Geminiviruses and Food Security: A Molecular Genetics Perspective for Sustainable Agriculture in Africa
Abstract
1. Introduction
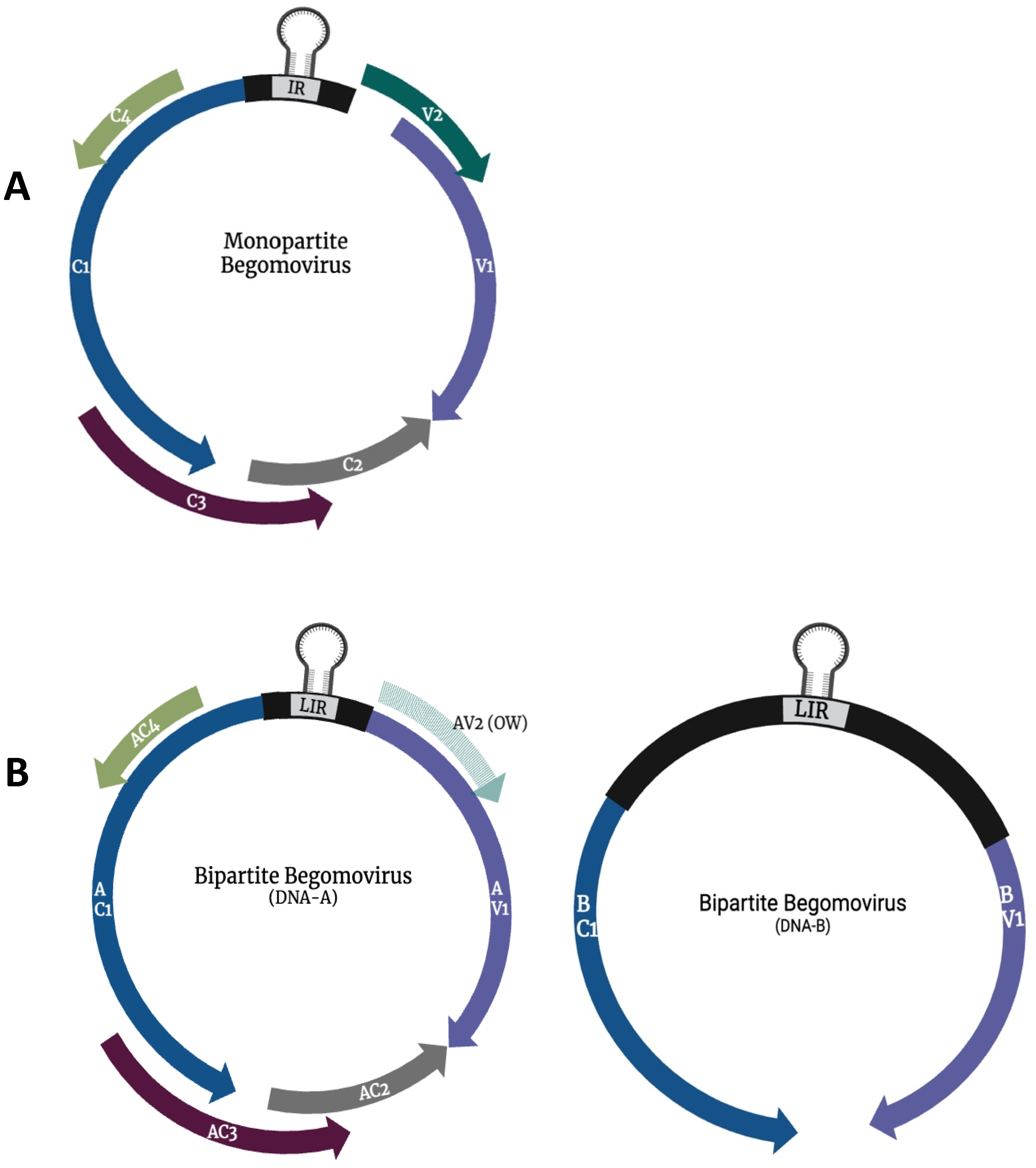
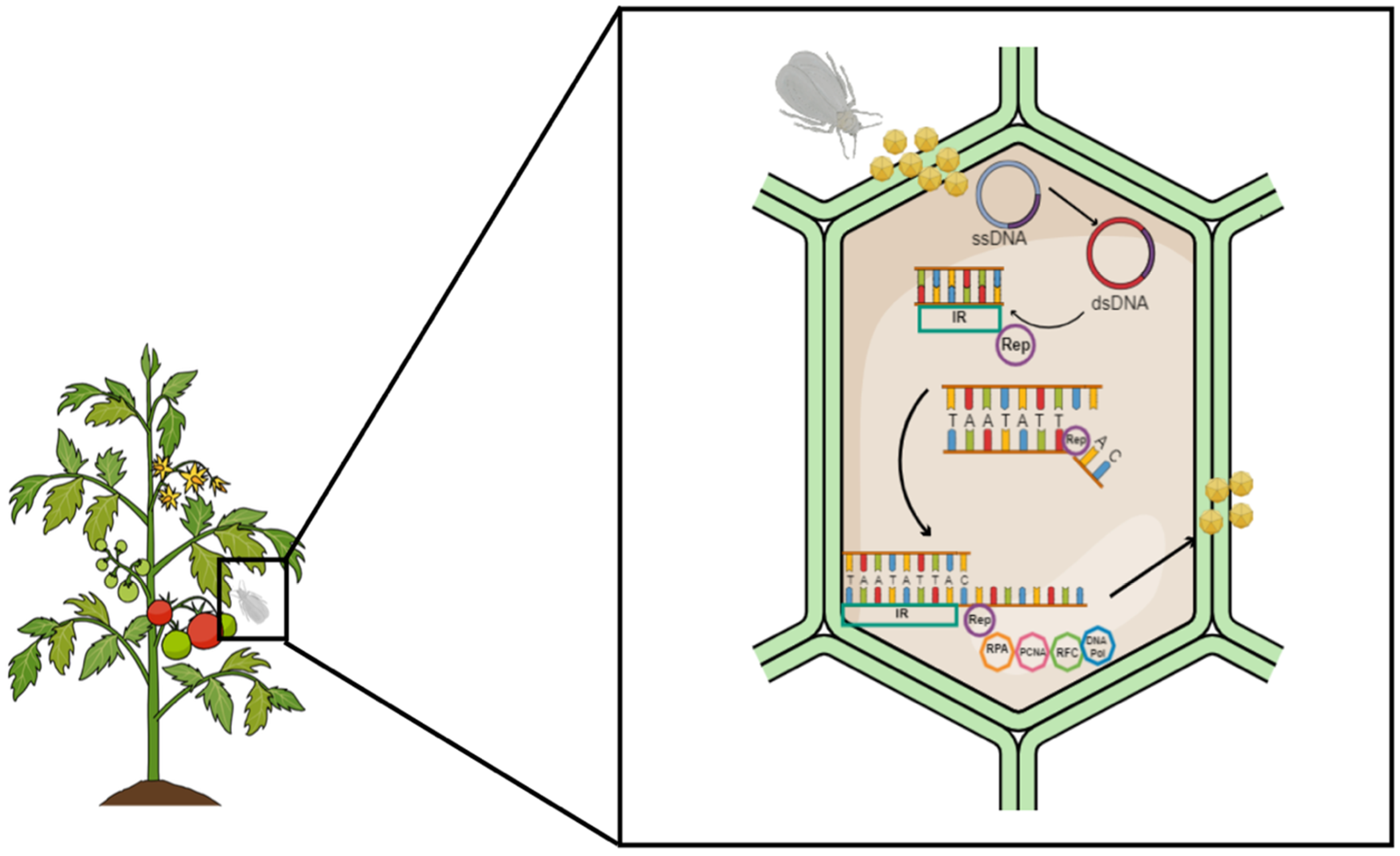
2. Geminivirus Replication
3. Secondary Effects of Geminivirus Infection (Particularly TYLCV)
4. CRISPR/Cas Protein Gene Modification
5. TALENs and ZFNs
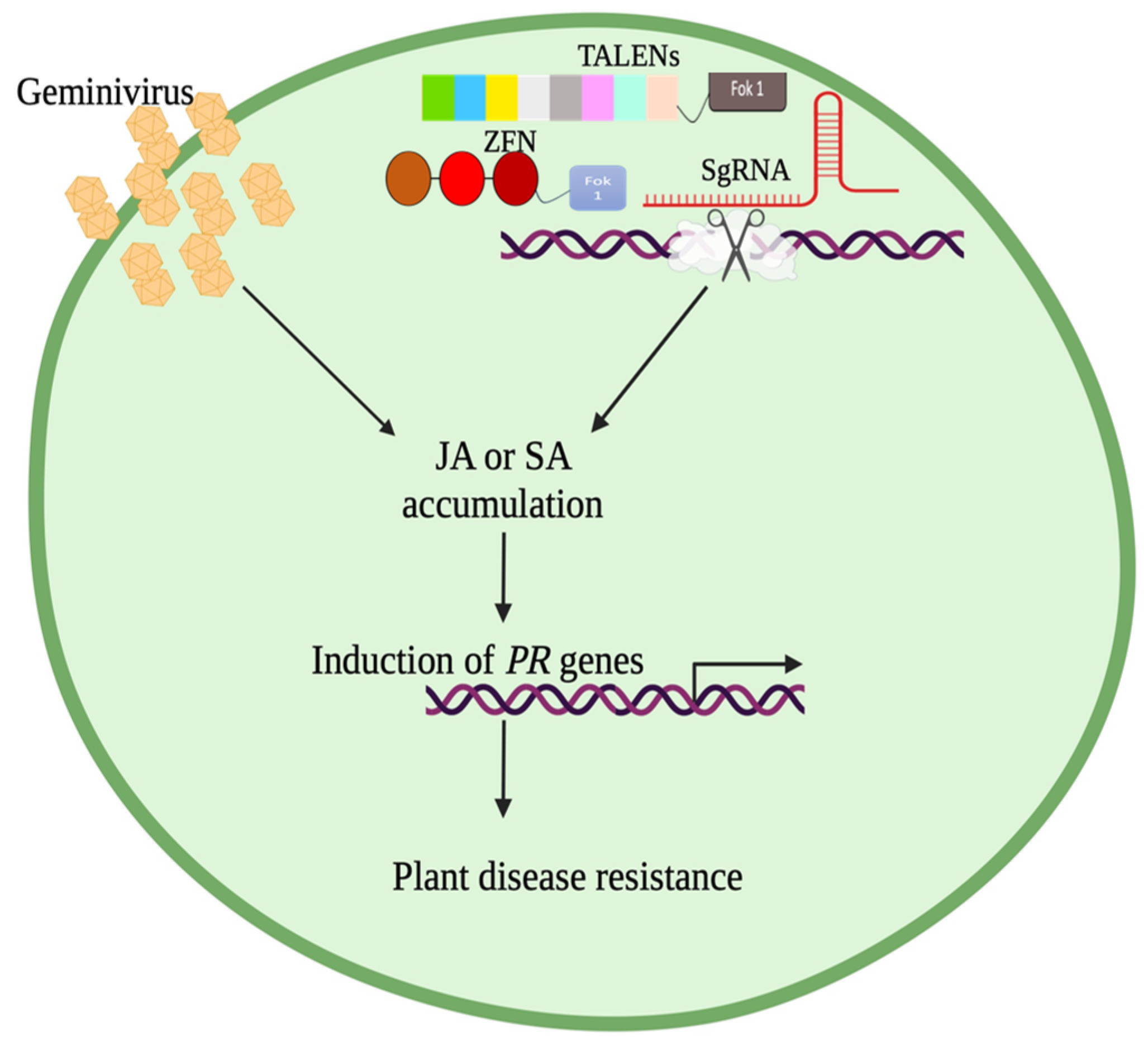
6. Geminivirus Resistance in Plants Using CRISPR/Cas9, TALENs, and ZFNs Technology
7. Enhancing Plant Resistance: A CRISPR-Cas or TALENs/ZFNs Approach to Overexpressing Salicylic Acid (SA) and Jasmonic Acid (JA)
8. Biotechnology for Sustainable Agriculture in Africa
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. The Sustainable Development Goals Report 2022; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Adeyeye, S.A.O.; Ashaolu, T.; Bolaji, O.T.; Abegunde, T.A.; Omoyajowo, A.O. Africa and the nexus of poverty, malnutrition and diseases. Crit. Rev. Food Sci. Nutr. 2021, 63, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organisation. An Introduction to the Basic Concepts of Food Security; Food and Agricultural Organisation: Rome, Italy, 2008. [Google Scholar]
- Pinstrup-Andersen, P. Food security: Definition and measurement. Food Sec. 2009, 1, 5–7. [Google Scholar] [CrossRef]
- Reincke, K.; Vilvert, E.; Fasse, A.; Graef, F.; Sieber, S.; Lana, M.A. Key factors influencing food security of smallholder farmers in Tanzania and the role of cassava as a strategic crop. Food Sec. 2018, 10, 911–924. [Google Scholar] [CrossRef]
- Food and Agricultural Organisation, United Nations Economic Commission for Africa and African Union Commission. Africa—Regional Overview of Food Security and Nutrition 2021: Statistics and Trends; FAO: Accra, Ghana, 2021. [Google Scholar]
- Anfoka, G.; Moshe, A.; Fridman, L.; Amrani, L.; Rotem, O.; Kolot, M.; Zeidan, M.; Czosnek, H.; Gorovits, R. Tomato Yellow Leaf Curl Virus infection mitigates the heat stress response of plants grown at high temperatures. Sci. Rep. 2016, 6, 19715. [Google Scholar] [CrossRef]
- Patil, B.L.; Kumar, L.; Hema, M.; Sreenivasulu, P.; Kumar, P.L.; Reddy, D.V.R. Tropical Food Legumes: Virus Diseases of Economic Importance and Their Control. Adv. Virus Res. 2014, 90, 431–505. [Google Scholar] [CrossRef]
- Picó, B.; Díez, M.J.; Nuez, F. Viral diseases causing the greatest economic losses to the tomato crop. II. The Tomato Yellow Leaf Curl Virus—A Review. Sci. Hortic. 1996, 67, 151–196. [Google Scholar] [CrossRef]
- Patil, B.L.; Fauquet, C.M. Cassava mosaic Geminiviruses: Actual knowledge and perspectives. Mol. Plant Pathol. 2009, 10, 685–701. [Google Scholar] [CrossRef]
- Legg, J.P.; Ogwal, S. Changes in the incidence of African Cassava Mosaic geminivirus and the abundance of its whitefly vector along south-north transects in Uganda. J. Appl. Entomol. 1998, 122, 169–178. [Google Scholar] [CrossRef]
- Legg, J.P. Emergence, spread and strategies for controlling the pandemic of cassava mosaic disease in eastern and central Africa. Crop Prot. 1999, 18, 627–637. [Google Scholar] [CrossRef]
- Chapman, S.; Kavanagh, T.; Baulcombe, D. Potato virus X as a vector for gene expression in plants. Plant J. 1992, 2, 549–557. [Google Scholar] [CrossRef]
- Donson, J.; Kearney, C.M.; Hilf, M.E.; Dawson, W.O. Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc. Natl. Acad. Sci. USA 1991, 88, 7204–7208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Gil-Humanes, J.; Wang, Y.; Liang, Z.; Shan, Q.; Ozuna, C.V.; Sánchez-León, S.; Baltes, N.J.; Starker, C.; Barro, F.; Gao, C.; et al. High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J. 2017, 89, 1251–1262. [Google Scholar] [CrossRef]
