Ethylene Is Crucial in Abscisic Acid-Mediated Modulation of Seed Vigor, Growth, and Photosynthesis of Salt-Treated Mustard
Abstract
1. Introduction
2. Results
2.1. Screening of Cultivars for ET Sensitivity by Measuring Growth, Photosynthesis, 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Synthase (ACS) Activity, and Ethylene Evolution
2.2. Effect of Eth, ABA, Flu, or AVG on Seed Germination of Mustard Grown under Salt Stress
2.3. Effect of Eth, ABA, Flu, or AVG on Growth and Photosynthesis in Mustard Grown under Salt Stress
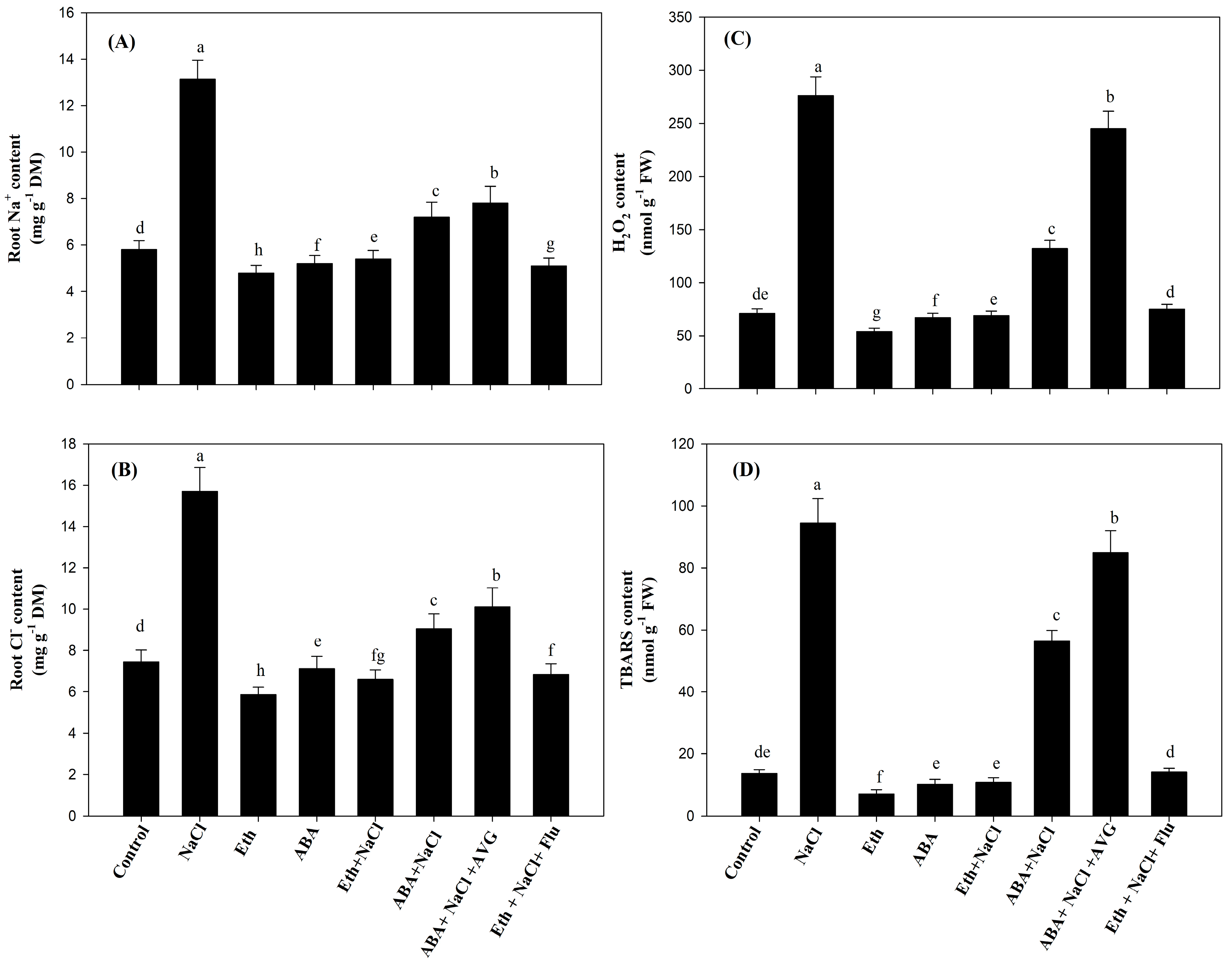
2.4. Effect of Eth, ABA, Flu, or AVG on the Content of Na+ and Cl− ions, H2O2, and Thiobarbituric Acid Reactive Substances (TBARS) in Mustard Grown under Salt Stress
2.5. Effects of Eth, ABA, Flu, or AVG on ROS Accumulation in Mustard Grown under Salt Stress
2.6. Effects of Eth, ABA, Flu, or AVG on S and N Assimilation and Antioxidant Enzyme Activity in Mustard Grown under Salt Stress
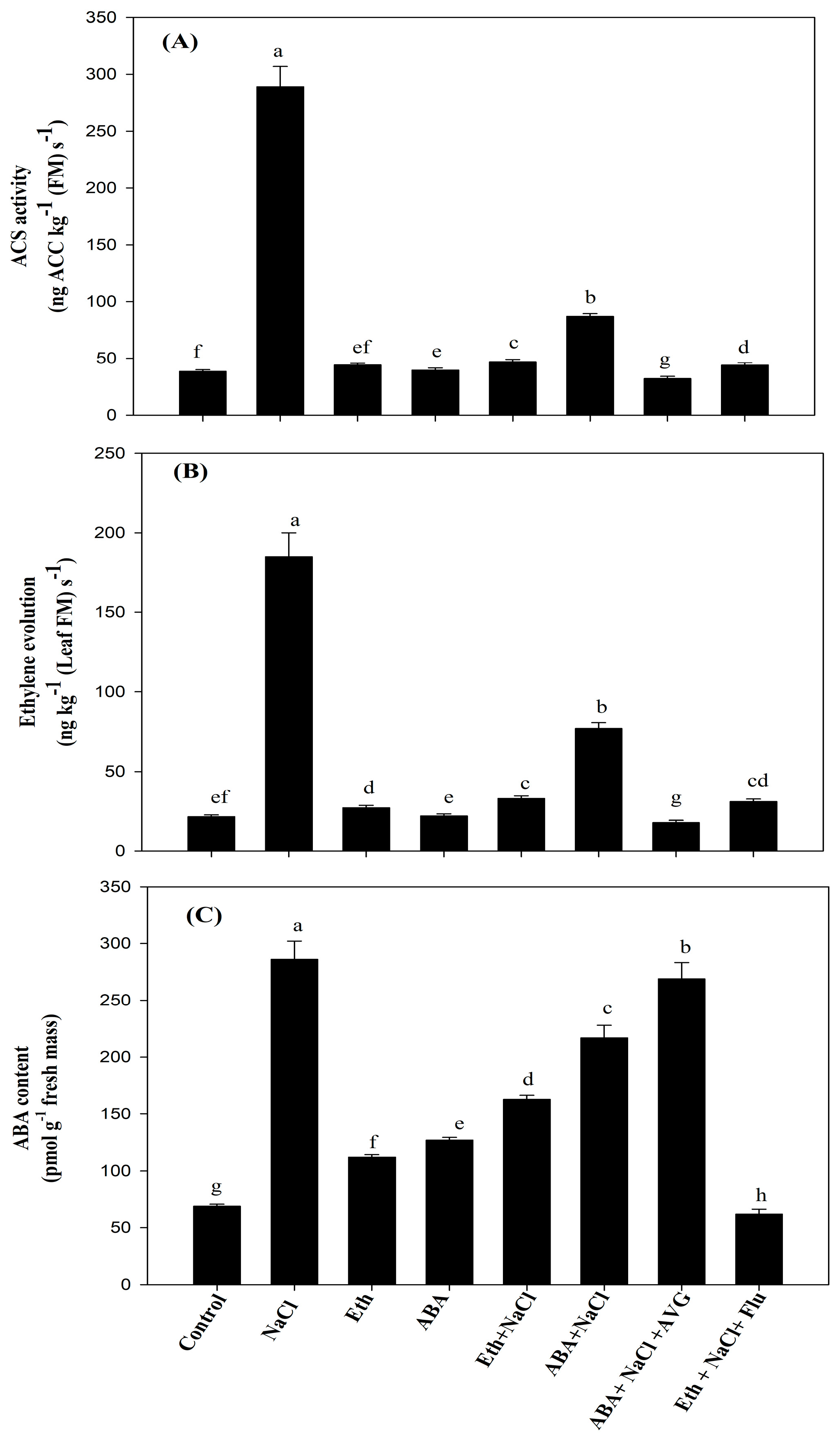
2.7. Effect of Eth, ABA, Flu, or AVG on ACS Activity, ET Evolution, and ABA Content in Mustard Grown under Salt Stress
2.8. Effect of Eth, ABA, Flu, or AVG on Stomatal Behavior in Mustard Grown under Salt Stress
2.9. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Seed Germination Parameters
4.3. Determination of Amylase Activity
4.4. Photosynthetic Traits
4.5. Determination of Growth Parameters
4.6. Determination of Sulfur Content
4.7. Determination of Cysteine Content
4.8. Determination of Glutathione Content
4.9. Determination of Nitrogen Content
4.10. Determination of Nitrate Reductase Activity
4.11. Assay of Antioxidant Enzymes
4.12. Ion Accumulation
Digestion of Plant Tissues
4.13. Determination of TBARS Content
4.14. Determination of H2O2 Content
4.15. Measurement of ACS Activity, Ethylene Evolution, and ABA Content
4.16. Physiological Measurements of Guard Cells
4.17. Histochemical Detection of ROS
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M.; et al. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotech. 2021, 329, 180–191. [Google Scholar] [CrossRef]
- Kumar, M. Crop plants and abiotic stresses. J. Biomol. Res. Ther. 2013, 3, e125. [Google Scholar] [CrossRef]
- Khan, S.; Sehar, Z.; Fatma, M.; Mir, I.R.; Iqbal, N.; Abbasi Tarighat, M.; Abdi, G.; Khan, N.A. Involvement of ethylene in melatonin-modified photosynthetic-N use efficiency and antioxidant activity to improve photosynthesis of salt-grown wheat. Physiol. Plant. 2022, 174, e13832. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Horvatinec, J. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Rather, B.A.; Sehar, Z.; Majid, A.; Jahan, B.; Mir, I.R.; Anjum, N.A.; Masood, A.; Khan, N.A. Ethylene and cellular redox management in plants. In The Plant Hormone Ethylene; Academic Press: Cambridge, MA, USA, 2023; pp. 141–170. [Google Scholar]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Rather, B.A.; Mir, I.R.; Masood, A.; Anjum, N.A.; Khan, N.A. Ethylene-nitrogen synergism induces tolerance to copper stress by modulating antioxidant system and nitrogen metabolism and improves photosynthetic capacity in mustard. Environ. Sci. Pollut. Res. 2022, 25, 49029–49049. [Google Scholar] [CrossRef]
