis. Soil Ecol. Lett. 2020, 2, 93–103. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2013, 27, 14–25. [Google Scholar] [CrossRef]
- Dadalto, J.P.; Fernandes, H.C.; Teixeira, M.M.; Cecon, P.R.; de Matos, A.T. Sistema de preparo do solo e sua influência na atividade microbiana. Eng. Agrícola 2015, 35, 506–513. [Google Scholar] [CrossRef]
- Lepcha, N.T.; Devi, N.B. Effect of land use, season, and soil depth on soil microbial biomass carbon of Eastern Himalayas. Ecol. Process. 2020, 9, 65. [Google Scholar] [CrossRef]
- Sauer, D.; Kuzyakov, Y.; Stahr, K. Spatial distribution of root exudates of five plant species as assessed by 14C labeling. J. Plant. Nutr. Soil Sci. 2006, 169, 360–362. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere micro-biome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y. Can phosphorus and nitrogen addition affect ammonia oxidizers in a high-phosphorus agricultural soil? Arch. Agron. Soil Sci. 2018, 64, 1728–1743. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Moscatelli, M.C.; Di Tizio, A.; Mancinelli, R.; Grego, S.; Marinari, S. Soil biochemical indicators as a tool to assess the short-term impact of agricultural management on changes in organic C in a Mediterranean environment. Ecol. Indic. 2009, 9, 518–527. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- Stott, D.E.; Andrews, S.S.; Liebig, M.A.; Wienhold, B.J.; Karlen, D.L. Evaluation of β-glucosidase activity as a soil quality indicator for the soil management assessment framework. Soil Sci. Soc. Am. J. 2010, 74, 107–119. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheschea, M.; Walker, F.; Eash, N. Long term tillage, cover crops, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzyme in a changing environment: Current knowledge and future decisions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Ros, G.H.; Chen, Y.; Yang, X.; Cui, Z.; Liu, X.; Jiang, R.; Zhang, F.; de Vries, W. Global meta-analysis of terrestrial nitrous oxide emissions and associated functional genes under nitrogen addition. Soil Biol. Biochem. 2022, 165, 108523. [Google Scholar] [CrossRef]
- Chen, M.; Chang, L.; Zhang, J.; Guo, F.; Vyzamal, J.; He, Q.; Chen, Y. Global nitrogen input on wetland ecosystem: The driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 2020, 4, 100063. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System; Embrapa: Brasília, Brazil, 2018; Available online: http://ainfo.cnptia.embrapa.br/digital/bitstream/item/181678/1/SiBCS-2018-ISBN-9788570358219-english.epub (accessed on 20 December 2023).
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Bayer, C.; Martin-Neto, L.; Mielniczuk, J.; Pillon, C.N.; Sangoi, L. Changes in soil organic matter fractions under subtropical no-till cropping systems. Soil Sci. Soc. Am. J. 2001, 65, 1473–1478. [Google Scholar] [CrossRef]
- Bongiovanni, M.D.; Lobartini, J.C. Particulate organic matter, carbohydrate, humic acid contents in soil macro- and microaggregates as affected by cultivation. Geoderma 2006, 136, 660–665. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: The use of the chloroform fumigation-incubation method in strongly acid soils. Soil Biol. Biochem. 1987, 19, 697–702. [Google Scholar] [CrossRef]
