Photosynthesis, Anatomy, and Metabolism as a Tool for Assessing Physiological Modulation in Five Native Species of the Brazilian Atlantic Forest
Abstract
1. Introduction
2. Results
2.1. Climatological Data
2.2. Studied Species
2.3. Gas Exchange and Chlorophyll a Fluorescence
2.4. Photosynthetic Pigments
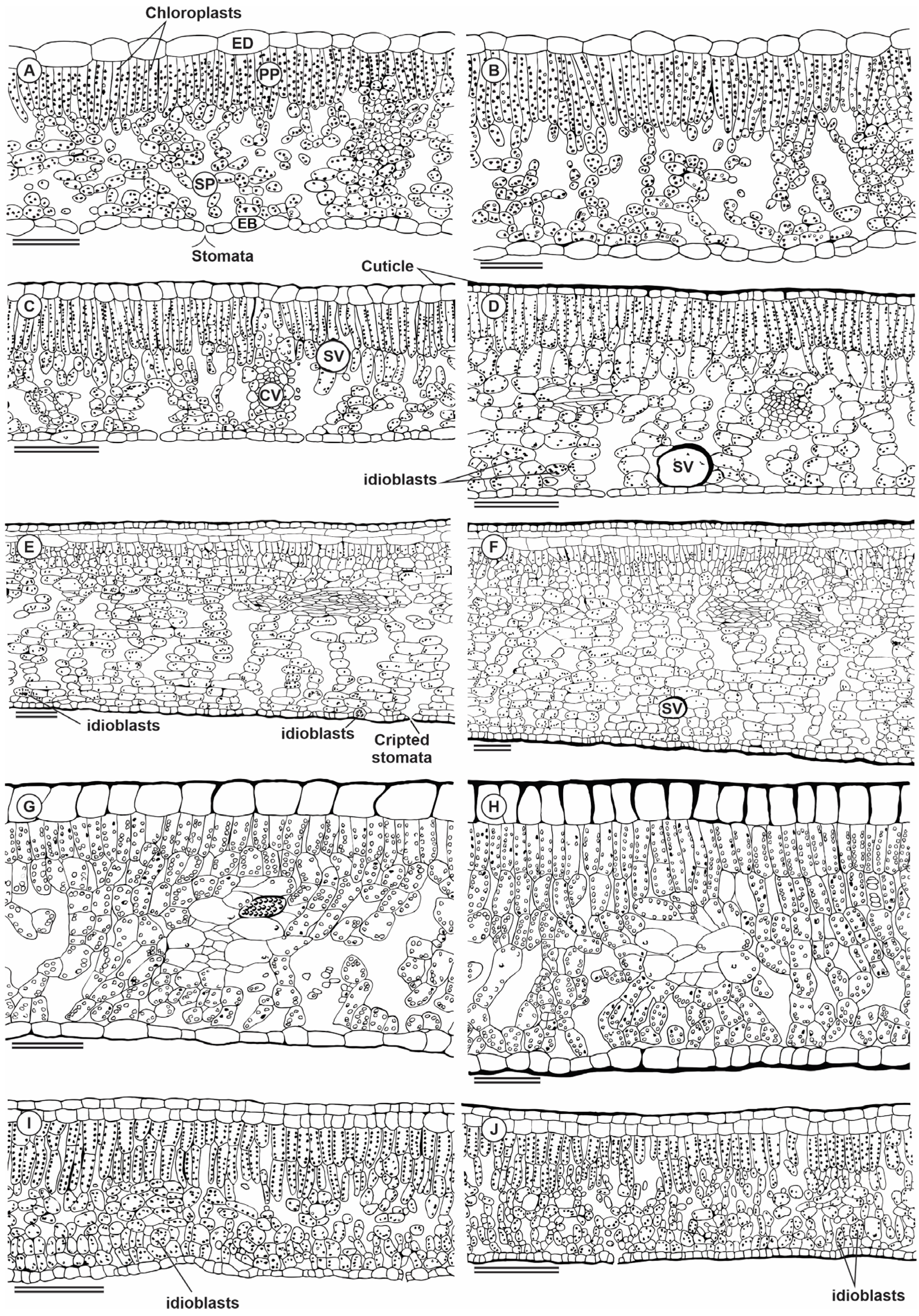
2.5. Plant Anatomy
2.6. Plant Metabolism
2.6.1. Paubrasilia echinata
2.6.2. Chorisia glaziovii
2.6.3. Clusia nemorosa
2.6.4. Licania tomentosa
2.6.5. Schinus terebinthifolia
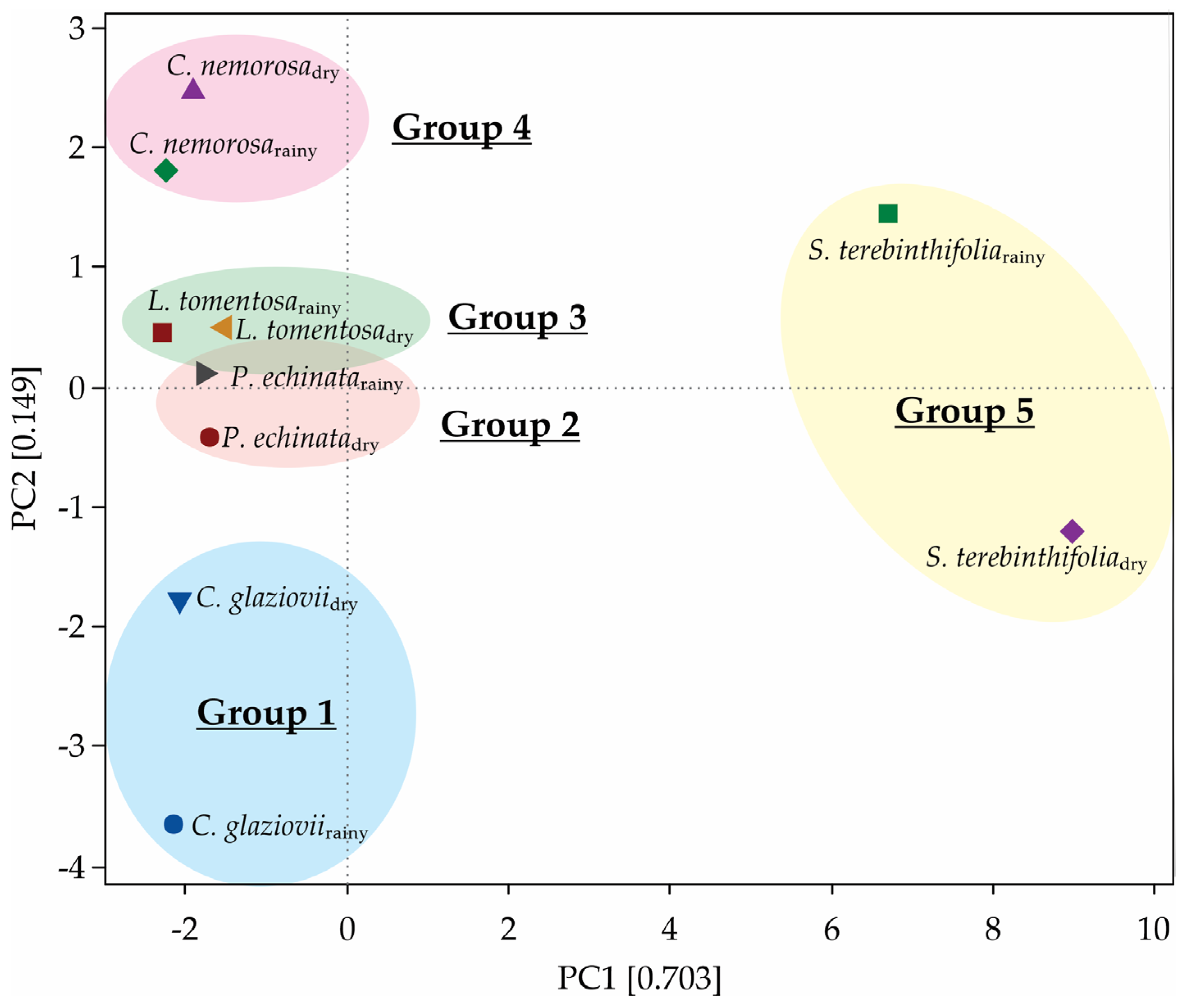
2.7. Principal Component Analysis
2.8. Plasticity Index
3. Discussion
4. Materials and Methods
4.1. Study Site and Plant Material
4.2. Gas Exchange and Chlorophyll a Fluorescence
4.3. Plant Anatomy
4.4. Metabolic Analysis and Metabolite Profile
4.5. Plasticity Index
4.6. Experimental Design and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patricia, L.; Morellato, C.; Haddad, C.F.B. Introduction: The Brazilian Atlantic Forest. Biotropica 2000, 32, 786–792. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- RBMA. A Mata Atlântica. Reserva da Biosfera da Mata Atlântica. 2024. Available online: https://rbma.org.br/n/a-mata-atlantica/ (accessed on 27 June 2024).
- Marques, M.C.M.; Trindade, W.; Bohn, A.; Grelle, C.E.V. The Atlantic Forest: An Introduction to the Megadiverse Forest of South America. In The Atlantic Forest; Marques, M.C.M., Grelle, C.E.V., Eds.; Springer, Cham, Switzerland, 2021.
- SOS_Mata_Atlântica. Atlas dos Remanescentes Florestais da Mata Atlântica Período 2021–2022; SOS Mata Atlântica: São Paulo, Brazil, 2023; Available online: https://acervo.socioambiental.org/acervo/documentos/atlas-dos-remanescentes-florestais-da-mata-atlantica-periodo-2021-2022 (accessed on 7 August 2023).