- Iqbal, N.; Sehar, Z.; Fatma, M.; Khan, S.; Alvi, A.F.; Mir, I.R.; Masood, A.; Khan, N.A. Melatonin reverses high-temperature-stress-inhibited photosynthesis in the presence of excess sulfur by modulating ethylene sensitivity in mustard. Plants 2023, 12, 3160. [Google Scholar] [CrossRef]
- Rather, B.A.; Mir, I.R.; Sehar, Z.; Anjum, N.A.; Masood, A.; Khan, N.A. The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol. Biochem. 2020, 155, 523–534. [Google Scholar] [CrossRef]
- Mir, I.R.; Gautam, H.; Anjum, N.A.; Masood, A.; Khan, N.A. Calcium and nitric oxide signaling in plant cadmium stress tolerance: A cross-talk. S. Afr. J. Bot. 2022, 150, 387–403. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Khan, N.A. Variation in photosynthesis and growth of mustard cultivars: Role of ethylene sensitivity. Sci. Hortic. 2012, 135, 1–6. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Saify, S.; Iqbal, N.; Khan, N.A. The ethylene biosynthetic enzymes, 1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS) and ACC oxidase (ACO): The less explored players in abiotic stress tolerance. Biomolecules 2024, 14, 90. [Google Scholar] [CrossRef]
- Sehar, Z.; Fatma, M.; Khan, S.; Mir, I.R.; Abdi, G.; Khan, N.A. Melatonin influences methyl jasmonate-induced protection of photosynthetic activity in wheat plants against heat stress by regulating ethylene-synthesis genes and antioxidant metabolism. Sci. Rep. 2023, 13, 7468. [Google Scholar] [CrossRef]
- Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.; Hussain, A.; Khan, N.A. Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 12650. [Google Scholar] [CrossRef] [PubMed]
- Sehar, Z.; Iqbal, N.; Fatma, M.; Rather, B.A.; Albaqami, M.; Khan, N.A. Ethylene suppresses abscisic acid, modulates antioxidant system to counteract arsenic-inhibited photosynthetic performance in the presence of selenium in mustard. Front. Plant Sci. 2022, 13, 852704. [Google Scholar] [CrossRef]
- Arraes, F.B.M.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.R.; Albuquerque, E.V.S.; Marin, S.R.R.; Grossi-de-Sa, M.F. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Gupta, R. Ethylene: A master regulator of salinity stress tolerance in plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Rather, B.A.; Masood, A.; Sehar, Z.; Anjum, N.A.; Khan, N.A. Abscisic acid in coordination with nitrogen alleviates salinity-inhibited photosynthetic potential in mustard by improving proline accumulation and antioxidant activity. Stresses 2021, 1, 162–180. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef]
- Nazareno, A.L.; Hernandez, B.S. A mathematical model of the interaction of abscisic acid, ethylene and methyl jasmonate on stomatal closure in plants. PLoS ONE 2017, 12, e0171065. [Google Scholar] [CrossRef] [PubMed]