- Fofana IB, F.; Sangaré, A.; Collier RTaylor, C.; Fauquet, C.M. A geminivirus-induced gene silencing system for gene function validation in cassava. Plant Mol. Biol. 2004, 56, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Muangsan, N.; Beclin, C.; Vaucheret, H.; Robertson, D. Geminivirus VIGS of endogenous genes requires SGS2/SDE1 and SGS3 and defines a new branch in the genetic pathway for silencing in plants. Plant J. 2004, 38, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Lett, M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J.; Consortium, I.R. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2021, 102, 12. [Google Scholar] [CrossRef]
- Varsani, A.; Navas-Castillo, J.; Moriones, E.; Hernández-Zepeda, C.; Idris, A.; Brown, J.K.; Zerbini, F.M.; Martin, D.P. Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 2014, 159, 2193–2203. [Google Scholar] [CrossRef]
- Zhou, X. Advances in understanding begomovirus satellites. Annu. Rev. Phytopathol. 2013, 51, 357–381. [Google Scholar] [CrossRef]
- Roumagnac, P.; Lett, J.M.; Fiallo-Olivé, E.; Fiallo-Olive, E.; Navas-Castillo, J.; Zerbini, F.M.; Martin, D.P.; Varsani, A. Establishment of five new genera in the family Geminiviridae: Citlodavirus, Maldovirus, Mulcrilevirus, Opunvirus, and Topilevirus. Arch. Virol. 2022, 167, 695–710. [Google Scholar] [CrossRef]
- Bejerman, N. Geminivirus–Vector Relationship. In Geminiviruses; Kumar, R., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- He, Y.-Z.; Wang, Y.-M.; Wang, X.-W. A plant DNA virus replicates in the salivary glands of the insect vector via the recruitment of host DNA synthesis machinery. Proc. Natl. Acad. Sci. USA 2020, 117, 16928–16937. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, B.; Unseld, S.; Ceulemans, H.; Russell, R.; Jekse, H. Geminate Structures of African Cassava Mosaic Virus. J. Virol. 2004, 78, 6758–6765. [Google Scholar] [CrossRef] [PubMed]
- Krupovic, M.; Ravantti, J.J.; Bamford, D.H. Geminiviruses: A tale of a plasmid becoming a virus. BMC Evol. Biol. 2009, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Olson, N.H.; Baker, T.S.; Faulkner, L.; Agbandje-McKenna, M.; Boulton, M.I.; Davies, J.W.; McKenna, R. Structure of the Maize Streak Virus geminate particle. Virology 2001, 279, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Fondong, V.N. Geminivirus proteins. Mol. Plant Pathol. 2013, 14, 635–649. [Google Scholar] [CrossRef]
- Rojas, M.R.; Hagen, C.; Lucas, W.J.; Gilbertson, R.L. Exploiting chinks in the plant’s armor: Evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 2005, 43, 361–394. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A. ICTVReport Consortium ICTVVirus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Lazarowitz, S.G.; Shepherd, R.J. Geminiviruses: Genome structure and gene function. CRC Crit. Rev. Plant Sci. 1992, 11, 327–349. [Google Scholar] [CrossRef]
- Pakkianathan, B.C.; Kontsedalov, S.; Lebedev, G.; Mahadav, A.; Zeidan, M.; Czosnek, H.; Ghanim, M. Replication of Tomato Yellow Leaf Curl Virus in its whitefly vector, Bemisia tabaci. J. Virol. 2015, 89, 9791–9803. [Google Scholar] [CrossRef]
- Saunders, K.; Lucy, A.; Stanley, J. DNA forms of the geminivirus African cassava mosaic virus consistent with a rolling circle mechanism of replication. Nucleic Acids Res. 1991, 19, 2325–2330. [Google Scholar] [CrossRef]
- Jeske, H.; Lütgemeier, M.; Preiss, W. DNA forms indicate rolling circle and recombination-dependent replication of Abutilon mosaic virus. EMBO J. 2001, 20, 6158–6167. [Google Scholar] [CrossRef]
- Rojas, M.R.; Jiang, H.; Salati, R.; Xoconostle-Cázares, B.; Sudarshana, M.R.; Lucas, W.J.; Gilbertson, R.L. Functional analysis of proteins involved in movement of the monopartite begomovirus, Tomato yellow leaf curl virus. Virology 2001, 291, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Kunik, T.; Palanichelvam, K.; Czosnek, H.; Citovsky, V.; Gafni, Y. Nuclear import of the capsid protein of tomato yellow leaf curl virus (TYLCV) in plant and insect cells. Plant J. 1998, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M.; Beachy, R.N.; Fauquet, C.M. The role of AV2 (“precoat”) and coat protein in viral replication and movement in tomato leaf curl geminivirus. Virology 1996, 224, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Padidam, M.; Beachy, R.N.; Fauquet, C.M. A phage single-stranded DNA (ssDNA) binding protein complements ssDNA accumulation of a geminivirus and interferes with viral movement. J. Virol. 1999, 73, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wei, H.; Tan, H.; Pan, S.; Liu, Q.; Bejarano, E.R.; Lozano-Duran, R. Plant DNA polymerases α and δ mediate replication of geminiviruses. Nat. Commun. 2021, 12, 2780. [Google Scholar] [CrossRef] [PubMed]