- de Souza, E.V.; Andrade, G.C.; de Araújo, H.H.; Dias-Pereira, J. Structural plasticity in leaves of Schinus terebinthifolius (Anacardiaceae) populations from three contrasting tropical ecosystems. J. Torrey Bot. 2022, 149, 187–193. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy—Meristems, Cells, and Tissues of Tre Plant Body—Their Structures, Function and Development, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; 624p. [Google Scholar]
- Tohge, T.; Ramos, M.S.; Nunes-Nesi, A.; Mutwil, M.; Giavalisco, P.; Steinhauser, D.; Schellenberg, M.; Willmitzer, L.; Persson, S.; Martinoia, E.; et al. Profiling the barley vacuole. Plant Physiol. 2011, 157, 1469–1482. [Google Scholar] [CrossRef]
- Dong, D.; Qi, C.; Zhang, J.; Deng, Q.; Xia, P.; Li, P.; Jia, C.; Zhao, B.; Zhang, N.; Guo, Y.-D. CsHSFA1d promotes drought stress tolerance by increasing the content of raffinose family oligosaccharides and scavenging accumulated reactive oxygen species in cucumber. Plant Cell Physiol. 2024, 65, 809–822. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Mengarda, L.H.G.; Milanez, C.R.D.; Silva, D.M.; Aguilar, M.A.G.; Cuzzuol, G.R.F. Morphological and physiological adjustments of brazilwood (Caesalpinia echinata Lam.) to direct solar radiation. Braz. J. Plant Physiol. 2012, 24, 161–172. [Google Scholar] [CrossRef]
- Bazzaz, F.A.; Carlson, R.W. Photosynthetic acclimation to variability in the light environment of early and late successional plants. Oecologia 1982, 54, 313–316. [Google Scholar] [CrossRef] [PubMed]
- INMET. Estações Climáticas, Gráficos. Diário. 2023. Available online: https://portal.inmet.gov.br/dadoshistoricos (accessed on 10 August 2023).
- Mengarda, L.H.G.; Souza, R.L.F.; Campostrini, E.; Reis, F.O.; Vendrame, W.A.; Cuzzuol, G.R.F. Light as an indicator of ecological succession in brazilwood (Caesalpinia echinata Lam.). Braz. J. Plant Physiol. 2009, 21, 55–64. [Google Scholar] [CrossRef]
- Baroni, A.M. Caracterização do Sistema Fotossintético em Folhas de Plantas Jovens de Caesalpinia echinata Lam. (Pau-Brasil). Ph.D. Thesis, São Paulo University, Sao Paulo, Brazil, 2005. [Google Scholar]
- Borges, L.A.; Sobrinho, M.S.; Lopes, A.V. Phenology, pollination, and breeding system of the threatened tree Caesalpinia echinata Lam. (Fabaceae), and a review of studies on the reproductive biology in the genus. Flora 2009, 204, 111–130. [Google Scholar] [CrossRef]
- Leal, A.J.B.; Dantas, I.C.; Chaves, T.P.; Felismino, D.C.; Vieira, K.V.M. Phytochemical and antimicrobial studies of Ceiba glaziovii Kuntze K. Schum. Biofar 2011, 5, 73–77. [Google Scholar]
- Albuquerque, U.P.; Medeiros, P.M.; Almeida, A.L.S.; Monteiro, J.M.; Lins Neto, E.M.F.; Melo, J.G.; Santos, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Garcia, M.; Holtum, J.A.M. On the nature of facultative and constitutive CAM: Environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. J. Exp. Bot. 2008, 59, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; Pasin, F.R.; Tsvetkova, I. Bioactive constituents of Brazilian red propolis. Evid. Based Complement. Alternat. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.M.; Lacerda, C.F.; Bezerra, M.A.; Barbosa, F.E.L.; Feitosa, H.O.; Sousa, C.H.C. Physiological responses of three woody species seedlings under water stress, in soil with and without organic matter. Rev. Árvore 2016, 40, 455–464. [Google Scholar] [CrossRef]
- Fernandes, J.; Castilho, R.O.; da Costa, M.R.; Wagner-Souza, K.; Kaplan, M.A.C.; Gattass, C.R. Pentacyclic triterpenes from Chrysobalanaceae species: Cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 2003, 190, 165–169. [Google Scholar] [CrossRef]
- Pilatti, D.M.; Fortes, A.M.T.; Jorge, T.C.M.; Boiago, N.P. Comparison of the phytochemical profiles of five native plant species in two different forest formations. Braz. J. Biol. 2018, 79, 233–242. [Google Scholar] [CrossRef]
- Nunes, D.P.; Scalon, S.P.Q.; Dresch, D.M.; Gordin, C.R.B. Photosynthetic and enzymatic metabolism of Schinus terebinthifolius Raddi seedlings under water deficit. Ciên. Agrotec. 2017, 41, 676–682. [Google Scholar] [CrossRef]