- Ghassemian, M.; Nambara, E.; Cutler, S.; Kawaide, H.; Kamiya, Y.; McCourt, P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 2000, 12, 1117–1126. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Yoo, S.D.; Sheen, J. Differential regulation of EIN3 stability by glucose and ethylene signaling in plants. Nature 2003, 425, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Beguerisse-Diaz, M.; Hernández-Gómez, M.C.; Lizzul, A.M.; Barahona, M.; Desikan, R. Compound stress response in stomatal closure: A mathematical model of ABA and ethylene interaction in guard cells. BMC Syst. Biol. 2012, 6, 146. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidant system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef]
- Benlloch-González, M.; Romera, J.; Cristescu, S.; Harren, F.; Fournier, J.M.; Benlloch, M. K+ starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J. Exp. Bot. 2010, 61, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, C.P. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 2008, 178, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, N.; Serizet, C.; Gosti, F.; Giraudat, J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 2000, 12, 1103–1115. [Google Scholar] [CrossRef]
- Cheng, W.H.; Chiang, M.H.; Hwang, S.G.; Lin, P.C. Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol. Biol. 2009, 71, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.A. Cross-talk between phytohormone signaling pathways under both optimal and stressful environmental conditions. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N.A., Nazar, R., Iqbal, N., Anjum, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Chapter 2; pp. 49–76. [Google Scholar]
- Kumar, D.; Hazra, S.; Datta, R.; Chattopadhyay, S. Transcriptome analysis of Arabidopsis mutants suggests a crosstalk between ABA, ethylene and GSH against combined cold and osmotic stress. Sci. Rep. 2016, 6, 36867. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. Foes or friends: ABA and ethylene interaction under abiotic stress. Plants 2021, 10, 448. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Sun, M.; Tuan, P.A.; Izydorczyk, M.S.; Ayele, B.T. Ethylene regulates post-germination seedling growth in wheat through spatial and temporal modulation of ABA/GA balance. J. Exp. Bot. 2020, 71, 1985–2004. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef]
- Bogatek, R.; Gniazdowska, A. Ethylene in seed development, dormancy and germination. Annu. Plant Rev. 2012, 44, 189–218. [Google Scholar]
- Ahammed, G.J.; Gantait, S.; Mitra, M.; Yang, Y.; Li, X. Role of ethylene crosstalk in seed germination and early seedling development: A review. Plant Physiol. Biochem. 2020, 151, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.; de Souza, G.A.; Pimenta, T.M.; Brito, F.A.; Picoli, E.A.; Zsögön, A.; Ribeiro, D.M. Salt stress inhibits germination of Stylosanthes humilis seeds through abscisic acid accumulation and associated changes in ethylene production. Plant Physiol. Biochem. 2018, 130, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Kim, H.; Bakshi, A.; Binder, B.M. The ethylene receptors ETHYLENE RESPONSE1 and ETHYLENE RESPONSE2 have contrasting roles in seed germination of Arabidopsis during salt stress. Plant Physiol. 2014, 165, 1353–1366. [Google Scholar] [CrossRef]
