In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Profile of Azara serrata Ruiz & Pav.
2.1.1. Phenolic Acids
2.1.2. Anthocyanins
2.2. Quantitation of Anthocyanins
2.3. Phytochemical and Antioxidant Activity
Comparative Determination of Total Anthocyanins, Phenols, Flavonoids, and Antioxidant Capacities
2.4. Enzyme Inhibitory Properties of Azara serrata. Ruiz & Pav.
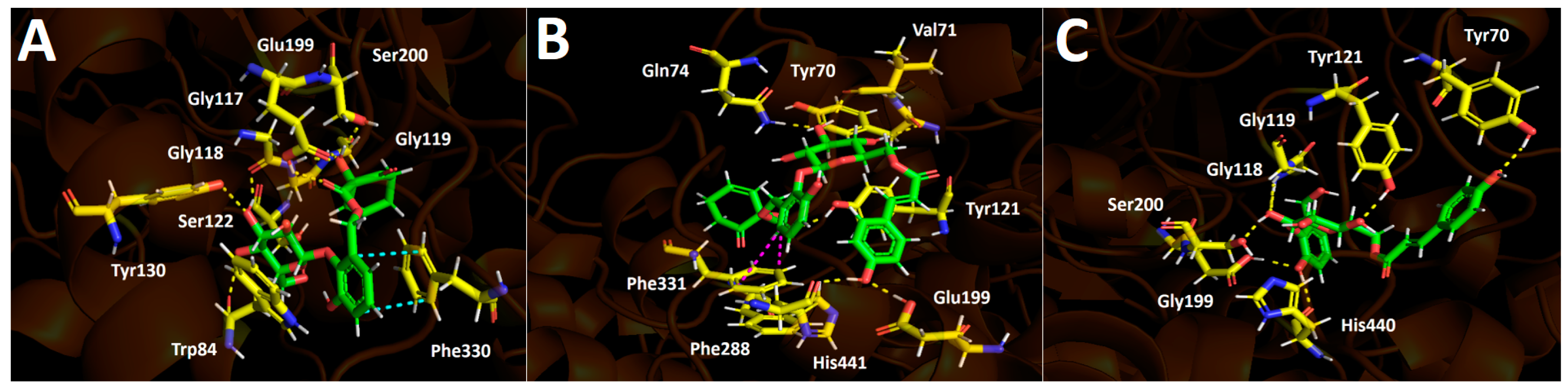
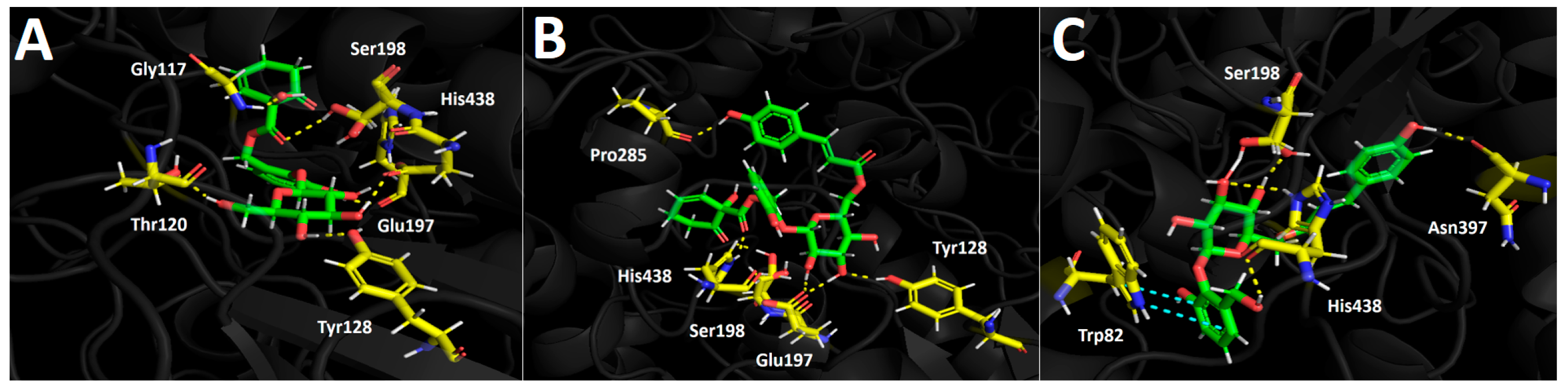
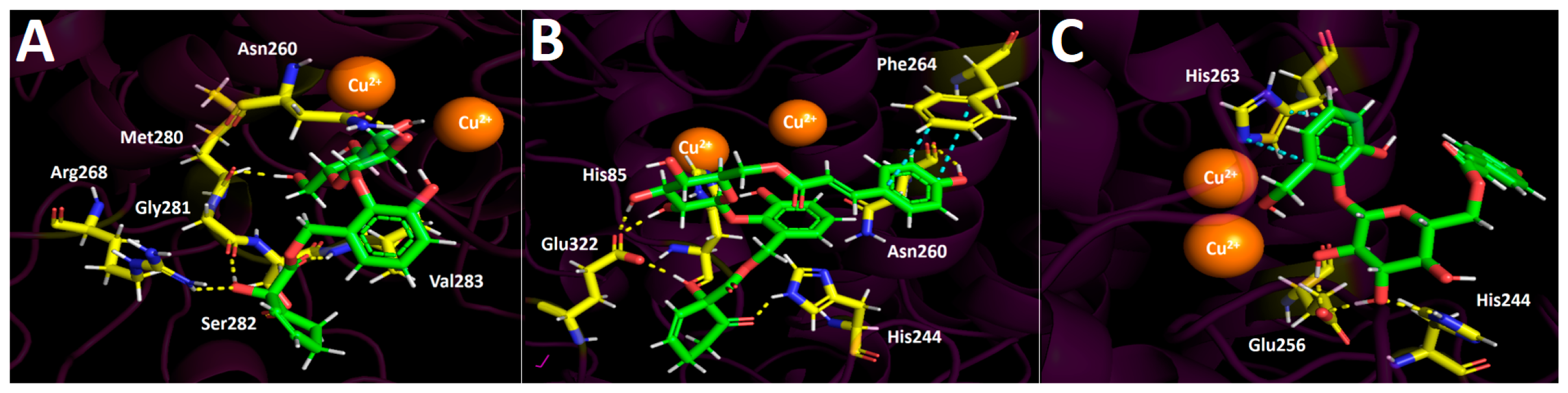
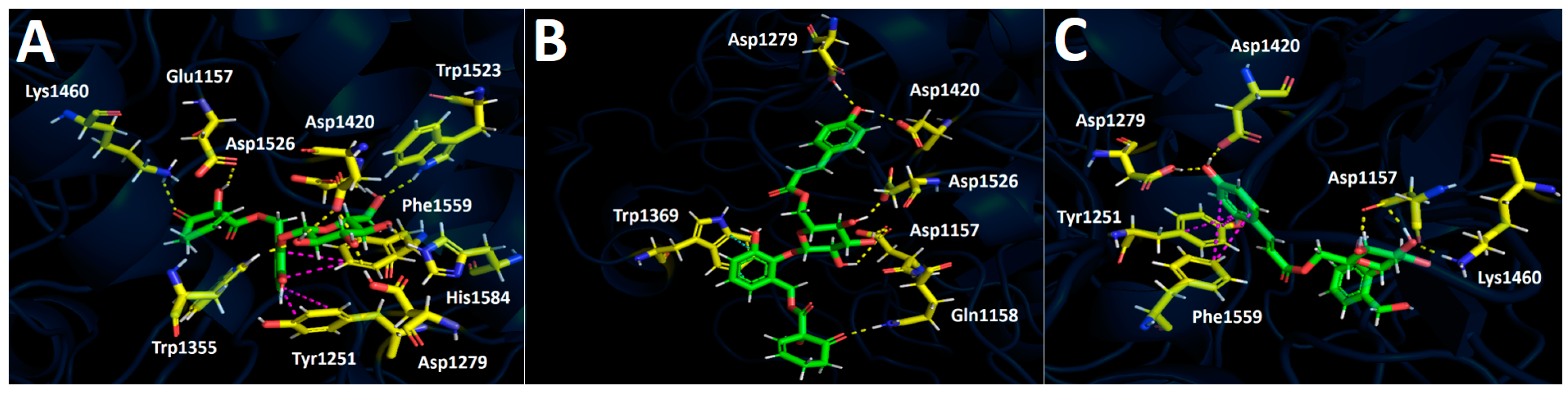
2.4.1. Docking Calculations
Acetylcholinesterase (TcAChE) Docking Results
Butyrylcholinesterase (hBuChE) Docking Results
Tyrosinase Docking Results
Lipase Docking Results
Glucosidase Docking Results
Amylase Docking Results
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Sample Preparation
3.3.1. Extract for Elucidation of the Phenolic Profiles
3.3.2. Enriched Anthocyanin Extract for Quantitation
3.3.3. Purified Anthocyanin Extract
3.4. Experimental Parameters
3.4.1. Ultra High Performance Liquid Chromatography (UHPLC) Diode Array Detector (DAD) Analysis for the Quantitation of the Anthocyanins
3.4.2. UHPLC Trapped Ion Mobility Spectrometry (TIMS) Time of Flight (TOF) Mass Spectrometry
3.5. Phytochemical Analysis
Determination of Total Phenol and Flavonoid Contents
3.6. Antioxidant Activity Assays
3.6.1. Ferric-Reducing Antioxidant Power (FRAP)
3.6.2. Free Radical Scavenging (DPPH)
3.6.3. ABTS Assay
3.6.4. ORAC Assay
3.7. Inhibition of Alpha-Amylase, Alpha-Glucosidase, and Lipase Enzymes
3.8. Inhibition of Acetylcholinesterase, Butyrylcholinesterase, and Tyrosinase Enzymes
3.9. Docking Simulations
3.10. Statistical Analyses
3.11. Metabolite Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed]
- Don, D.; Johnst, I.M.; Fundación, R.A. Philippi de Estudios Naturales Santiago de Chile. Available online: https://fundacionphilippi.cl/catalogo/azara-petiolaris/ (accessed on 26 July 2024).
