An Effective Somatic-Cell Regeneration and Genetic Transformation Method Mediated by Agrobacterium tumefaciens for Portulaca oleracea L.
Abstract
1. Introduction
2. Results
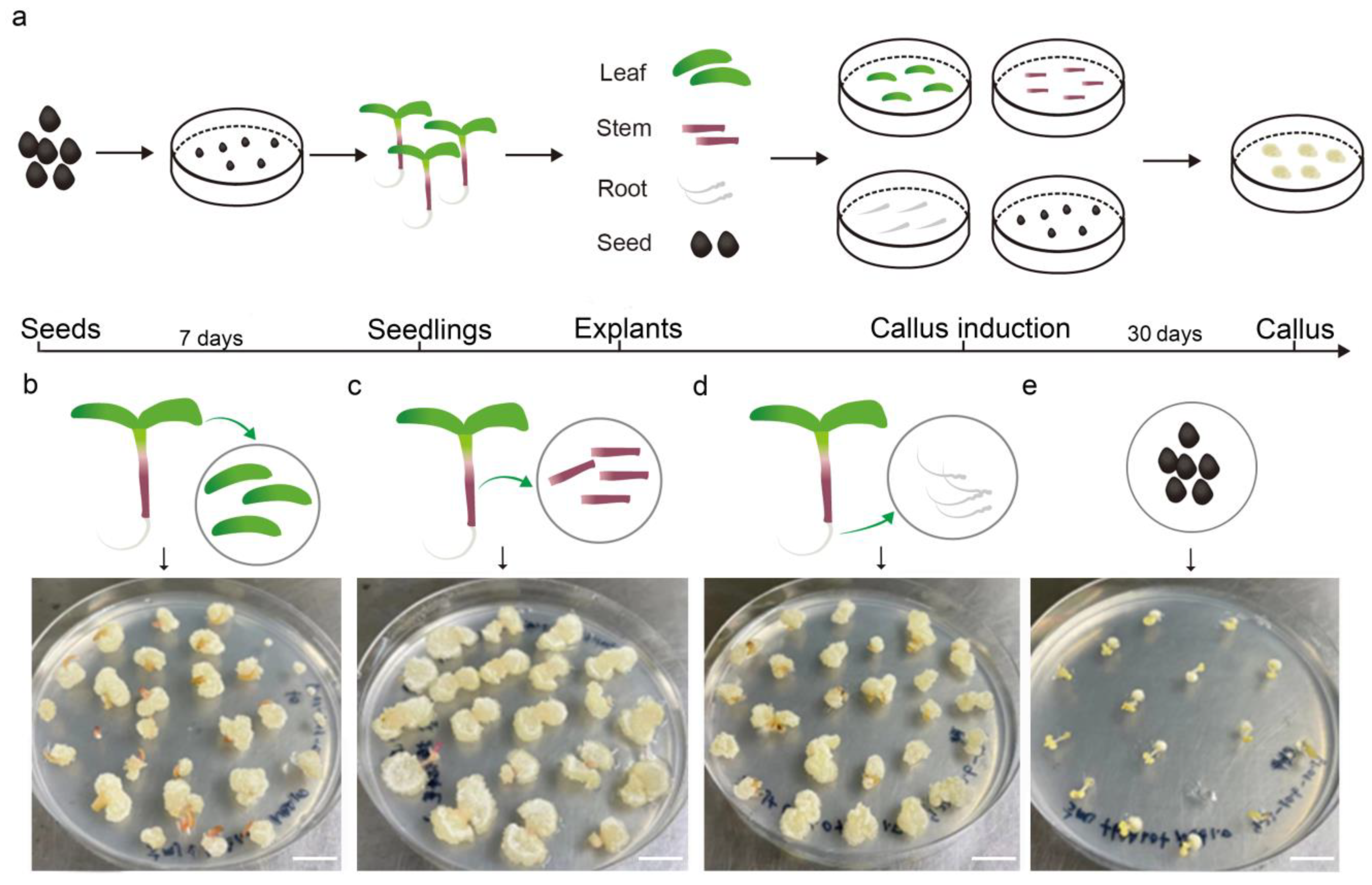
2.1. Callus Induction of Different Parts of Purslane and Medium Optimization
2.2. Shoot Regeneration and Medium Optimization
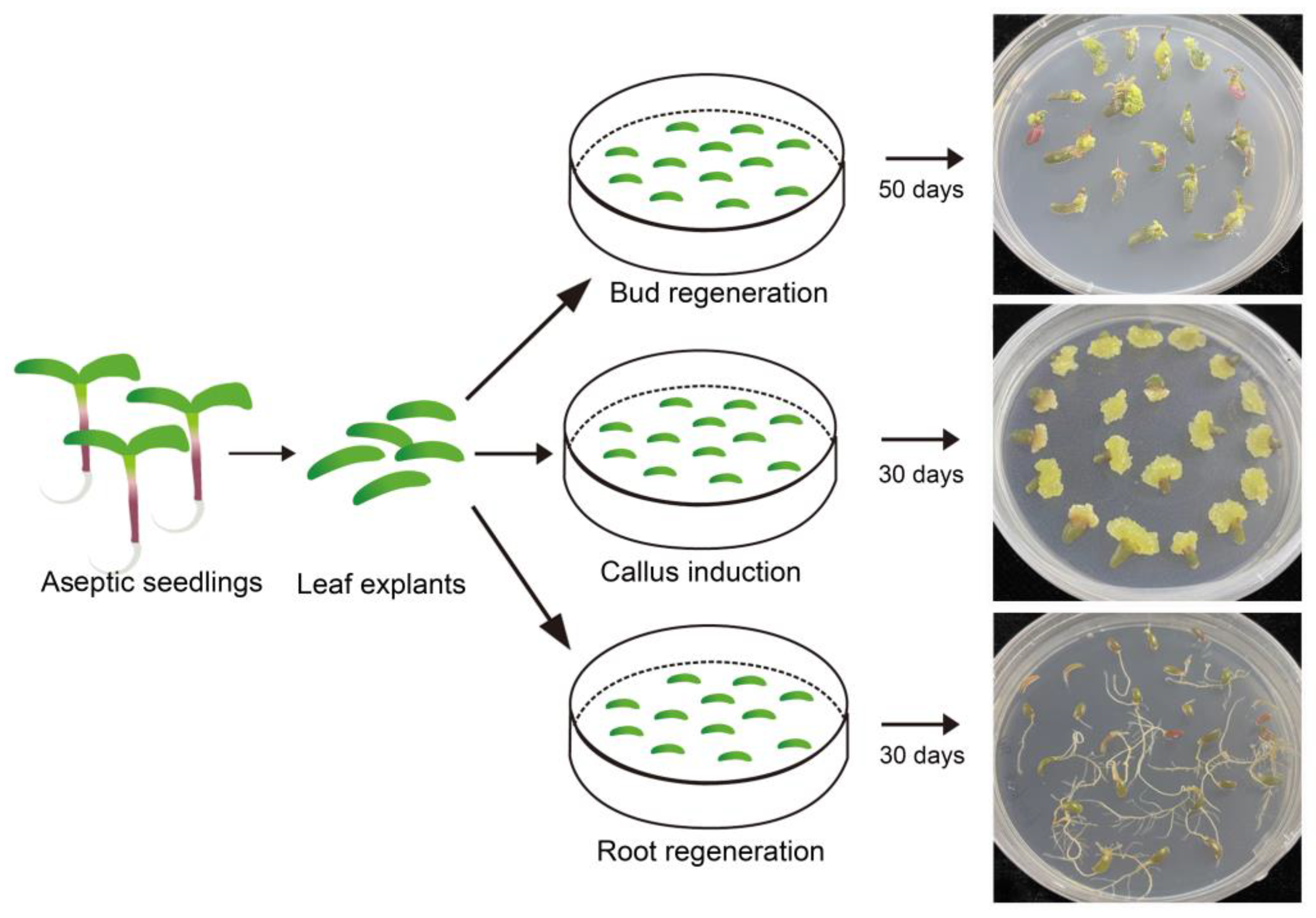
2.3. The Developmental Directions of Leaf Explants Vary on Different Media
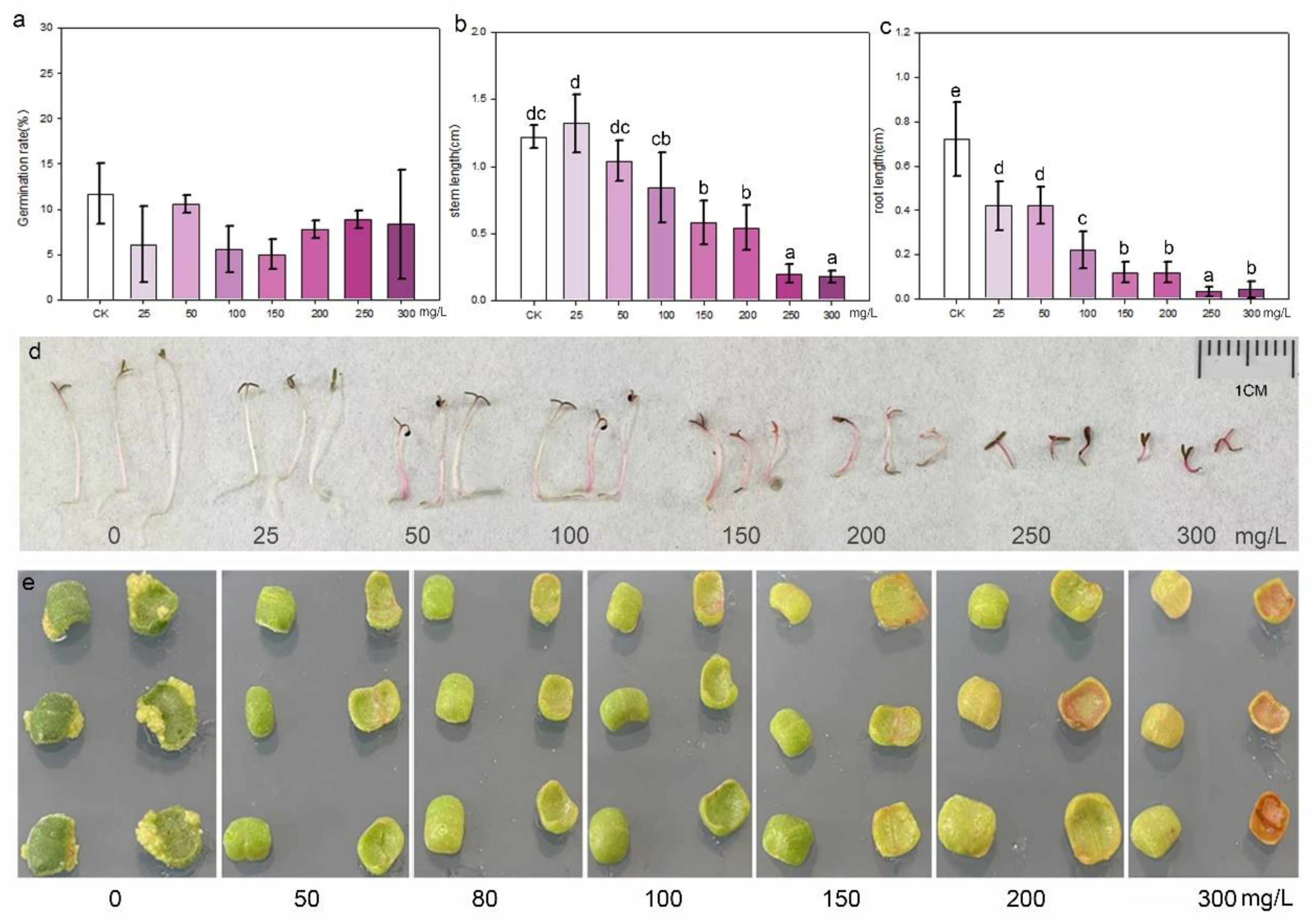
2.4. Sensitivity of Seedlings and Explants to Antibiotics
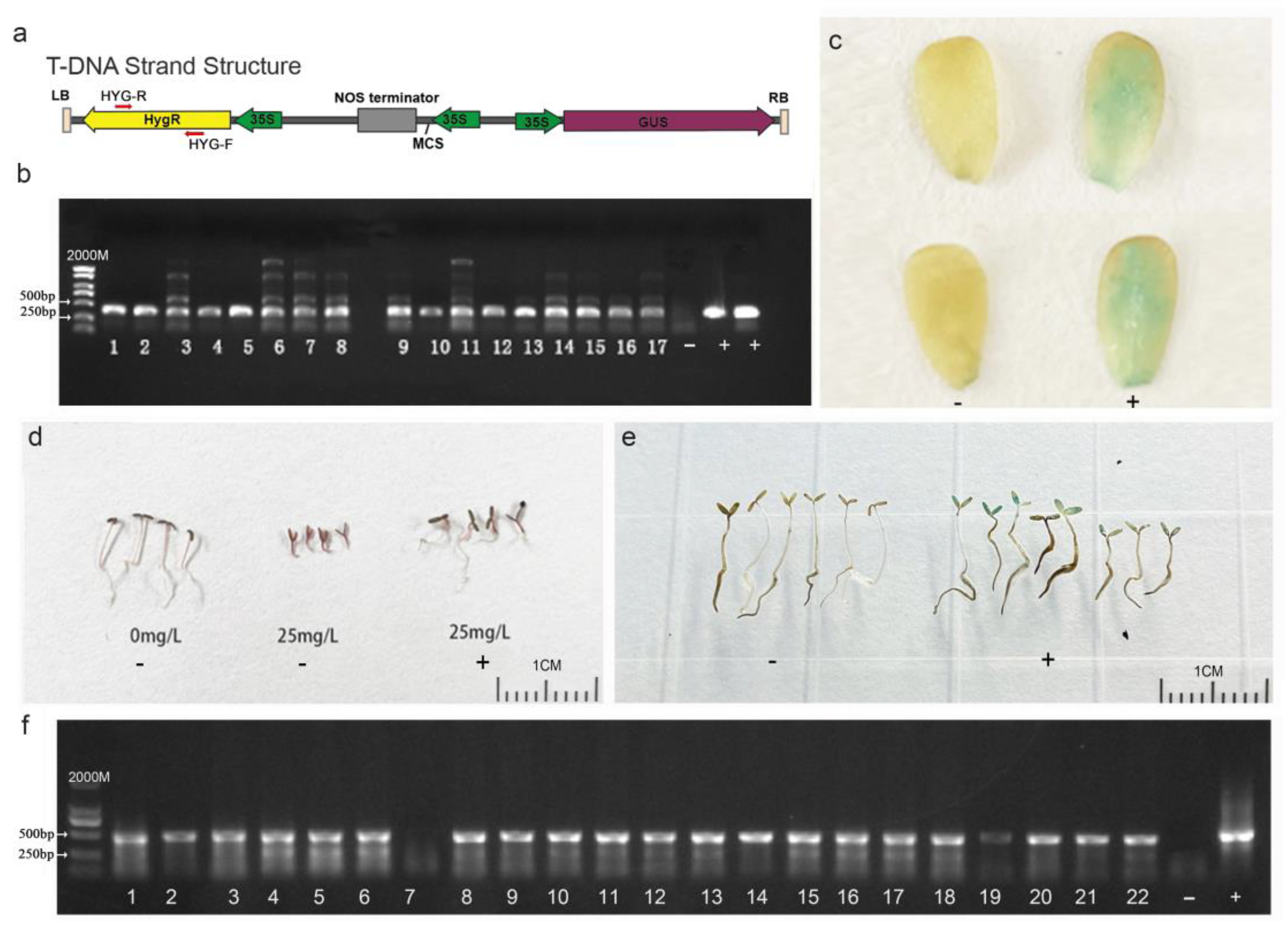
2.5. Genetic Transformation Workflow Mediated by Agrobacterium tumefaciens
2.6. Transgenic Plant Identification
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Species Identification
4.2. Sterilization and Aseptic Seedling Preparation
4.3. Callus Induction and Somatic-Cell Regeneration
4.4. Identification of the Sensitivity to Different Antibiotics
4.5. Agrobacterium tumefaciens Culture and Plant Transformation
4.6. GUS Staining and Polymerase Chain Reaction (PCR) Identification
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.; Ali, M.E.; Rahman, M.M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xia, T.; Jiang, Y.; Wang, N.; Lai, L.; Xu, S.; Yue, X.; Xin, H. A review on ethnopharmacology, phytochemistry, pharmacology and potential uses of Portulaca oleracea L. J. Ethnopharmacol. 2024, 319, 117211. [Google Scholar] [CrossRef]
- Liu, L.; Howe, P.; Zhou, Y.F.; Xu, Z.Q.; Hocart, C.; Zhan, R. Fatty acids and beta-carotene in Australian purslane (Portulaca oleracea) varieties. J Chromatogr. 2000, 893, 207–213. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Tan, D.X.; Manchester, L.C.; Reiter, R.J. Purslane: A plant source of omega-3 fatty acids and melatonin. J. Pineal. Res. 2005, 39, 331–332. [Google Scholar] [CrossRef]