- Białecka, B.; Kępczyński, J. Germination, α-, β-amylase and total dehydrogenase activities of Amaranthus caudatus seeds under water stress in the presence of ethephon or gibberellin A3. Acta Biol. Crac. Ser. Bot. 2010, 52, 7–12. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Per, T.S.; Khan, N.A. Ethephon increases photosynthetic-nitrogen use efficiency, proline and antioxidant metabolism to alleviate decrease in photosynthesis under salinity stress in mustard. Plant Signal. Behav. 2017, 12, e1297000. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Khan, N.A. The influence of exogenous ethylene on growth and photosynthesis of mustard (Brassica juncea) following defoliation. Sci. Hortic. 2005, 105, 499–505. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963. [Google Scholar] [CrossRef]
- Azoulay-Shemer, T.; Schulze, S.; Nissan-Roda, D.; Bosmans, K.; Shapira, O.; Weckwerth, P.; Schroeder, J. A role for ethylene signaling and biosynthesis in regulating and accelerating CO2-and ABA-mediated stomatal movements in Arabidopsis. New Phytol. 2023, 6, 2460–2475. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, L.; Depaepe, T.; Bertrand, S.; Van Der Straeten, D. The ethylene precursor ACC affects early vegetative development independently of ethylene signaling. Front. Plant Sci. 2019, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Czékus, Z.; Poór, P.; Ördög, A. Ethylene-dependent regulation of oxidative stress in the leaves of fusaric acid-treated tomato plants. Plant Physiol. Biochem. 2023, 196, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon mitigates nickel stress by modulating antioxidant system, glyoxalase system and proline metabolism in Indian mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Mir, M.R.; Nazar, R.; Singh, S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Chang, C.; Wang, B.; Shi, L.; Li, Y.; Duo, L.; Zhang, W. Alleviation of salt stress-induced inhibition of seed germination in cucumber (Cucumis sativus L.) by ethylene and glutamate. J. Plant Physiol. 2010, 167, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.J.; Liu, C.W.; Yiu, J.C. Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol. Biochem. 2007, 45, 822–833. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Chory, J. Signals from chloroplasts converge to regulate nuclear gene expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Amzallag, G.N.; Lerner, H.R.; Poljakoff-Mayber, A. Exogenous ABA as a modulator of the response of sorghum to high salinity. J. Exp. Bot. 1990, 41, 1529–1534. [Google Scholar] [CrossRef]