- Sagareishvili, T.G.; Alaniya, M.D.; Kemertelidze, P. A new flavonol glycoside from Azara microphylla. Chem. Nat. Compd. 1983, 19, 275–278. [Google Scholar] [CrossRef]
- Giordano, A.; Retamal, M.; Leyton, F.; Martínez, P.; Bridi, R.; Velásquez, P.; Montenegro, G. Bioactive polyphenols and antioxidant capacity of Azara petiolaris and Azara integrifolia Honeys. CyTA—J. Food 2018, 16, 484–489. [Google Scholar] [CrossRef]
- Paz, C.; González-Chavarría, I.; Freire, E.; Ortiz, L.; Karpiński, T.M.; Duprat, F.; Baggio, R. Phytochemical and crystallographic studies of Azara dentata extracts and its cytotoxic effects on human breast cancer cell, MCF-7. J. Chil. Chem. Soc. 2020, 65, 4891–4894. [Google Scholar] [CrossRef]
- Ramos, L.C.; Palacios, J.; Barrientos, R.E.; Gómez, J.; Castagnini, J.M.; Barba, F.J.; Tapia, A.; Paredes, A.; Cifuentes, F.; Simirgiotis, M.J. UHPLC-MS Phenolic Fingerprinting, Aorta Endothelium Relaxation Effect, Antioxidant, and Enzyme Inhibition Activities of Azara dentata Ruiz & Pav Berries. Foods 2023, 12, 643. [Google Scholar] [CrossRef]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 2011, 72, 1497–1509. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef]
- Siddiqi, K. Non-Communicable diseases. In Public Health; Walley, J., Wright, J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 287–308. ISBN 9780199238934. [Google Scholar]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Bridle, P.; Stott, K.G.; Timberlake, C.F. Anthocyanins in Salix species: A new anthocyanin in salix purpurea bark. Phytochemistry 1973, 12, 1103–1106. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; García-Estévez, I.; Rivas-Gonzalo, J.C.; La Rodríguez de Cruz, D.; Escribano-Bailón, M.T. Anthocyanins of the anthers as chemotaxonomic markers in the genus Populus L. Differentiation between Populus nigra, Populus alba and Populus tremula. Phytochemistry 2016, 128, 35–49. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, C.; Karrar, E.; Du, M.; Jin, Q.; Wang, X. Analysis of Chemical Composition and Antioxidant Activity of Idesia polycarpa Pulp Oil from Five Regions in China. Foods 2023, 12, 1251. [Google Scholar] [CrossRef]
- Bochi, V.C.; Godoy, H.T.; Giusti, M.M. Anthocyanin and other phenolic compounds in Ceylon gooseberry (Dovyalis hebecarpa) fruits. Food Chem. 2015, 176, 234–243. [Google Scholar] [CrossRef][Green Version]
- Ajay, S.; Panicker, J.S.; Krishnan, R.R.; Prema, K.H. Greener Extraction of Anthocyanin Pigment from Syzygium samarangenese and Flacourtia jangomas: An Alternative to Synthetic pH Indicators. Waste Biomass Valorization 2023, 15, 1175–1184. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Feistel, F.; Paetz, C.; Menezes, R.C.; Veit, D.; Schneider, B. Acylated Quinic Acids Are the Main Salicortin Metabolites in the Lepidopteran Specialist Herbivore Cerura vinula. J. Chem. Ecol. 2018, 44, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, Y.P.; Sung, S.H.; Kim, Y.C. Inhibitory activity of phenolic glycosides from the fruits of Idesia polycarpa on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Planta Medica 2007, 73, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.B.; Kim, H.W.; Lee, M.; Lee, H.H.; Kim, S.H.; Kang, S.K.; Sung, S.H. Isolation and structure elucidation of (−)-Idescarparide, a new spiro compound from Idesia polycarpa. Tetrahedron Lett. 2014, 55, 5447–5449. [Google Scholar] [CrossRef]
- Ward, J.L.; Wu, Y.; Harflett, C.; Onafuye, H.; Corol, D.; Lomax, C.; Macalpine, W.J.; Cinatl, J.; Wass, M.N.; Michaelis, M.; et al. Miyabeacin: A new cyclodimer presents a potential role for willow in cancer therapy. Sci. Rep. 2020, 10, 6477. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- He, J.; Carvalho, A.R.F.; Mateus, N.; de Freitas, V. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines. J. Agric. Food Chem. 2010, 58, 9249–9258. [Google Scholar] [CrossRef]
- Cruz, L.; Petrov, V.; Teixeira, N.; Mateus, N.; Pina, F.; de Freitas, V. Establishment of the chemical equilibria of different types of pyranoanthocyanins in aqueous solutions: Evidence for the formation of aggregation in pyranomalvidin-3-O-coumaroylglucoside-(+)-catechin. J. Phys. Chem. B 2010, 114, 13232–13240. [Google Scholar] [CrossRef]
- Schwarz, M.; Jerz, G.; Winterhalter, P. Isolation and structure of Pinotin A, a new anthocyanin derivative from Pinotage wine. Vitis 2003, 42, 105–106. [Google Scholar]
- Mateus, N.; Silva, A.M.S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; de Freitas, V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef]
- Hillebrand, S.; Schwarz, M.; Winterhalter, P. Characterization of anthocyanins and pyranoanthocyanins from blood orange Citrus sinensis (L.) Osbeck juice. J. Agric. Food Chem. 2004, 52, 7331–7338. [Google Scholar] [CrossRef]
- Schwarz, M.; Wray, V.; Winterhalter, P. Isolation and identification of novel pyranoanthocyanins from black carrot (Daucus carota L.) juice. J. Agric. Food Chem. 2004, 52, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.M.; Fossen, T.; Torskangerpoll, K.; Fossen, A.; Hauge, U. Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5-carboxypyranopelargonidin. Phytochemistry 2004, 65, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Andersen, Ø.M. Anthocyanins from red onion, Allium cepa, with novel aglycone. Phytochemistry 2003, 62, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Laaksonen, O.; Yang, W.; Zhang, B.; Yang, B. Pyranoanthocyanins in bilberry (Vaccinium myrtillus L.) wines fermented with Schizosaccharomyces pombe and their evolution during aging. Food Chem. 2020, 305, 125438. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. Genetic Differentiation in Anthocyanin Content among Berry Fruits. Curr. Issues Mol. Biol. 2021, 43, 36–51. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui Aristotelia chilensis (Molina) Stuntz a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- INTA. Portal Antioxidantes. 2024. Available online: https://portalantioxidantes.com/orac-base-de-datos-actividad-antioxidante-y-contenido-de-polifenoles-totales-en-frutas/ (accessed on 26 July 2024).