- Castellano, M.M.; Sanz-Burgos, A.P.; Gutierrez, C. Initiation of DNA replication in an eukaryotic rolling-circle replicon: Identification of multiple DNA-protein complexes at the geminivirus origins. J. Mol. Biol. 1999, 290, 639–652. [Google Scholar] [CrossRef]
- Sanz-Burgos, A.P.; Gutiérrez, C. Organization of the cis-acting element required for wheat dwarf geminivirus DNA replication and visualization of a rep protein-DNA complex. Virology 1998, 243, 119–129. [Google Scholar] [CrossRef][Green Version]
- Orozco, B.M.; Hanley-Bowdoin, L. Conserved sequence and structural motifs contribute to the DNA binding and cleavage activities of a geminivirus replication protein. J. Biol. Chem. 1998, 273, 24448–24456. [Google Scholar] [CrossRef]
- Krenz, B.; Neugart, F.; Kleinow, T.; Jeske, H. Self-interaction of Abutilon Mosaic Virus replication initiator protein (Rep) in plant cell nuclei. Virus Res. 2011, 161, 194–197. [Google Scholar] [CrossRef]
- Horvath, G.V.; Pettko-Szandtner, A.; Nikovics, K.; Bilgin, M.; Boulton, M.; Davies, J.W.; Gutierrez, C.; Dudits, D. Prediction of functional regions of the maize streak virus replication-associated proteins by protein–protein interaction analysis. Plant Mol. Biol. 1998, 38, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Sanz-Burgos, A.P.; Ramirez-Parra, E.; Castellano, M.M.; Gutierrez, C. Interaction of geminivirus Rep protein with replication factor C and its potential role during geminivirus DNA replication. Virology 2002, 302, 83–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fontes, E.P.; Eagle, P.A.; Sipe, P.S.; Luckow, V.A.; Hanley-Bowdoin, L. Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 1994, 269, 8459–8846. [Google Scholar] [CrossRef]
- Laufs, J.; Trauts, W.; Heyraud, F.; Matzeit, V.; Rogers, S.G.; Schell, J.; Groneborn, B. In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of Tomato Yellow Leaf Curl Virus. Proc. Natl. Acad. Sci. USA 1995, 92, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Orozco, B.M.; Hanley-Bowdoin, L. A DNA structure is required for geminivirus replication origin function. J. Virol. 1996, 70, 148–258. [Google Scholar] [CrossRef] [PubMed]
- Eagle, P.A.; Orozco, B.M.; Hanley-Bowdoin, L. A DNA sequence required for geminivirus replication also mediates transcriptional replication. Plant Cell 1994, 6, 1157–1170. [Google Scholar] [CrossRef]
- Hofer, J.M.; Dekker, E.L.; Reynolds, H.V.; Woolston, C.J.; Cox, B.S.; Mullineaux, P.M. Coordinate regulation of replication and virion sense gene expression in wheat dwarf virus. Plant Cell 1992, 4, 213–223. [Google Scholar] [CrossRef]
- Elmer, J.S.; Brand, L.; Sunter, G.; Gardiner, W.E.; Bisaro, D.M.; Rogers, S.G. Genetic analysis of the Tomato Golden Mosaic Virus. II. Requirement for the product of the highly conserved ALI coding sequence for replication. Nucleic Acids Res. 1988, 16, 7043–7060. [Google Scholar] [CrossRef]
- Singh, D.K.; Islam, M.N.; Choudhury, N.R.; Karjee, S.; Mukherjee, S.K. The 32 kDa subunit of replication protein A (RPA) participates in the DNA replication of Mung Bean Yellow Mosaic India Virus (MYMIV) by interacting with the viral Rep protein. Nucleic Acids Res. 2007, 35, 755–770. [Google Scholar] [CrossRef]
- Ringler, C.; Zhu, T.; Cai, X.; Koo, J.; Wang, D. Climate change impacts on food insecurity in Sub-Saharan Africa. In Insights from Comprehensive Climate Change Scenarios; International Food Policy Research Institute: Washington, DC, USA, 2010. [Google Scholar]