- Khadri, M.; Tejera, N.A.; Lluch, C. Sodium chloride-aba interaction in two common bean (Phaseolus vulgaris) cultivars differing in salinity tolerance. Environ. Exp. Bot. 2007, 60, 211–218. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Ullah, N.; Khan, H.; Jahangir, M.; Flowers, T.J. Exogenous abscisic acid (ABA) and silicon (Si) promote salinity tolerance by reducing sodium (Na+) transport and bypass flow in rice (‘Oryza sativa’ indica). Aust. J. Crop Sci. 2013, 7, 1219–1226. [Google Scholar]
- Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; Khan, N.A. Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303. [Google Scholar] [CrossRef]
- Masood, A.; Iqbal, N.; Khan, N.A. Role of ethylene in the alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012, 35, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Park, S.H.; La, V.H.; Bae, D.W.; Kim, T.H. Drought-induced xylem sulfate activates the ABA-mediated regulation of sulfate assimilation and glutathione redox in Brassica napus leaves. Metabolites 2022, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.J.; Wang, Z.; Zhao, Q.; Mao, J.L.; Speiser, A.; Wirtz, M.; Hell, R.; Zhu, J.K.; Xiang, C.B. Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 2014, 77, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Khan, M.I.R.; Fatma, M.; Asgher, M.; Per, T.S.; Khan, N.A. Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 2016, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.R.; Rather, B.A.; Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide in co-ordination with nitrogen reverses cadmium-inhibited photosynthetic activity by interacting with ethylene synthesis, strengthening the antioxidant system, and nitrogen and sulfur assimilation in mustard (Brassica juncea L.). Sci. Hortic. 2023, 314, 111958. [Google Scholar] [CrossRef]
- Wu, J.; Seng, S.; Sui, J.; Vonapartis, E.; Luo, X.; Gong, B.; Yi, M. Gladiolus hybridus ABSCISIC ACID INSENSITIVE 5 (Gh ABI5) is an important transcription factor in ABA signaling that can enhance Gladiolus corm dormancy and Arabidopsis seed dormancy. Front. Plant Sci. 2015, 6, 960. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.G.; Jenner, C.F.; Paleg, L.G. The metabolism of soluble nucleotides in wheat aleurone layers treated with gibberellic acid. Plant Physiol. 1972, 49, 404–410. [Google Scholar] [CrossRef][Green Version]
- Usuda, H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Chesnin, L.; Yien, C.H. Turbidimetric determination of available sulfates. Soil Sci. Soc. Am. J. 1951, 15, 149–151. [Google Scholar] [CrossRef]
- Gaitonde, M.K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967, 104, 627–633. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–554. [Google Scholar] [PubMed]
- Lindner, R.C. Rapid analytical method for some of the more common organic substances of plant and soil. Plant Physiol. 1944, 19, 76–84. [Google Scholar] [CrossRef]