- Assefa, S.T.; Yang, E.-Y.; Chae, S.-Y.; Song, M.; Lee, J.; Cho, M.-C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef]
- van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; van de Lisdonk, E.H.; Rutten, G.E.; van Weel, C. Alpha-Glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef]
- Liu, K.; Luo, M.; Wei, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Rolinski, M.; Fox, C.; Maidment, I.; McShane, R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst. Rev. 2012, 2012, CD006504. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; Rossignol, D.A. Treatments for biomedical abnormalities associated with autism spectrum disorder. Front. Pediatr. 2014, 2, 66. [Google Scholar] [CrossRef]
- Hopfstock, P.; Punyasiri, P.A.N.; Kiene, M.; Kottawa-Arachchi, J.D.; Gök, R.; Winterhalter, P. Characterization and Quantitation of Anthocyanins of the Pigmented Tea Cultivar TRI 2043 (Camellia sinensis L.) from Sri Lanka. Separations 2024, 11, 157. [Google Scholar] [CrossRef]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical Profiling, Antioxidant, Anticholinesterase, and Antiprotozoal Potentials of Artemisia copa Phil. (Asteraceae). Front. Pharmacol. 2020, 11, 594174. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Molina, N.; Parada, J.; Simirgiotis, M.; Montecinos-González, R. The Potential of Using Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound-Assisted Extraction to Fight against Aging-Related Diseases. Foods 2024, 13, 269. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sánchez, M.; Hillmann-Eggers, M.; Gómez-Serranillos, M.P.; Vargas-Arana, G.; Simirgiotis, M.J. UHPLC-MS Chemical Fingerprinting and Antioxidant, Enzyme Inhibition, Anti-Inflammatory In Silico and Cytoprotective Activities of Cladonia chlorophaea and C. gracilis (Cladoniaceae) from Antarctica. Antioxidants 2022, 12, 10. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Frisch, M.J.; Hiscocks, J. Gaussian 09; Revision B. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Release, S. 2: Maestro, Version 11.8; Schrödinger, LLC: New York, NY, USA, 2018. [Google Scholar]
- Greenblatt, H.M.; Kryger, G.; Lewis, T.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with (-)—Galanthamine at 2.3 A resolution. FEBS Lett. 1999, 463, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl-and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Terzyan, S.; Wang, C.S.; Downs, D.; Hunter, B.; Zhang, X.C. Crystal structure of the catalytic domain of human bile salt activated lipase. Protein Sci. 2000, 9, 1789–1790. [Google Scholar] [CrossRef]
- Ren, L.; Qin, X.; Cao, X.; Wang, L.; Bai, F.; Bai, G.; Shen, Y. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2011, 2, 827–836. [Google Scholar] [CrossRef]
- Nahoum, V.; Roux, G.; Anton, V.; Rougé, P.; Puigserver, A.; Bischoff, H.; Henrissat, B.; Payan, F. Crystal structures of human pancreatic alpha-amylase in complex with carbohydrate and proteinaceous inhibitors. Biochem. J. 2000, 346, 201–208. [Google Scholar] [CrossRef]
- Westbrook, J. RCSB Protein Data Bank. Structural biology views for basic and applied research. Acta Crystallogr. 2017, A73, a308. [Google Scholar] [CrossRef]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Silman, I.; Harel, M.; Axelsen, P.; Raves, M.; Sussman, J.L. Three-Dimensional structures of acetylcholinesterase and of its complexes with anticholinesterase agents. Biochem. Soc. Trans. 1994, 22, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.H.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase Inhibition Activity, Alkaloid Profiling and Molecular Docking of Chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Silva, N.d.F.; Andrade, E.H.A.; Gratieri, T.; Setzer, W.N.; Maia, J.G.S.; Da Silva, J.K.R. Tyrosinase inhibitory activity, molecular docking studies and antioxidant potential of chemotypes of Lippia origanoides (Verbenaceae) essential oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, Y.; Ran, M.; Li, Q.; Ruan, Z.; Jin, N. Inhibition of Tyrosinase by Mercury Chloride: Spectroscopic and Docking Studies. Front. Pharmacol. 2020, 11, 81. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; Delano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]