- United Nations International Children’s Emergency Fund. More than Twenty Million Children Suffering in the Horn of Africa as Drought Intensifies; United Nations International Children’s Emergency Fund: New York, NY, USA, 2022. [Google Scholar]
- Toreti, A.; Bavera, D.; Acosta Navarro, J.; Cammalleri, C.; de Jager, A.; Di Ciollo, C.; Hrast Essenfelder, A.; Maetens, W.; Magni, D.; Masante, D.; et al. Drought in East Africa August 2022; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- United Nations, International Organization for Migration (UN IOM). 15 Million People Face Humanitarian Crisis Due to Drought in the Horn of Africa; United Nations, International Organization for Migration (UN IOM): Geneva, Switzerland, 2022. [Google Scholar]
- Xu, P.; Chen, F.; Mannas, J.P.; Feldman, T.; Sumner, L.W.; Roossinck, M.J. Virus infection improves drought tolerance. New Phytol. 2008, 180, 911–992. [Google Scholar] [CrossRef]
- Moshe, A.; Gorovits, R.; Liu, Y.; Czosnek, H. Tomato plant cell death induced by inhibition of HSP90 is alleviated by Tomato yellow leaf curl virus infection. Mol. Plant Pathol. 2016, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, acc4353. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Hola, D.; Fischer, L.; Jedelsky, P.L.; Hnilicka, F.; Wilhelmova, N.; Rothova, O.; Kocova, M.; Prochazkova, D.; Honnerova, J. The physiology and proteomics of drought tolerance in maize: Early stomatal closure as a cause of lower tolerance to short-term dehydration. PLoS ONE 2012, 7, e38017. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Cao, W.; Wang, J.; Yu, J.; Yang, Z.; Huang, B. Characterization and functional analysis of FaHsfC1b from Festuca arundinacea conferring heat tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Larkindale, J.; Lee, U.; Von Koskull-Doring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (HSF) family: Structure, function, and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Xing, D.; Gao, C. Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J. Exp. Bot. 2009, 60, 2073–2091. [Google Scholar] [CrossRef]
- Corrales-Gutierrez, M.; Medina-Puche, L.; Yu, Y.; Wang, L.; Ding, X.; Luna, A.P.; Bejarano, E.R.; Castillo, A.G.; Lozano-Duran, R. The C4 protein from the geminivirus Tomato Yellow Leaf Curl Virus confers drought tolerance in Arabidopsis through an ABA-independent mechanism. Plant Biotechnol. J. 2020, 18, 1121–1123. [Google Scholar] [CrossRef]
- Lui, L.; Saunders, K.; Thomas, C.L.; Davies, J.W.; Stanley, J. Bean yellow dwarf virus RepA but not Rep, binds to maize retinoblastoma protein and the virus tolerates mutation in the consensus binding motif. Virology 1999, 256, 270–279. [Google Scholar] [CrossRef][Green Version]
- Baltes, N.J.; Gil-Humanes, J.; Cermak, T.; Atkins, P.A.; Voytas, D.F. DNA replicons for plant genome engineering. Plant Cell 2014, 26, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fu, C.; Zhou, J.; Hui, F.; Wang, Q.; Wang, F.; Wang, G.; Xu, Z.; Che, L.; Yuan, D.; et al. Highly efficient genome editing using geminivirus-based CRISPR/Cas9 system in cotton plant. Cells 2022, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xing, M.; Liu, X.; Fang, Z.; Yang, L.; Zhang, Y.; Wang, Y.; Zhuang, M.; Lv, H. An efficient virus-induced gene silencing (VIGS) system for functional genomics in Brassicas using a cabbage leaf curl virus (CaLCuV)-based vector. Planta 2020, 252, 42. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, J.; Kim, H.K.; Kim, J.Y.; Kim, M.S.; Cho, Y.G.; Bae, S.; Kang, K.K.; Jung, Y.J. Genome editing of golden SNP-carrying lycopene epsilon-cyclase (LcyE) gene using the CRISPR-Cas9/HDR and geminiviral replicon system in rice. Int. J. Mol. Sci. 2022, 23, 10383. [Google Scholar] [CrossRef]