- Kuo, T.M.; Warner, R.L.; Kleinhofs, A. In vitro stability of nitrate reductase from barley leaves. Phytochemistry 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Mir, I.R.; Rather, B.A.; Masood, A.; Anjum, N.A.; Khan, N.A. Nitrogen sources mitigate cadmium phytotoxicity differentially by modulating cellular buffers, N-assimilation, non-protein thiols, and phytochelatins in mustard (Brassica juncea L.). J. Soil Sci. Plant Nutr. 2022, 22, 3847–3867. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Avni, A.; Bailey, B.A.; Mattoo, A.K.; Anderson, J.D. Induction of ethylene biosynthesis in Nicotiana tabacum by a Trichoderma viride xylanase is correlated to the accumulation of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase transcripts. Plant Physiol. 1994, 106, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.T.; Kao, C.H. Nitric oxide counteracts the senescence of rice leaves induced by abscisic acid. J. Plant Physiol. 2003, 160, 871–879. [Google Scholar] [CrossRef]
- Mir, I.R.; Rather, B.A.; Masood, A.; Majid, A.; Sehar, Z.; Anjum, N.A.; Khan, N.A. Soil sulfur sources differentially enhance cadmium tolerance in Indian mustard (Brassica juncea L.). Soil Syst. 2021, 5, 29. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide 780 and H2O2 accumulation in Brassica juncea seedlings. Biol. Protoc. 2014, 4, e1108. [Google Scholar]



| Cultivar | Ethephon (mmol) | Plant Dry Mass (g plant−1) | Leaf Area (cm2 plant−1) | Net Photosynthesis (µmol CO2 m−2 s−1) | Stomatal Conductance (mmol CO2 m−2 s−1) | Intercellular CO2 Concentration (µmol CO2 mol−1) | ACS Activity (ng ACC kg−1 (FM) s−1) | Ethylene (ng kg−1 (FM) s−1) |
|---|---|---|---|---|---|---|---|---|
| PM-27 | 0 (control) | 1.14 ± 0.10 o | 100.5 ± 7.1 n | 9.7 ± 1.0 j | 268.1 ± 11.5 m | 221.9 ± 25.4 m | 32.1 ± 2.0 h | 19.6 ± 1.5 g |
| 0.5 | 1.38 ± 0.15 l | 108.3 ± 8.1 j | 10.1 ± 1.4 j | 286.6 ± 12.6 l | 239.5 ± 27.5 ij | 33.4 ± 2.4 g | 20.8 ± 1.8 ef | |
| 1.0 | 1.57 ± 0.19 jk | 115.5 ± 9.1 gh | 11.6 ± 1.8 hi | 317.1 ± 13.3 i | 267.5 ± 29.8 g | 35.4 ± 3.3 f | 22.7 ± 1.9 de | |
| 2.0 | 1.68 ± 0.1 j | 121.5 ± 8.3 ef | 13.8 ± 1.6 f | 359.6 ± 12.3 g | 295.8 ± 28.3 d | 38.6 ± 3.2 cd | 25.1 ± 2.6 c | |
| PM-28 | 0 (control) | 1.11 ± 0.10 o | 100.7 ± 6.4 n | 8.3 ± 1.0 k | 255.2 ± 10.4 p | 219.6 ± 23.4 m | 31.3 ± 1.8 h | 18.5 ± 1.6 gh |
| 0.5 | 1.32 ± 0.11 m | 107.3 ± 6.8 j | 8.6 ± 1.3 jk | 271.4 ± 11.6 m | 234.5 ± 24.6 jk | 32.6 ± 1.9 gh | 19.4 ± 1.8 g | |
| 1.0 | 1.39 ± 0.15 l | 112.3 ± 7.6 i | 9.6 ± 1.7 j | 283.3 ± 12.6 l | 256.6 ± 25.gh | 33.6 ± 2.4 fg | 20.3 ± 1.9 f | |
| 2.0 | 1.48 ± 0.14 k | 117.1 ± 7.3 g | 11.4 ± 1.4 i | 324.6 ± 12.7 h | 285.9 ± 25.6 ef | 36.7 ± 2.1 de | 22.5 ± 2.1 de | |
| Pusa Agrani | 0 (control) | 1.23 ± 0.10 n | 102.6 ± 5.9 lm | 11.4 ± 1.0 i | 298.3 ± 11.7 k | 224.4 ± 25.lm | 33.2 ± 2.2 g | 21.5 ± 1.4 ef |
| 0.5 | 1.51 ± 0.07 k | 113.3 ± 4.6 hi | 12..1 ± 1.4 h | 329.4 ± 12.8 h | 244.8 ± 27.8 i | 35.1 ± 3.1 f | 23.1 ± 1.8 d | |
| 1.0 | 1.78 ± 0.17 hi | 124.2 ± 9.3 de | 13.8 ± 1.8 f | 360.3 ± 13.8 fg | 278.8 ± 30.2 f | 36.7 ± 3.6 ef | 25.3 ± 2.0 c | |
| 2.0 | 1.92 ± 0.14 g | 130.3 ± 8.1 c | 16.9 ± 1.7 c | 419.7 ± 10.6 c | 315.7 ± 29.6 c | 40.5 ± 3.3 c | 28.6 ± 2.6 b | |