| Peak Number | Retention Time [min] | Compound Name | UV/Vis Max. [nm] | Molecular Formula | Theoretical Mass [m/z] | Detected Mass [m/z] | Mass Error [ppm] | Fragment Ion MS/MS [m/z] | CCS [Å2] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.20 | unknown | C12H16O8 | 287.0772 | 287.0769 | 1.3 | 125, 83 | 169.4 | |
| 2 | 4.20 | caffeoyl glucaric acid | 212–324 | C15H16O11 | 371.0620 | 371.0616 | 1.0 | 209, 191 | 185.3 |
| 3 | 4.82 | unknown | C16H19NO9 | 368.0987 | 368.0981 | 1.7 | 160, 119 | 174.9 | |
| 4 | 5.01 | gallic acid glucoside isomer | C13H16O10 | 331.0671 | 331.0668 | 0.8 | 285, 123 | 168.3 | |
| 5 | 5.68 | caffeoyl glucaric acid isomer | 198–278 | C15H16O11 | 371.0620 | 371.0614 | 1.5 | 209, 191 | 171.6 |
| 6 | 6.28 | pyrocatechuic acid glucoside | 216–284 | C13H16O9 | 315.0722 | 315.0719 | 0.9 | 153 | 171.6 |
| 7 | 6.69 | gallic acid glucoside isomer | C13H16O10 | 331.0671 | 331.0663 | 2.2 | 313, 169 | 169.3 | |
| 8 | 6.93 | idesin/salirepin isomer | 196–276 | C13H18O8 | 301.0929 | 301.0928 | 0.5 | 139 | 165.6 |
| 9 | 7.37 | unknown | C16H14O11 | 381.0463 | 381.0476 | −3.5 | 259, 241, 139 | 175.5 | |
| 10 | 7.52 | unknown | C18H24O12 | 431.1187 | 431.1195 | 1.9 | 137, 93 | 187.5 | |
| 11 | 7.80 | idesin/salirepin isomer | C13H18O8 | 301.0929 | 301.0929 | 0.1 | 121, 59 | 164.3 | |
| 12 | 8.06 | pyrocatechuic acid glucoside | C13H16O9 | 315.0722 | 315.0719 | 0.8 | 153, 109 | 166.1 | |
| 13 | 8.54 | unknown | C14H20O9 | 331.1035 | 331.1031 | 1.0 | 285, 123 | 173.0 | |
| 14 | 8.71 | caffeoyl glucaric acid isomer | 218–324 | C15H16O11 | 371.0620 | 371.0617 | 0.8 | 209, 191 | 174.2 |
| 15 | 8.97 | chlorogenic acid isomer | 216–324 | C16H18O9 | 353.0878 | 353.0878 | 1.5 | 191, 135 | 170.9 |
| 16 | 9.36 | unknown | C18H24O13 | 447.1144 | 447.1140 | 1.0 | 152, 108 | 190.1 | |
| 17 | 9.74 | caffeoyl threonic acid isomer | 216–326 | C13H14O8 | 297.0616 | 297.0615 | 0.2 | 179, 135 | 159.3 |
| 18 | 9.85 | arbutin | 194–272 | C12H16O7 | 271.0823 | 271.0823 | 0.3 | 109 | 156.5 |
| 19 | 10.07 | idesin/salirepin isomer | 196–274 | C13H18O8 | 301.0929 | 301.0928 | 0.2 | 139, 121 | 162.6 |
| 20 | 11.18 | caffeoyl threonic acid isomer | 218–328 | C13H14O8 | 297.0616 | 297.0615 | 0.5 | 179, 135 | 159.3 |
| 21 | 11.62 | coumaroylquinic acid | C16H18O8 | 337.0929 | 337.0925 | 1.3 | 163, 119 | 179.3 | |
| 22 | 12.31 | caffeic acid glucoside | C15H18O9 | 341.0878 | 341.0877 | 0.2 | 179, 135 | 177.8 | |
| 23 | 12.78 | chlorogenic acid isomer | 216–326 | C16H18O9 | 353.0878 | 353.0874 | 1.0 | 191, 127 | 184.4 |
| 24 | 13.67 | feruloylquinic acid isomer | 218–324 | C17H20O9 | 367.1035 | 367.1030 | 1.3 | 191, 134, 117 | 179.1 |
| 25 | 14.04 | chlorogenic acid isomer | 216–326 | C16H18O9 | 353.0878 | 353.0877 | 0.2 | 191, 135, | 184.4 |
| 26 | 15.96 | caffeoyl threonic acid isomer | 218–324 | C13H14O8 | 297.0616 | 297.0617 | −0.5 | 179, 135, 75 | 158.2 |
| 27 | 16.47 | unknown | C20H32O10 | 431.1923 | 431.1917 | 1.3 | 299, 71, 59 | 199.9 | |
| 28 | 16.68 | unknown | C18H28O9 | 387.1661 | 387.1663 | −0.6 | 369, 207, 163 | 186.8 | |
| 29 | 18.62 | feruloylquinic acid isomer | 218–322 | C17H20O9 | 367.1035 | 367.1032 | 0.7 | 191, 134, 93 | 197.2 |
| 30 | 19.05 | verbasoside | C20H30O12 | 461.1664 | 461.1670 | −1.1 | 191, 131, 89 | 205.8 | |