- Castellazzi, M.S.; Brookes, P.C.; Jenkinson, D.S. Distribution of microbial biomass down soil profiles under regenerating woodland. Soil Biol. Biochem. 2004, 36, 1485–1489. [Google Scholar] [CrossRef]
- Joergensen, R.G.; MUELLER, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Swift, R. Organic matter characterization. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun Soil Sci Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Gianello, C.; Bremner, J.M. Comparison of chemical methods of assessing potentially available organic nitrogen in soil. Commun Soil Sci Plant Anal. 1986, 17, 215–236. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2–Microbiological and Biochemical Properties; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Lopes, A.A.C.; de Sousa, D.M.G.; dos Reis Junior, F.B.; Mendes, I.C. Air-drying and long-term storage effects on β-glucosidase, acid phosphatase and arylsulfatase activities in a tropical Savannah Oxisol. Appl. Soil Ecol 2015, 93, 68–77. [Google Scholar] [CrossRef]
- Sato, J.H.; de Carvalho, A.M.; Figueiredo, C.C.; Coser, T.R.; Sousa, T.R.; Vilela, L.; Marchão, R.L. Nitrous oxide fluxes in a Brazilian clayey oxisol after 24 years of integrated crop-livestock management. Nutr. Cycl. Agroecosyst. 2017, 108, 55–68. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Zanatta, J.A.; Vieira, F.C.B.; Pilcolo, M.C.; Dieckow, J.; Six, J. Soil nitrous oxide emissions as affected by long-term tillage, cropping systems and nitrogen fertilization in Southern Brazil. Soil Tillage Res. 2015, 146, 213–222. [Google Scholar] [CrossRef]
- Hair, J.F. Multivariate Data Analysis: An Overview. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 904–907. [Google Scholar]
| 2016 | |||
| Management System | TC | PC | MAC |
| g kg−1 | |||
| CCF1 | 33.50 b | 2.80 b | 30.70 a |
| CCF2 | 33.27 b | 3.86 ab | 29.41 a |
| ICLF1 | 33.15 b | 4.07 ab | 29.07 a |
| ICLF2 | 39.15 a | 6.45 a | 32.70 a |
| 2017 | |||
| CCF1 | 27.92 ab | 2.65 a | 25.27 ab |
| CCF2 | 25.82 b | 2.52 a | 23.30 b |
| ICLF1 | 27.35 b | 2.82 a | 24.52 b |
| ICLF2 | 30.92 a | 3.40 a | 27.85 a |
| 2016 | ||||
| Management System | TN | PN | MAN | AN |
| g kg−1 | mg kg−1 | |||
| CCF1 | 3.45 b | 0.35 a | 2.82 b | 58.81 a |
| CCF2 | 5.15 a | 0.37 a | 4.45 a | 53.73 a |
| ICLF1 | 3.95 ab | 0.35 a | 3.32 ab | 37.07 a |
| ICLF2 | 3.85 b | 0.43 a | 3.32 ab | 65.72 a |
| 2017 | ||||
| CCF1 | 2.35 a | 0.35 a | 2.00 a | 29.39 b |
| CCF2 | 2.50 a | 0.38 a | 2.12 a | 66.80 a |
| ICLF1 | 2.22 a | 0.35 a | 1.87 a | 30.52 b |
| ICLF2 | 2.50 a | 0.42 a | 2.08 a | 33.06 b |
| 2016 | |||
| Management System | C-FA | C-HA | C-HUM |
| g kg−1 | |||
| CCF1 | 6.99 a | 1.57 a | 8.83 a |
| CCF2 | 7.10 a | 1.55 a | 9.41 a |
| ICLF1 | 7.11 a | 2.24 a | 9.65 a |
| ICLF2 | 6.69 a | 1.48 a | 7.82 a |
| 2017 | |||
| CCF1 | 7.62 a | 2.76 a | 11.17 a |
| CCF2 | 7.95 a | 2.42 a | 10.62 a |
| ICLF1 | 6.92 a | 1.62 b | 10.38 a |
| ICLF2 | 7.36 a | 1.64 b | 9.85 a |
| 2016 | ||||
| Management System | MBC | MBN | MQC | MQN |