- Ghorani, V.; Saadat, S.; Khazdair, M.R.; Gholamnezhad, Z.; El-Seedi, H.; Boskabady, M.H. Phytochemical characteristics and anti-inflammatory, immunoregulatory, and antioxidant effects of Portulaca oleracea L.: A Comprehensive Review. Evid. Based Complement. Alternat. Med. 2023, 2023, 2075444. [Google Scholar] [CrossRef]
- Narimani, B.; Amini, M.R.; Sheikhhossein, F.; Akhgarjand, C.; Gholizadeh, M.; Askarpour, M.; Hekmatdoost, A. The effects of purslane consumption on blood pressure, body weight, body mass index, and waist circumference: A systematic review and meta-analysis of randomised controlled. J. Nutr. Sci. 2023, 12, e129. [Google Scholar] [CrossRef]
- Matthews, J.F.; Ketron, D.W.; Zane, S.F. The biology and taxonomy of the Portulaca oleracea L. (Portulacaceae) complex in North America. Rhodora 1993, 95, 166–183. [Google Scholar]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.; Abdul Hamid, A.; Aslani, F. Screening of purslane (Portulaca oleracea L.) accessions for high salt tolerance. Sci. World J. 2014, 2014, 627916. [Google Scholar] [CrossRef]
- Ferrari, R.C.; Cruz, B.C.; Gastaldi, V.D.; Storl, T.; Ferrari, E.C.; Boxall, S.F.; Hartwell, J.; Freschi, L. Exploring C4-CAM plasticity within the Portulaca oleracea complex. Sci. Rep. 2020, 10, 14237. [Google Scholar] [CrossRef]
- Gilman, I.S.; Moreno-Villena, J.J.; Lewis, Z.R.; Goolsby, E.W.; Edwards, E.J. Gene co-expression reveals the modularity and integration of C4 and CAM in Portulaca. Plant Physiol. 2022, 189, 735–753. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Yan, G.; Hua, L.; Liu, H.; Huang, W.; Liang, Z.; Chao, Q.; Hibberd, J.M.; Jiao, Y.; et al. Gene duplications facilitate C4-CAM compatibility in common purslane. Plant Physiol. 2023, 193, 2622–2639. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.J.; Gelvin, S.B.; Jackson, D.P.; Kausch, A.P.; et al. Advancing crop transformation in the era of genome editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