| 31 | 20.00 | unknown | C15H16N2O6 | 319.0936 | 319.0938 | −0.7 | 142, 130, 116 | 181.5 | |
| 32 | 20.32 | unknown | C19H26O10 | 413.1453 | 413.1445 | 2.0 | 161, 133 | 200.7 | |
| 33 | 21.78 | idescarpin/HCH-salirepin isomer | C20H24O11 | 439.1246 | 439.1249 | −0.7 | 301, 283, 139 | 193.1 | |
| 34 | 22.50 | idescarpinol/HCH-salirepinol | C20H26O11 | 441.1402 | 441.1400 | 0.4 | 301, 283 157, 139 | 194.3 | |
| 35 | 23.03 | unknown | 218–328 | C21H26O10 | 437.1453 | 437.1448 | 1.2 | 161, 133 | 215.9 |
| 36 | 24.90 | idescarpin/HCH-salirepin isomer | 196–278 | C20H24O11 | 439.1246 | 439.1244 | 0.4 | 301, 139 | 217.8 |
| 37 | 25.98 | trichocarposide isomer | 220–320 | C22H24O9 | 431.1348 | 431.1351 | −0.7 | 307, 145, 123 | 219.2 |
| 38 | 26.42 | unknown | 220–314 | C21H26O9 | 421.1504 | 421.1500 | 1.0 | 145, 117 | 213.1 |
| 39 | 26.61 | populoside | 220–324 | C22H24O10 | 447.1297 | 447.1295 | 0.4 | 323, 179, 161, 132 | 201.8 |
| 40 | 26.99 | unknown | 220–326 | C21H28O10 | 439.1610 | 439.1616 | −1.3 | 161, 133 | 211.5 |
| 41 | 27.48 | trichocarposide isomer | 220–312 | C22H24O9 | 431.1348 | 431.1350 | −0.5 | 161, 133 | 202.4 |
| 42 | 27.80 | unknown | 220–312 | C21H26O9 | 421.1504 | 421.1508 | −0.9 | 137 | 206.4 |
| 43 | 28.18 | salicortin isomer | 220–312 | C21H28O9 | 423.1661 | 423.1657 | 0.8 | 145, 117 | 215.1 |
| 44 | 29.48 | salicortin isomer | 220–312 | C21H28O9 | 423.1661 | 423.1654 | 1.5 | 145, 117 | 207.5 |
| 45 | 29.89 | flacourtin/salireposide | C20H22O9 | 405.1191 | 405.1189 | 0.5 | 135, 91 | 202.1 | |
| 46 | 30.18 | trichocarposide isomer | 220–312 | C22H24O9 | 431.1348 | 431.1345 | 0.7 | 161, 132 | 214.1 |
| 47 | 30.80 | salicortin isomer | 220–312 | C21H28O9 | 423.1661 | 423.1658 | 0.7 | 145, 117 | 215.4 |
| 48 | 31.22 | idescarpin/HCH-salirepin isomer | 196–276 | C20H24O11 | 439.1246 | 439.1236 | 2.3 | [2M − H] 879.2552, 301, 139 | 193.1 |
| 49 | 31.34 | HCH-idescarpin/di-HCH-salirepin isomer | C27H30O14 | 577.1563 | 577.1558 | 0.8 | 439, 421, 301, 139 | 222.4 | |
| 50 | 31.96 | cou-idesin/salirepin | C22H24O10 | 447.1297 | 447.1295 | 0.4 | 301, 139 | 201.8 | |
| 51 | 32.31 | trichocarposide isomer | 220–326 | C22H24O9 | 431.1348 | 431.1351 | −0.9 | 161, 133 | 213.8 |
| 52 | 32.52 | cou-arbutin | 220–312 | C21H22O9 | 417.1191 | 417.1189 | 0.4 | 307, 163, 145 | 208.5 |
| 53 | 32.7 | cou-salicin | 220–326 | C22H24O9 | 431.1348 | 431.1340 | 1.8 | 161, 133 | 211.9 |
| 54 | 32.96 | ac-salicortin | 220–314 | C23H30O10 | 465.1766 | 465.1763 | 0.8 | 423, 405, 163, 145 | 220.7 |
| 55 | 33.62 | ac-cou-idesin/salirepin isomer | 220–312 | C24H26O11 | 489.1402 | 489.1401 | 0.3 | 343, 301, 139 | 210.2 |
| 56 | 34.51 | unknown coumaroyl species | 222–310 | C22H24O8 | 415.1398 | 415.1398 | 0.1 | 163, 145, 117 | 211.9 |
| 57 | 34.79 | ac-cou-idesin/salirepin isomer | C24H26O11 | 489.1402 | 489.1402 | 0.0 | 343, 301, 139 | 217.5 | |
| 58 | 35.02 | unknown coumaroyl species | C22H24O8 | 415.1398 | 415.1395 | 0.7 | 163, 145, 117 | 209.8 | |
| 59 | 35.64 | idescarpin/HCH-salirepin isomer | 196–276 | C20H24O11 | 439.1246 | 439.1249 | −0.7 | [2M − H] 879.2552, 301, 139 | 193.1 |
| 60 | 35.85 | unknown | 222–310 | C23H26O8 | 429.1555 | 429.1552 | 0.6 | 145, 117 | 219.6 |
| 61 | 36.72 | unknown | C31H34O14 | 629.1876 | 629.1864 | 1.8 | 157 | 232.2 | |
| 62 | 36.78 | idesin/salirepin salicylate | C20H22O10 | 421.1140 | 421.1135 | 1.3 | 137, 93 | 193.4 | |
| 63 | 37.44 | cou-idescarpin/HCH-salirepin | C29H30O13 | 585.1614 | 585.1613 | 0.1 | 439, 301, 139 | 220.7 | |
| 64 | 37.85 | ac-cou-idescarpin/HCH-salirepin isomer | 196–220 | C31H32O14 | 627.1719 | 627.1721 | −0.3 | 481, 343, 301 | 232.1 |
| 65 | 38.30 | ac-cou-idescarpin/HCH-salirepin isomer | 196–220 | C31H32O14 | 627.1719 | 627.1711 | 1.3 | 481, 343, 301 | 239.9 |