- Dahan-Meir, T.; Filler-Hayut, S.; Melamed-Bessudo, C.; Bocobza, S.; Czosnek, H.; Aharoni, A.; Levy, A.A. Efficient in planta gene targeting in tomato using geminiviral replicons and the CRISPR/Cas9 system. Plant J. 2018, 95, 5–16. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, L.; Xin, Q.; Zhang, X.; Song, Y.; Wang, P.; Hong, D.; Fan, Z.; Yang, G. Optimizing glyphosate tolerance in rapeseed by CRISPR/Cas9-based geminiviral donor DNA replicon system with Csy4-based single-guide RNA processing. J. Exp. Bot. 2021, 72, 4796–4808. [Google Scholar] [CrossRef]
- Fauser, F.; Roth, N.; Pacher, M.; Ilg, G.; Sanchez-Fernandez, R.; Biesgen, C. In planta gene targeting. Proc. Natl. Acad. Sci. USA 2012, 109, 7535–7540. [Google Scholar] [CrossRef]
- Schiml, S.; Fauser, F.; Puchta, H. The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J. 2014, 80, 1139–1150. [Google Scholar] [CrossRef]
- Vu, T.V.; Sivankalyani, V.; Kim, E.J.; Doan DT, H.; Tran, M.T.; Kim, J.; Sung, Y.W.; Park, M.; Kang, Y.J.; Kim, J.Y. Highly efficient homology-directed repair using CRISPR/Cpf1-geminiviral replicon in tomato. Plant Biotechnol. J. 2020, 18, 2133–2143. [Google Scholar] [CrossRef]
- Cermak, T.; Baltes, N.J.; Cegan, R.; Zhang, Y.; Voytas, D.F. High-frequency, precise modification of the tomato genome. Genome Biol. 2015, 16, 232. [Google Scholar] [CrossRef] [PubMed]
- Olivares, F.; Loyola, R.; Olmedo, B.; Miccono, M.D.; Aguirre, C.; Vergara, R.; Riquelme, D.; Madrid, G.; Plantat, P.; Mora, R.; et al. CRISPR/Cas9 targeted editing of genes associated with fungal susceptibility in Vitis vinifera L. cv. Thompson seedless using geminivirus-derived replicons. Front. Plant Sci. 2021, 12, 791030. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Baltes, N.J.; Voytas, D.F.; Douches, D.S. Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases. Front. Plant Sci. 2016, 7, 1045. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Han, T.; Liu, G.; Chen, T.; Wang, Y.; Yu, A.Y.; Liu, Y. A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Sci. Rep. 2015, 5, 14926. [Google Scholar] [CrossRef] [PubMed]
- Cermak, T.; Curtin, S.J.; Čegan, R.; Kono, T.J.; Konečná, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; Voytas, D.F. A multipurpose toolkit to enable advanced genome engineering in plants. Plant Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Acha, G.; Vergara, R.; Muñoz, M.; Mora, R.; Aguirre, C.; Muñoz, M.; Kalazich, J.; Prieto, H. A traceable DNA-replicon derived vector to speed up gene editing in potato: Interrupting genes related to undesirable postharvest tuber traits as an example. Plants 2021, 10, 1882. [Google Scholar] [CrossRef]
- Kimm, Y.; Kweon, J.; Kim, J.-S. TALENs and ZFN are associated with different mutation signatures. Nat. Methods 2013, 10, 185. [Google Scholar] [CrossRef]
- Sera, T. Inhibition of virus DNA replication by artificial zinc finger proteins. Virol. J. 2005, 79, 2614–2619. [Google Scholar] [CrossRef]
- Takenaka, K.; Koshino-Kimura, Y.; Aoyama, Y.; Sera, T. Inhibition of Tomato Yellow Leaf Curl Virus replication by artificial zinc-finger proteins. Nucleic Acids Symp. Ser. 2008, 51, 429–430. [Google Scholar] [CrossRef][Green Version]
- Koshino-Kimura, Y.; Takenaka, K.; Domoto, F.; Aoyama, Y.; Sera, T. Generation of plants resistance to Tomato Yellow Leaf Curl Virus by using artificial zinc finger proteins. Nucleic Acids Symp. Ser. 2008, 52, 189–190. [Google Scholar] [CrossRef]
- Mori, T.; Takenaka, K.; Domoto, F.; Aoyama, Y.; Sera, T. Inhibition of binding of Tomato Yellow Curl Virus rep to its replication origin by artificial zinc-finger protein. Mol. Biotechnol. 2013, 54, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, F.; Cai, J.; Chen, W.; Zhao, N.; Sun, Y.; Guo, Y.; Yang, X.; Wu, X. Artificial TALE as a convenient protein platform for engineering broad-spectrum resistance to Begomoviruses. Viruses 2015, 7, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Khan, S.H.; Sadia, B.; Jamil, A.; Mansoor, S. TALE-mediated inhibition of replication of begomoviruses. Int. J. Agric. Biol. 2018, 20, 109–118. [Google Scholar] [CrossRef]