- Bevan, M.W.; Flavell, R.B.; Chilton, M.D. A chimaeric antibiotic resistance gene as a selectable marker for plant cell transformation. Nature 1983, 304, 184–187. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Gao, C.; Nielsen, K.K. Comparison between Agrobacterium-mediated and direct gene transfer using the gene gun. Biolistic DNA Deliv. Methods Protoc. 2013, 940, 3–16. [Google Scholar]
- Hwang, H.H.; Yu, M.; Lai, E.M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef] [PubMed]
- Tzfira, T.; Citovsky, V. Agrobacterium-mediated genetic transformation of plants: Biology and biotechnology. Curr. Opin. Biotechnol. 2006, 17, 147–154. [Google Scholar] [CrossRef]
- Cheng, M.; Lowe, B.A.; Spencer, T.M.; Ye, X.; Armstrong, C.L. Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell. Dev. Biol. 2004, 40, 31–45. [Google Scholar] [CrossRef]
- Frary, A.; Earle, E.D. An examination of factors affecting the efficiency of Agrobacterium-mediated transformation of tomato. Plant Cell Rep. 1996, 16, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hinchee, M.A.; Connor-Ward, D.V.; Newell, C.A.; McDonnell, R.E.; Sato, S.J.; Gasser, C.S.; Fischhoff, D.A.; Re, D.B.; Fraley, R.T.; Horsch, R.B. Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. BioTechnology 1988, 6, 915–922. [Google Scholar] [CrossRef]
- Sedaghati, B.; Haddad, R.; Bandehpour, M. Efficient plant regeneration and Agrobacterium-mediated transformation via somatic embryogenesis in purslane (Portulaca oleracea L.): An important medicinal plant. Plant Cell Tiss. Organ Cult. 2019, 136, 231–245. [Google Scholar] [CrossRef]
- Sedaghati, B.; Haddad, R.; Bandehpour, M. Purslane (Portulaca oleracea L.) as a novel green-bioreactor for expression of human serum albumin (HSA) gene. Transgenic Res. 2022, 31, 369–380. [Google Scholar] [CrossRef]
- Hada, A.; Krishnan, V.; Mohamed Jaabir, M.; Kumari, A.; Jolly, M.; Praveen, S.; Sachdev, A. Improved Agrobacterium tumefaciens-mediated transformation of soybean [Glycine max (L.) Merr.] following optimization of culture conditions and mechanical techniques. In Vitro Cell. Dev. Biol. 2018, 54, 672–688. [Google Scholar] [CrossRef]