| 66 | 38.78 | diac-cou-idescarpin/HCH-salirepin | 222 | C33H34O15 | 669.1825 | 669.1824 | 0.2 | 523, 481, 343 | 242.8 |
| Anthocyanins and derivates | |||||||||
| 67 | 13.71 | delphinidin-glucoside | 522 | C21H21O12 | 465.1028 | 465.1023 | 1.0 | 303 | 205.2 |
| 68 | 14.07 | delphinidin-rutinoside | 522 | C27H31O16 | 611.1607 | 611.1608 | −0.3 | 303 | 236.0 |
| 69 | 15.52 | cyanidin-glucoside | 514 | C21H21O11 | 449.1078 | 449.1075 | 0.7 | 287 | 201.5 |
| 70 | 16.29 | cyanidin-rutinoside | 516 | C27H31O15 | 595.1657 | 595.1660 | −0.4 | 287 | 232.7 |
| 71 | 16.85 | delphinidin-arabinose | C20H19O11 | 435.0922 | 435.0921 | 0.3 | 303 | 200.5 | |
| 72 | 17.12 | pelargonidin-glucoside | 510 | C21H21O10 | 433.1129 | 433.1129 | 0.0 | 271 | 199.1 |
| 73 | 18.68 | cyanidin-arabinose | 514 | C20H19O10 | 419.0973 | 419.0970 | 0.7 | 287 | 196.9 |
| 74 | 20.99 | pyr-(delphinidin-rutinoside) | 372–514 | C47H51O26 | 1031.2663 | 1031.2664 | −0.1 | 723, 439, 393 | 291.7 |
| 75 | 21.48 | pyr-(delphinidin-glucoside) | 372–512 | C41H41O22 | 885.2085 | 885.2084 | −0.3 | 723, 439, 393 | 272.7 |
| 76 | 21.74 | pyr-(delphinidin-arabinoside) | 372–512 | C40H39O21 | 855.1978 | 855.1974 | 0.5 | 723, 439, 393 | 269.2 |
| 77 | 21.90 | pyr-ac-(delphinidin-glucoside) | C43H43O23 | 927.2190 | 927.2192 | −0.3 | 765, 439, 393 | 279.9 | |
| 78 | 22.71 | pyr-(cyanidin-rutinoside) | 356–508 | C47H51O25 | 1015.2174 | 1015.2713 | 0.1 | 707, 423, 377 | 289.2 |
| 79 | 23.26 | pyr-(cyanidin-glucoside) | 354–506 | C41H41O21 | 869.2137 | 869.2135 | −0.2 | 707, 423, 377 | 269.2 |
| 80 | 23.50 | pyr-(cyanidin-arabinoside) | 354–508 | C40H39O20 | 839.2029 | 839.2032 | −0.3 | 707, 423, 377 | 266.1 |
| 81 | 24.38 | pyr-(pelargonidin-glucoside) | C41H41O20 | 853.2186 | 853.2179 | 0.8 | 691, 407, 361 | 267.4 | |
| 82 | 24.64 | pyr-base-(cyanidin-rutinoside) | C33H33O16 | 685.1763 | 685.1766 | −0.4 | 377 | 240.3 | |
| 83 | 25.08 | pyr-base-(cyanidin-glucoside) | C27H23O12 | 539.1184 | 539.1183 | 0.2 | 377 | 219.0 | |
| 84 | 25.52 | pyr-base-(cyanidin-arabinoside) | C26H21O11 | 509.1078 | 509.1071 | 1.5 | 377 | 214.8 | |
| 85 | 27.18 | pyr-ac-(cyanidin-glucoside) | C43H43O22 | 911.2240 | 911.2244 | −0.4 | 749, 423, 377 | 282.4 | |
| 86 | 27.50 | pyr-diac-(cyanidin-glucoside) | C45H45O23 | 953.2346 | 953.2345 | 0.1 | 791, 423, 377 | 283.4 | |
| 87 | 30.58 | pyr-ac-cou-(delphinidin-rutinoside) | 376–514 | C58H59O29 | 1219.3137 | 1219.3132 | 0.4 | 911, 765, 439, 393 | 325.4 |
| 88 | 30.84 | pyr-cou-(delphinidin-glucoside) | C50H47O24 | 1031.2452 | 1031.2450 | 0.2 | 869, 723, 439, 393 | 299.6 | |
| 89 | 30.98 | pyr-ac-cou-(cyanidin-rutinoside) | C58H59O28 | 1203.3187 | 1203.3186 | 0.1 | 895, 749, 423, 377 | 322.7 | |
| 90 | 31.17 | pyr-ac-cou-(delphinidin-glucoside) | 374–514 | C52H49O25 | 1073.2557 | 1073.2562 | −0.4 | 911, 765, 439, 393 | 307.4 |
| 91 | 31.28 | pyr-cou-(cyanidin-glucoside) | C50H47O23 | 1015.2503 | 1015.2500 | 0.2 | 853, 707, 423, 377 | 296.1 | |
| 92 | 31.52 | pyr-ac-cou-(cyanidin-glucoside) | 356–510 | C52H49O24 | 1057.2608 | 1057.2607 | 0.1 | 895, 749, 423, 377 | 303.9 |
| 93 | 31.84 | pyr-ac-cou-(cyanidin-arabinose) | C51H47O23 | 1027.2503 | 1027.2490 | 1.3 | 895, 749, 423, 377 | 301.8 | |
| 94 | 32.18 | pyr-ac-cou-(delphinidin-glucoside) | C52H49O25 | 1073.2557 | 1073.2546 | 1.0 | 911, 765, 439, 393 | 308.9 | |
| 95 | 32.70 | pyr-ac-cou-(cyanidin-glucoside) | 352–508 | C52H49O24 | 1057.2608 | 1057.2596 | 1.2 | 895, 749, 423, 377 | 306.9 |
| 96 | 33.29 | pyr-ac-cou-(pelargonidin-glucoside) | C52H49O23 | 1041.2659 | 1041.2651 | 0.8 | 879, 733, 407, 361 | 307.3 | |
| 97 | 33.47 | pyr-diac-cou-(delphinidin-glucoside) | C54H51O26 | 1115.2663 | 1115.2668 | −0.4 | 953, 807, 439, 393 | 311.4 | |
| 98 | 34.15 | pyr-diac-cou-(cyanidin-glucoside) | C54H51O25 | 1099.2714 | 1099.2720 | −0.5 | 937, 791, 423, 377 | 308.7 | |