- Dosanjos, L.; Oliva, M.A.; Kuki, K.N.; Mielke, M.S.; Ventrella, M.C.; Galvão, M.F.; Pinto, L.R. Key leaf traits indicative of photosynthetic plasticity in tropical tree species. Trees 2015, 29, 247–258. [Google Scholar] [CrossRef]
- Valladares, F.; Balaguer, L.; Martinez-Ferri, E.; Perez-Corona, E.; Manrique, E. Plasticity, instability and canalization: Is the phenotypic variation in seedlings of sclerophyll oaks consistent with the environmental unpredictability of Mediterranean ecosystems? New Phytol. 2002, 156, 457–467. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Lee, D.W.; Oberbauer, S.F.; Johnson, P.; Baskaran, K.; Mansor, M.; Mohamed, H.; Yap, S.K. Effects of irradiance and spectral quality on leaf structure and funcion in seedlings of two Southeast Asian Hopea (Dipterocarpaceae) species. Am. J. Bot. 2000, 87, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Barata-Luís, R.M.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Endres, L. Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass Bioenergy 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars (Olea europaea L.) tolerant to drought stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.A.; Pearson, J.; Hall, A.J. Osmotic adjustment and yield maintenance under drought in sunflower. Field Crops Res. 2002, 75, 235–246. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.Q.Q.; Martins, A.O.; Jarma-Orozco, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Biochem. 2021, 168, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Antunes, W.C.; Mendes, K.R.; Chaves, A.R.M.; Ometto, J.P.; Jarma-Orozco, A.; Pompelli, M.F. Spondias tuberosa trees grown in tropical, wet environments are more susceptible to drought than those grown in arid environments. Rev. Col. Ciên. Hortic. 2016, 10, 9–27. [Google Scholar] [CrossRef]
- Boeger, M.R.T.; Espíndola Júnior, A.; Maccari Júnior, A.; Reissmann, C.B.; Alves, A.C.A.; Rickli, F.L. Structural variation of leaves of medicinal species associated with “erva-mate”, under different light intensities. Floresta 2009, 39, 215–225. [Google Scholar] [CrossRef]
- Dias, J.; Pimenta, J.A.; Medri, M.E.; Boeger, M.R.T.; Freitas, C.T.D. Physiological aspects of sun and shade leaves of Lithraea molleoides (Vell.) Engl. (Anacardiaceae). Braz. Arch. Biol. Technol. 2007, 50, 91–99. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Martins, S.C.; Celin, E.F.; Ventrella, M.C.; DaMatta, F.M. What is the influence of ordinary epidermal cells and stomata on the leaf plasticity of coffee plants grown under full-sun and shady conditions? Braz. J. Biol. 2010, 70, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.D.C. Morfologia, Anatomia, Histoquímica e Aspectos Fisiológicos da Lâmina Foliar de Espécies de Clusia (Clusiaceae). Master’s Thesis, University of Brasilia, Brasília, Brazil, 2007. [Google Scholar]
- Hertel, M.F.; Araújo, H.H.; Stolf-Moreira, R.; Pereira, J.D.; Pimenta, J.A.; Bianchini, E.; Oliveira, H.C. Different leaf traits provide light-acclimation responses in two neotropical woody species. Theor. Exp. Plant Physiol. 2021, 33, 313–327. [Google Scholar] [CrossRef]
- Araújo, I.; Marimon, B.S.; Scalon, M.C.; Cruz, W.J.A.; Fauset, S.; Vieira, T.C.S.; Galbraith, D.R.; Gloor, M.U. Intraspecific variation in leaf traits facilitates the occurrence of trees at the Amazonia–Cerrado transition. Flora 2021, 279, 151829. [Google Scholar] [CrossRef]
- Ting, I.P.; Hann, J.; Holbrook, N.M.; Putz, F.E.; Sternberg, L.S.L.; Price, D.; Goldstein, G. Photosynthesis in hemiepiphytic species of Clusia and Ficus. Oecologia 1987, 74, 339–346. [Google Scholar] [CrossRef]
- Luján, M.; Leverett, A.; Winter, K. Forty years of research into crassulacean acid metabolism in the genus Clusia: Anatomy, ecophysiology and evolution. Ann. Bot. 2023, 132, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Olivares, F.; Ball, E.; Lüttge, U.; Haag-Kerwer, A. In situ studies of Crassulacean acid metabolism in several sympatric species of tropical trees of the genus Clusia. New Phytol. 1994, 126, 203–211. [Google Scholar] [CrossRef]