- Ali, Z.; Abulfaraj, A.; Idris, A.; Ali, S.; Tashkandi, M.; Magdy, M.M. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 2015, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, M.; Ali, Z.; Aljedaani, F.; Shami, A.; Mahfouz, M.M. Engineering resistance against Tomato Yellow Leaf Curl Virus via the CRISPR/Cas9 system in tomato. Plant Signal. Behav. 2018, 13, e1525996. [Google Scholar] [CrossRef]
- Rajabu, C.A.; Kennedy, G.G.; Ndunguru, J.; Ateka, E.M.; Tairo, F.; Hanley-Bowdoin, L.; Ascencio-Ibanez, J.T. Lania: A small, fast growing tomato variety is an excellent model system for studying geminiviruses. J. Virol. Methods 2018, 256, 89–99. [Google Scholar] [CrossRef]
- Verlaan, M.G.; Hutton, S.F.; Ibrahem, R.M.; Kormelink, R.; Visser, R.G.F.; Scott, J.W.; Edwards, J.D.; Bai, Y.; Mcdowell, J.M. The Tomato Yellow Leaf Curl Virus resistance genes Ty-1 and Ty-3 are allelic and code for DFDGD-class RNA–dependent RNA polymerases. PLoS Genet. 2013, 9, e1003399. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Ohnishi, J.; Saito, A.; Ohyama, A.; Nunome, T.; Miyatake, K.; Fukuoka, H. An NB-LRR gene, TYNBS1, is responsible for resistance mediated by the Ty-2 Begomovirus resistance locus of tomato. Theor. Appl. Genet. 2018, 131, 1345–1362. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Zhao, L.; Zhou, R.; Yu, W.; Zhao, T. Application of whole genome resequencing in mapping of a Tomato Yellow Leaf Curl Virus resistance gene. Sci. Rep. 2018, 8, 9592. [Google Scholar] [CrossRef]
- Ren, Y.; Tao, X.; Li, D.; Yang, X.; Zhou, X. ty-5 confers broa-spectrum resistance in geminiviruses. Viruses 2022, 14, 1804. [Google Scholar] [CrossRef]
- Voorburg, C.M.; Yan, Z.; Bergua-Vidal, M.; Wolters, A.-M.A.; Bai, Y.; Kormelink, R. Ty-1, a universal resistance gene against geminiviruses that is compromised by co-replication of a betasatellite. Mol. Plant Pathol. 2020, 21, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Si, X.; Zhang, Y.; Zhang, H.; Zhang, F.; Gao, C. Conferring DNA virus resistance with high specificity in plants using virus-inducible genome-editing system. Genome Biol. 2018, 19, 197. [Google Scholar] [CrossRef]
- Pramanik, D.; Shelake, R.M.; Park, J.; Kim, M.J.; Hwang, I.; Park, Y.; Kim, J.Y. CRISPR/Cas9-mediated generation of pathogen-resistant tomato against Tomato Yellow Leaf Curl Virus Powdery Mildew. Int. J. Mol. Sci. 2021, 22, 1878. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and Phytohormones: Working in tandem for plant growth and development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, Y.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Salicylic acid-induced differential resistance to the Tomato Yellow Leaf Curl Virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Bowling, S.A.; Guo, A.; Cao, H.; Gordon, A.S.; Klessig, D.F.; Dong, X. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 1994, 6, 1845–1857. [Google Scholar] [CrossRef]
- Ascencio-Ibáñez, J.T.; Sozzani, R.; Lee, T.-J.; Chu, T.-M.; Wolfinger, R.D.; Cella, R.; Hanley-Bowdoin, L. Global analysis of arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol. 2008, 148, 436–454. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Teng, K.; Lai, J.; Zhang, Y.; Huang, Y.; Li, Y.; Liang, L.; Wang, Y.; Chu, C.; et al. Up-regulation of LSB1/GDU3 affects geminivirus infection by activating the salicylic acid pathway. Plant J. 2010, 62, 12–23. [Google Scholar] [CrossRef]
- Wang, P.; Sun, S.; Liu, K.; Peng, R.; Li, N.; Hu, B.; Wang, L.; Wang, H.; Afzal, A.J.; Geng, X. Physiological and transcriptomic analyses revealed gene networks involved in heightened resistance against Tomato Yellow Leaf Curl Virus infection in salicylic acid and jasmonic acid-treated tomato plants. Front. Microbiol. 2022, 13, 970139. [Google Scholar] [CrossRef]