| 99 | 34.29 | pyr-ac-cou-(delphinidin-glucoside) | C52H49O25 | 1073.2557 | 1073.2560 | −0.3 | 911, 765, 439, 393 | 304.8 | |
| 100 | 34.39 | pyr-ac-cou-(cyanidin-glucoside) | C52H49O24 | 1057.2608 | 1057.2608 | 0.5 | 895, 749, 423, 377 | 304.2 | |
| Peak Number | Retention Time [min] | Compound Name | µg/g DW Berries |
|---|---|---|---|
| 67 | 5.32 | delphinidin-glucoside | 81.20 ± 8.00 |
| 68 | 6.22 | delphinidin-rutinoside | 20.45 ± 1.90 |
| 69 | 6.66 | cyanidin-glucoside | 115.8 ± 9.38 |
| 70 | 7.76 | cyanidin-rutinoside | 21.37 ± 1.58 |
| 71 | 8.13 | delphinidin-arabinoside | 7.59 ± 0.43 |
| 73 | 9.67 | cyanidin-arabinoside | 7.99 ± 0.35 |
| 74 | 11.07 | pyr-delphinidin-rutinoside | 14.65 ± 1.42 |
| 75 | 11.42 | pyr-delphinidin-glucoside | 37.73 ± 3.13 |
| 76 | 11.62 | pyr-delphinidin-arabinoside | 4.23 ± 0.35 |
| 78 | 12.81 | pyr-cyanidin-rutinoside | 17.37 ± 1.18 |
| 79 | 13.15 | pyr-cyanidin-glucoside | 75.55 ± 4.67 |
| 80 | 13.41 | pyr-cyanidin-arabinoside | 4.62 ± 0.20 |
| 87 | 20.66 | pyr-ac-coumaroyl-delphinidin-rutinoside | 3.21 ± 0.36 |
| 90 | 21.49 | pyr-ac-coumaroyl-delphinidin-glucoside | 9.61 ± 0.69 |
| 92 | 22.29 | pyr-ac-coumaoryl-cyanidin-glucoside | 18.66 ± 1.18 |
| 95 | 23.58 | pyr-ac-coumaroyl-cyanidin-glucoside | 1.47 ± 0.11 |
| sum | 441.49 ± 34.93 |
| Sample | A. serrata Fruits |
|---|---|
| TAC a | 500.40 ± 9.67 |
| TFC b | 2400.64 ± 94.45 |
| TPC c | 5700.410 ± 47.24 |
| DPPH d | 350.301 ± 10.56 |
| ABTS d | 490.626 ± 7.87 |
| ORAC d | 387.21 ± 5.27 |
| FRAP d | 426.96 ± 15.23 |
| Assay | hBuChE | TcAChE | Tyrosinase | Lipase | Glucosidase | Amylase |
|---|---|---|---|---|---|---|
| A. serrata extract | 12.24 ± 0.03 | 3.92 ± 0.23 | 11.12 ± 0.10 | 32.43 ± 0.0 | 371.6 ± 0.0 | 7.23 ± 0.0 |
| Galantamine | 3.81 ± 0.02 | 0.57 ± 0.03 | - | - | - | - |
| Acarbose | - | - | - | - | 7.25 ± 0.0 | 6.56 ± 0.0 |
| Orlistat | - | - | - | 1.72 ± 0.0 | - | - |
| Kojic acid | - | - | 0.73 ± 0.04 | - | - | - |
| Compound | Binding Energy (kcal/mol) Acetylcholinesterase | Binding Energy (kcal/mol) Butyrylcholinesterase | Binding Energy (kcal/mol) Tyrosinase | Binding Energy (kcal/mol) Lipase | Binding Energy (kcal/mol) Glucosidase | Binding Energy (kcal/mol) Amylase |
|---|---|---|---|---|---|---|
| Idescarpin | −17.847 | −15.512 | −11.054 | −12.127 | −12.160 | −9.210 |
| Coumaroyl idescarpin | −16.071 | −15.115 | −10.050 | −12.831 | −11.933 | −10.150 |
| Coumaroyl idesin | −18.210 | −12.103 | −10.009 | −12.605 | −11.032 | −11.074 |
| Galantamine | −12.989 | −7.125 | ---- | ---- | ---- | ---- |
| Acarbose | ---- | ---- | ---- | ---- | −18.591 | −12.626 |
| Orlistat | ---- | ---- | ---- | −8.334 | ---- | |
| Kojic acid | ---- | ---- | −6.050 | ---- | ---- | ---- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopfstock, P.; Romero-Parra, J.; Winterhalter, P.; Gök, R.; Simirgiotis, M. In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile. Plants 2024, 13, 2756. https://doi.org/10.3390/plants13192756
Hopfstock P, Romero-Parra J, Winterhalter P, Gök R, Simirgiotis M. In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile. Plants. 2024; 13(19):2756. https://doi.org/10.3390/plants13192756
Chicago/Turabian StyleHopfstock, Philipp, Javier Romero-Parra, Peter Winterhalter, Recep Gök, and Mario Simirgiotis. 2024. "In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile" Plants 13, no. 19: 2756. https://doi.org/10.3390/plants13192756
APA StyleHopfstock, P., Romero-Parra, J., Winterhalter, P., Gök, R., & Simirgiotis, M. (2024). In Vitro Inhibition of Enzymes and Antioxidant and Chemical Fingerprinting Characteristics of Azara serrata Ruiz & Pav. Fruits, an Endemic Plant of the Valdivian Forest of Chile. Plants, 13(19), 2756. https://doi.org/10.3390/plants13192756