- Lian, Z.; Nguyen, C.D.; Liu, L.; Wang, G.; Chen, J.; Wang, S.; Yi, G.; Wilson, S.; Ozias-Akins, P.; Gong, H.; et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 2022, 20, 1622–1635. [Google Scholar] [CrossRef]
- Gonzalez, J.; Friero, E.; Jouve, N. Influence of genotype and culture medium on callus formation and plant regeneration from immature embryos of Triticum turgidum Desf. cultivars. Plant Breed. 2001, 120, 513–517. [Google Scholar] [CrossRef]
- Miki, B.; McHugh, S. Selectable marker genes in transgenic plants: Applications, alternatives and biosafety. J. Biotechnol. 2004, 107, 193–232. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, J.S.; Stewart, J.C.N. Advanced genetic tools for plant biotechnology. Nat. Rev. Genet. 2013, 14, 781–793. [Google Scholar] [CrossRef]
- Reed, J.; Privalle, L.; Powell, M.L.; Meghji, M.; Dawson, J.; Dunder, E.; Sutthe, J.; Wenck, A.; Launis, K.; Kramer, C.J.I.V.C.; et al. Phosphomannose isomerase: An efficient selectable marker for plant transformation. In Vitro Cell. Dev. Biol. 2001, 37, 127–132. [Google Scholar] [CrossRef]
- Twyman, R.; Stöger, E.; Kohli, A.; Capell, T.; Christou, P. Selectable and screenable markers for rice transformation. In Testing for Genetic Manipulation in Plants; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–17. [Google Scholar]
- Xia, Y.; Cao, Y.; Ren, Y.; Ling, A.; Du, K.; Li, Y.; Yang, J.; Kang, X. Effect of a suitable treatment period on the genetic transformation efficiency of the plant leaf disc method. Plant Methods 2023, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Husaini, A.M. Pre- and post-agroinfection strategies for efficient leaf disk transformation and regeneration of transgenic strawberry plants. Plant Cell Rep. 2010, 29, 97–110. [Google Scholar] [CrossRef]