- Gama, V.N.; Macieira, B.P.B.; Zanetti, L.V.; Fantinato, D.E.; Xavier, T.M.T.; Pezzopane, J.E.M.; Cuzzuol, G.R.F. Ecophysiological responses of medium morphotype of Paubrasilia echinata Lam.—Gagnon, H.C.Lima & G.P.Lewis raised under full sunlight and natural shade. Rev. Arvore 2019, 43, 1–13. [Google Scholar] [CrossRef]
- Ivancich, H.S.; Lencinas, M.V.; Pastur, G.J.M.; Esteban, R.M.S.; Hernández, L.; Lindstrom, I. Foliar anatomical and morphological variation in Nothofagus pumilio seedlings under controlled irradiance and soil moisture levels. Tree Physiol. 2014, 32, 554–564. [Google Scholar] [CrossRef]
- Leroux, O. Collenchyma: A versatile mechanical tissue with dynamic cell walls. Ann. Bot. 2012, 110, 1083–1098. [Google Scholar] [CrossRef]
- Cao, K.F. Leaf anatomy and clorophyll content of twelve woody species in contrasting light conditions in a Bornean heath forest. Can. J. Bot. 2000, 78, 1245–1253. [Google Scholar] [CrossRef]
- Rossato, D.R.; Kolb, R.M. Gochnatia polymorpha (Less.) Cabrera (Asteraceae) changes in leaf structure due to differences in light and edaphic conditions. Acta Bot. Bras. 2010, 2, 605–612. [Google Scholar] [CrossRef]
- Cutter, E.G. Anatomia Vegetal—Parte I Células e Tecidos, 2nd ed.; Roca: São Paulo, Brazil, 1986. [Google Scholar]
- Rossatto, D.R.; Kolb, R.M. An evergreen neotropical savanna tree (Gochnatia polymorpha, Asteraceae) produces different dry- and wet-season leaf types. Aust. J. Bot. 2009, 57, 439. [Google Scholar] [CrossRef]
- Silva, C.R.A.; Silva, M.S.; Ferreira, L.M.S.L.; Leite, K.R.B.; Silva, L.B. Morphological and anatomical aspects of the leaves of Rhizophora mangle L. (Rhizophoraceae) under different lighting conditions. Rev. Biol. Neotrop. 2015, 12, 74–80. [Google Scholar] [CrossRef]
- González-Valenzuela, L.; Renard, J.; Depège-Fargeix, N.; Ingram, G. The plant cuticle. Curr. Biol. 2023, 33, PR210–PR214. [Google Scholar] [CrossRef]
- Zani, L.B.; Macieira, B.P.B.; Corte, V.B.; Milanez, C.R.D.; Cuzzuol, G.R.F. The vessel elements and hemicelluloses as the most plastic structural components of the brazilwood ontogeny (Caesalpinia echinata Lam.) medium morphotype. Braz. J. Bot. 2017, 40, 793–800. [Google Scholar] [CrossRef]
- Marques, A.R.; Garcia, Q.S.; Rezende, J.L.P.; Fernandes, G.W. Variations in leaf characteristics of two species of Miconia in the Brazilian cerrado under different light intensities. Trop. Ecol. 2000, 41, 47–60. [Google Scholar]
- Carey, G. Comparative anatomy of leaves from species in two habitats around Sidney. Proc. Linn. Soc. N. S. W. 1938, 63, 439–450. [Google Scholar]
- Popp, M.; Kramer, D.; Lee, H.; Diaz, M.; Ziegler, H.; Lüttge, U. Crassulacean acid metabolism in tropical dicotyledonous trees of the genus Clusia. Trees 1987, 1, 238–247. [Google Scholar] [CrossRef]
- Franco, A.C.; Ball, E.; Lüittge, X.J. Differential effects of drought and light levels on accumulation of citric and malic acids during CAM in Clusia. Plant Cell Environ. 1992, 15, 821–829. [Google Scholar] [CrossRef]
- Borland, A.M.; Tecsi, L.I.; Leegood, R.C.; Walker, R.P. Inducibility of crassulacean acid metabolism (CAM) in Clusia species; physiological/biochemical characterisation and intercellular localization of carboxylation and decarboxylation processes in three species which exhibit different degrees of CAM. Planta 1998, 205, 342–351. [Google Scholar] [CrossRef]
- Peterbauer, T.; Richter, A. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci. Res. 2001, 11, 185–197. [Google Scholar]
- Chazdon, R.L.; Kaufmann, S. Plasticity of leaf anatomy of two rain forest shrubs in relation to photosynthetic light acclimation. Funct. Ecol. 1993, 7, 385–394. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, K.; Chen, L. Response of photosynthetic plasticity of Paeonia suffruticosa to changed light environments. Environ. Exp. Bot. 2003, 49, 121–133. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Bode, R.; Dahal, K.; Busch, F.A.; Possmayer, M.; Szyszka, B.; Rosso, D.; Ensminger, I.; Krol, M.; Ivanov, A.G.; et al. Shedding some light on cold acclimation, cold adaptation, and phenotypic plasticity. Botany 2012, 91, 127–136. [Google Scholar] [CrossRef]