| mg kg−1 | % | |||
| CCF1 | 488.33 c | 118.9 a | 1.47 b | 3.42 a |
| CCF2 | 855.86 ab | 47.35 c | 2.60 a | 0.91 c |
| ICLF1 | 595.72 bc | 61.20 bc | 1.80 ab | 1.57 bc |
| ICLF2 | 939.89 a | 89.86 ab | 2.40 ab | 2.54 ab |
| 2017 | ||||
| CCF1 | 185.06 c | 26.75 b | 0.66 b | 1.14 a |
| CCF2 | 247.42 b | 30.35 ab | 0.96 a | 1.22 a |
| ICLF1 | 256.54 b | 28.58 ab | 0.94 a | 1.28 a |
| ICLF2 | 360.4 a | 32.67 a | 1.16 a | 1.31 a |
| Cumulative N2O Emissions | ||
|---|---|---|
| Management System | 2015/2016 | 2016/2017 |
| kg ha−1 | ||
| CCF1 | 0.71 a | 0.16 a |
| CCF2 | 0.85 a | 0.24 a |
| ICLF1 | 0.36 b | 0.11 a |
| ICLF2 | 0.51 b | 0.18 a |
| Management System a | Annual Sequence of Crops (1991–2017 b) |
|---|---|
| Continuous crop (CCF1 c) | S-S-M-S-M-S-M-S-S-Pg-S-Pg-S-S-Sb-S-S-M-S-M-S-M-S-S-Sb-M-S-S |
| Continuous crop (CCF2) | S-S-M-S-M-S-M-S-S-Pg-S-Pg-S-S-Sb-S-S-M-S-M-S-M-S-S-Sb-M-S-S |
| Crop–livestock (ICLF1) | S-S-M-S-Ag-Ag-Ag-Ag-S-Pg-S-Pg-S/Ub-Ub-Ub-S-Pg-P-P-S-P-S-Pg/P-M-S-S |
| Crop–livestock (ICLF2) | S-S-M-S-Ag-Ag-Ag-Ag-S-Pg-S-Pg-S/Ub-Ub-Ub-S-Pg-P-P-S-P-S-Pg/P-M-S-S |
| Management System | Al | Ca | H + Al | SOM | K | P | pH |
|---|---|---|---|---|---|---|---|
| cmolc dm−3 | % | mg L−1 | |||||
| CCF1 | 0.044 | 4.06 | 3.81 | 4.06 | 111.25 | 3.82 | 5.65 |
| CCF2 | 0.020 | 5.49 | 3.02 | 4.59 | 139 | 7.81 | 5.51 |
| ICLF1 | 0.032 | 3.56 | 4.29 | 3.78 | 108 | 12.30 | 5.35 |
| ICLF2 | 0.028 | 5.36 | 3.12 | 3.58 | 169.75 | 27.62 | 5.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, A.M.; Ramos, M.L.G.; Dos Santos, D.C.R.; de Oliveira, A.D.; de Carvalho Mendes, I.; Silva, S.B.; de Sousa, T.R.; Dantas, R.d.A.; Silva, A.M.M.; Marchão, R.L. Understanding the Relations between Soil Biochemical Properties and N2O Emissions in a Long-Term Integrated Crop–Livestock System. Plants 2024, 13, 365. https://doi.org/10.3390/plants13030365
de Carvalho AM, Ramos MLG, Dos Santos DCR, de Oliveira AD, de Carvalho Mendes I, Silva SB, de Sousa TR, Dantas RdA, Silva AMM, Marchão RL. Understanding the Relations between Soil Biochemical Properties and N2O Emissions in a Long-Term Integrated Crop–Livestock System. Plants. 2024; 13(3):365. https://doi.org/10.3390/plants13030365
Chicago/Turabian Stylede Carvalho, Arminda Moreira, Maria Lucrécia Gerosa Ramos, Divina Cléia Resende Dos Santos, Alexsandra Duarte de Oliveira, Ieda de Carvalho Mendes, Stefany Braz Silva, Thais Rodrigues de Sousa, Raíssa de Araujo Dantas, Antonio Marcos Miranda Silva, and Robélio Leandro Marchão. 2024. "Understanding the Relations between Soil Biochemical Properties and N2O Emissions in a Long-Term Integrated Crop–Livestock System" Plants 13, no. 3: 365. https://doi.org/10.3390/plants13030365
APA Stylede Carvalho, A. M., Ramos, M. L. G., Dos Santos, D. C. R., de Oliveira, A. D., de Carvalho Mendes, I., Silva, S. B., de Sousa, T. R., Dantas, R. d. A., Silva, A. M. M., & Marchão, R. L. (2024). Understanding the Relations between Soil Biochemical Properties and N2O Emissions in a Long-Term Integrated Crop–Livestock System. Plants, 13(3), 365. https://doi.org/10.3390/plants13030365