- Gallagher, S.R. GUS Protocols: Using the GUS Gene as a Reporter of Gene Expression; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Barroso, T.L.C.T.; de Barros Alexandre, J.; da Cruz, E.P.; Dias, A.R.G.; Forster-Carneiro, T.; Bastos, C.P. An updated-on applications and future perspectives for the valorization of purslane (Portulaca oleracea): A comprehensive review and bibliometric analysis. Eur. Food Res. Technol. 2024, 250, 1285–1306. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, L.; Zhu, Q.-H.; Fan, L.; Guo, L. Twenty years of plant genome sequencing: Achievements and challenges. Trends Plant Sci. 2022, 27, 391–401. [Google Scholar] [CrossRef]
- Moreno-Villena, J.J.; Zhou, H.; Gilman, I.S.; Tausta, S.L.; Cheung, C.Y.M.; Edwards, E.J. Spatial resolution of an integrated C4+CAM photosynthetic metabolism. Sci. Adv. 2022, 8, eabn2349. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Horsch, R.; Fry, J.; Hoffmann, N.; Wallroth, M.; Eichholtz, D.; Rogers, S.; Fraley, R. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef]
- Horsch, R.B.; Klee, H.J. Rapid assay of foreign gene expression in leaf discs transformed by Agrobacterium tumefaciens: Role of T-DNA borders in the transfer process. Proc. Natl. Acad. Sci. USA 1986, 83, 4428–4432. [Google Scholar] [CrossRef]
- Ishida, Y.; Saito, H.; Ohta, S.; Hiei, Y.; Komari, T.; Kumashiro, T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat. Biotechnol. 1996, 14, 745–750. [Google Scholar] [CrossRef]
- Rogers, S.G.; Horsch, R.B.; Fraley, R.T. Gene transfer in plants: Production of transformed plants using Ti plasmid vectors. Methods Enzymol. 1986, 118, 627–640. [Google Scholar]
- Franklin, G.; Sheeba, C.; Lakshmi Sita, G. Regeneration of eggplant (Solanum melongena L.) from root explants. In Vitro Cell. Dev. Biol. 2004, 40, 188–191. [Google Scholar] [CrossRef]
- Ishizaki, T.; Komai, F.; Masuda, K. Screening for strongly regenerative genotypes of spinach in tissue culture using subcultured root explants. Plant Cell Tiss. Organ Cult. 2001, 67, 251–255. [Google Scholar] [CrossRef]