- Aidar, M.P.M.; Godoy, J.R.L.; Bergmann, J.; Joly, C.A. Atlantic Forest succession and mycorrhiza colonization over calcareous soil—PETAR, SP. Braz. J. Bot. 2001, 24, 455–469. [Google Scholar] [CrossRef]
- Budowski, A. Distribution of tropical rain forest species in the light of successional progress. Turrialba 1965, 15, 40–42. [Google Scholar]
- Cuzzol, G.R.F.; Milanez, C.R.D. Morphological and physiological adjustments in juvenile tropical trees under constrastaing sunlight irradiance. In Photosynthesis—Fundamental Aspects; Najafpour, M.M., Ed.; InTech—Open Access Publisher: London, UK, 2012; Volume 1, pp. 501–518. [Google Scholar]
- Poorter, L. Growth responses of 15-rain forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 1999, 13, 396–410. [Google Scholar] [CrossRef]
- Popma, J.; Bongers, F. Acclimation of seedlings of three Mexican tropical rain forest tree species to a change in light availability. J. Trop. Ecol. 1991, 7, 85–97. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Ash, J.E.; Hopkins, M.S.; Grahan, A. Influence of seed size and seedling ecological attributes on shade-tolerance of rain-forest tree species in northen Queensland. J. Ecol. 1994, 82, 149–163. [Google Scholar] [CrossRef]
- Souza, R.P.; Válio, I.F.M. Seedling growth of fifteen Brazilian tropical tree species differing in successional status. Braz. J. Bot. 2003, 26, 35–47. [Google Scholar] [CrossRef]
- Junchum, F.S.; Costa, M.A.; Amorim, A.M.; Corrêa, R.X. Phylogenetic relationships among morphotypes of Caesalpinia echinata Lam. (Caesalpinioideae: Leguminosae) evidenced by trnL intron sequences. Naturwissenschaften 2008, 95, 1085–1091. [Google Scholar] [CrossRef]
- Dos Santos, O.O.; Mendes, K.R.; Martins, S.V.C.; Batista-Silva, W.; dos Santos, M.A.; Figueirôa, J.M.; Souza, E.R.; Fernandes, D.; Araújo, W.L.; Pompelli, M.F. Physiological parameters and plasticity as key factors to understand pioneer and late successional species in the Atlantic Rainforest. Acta Physiol. Plant 2019, 41, 145. [Google Scholar] [CrossRef]
- Proclima. Programa de Monitoramento Climático em Tempo Real da Região Nordeste. Available online: http://proclima.cptec.inpe.br/balanco_hidrico/balancohidrico.shtml (accessed on 16 June 2023).
- Corte-Real, N.; Miranda, P.V.V.C.; Endres, L.; Souza, E.R.; Pompelli, M.F. Tolerance to salinity in Jatropha curcas are genotype-dependent. Braz. J. Dev. 2019, 5, 22169–22199. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; DaMatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.R.; Batista-Silva, W.; Dias-Pereira, J.; Pereira, M.P.S.; Souza, E.V.; Serrão, J.E.; Granja, J.A.A.; Pereira, E.C.; Gallacher, D.J.; Mutti, P.R.; et al. Leaf plasticity across wet and dry seasons in Croton blanchetianus (Euphorbiaceae) at a tropical dry forest. Sci. Rep. 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.O.; Lichtenstein, G.; Alseekh, S.; Rosado-Souza, L.; Conte, M.; Suguiyama, V.F.; Lira, B.S.; Fanourakis, D.; Usadel, B.; Bhering, L.L.; et al. The genetic architecture of photosynthesis and plant growth-related traits in tomato. Plant Cell Environ. 2017, 41, 327–341. [Google Scholar] [CrossRef]
- Fernie, A.R.; Roscher, A.; Ratcliffe, R.G.; Kruger, N.J. Fructose 2,6-bisphosphate activates pyrophosphate: Fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling heterotrophic cells. Planta 2001, 212, 250–263. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Carrari, F.; Gibon, Y.; Sulpice, R.; Lytovchenko, A.; Fisahn, J.; Graham, J.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J. 2007, 50, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed]








| Species | Photosynthetic Metabolism | Successional Group | Economic Importance | Tree Information | References |
|---|---|---|---|---|---|