| Pusa Jagannath | 0 (control) | 1.73 ± 0.10 j | 103.7 ± 7.3 l | 12.1 ± 0.96 h | 311.8 ± 11.9 j | 224.6 ± 29.5 l | 35.1 ± 2.4 f | 23.4 ± 1.5 d |
| 0.5 | 2.13 ± 0.07 f | 117.8 ± 8.7 g | 13.1 ± 11.6 g | 356.6 ± 12.5 g | 249.5 ± 31.3 hi | 37.5 ± 3.3 de | 25.3 ± 2.0 c | |
| 1.0 | 2.54 ± 0.07 d | 128.4 ± 12.7 d | 14.9 ± 1.9 e | 386.2 ± 15.1 de | 289.7 ± 33.7 e | 39.2 ± 3.8 cd | 27.6 ± 2.6 bc | |
| 2.0 | 2.75 ± 0.47 c | 134.9 ± 12.5 b | 18.6 ± 1.7 ab | 457.6 ± 13.3 b | 345.4 ± 33.4 b | 43.5 ± 3.6 ab | 31.4 ± 2.7 a | |
| Pusa Vijay | 0 (control) | 1.82 ± 0.09 h | 106.2 ± 7.6 k | 12.2 ± 1.3 h | 312.8 ± 12.6 j | 226.1 ± 31.1 l | 35.7 ± 3.0 ef | 23.4 ± 1.5 d |
| 0.5 | 2.27 ± 0.26 e | 123.5 ± 11.5 e | 13.5 ± 1.7 f | 365.6 ± 13.1 f | 255.9 ± 33.7 h | 38.6 ± 3.7 cd | 25.6 ± 2.1 c | |
| 1.0 | 3.09 ± 0.34 b | 135.4 ± 15.6 b | 15.6 ± 1.9 d | 396.7 ± 17.9 d | 299.1 ± 36.4 d | 40.3 ± 4.1 c | 28.5 ± 2.6 b | |
| 2.0 | 3.26 ± 0.31 a | 141.5 ± 14.2 a | 19.4 ± 1.56 a | 469.6 ± 14.6 a | 358.3 ± 35.8 a | 45.3 ± 4.0 a | 32.3 ± 2.8 a |
| Treatment | Germination Percentage (%) | Germination Index (%) | Vigor Index (%) | Amylase Activity (U g−1 Protein min−1) | H2O2 Content (nmol g−1 FW) | APX Activity (U g−1 Protein min−1) | GR Activity (U g−1 Protein min−1) |
|---|---|---|---|---|---|---|---|
| Control | 72 ± 1.9 b | 21.2 ± 0.67 b | 3.2 ± 0.10 b | 82.5 ± 2.4 b | 46.3 ± 1.2 f | 0.68 ± 0.02 g | 0.27 ± 0.01 f |
| NaCl | 46 ± 1.2 e | 10.5 ± 0.299 e | 0.9 ± 0.02 e | 44.7 ± 1.2 e | 198.4 ± 5.4 a | 1.07 ± 0.03 e | 0.33 ± 0.02 e |
| Eth | 82 ± 2.4 a | 24.8 ± 0.72 a | 3.6 ± 0.11 a | 105.6 ± 3.3 a | 31.5 ± 0.8 h | 2.25 ± 0.06 c | 0.46 ± 0.03 c |
| ABA | 21 ± 0.4 f | 6.3 ± 0.72 f | 0.7 ± 0.02 f | 39.4 ± 1.0 f | 38.2 ± 0.9 g | 2.06 ± 0.06 d | 0.36 ± 0.01 de |
| Eth + NaCl | 58 ± 1.5 d | 14.2 ± 0.68 d | 1.1 ± 0.04 d | 66.8 ± 2.1 d | 53.7 ± 1.4 e | 3.41 ± 0.08 a | 0.67 ± 0.03 a |
| ABA + NaCl | 13 ± 0.1 g | 1.7 ± 0.04 g | 0.5 ± 0.01 g | 38.9 ± 0.6 fg | 71.8 ± 1.9 c | 2.21 ± 0.06 cd | 0.53 ± 0.01 bn |
| ABA + NaCl +AVG | 7 ± 0.3 h | 1.3 ± 0.05 h | 0.3 ± 0.01 h | 23.2 ± 1.0 h | 81.2 ± 1.9 b | 0.84 ± 0.02 f | 0.29 ± 0.01 fg |
| Eth + NaCl + Flu | 60 ± 1.4 c | 15.2 ± 0.39 c | 1.5 ± 0.03 c | 76.5 ± 1.8 c | 62.3 ± 1.4 d | 2.82 ± 0.09 b | 0.56 ± 0.01 b |
| Treatment | Plant Dry Mass (g plant−1) | Leaf Area (cm2 Plant−1) | Net Photosynthesis (µmol CO2 m−2 s−1) | Stomatal Conductance (mmol CO2 m−2 s−1) | Intercellular CO2 Concentration (µmol CO2 mol−1) | Fv/Fm | Rubisco Activity (µmol CO2 mg−1 Protein min−1) |
|---|---|---|---|---|---|---|---|
| Control | 2.42 ± 0.23 f | 102.1 ± 5.76 e | 14.1 ± 1.12 e | 306 ± 23.7 f | 221 ± 23.1 f | 0.72 ± 0.04 f | 0.84 ± 0.069 f |
| NaCl | 1.32 ± 0.12 h | 49.2 ± 2.98 g | 5.8 ± 0.63 g | 214 ± 13.9 h | 131 ± 14.2 h | 0.42 ± 0.02 h | 0.32 ± 0.018 h |
| Eth | 3.54 ± 0.3 a | 138.1 ± 8.42 a | 19.5 ± 1.65 a | 456 ± 27.4 a | 348 ± 24.7 a | 0.93 ± 0.05 a | 1.39 ± 0.11 a |
| ABA | 3.19 ± 0.30 b | 127.3 ± 7.81 b | 18.3 ± 1.47 b | 410 ± 29.6 b | 302 ± 26.1 b | 0.87 ± 0.05 b | 1.24 ± 0.079 b |