- Us-Camas, R.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Peña, C. In vitro culture: An epigenetic challenge for plants. Plant Cell Tiss. Organ Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Yang, J.; Seong, E.S.; Kim, M.; Ghimire, B.; Kang, W.; Yu, C.Y.; Li, C.H. Direct somatic embryogenesis from pericycle cells of broccoli (Brassica oleracea L. var. Italica) root explants. Plant Cell Tiss. Organ Cult. 2010, 100, 49–58. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.M.; Rahman, M. Micro-cloning in commercially important six bamboo species for mass propagation and at a large-scale cultivation. Plant Tissue Cult. Biotech. 2005, 15, 103–111. [Google Scholar]
- Huang, T.; Zhang, H.; Zhao, R.; Zhu, Z. Establishing an efficient regeneration system for tissue culture in Bougainvillea buttiana ‘Miss Manila’. Plants 2022, 11, 2372. [Google Scholar] [CrossRef]
- Zhong, W.; Zhou, J.; Tang, D.; Huang, Y.; Liu, F.; Zhang, M.; Wang, G.; Wu, S.; He, Y.; Tang, J. Establishment of tissue culture system of Actinidia deliciosa cultivar “Guichang”. J. Chem. 2021, 1, 9951949. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Guan, L.; Li, Z.; Wang, H.; Luo, J. Optimization of high-efficiency tissue culture regeneration systems in gray poplar. Life 2023, 13, 1896. [Google Scholar] [CrossRef]
- Gopitha, K.; Bhavani, A.L.; Senthilmanickam, J. Effect of the different auxins and cytokinins in callus induction, shoot, root regeneration in sugarcane. J. Pharma. Bio. Sci. 2010, 1, 1–7. [Google Scholar]
- Gaba, V.P. Plant growth regulators in plant tissue culture and development. In Plant Development and Biotechnology; CRC Press: Boca Raton, FL, USA, 2005; pp. 87–99. [Google Scholar]
- Joyce, P.; Kuwahata, M.; Turner, N.; Lakshmanan, P. Selection system and co-cultivation medium are important determinants of Agrobacterium-mediated transformation of sugarcane. Plant Cell Rep. 2010, 29, 173–183. [Google Scholar] [CrossRef]
- Pitzschke, A.; Hirt, H. New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J. 2010, 29, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Uranbey, S.; Sevimay, C.; Kaya, M.; Ipek, A.; Sancak, C.; Başalma, D.; Er, C.; Özcan, S. Influence of different co-cultivation temperatures, periods and media on Agrobacterium tumefaciens-mediated gene transfer. Biol. Plant. 2005, 49, 53–57. [Google Scholar] [CrossRef]