| Paubrasilia echinata Lam.—Gagnon, H.C.Lima & G.P.Lewis | C3 | Late Successional | Ornamental tree; its heartwood is used as dyestuff and to manufacture high-quality bows of stringed instruments, and the seeds have a pro-inflammatory activity. Used in silvicultural systems. | It is an endangered semideciduous tree species with a 10–15 m tall stalk 40–70 cm in diameter. Bipinnate leaves 10 to 15 cm in length and 5–6 pairs of pinnae. Some authors consider P. echinata as late successional [11,14]; however, Baroni [15] describes it as a pioneer. | Mengarda et al. [11], Borges et al. [16], Baroni [15] |
| Chorisia glaziovii (Kuntze) E. Santos) | C3 | Pioneer | Extract from stem bark shows significant antimicrobial activity. Stem bark or leaves are used in folk medicine in the treatment of inflammation, diabetes, rheumatism. | A medium-sized tree 6–15 m tall, with composite leaves 3 cm in length. | Leal et al. [17], Albuquerque et al. [18] |
| Clusia nemorosa (G. Mey) | Many species facultative and constitutive metabolism CAM | Pioneer | It has pharmacological potential due to its antibacterial, antifungal, antiviral, anti-inflammatory, hepatoprotective, and antioxidant properties. Leaf extracts have anti-inflammatory and antiproliferative effects. Widely used in folk medicine. | C. nemorosa is found from the Brazilian Northern coast to Rio de Janeiro, and in forests and “Campo Rupestre” vegetation from the Amazon to Bahia. It is a 20 m tall tree; it has oblong and cartilage leaves. Clusia spp. are considered pioneers or facilitators for other species in the community. | Winter et al. [19], Trusheva et al. [20] |
| Licania tomentosa (Benth) Fritsch. | C3 | Late Successional | This species has ethnopharmacological potential because it has numerous therapeutic attributes. Seed extracts of L. tomentosa have been demonstrated to exert inhibitory activity against the herpes simplex virus, and extracts of its leaves and fruits have been shown to have anti-cancer properties against leukemia cell strains. | It is a tree that has chemical features such as flavonoids, terpenoids (triterpenes and diterpenes), steroids, and tannins. | Rocha et al. [21], Fernandes et al. [22] |
| Schinus terebinthifolia Raddi | C3 | Pioneer | Leaves, fruit, and bark have antimicrobial, analgesic, anti-inflammatory, antioxidant, anti-allergic, anti-free radical, and insecticidal activities. The fruits are rich in tannins, flavonoids, and essential oil and are used as cosmetology components, and in the perfume industry, presenting phenolic compounds and hydrolyzable tannins. It is used in protective reforestation. | It can be used in the recovery of degraded areas, as it demonstrates rapid growth and has a very expansive root system that facilitates water uptake from the deeper layers of the soil. The species has a remarkable anatomical plasticity. This characteristic is pivotal for its use in the early stages of ecological succession in land restoration. Also, it provides the species with an adaptive advantage in a climate change scenario. Its antioxidant enzyme activity is increased both in the aerial part and the root in response to water deficit. | Some authors consider this species as a pioneer, like Pilatti et al. [23] and Nunes et al. [24], while Dos Anjos et al. [25] consider it as early secondary |
| Source of Variation | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Species | 9 | 1.702 | 0.189 | 74.838 | <0.001 |
| Months | 5 | 0.013 | 0.003 | 0.988 | 0.435 |
| Residual | 45 | 0.114 | 0.003 | ||
| Total | 59 | 1.828 | 0.031 |
| Species | Mean (SE) (cm2 g−1) |
|---|---|
| Paubrasilia echinata | 10.9 ± 0.5 b |
| Chorisia glaziovii | 15.1 ± 0.3 a |
| Clusia nemorosa | 5.1 ± 0.3 c |
| Licania tomentosa | 9.9 ± 0.2 b |
| Schinus terebinthifolius | 9.8 ± 0.4 b |
| Physiological Features | P. echinata | C. glaziovii | C. nemorosa | L. tomentosa | S. terebinthifolia | Mean Value |
|---|---|---|---|---|---|---|
| Net photosynthesis (PN) | 0.773 | 0.839 | 0.765 | 0.775 | 0.798 | 0.790 |