- Miozzi, L.; Napoli, C.; Sardo, L.; Accotto, G.P. Transcriptomics of the interaction between the monopartite phloem-limited geminivirus Tomato Yellow Leaf Curl Sardinia Virus and Solanum lycopersicum highlights a role for plant hormones, autophagy and plant immune system fine tuning during infection. PLoS ONE 2014, 9, e89951. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qian, L.; Wang, X.; Shao, R.; Hong, Y.; Liu, S.; Wang, X. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-C.; Pan, L.-L.; Ying, F.-Z.; Li, P.; Wang, X.-W.; Liu, S.-S. Jasmonic acid-related resistance in tomato mediates interactions between whitefly and whitefly- transmitted virus. Sci. Rep. 2017, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H.; Tee, C.; van Loon, J.J.A.; Dickle, M.; et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Durán, R.; Rosas-Díaz, T.; Gusmaroli, G.; Luna, A.P.; Taconnat, L.; Deng, X.W.; Bejarano, E.R. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 2011, 23, 1014–1032. [Google Scholar] [CrossRef]
- La Camera, S.; Balague, C.; Gobel, C.; Geoffroy, P.; Legrand, M.; Feussner, I.; Roby, D.; Heitz, T. The Arabidopsis patatin-like protein 2 (PLP2) plays an essential role in cell death execution. It differentially affects oxylipin biosynthesis and resistance to pathogens. Plant Cell 2009, 22, 469–481. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Bahn, S.C.; Pan, X.; Li, M.; Vu, H.S.; Roth, M.R.; Scheu, B.; Welti, R.; Hong, Y.; et al. The Patatin-Containing Phospholipase A pPLAIIα modulates oxylipin formation and water loss in Arabidopsis thaliana. Mol. Plant 2012, 5, 452–460. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables; Working Paper No. ESA/P/WP.241; The Department of Economic and Social Affairs of the United Nations: New York, NY, USA, 2015. [Google Scholar]
- Knox, J.; Hess, T.; Daccache, A.; Wheeler, T. Climate change impacts on crop productivity in Africa and South Asia. Environ. Res. Lett. 2012, 7, 034032. [Google Scholar] [CrossRef]
- Tripathi, J.N.; Ntui, V.O.; Tripathi, L. Precision genetics tools for genetic improvement of banana. Plant Genome 2024, 17, e20416. [Google Scholar] [CrossRef]
- Jhu, M.Y.; Ellision, E.E.; Sinha, N.R. CRISPR gene editing to improve crop reistance to parastic plants. Front. Genome Ed. 2023, 25, 1289416. [Google Scholar] [CrossRef]
- Gobena, D.; Shimels, M.; Rich, P.J.; Ruyter-Spira, C.; Bouwmeester, H.; Kanuganti, S.; Mengiste, T.; Ejeta, G. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proc. Natl. Acad. Sci. USA 2017, 114, 4471–4476. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yang, Y.; Futrell, S.; Kelly, E.A.; Lorts, C.M.; Nebie, B.; Runo, S.; Yang, J.; Alveraz, S.; Lasky, J.R.; et al. CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase (CCD) genes in sorghum alters strigolactone biosynthesis and plant biotic interactions. Phytobiomes J. 2023, 7, 339–351. [Google Scholar] [CrossRef]
- Legg, J.P.; Lava Kumar, P.; Makeshkumar, T.; Tripathi, L.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. Cassava virus diseases: Biology, epidemiology, and management. Adv. Virus Res. 2015, 91, 85–142. [Google Scholar] [CrossRef] [PubMed]
- Chatukuta, P.; Rey ME, C. A cassava protoplast system for screening genes associated with the response to South African cassava mosaic virus. Virol. J. 2020, 17, 184. [Google Scholar] [CrossRef]
- Legg, J.P.; Kumar, L.P.; Mahuku, G.; Wosula, E.; Stavalone, L.; Terry, E.; Bosque-Perez, N. Viruses affecting African crops and their vectors. In Critical Issues in Plant Health: 50 Years of Research in African Agriculture; Neuenschwander, P., Tamò, M., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 1–40. [Google Scholar]
- Mehta, D.; Stürchler, A.; Anjanappa, R.B.; Zaidi, S.S.; Hirsch-Hoffmann, M.; Gruissem, W.; Vanderschuren, H. Linking CRISPR-Cas9 interference in cassava to the evolution of editing-resistant geminiviruses. Genome Biol. 2019, 20, 80. [Google Scholar] [CrossRef]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, M.; Pan, R.; Zhang, T.; Lu, X.; Zhen, X.; Che, Y.; Li, R.; Liu, J.; Chen, Y.; et al. Engineering bacterial blight resistance plants through CRISPR/Cas9-targeted editing of the MeSWEET10a promoter in cassava. bioRxiv 2022, 1–15. [Google Scholar] [CrossRef]