- Hansen, G.; Wright, M.S. Recent advances in the transformation of plants. Trends Plant Sci. 1999, 4, 226–231. [Google Scholar] [CrossRef]
- Jones, H.D. Wheat transformation: Current technology and applications to grain development and composition. J. Cereal Sci. 2005, 41, 137–147. [Google Scholar] [CrossRef]
- Guo, M.; Bian, X.; Wu, X.; Wu, M. Agrobacterium-mediated genetic transformation: History and progress. In Genetic Transformation; InTech: Rijeka, Croatia, 2011; pp. 1–28. [Google Scholar]
- Chetty, V.J.; Ceballos, N.; Garcia, D.; Narváez-Vásquez, J.; Lopez, W.; Orozco-Cárdenas, M.L. Evaluation of four Agrobacterium tumefaciens strains for the genetic transformation of tomato (Solanum lycopersicum L.) cultivar Micro-Tom. Plant Cell Rep. 2013, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Komari, T. Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 2008, 3, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Gelvin, S.B. Integration of Agrobacterium T-DNA into the plant genome. Annu. Rev. Genet. 2017, 51, 195–217. [Google Scholar] [CrossRef]
- Cheng, Z.M.; Schnurr, J.; Kapaun, J. Timentin as an alternative antibiotic for suppression of Agrobacterium tumefaciens in genetic transformation. Plant Cell Rep. 1998, 17, 646–649. [Google Scholar] [CrossRef]


| Accession No. | City | Province | District | Shoot Regeneration |
|---|---|---|---|---|
| 0# | Guangzhou | Guangdong | South China | Yes |
| 8# | Dehong | Yunnan | Southwest China | Yes |
| 10# | Baise | Guangxi | South China | Yes |
| 17# | Jiulongpo | Chongqing | Southwest China | Yes |
| 21# | Shangqiu | Henan | Central China | Yes |
| 33# | Xi’an | Shanxi | North China | Yes |
| 38# | Guiping | Guangxi | South China | Yes |
| 43# | Jiamusi | Heilongjiang | Northeast China | Yes |
| 46# | Yulin | Guangxi | South China | Yes |