| Eth + NaCl | 2.94 ± 0.28 c | 122.4 ± 7.77 b | 18.1 ± 1.55 b | 386 ± 26.6 c | 247 ± 23.5 c | 0.85 ± 0.05 c | 1.21 ± 0.93 c |
| ABA + NaCl | 2.79 ± 0.27 d | 113.3 ± 7.14 c | 16.5 ± 1.47 c | 361 ± 25.8 d | 226 ± 24.3 d | 0.74 ± 0.04 d | 1.14 ± 0.057 d |
| ABA + NaCl +AVG | 1.41 ± 0.13 g | 51.8 ± 2.97 f | 6.8 ± 0.76 f | 232 ± 14.3 g | 140 ± 15.2 g | 0.45 ± 0.03 g | 0.42 ± 0.019 g |
| Eth + NaCl + Flu | 2.68 ± 0.26 e | 106.8 ± 5.92 d | 15.4 ± 1.19 d | 312± 25.4 e | 238 ± 22.8 e | 0.79 ± 0.05 e | 1.13 ± 0.12 e |
| Treatments | Sulfur Content | Cysteine Content | GSH Content | Nitrogen Content (mg g−1 Dry Mass) | NR Activity (nmol NO2 g−1 FW h−1) | APX Activity | GR Activity |
|---|---|---|---|---|---|---|---|
| (mg g−1 Dry Mass) | (U mg−1 Protein min−1) | ||||||
| Control | 7.45 ± 0.46 f | 18.9 ± 1.41 g | 349 ± 21 g | 19.6 ± 1.21 d | 312 ± 11.9 d | 0.72 ± 0.033 h | 0.21 ± 0.019 h |
| NaCl | 6.10 ± 0.49 h | 34.2 ± 2.33 e | 412 ± 29 d | 11.5 ± 0.97 g | 218 ± 9.12 g | 1.42 ± 0.067 e | 0.31 ± 0.023 e |
| Eth | 11.53 ± 0.87 a | 45.1 ± 2.51 b | 442 ± 37 b | 28.3 ± 1.81 a | 412 ± 15.6 a | 2.23 ± 0.11 c | 0.49 ± 0.033 c |
| ABA | 9.32 ± 0.71 d | 37.2 ± 2.37 d | 367 ± 24 e | 23.2 ± 1.56 b | 368 ± 14.8 c | 1.32 ± 0.050 f | 0.28 ± 0.017 f |
| Eth + NaCl | 10.13 ± 1.08 b | 52.7 ± 2.67 a | 452 ± 43 a | 21.2 ± 1.37 bc | 372 ± 13.8 b | 2.60 ± 0.127 a | 0.57 ± 0.039 a |
| ABA + NaCl | 8.11 ± 0.75 e | 38 ± 2.32 c | 427 ± 34 c | 16.7± 1.06 e | 301 ± 11.6 e | 1.85 ± 0.061 d | 0.38 ± 0.031 d |
| ABA + NaCl + AVG | 6.71 ± 0.37 g | 31.6 ± 2.04 ef | 359 ± 22 f | 15.5 ± 1.01 f | 245 ± 10.1 f | 0.98 ± 0.041 g | 0.23 ± 0.022 g |
| Eth + NaCl + Flu | 9.36 ± 1.21 c | 51.3 ± 2.61 a | 451 ± 38 a | 20.9 ± 1.19 c | 369 ± 15.14 bc | 2.34 ± 0.116 b | 0.54 ± 0.035 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masood, A.; Khan, S.; Mir, I.R.; Anjum, N.A.; Rasheed, F.; Al-Hashimi, A.; Khan, N.A. Ethylene Is Crucial in Abscisic Acid-Mediated Modulation of Seed Vigor, Growth, and Photosynthesis of Salt-Treated Mustard. Plants 2024, 13, 2307. https://doi.org/10.3390/plants13162307
Masood A, Khan S, Mir IR, Anjum NA, Rasheed F, Al-Hashimi A, Khan NA. Ethylene Is Crucial in Abscisic Acid-Mediated Modulation of Seed Vigor, Growth, and Photosynthesis of Salt-Treated Mustard. Plants. 2024; 13(16):2307. https://doi.org/10.3390/plants13162307
Chicago/Turabian StyleMasood, Asim, Sheen Khan, Iqbal R. Mir, Naser A. Anjum, Faisal Rasheed, Abdulrahman Al-Hashimi, and Nafees A. Khan. 2024. "Ethylene Is Crucial in Abscisic Acid-Mediated Modulation of Seed Vigor, Growth, and Photosynthesis of Salt-Treated Mustard" Plants 13, no. 16: 2307. https://doi.org/10.3390/plants13162307
APA StyleMasood, A., Khan, S., Mir, I. R., Anjum, N. A., Rasheed, F., Al-Hashimi, A., & Khan, N. A. (2024). Ethylene Is Crucial in Abscisic Acid-Mediated Modulation of Seed Vigor, Growth, and Photosynthesis of Salt-Treated Mustard. Plants, 13(16), 2307. https://doi.org/10.3390/plants13162307