- Juma, B.S.; Mukami, A.; Mweu, C.; Ngugi, M.P.; Mbinda, W. Targeted mutagenesis of the CYP79D1 gene via CRISPR/Cas9-mediated genome editing results in lower levels of cyanide in cassava. Front. Plant Sci. 2022, 13, 1009860. [Google Scholar] [CrossRef]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS- and PTST1-edited cassava for modified starch. Sci. Adv. 2018, 4, eaat6086. [Google Scholar] [CrossRef]
- Ning, J.; He, W.; Wu, L.; Chang, L.; Hu, M.; Fu, Y.; Liu, F.; Sun, H.; Gu, P.; Ndiondjop, M.N.; et al. The MYB transcription factor Seed Shattering 11 controls seed shattering by repressing lignin synthesis in African rice. Plant Biotechnol. J. 2023, 21, 931–942. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Y.; Ma, L.; Guo, Y.; Ran, Y. Efficient Genome Editing in Setaria italica Using CRISPR/Cas9 and Base Editors. Front. Plant Sci. 2022, 12, 815946. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, E.; Coussens, G.; Aesaert, S.; Neyt, P.; Anami, S.; Lijsebettens, M.V. Modulation of energy homeostasis in maize and Arabidopsis to develop lines tolerant to drought, genotoxic and oxidative stresses. Afr. Focus. 2017, 30, 66–76. [Google Scholar] [CrossRef]
| Virus Used | Targeted Gene | Plant | Year | Ref |
|---|---|---|---|---|
| Bean yellow dwarf virus (BeYDV) a | Carotenoid isomerase (CRTISO) Phytoene synthase 1 (PSY1) | Tomato | 2018 | [74] |
| BeYDV a | GhCLA1 | Gossypium hirsutum (Upland cotton) | 2022 | [71] |
| BeYDV a | SlHKT1;2 | Tomato | 2020 | [78] |
| BeYDV a | Lycopene epsilon-cyclase (LcyE) | Rice | 2022 | [73] |
| BeYDV a | Anthocyanin mutant 1 (ANT1) | Tomato | 2015 | [79] |
| BeYDV a | Auxin induced in root culture 12 (VviAIR12) Sugar will eventually be exported transporter 4 (VvSWEET4) Lesion initiation 2 (VviLIN2) Dimerization partner-E2F-like 1 (VviDEL1) | Vitis vinifera L. (Grape) | 2021 | [80] |
| BeYDV a | Acetolactate synthase 1 (ALC1) | Solanum tuberosum L. (potato) | 2016 | [81] |
| Cabbage leaf curl virus (CaLCV) b | Phytoene desaturase 3 (NbPDS3) NbIspH | N. Benthamiana | 2015 | [82] |
| BeYDV a | Solyc06g074350 Solyc02g085500 Solyc02g090730 Solyc11g071810 Solyc06g074240 Solyc02g07739 Ubiquitin 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) Mildew locus O [TaMLO] Hordeum vulgare; HvMLO | Tomato | 2017 | [83] |
| Wheat | ||||
| Barley | ||||
| BeYDV a | Vacuolar invertase 1 (StvacINV1) Beta-amylase 1 (StBAM1) Polyphenol oxidase 1 and 2 (StPPO1 and StPPO2) | Potato | 2021 | [84] |
| BeYDV a | Enolpyruvylshikimate-3-phosphate synthase (EPSPS) | Brassica napus L. (Rapeseed) | 2021 | [75] |
| Wheat dwarf virus (WDV) a | Mildew locus O (MLO) EPSPS | Triticum sp. (wheat) | 2017 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenda, M.F.; Masamba, P.; Allie, F.; Kappo, A.P. Geminiviruses and Food Security: A Molecular Genetics Perspective for Sustainable Agriculture in Africa. Plants 2024, 13, 2768. https://doi.org/10.3390/plants13192768
Zenda MF, Masamba P, Allie F, Kappo AP. Geminiviruses and Food Security: A Molecular Genetics Perspective for Sustainable Agriculture in Africa. Plants. 2024; 13(19):2768. https://doi.org/10.3390/plants13192768
Chicago/Turabian StyleZenda, Minenhle Felicia, Priscilla Masamba, Farhahna Allie, and Abidemi Paul Kappo. 2024. "Geminiviruses and Food Security: A Molecular Genetics Perspective for Sustainable Agriculture in Africa" Plants 13, no. 19: 2768. https://doi.org/10.3390/plants13192768
APA StyleZenda, M. F., Masamba, P., Allie, F., & Kappo, A. P. (2024). Geminiviruses and Food Security: A Molecular Genetics Perspective for Sustainable Agriculture in Africa. Plants, 13(19), 2768. https://doi.org/10.3390/plants13192768