| 47# | Yingtan | Jiangxi | East China | Yes |
| 62# | Zhoukou | Henan | Central China | Yes |
| 64# | Zhenghzhou | Henan | Central China | Yes |
| 65# | Ledong | Hainan | South China | Yes |
| 78# | Datong | Shanxi | North China | Yes |
| 102# | Hechuan | Chongqing | Southwest China | Yes |
| 106# | Zhanjiang | Guangdong | South China | Yes |
| 115# | Suihua | Heilongjiang | Northeast China | Yes |
| 116# | Huainan | Anhui | East China | Yes |
| 117# | Liuyang | Hunan | Central China | Yes |
| 118# | Liuyang | Hunan | Central China | No |
| 119# | Suzhou | Anhui | East China | Yes |
| 320# | Baicheng | Jilin | Northeast China | Yes |
| 326# | Zhoukou | Henan | Central China | Yes |
| 313# | Yuxi | Yunan | Southwest China | Yes |
| 325# | Hefei | Anhui | East China | Yes |
| 500# | Guangzhou | Guangdong | South China | Yes |
| ZP# | Huai’an | Jiangsu | East China | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhao, X.; Fang, J.; Yang, Q.; Li, P.; Yan, J. An Effective Somatic-Cell Regeneration and Genetic Transformation Method Mediated by Agrobacterium tumefaciens for Portulaca oleracea L. Plants 2024, 13, 2390. https://doi.org/10.3390/plants13172390
Xu M, Zhao X, Fang J, Yang Q, Li P, Yan J. An Effective Somatic-Cell Regeneration and Genetic Transformation Method Mediated by Agrobacterium tumefaciens for Portulaca oleracea L. Plants. 2024; 13(17):2390. https://doi.org/10.3390/plants13172390
Chicago/Turabian StyleXu, Mengyun, Xinyu Zhao, Jiahui Fang, Qinwen Yang, Ping Li, and Jian Yan. 2024. "An Effective Somatic-Cell Regeneration and Genetic Transformation Method Mediated by Agrobacterium tumefaciens for Portulaca oleracea L." Plants 13, no. 17: 2390. https://doi.org/10.3390/plants13172390
APA StyleXu, M., Zhao, X., Fang, J., Yang, Q., Li, P., & Yan, J. (2024). An Effective Somatic-Cell Regeneration and Genetic Transformation Method Mediated by Agrobacterium tumefaciens for Portulaca oleracea L. Plants, 13(17), 2390. https://doi.org/10.3390/plants13172390