| Stomatal conductance (gs) | 0.837 | 0.877 | 0.879 | 0.815 | 0.638 | 0.809 |
| Variable, maximum fluorescence (Fv/Fm) | 0.233 | 0.406 | 0.145 | 0.181 | 0.122 | 0.218 |
| Current PSII efficiency (ΦPSII) | 0.981 | 0.820 | 0.795 | 0.814 | 0.836 | 0.849 |
| Electron transport rate (ETR) | 0.987 | 0.954 | 0.704 | 0.713 | 0.788 | 0.829 |
| Active radiation dissipated as heat (D) | 0.678 | 0.722 | 0.647 | 0.689 | 0.795 | 0.706 |
| Chlorophyll “a” | 0.894 | 0.938 | 0.392 | 0.259 | 0.539 | 0.605 |
| Chlorophyll “b” | 0.894 | 0.926 | 0.454 | 0.601 | 0.553 | 0.686 |
| Chlorophyll “a + b” | 0.855 | 0.935 | 0.366 | 0.243 | 0.539 | 0.588 |
| Total carotenoids | 0.830 | 0.454 | 0.430 | 0.314 | 0.436 | 0.493 |
| Mean Value | 0.796 ± 0.069 a | 0.787 ± 0.064 a | 0.558 ± 0.074 b | 0.540 ± 0.082 b | 0.604 ± 0.069 ab | 0.657 ± 0.061 B |
| Anatomical features | ||||||
| Abaxial epidermis surface thickness | 0.462 | 0.429 | 0.857 | 0.880 | 0.948 | 0.715 |
| Adaxial epidermis surface thickness | 0.252 | 0.464 | 0.674 | 0.529 | 0.822 | 0.548 |
| Palisade parenchyma thickness | 0.531 | 0.345 | 0.289 | 0.888 | 0.707 | 0.552 |
| Spongy parenchyma thickness | 0.250 | 0.417 | 0.282 | 0.671 | 0.650 | 0.454 |
| Total leaf thickness | 0.305 | 0.284 | 0.262 | 0.370 | 0.427 | 0.329 |
| Specific leaf area (SLA) | 0.495 | 0.718 | 0.547 | 0.356 | 0.670 | 0.557 |
| Ordinary cell density | 0.116 | 0.440 | 0.106 | 0.201 | 0.394 | 0.251 |
| Stomatal density | 0.396 | 0.500 | 0.174 | 0.349 | 0.457 | 0.375 |
| Stomatal index | 0.332 | 0.397 | 0.158 | 0.226 | 0.483 | 0.319 |
| Mean Value | 0.349 ± 0.045 a | 0.444 ± 0.040 a | 0.372 ± 0.087 a | 0.497 ± 0.087 a | 0.617 ± 0.064 a | 0.456 ± 0.050 B |
| Metabolic features | ||||||
| α-Ketoglutarate | 0.721 | 0.636 | 0.466 | 0.569 | 0.851 | 0.649 |
| 3-P-Glycerate | 0.547 | 0.582 | 0.639 | 0.730 | 0.487 | 0.597 |
| Citrate | 0.958 | 0.972 | 0.767 | 0.841 | 0.869 | 0.881 |
| Dehydroascorbate | 0.801 | 0.910 | 0.994 | 0.678 | 0.730 | 0.823 |
| Fumarate | 0.504 | 0.864 | 1.000 | 0.673 | 0.577 | 0.723 |
| Gluconate | 0.774 | 0.975 | 0.987 | 0.745 | 0.850 | 0.866 |
| Glycerate | 0.844 | 0.961 | 0.970 | 0.951 | 0.988 | 0.943 |
| Isocitrate | nd | nd | nd | nd | 0.817 | 0.817 |
| Malate | 0.812 | 0.621 | 0.653 | 0.757 | 0.760 | 0.721 |
| Malonate | 0.802 | 0.684 | 1.000 | nd | 0.950 | 0.859 |
| Palmitate | 0.648 | 0.885 | 0.590 | 0.774 | 0.767 | 0.733 |
| Succinate | 0.908 | 0.997 | 0.992 | 0.780 | 0.960 | 0.927 |
| Fructose | 0.729 | 0.787 | 0.995 | 0.759 | 0.803 | 0.815 |
| Fructose-6-phosphate | nd | 0.609 | nd | 0.625 | 0.753 | 0.662 |
| Glucose | 0.510 | 0.990 | 0.386 | 0.809 | 0.692 | 0.678 |
| Glucose-6-phosphate | 0.809 | 0.909 | 0.612 | 1.000 | 0.538 | 0.774 |
| Maltose | 0.465 | 0.674 | 0.887 | 0.824 | 0.784 | 0.727 |
| Mannose | 0.621 | 0.899 | 0.686 | 0.699 | 0.960 | 0.773 |
| Pyruvate | 0.452 | 0.771 | 0.999 | 0.538 | nd | 0.690 |
| Sucrose | 0.680 | 0.798 | 0.693 | 0.788 | nd | 0.740 |
| Aminobutyric acid (GABA) | 0.603 | 0.646 | 0.578 | 0.543 | 0.992 | 0.672 |
| Asparagine | 0.862 | 0.935 | 0.999 | 0.595 | 0.554 | 0.789 |
| Aspartate | 0.834 | 0.966 | 0.996 | 0.999 | 0.992 | 0.957 |
| β-alanine | 0.824 | 0.825 | 0.995 | 0.618 | 0.554 | 0.763 |
| Citrulline | 0.501 | 0.575 | 1.000 | 0.713 | 1.000 | 0.758 |
| Cysteine | 0.768 | 0.758 | 0.999 | 0.709 | 0.950 | 0.837 |
| Glutamine | 0.770 | 0.929 | 0.729 | 0.824 | 0.745 | 0.800 |
| Glutamate | 0.814 | 0.454 | 0.626 | 0.707 | 0.731 | 0.666 |
| Glycine | 0.725 | 0.892 | 0.999 | 0.870 | 0.707 | 0.839 |
| Histidine | 0.649 | 0.992 | 0.895 | 0.838 | 0.839 | 0.843 |
| Homoserine | 0.901 | 0.877 | 0.904 | 0.685 | 0.990 | 0.871 |
| Hydroxyproline | 0.706 | 0.991 | 0.999 | 0.732 | 0.944 | 0.874 |
| Isoleucine | 0.541 | 1.000 | 0.673 | 0.761 | 0.986 | 0.792 |
| Leucine | 0.903 | 0.884 | 0.973 | 0.734 | 0.927 | 0.884 |
| Methionine | 0.621 | 0.652 | 0.538 | 0.604 | 0.817 | 0.646 |
| Ornithine | 0.569 | nd | 0.881 | 0.816 | 0.891 | 0.789 |
| Phenylalanine | 0.822 | 0.896 | 0.982 | 0.975 | 0.792 | 0.893 |
| Serine | 0.504 | 0.563 | 0.616 | 0.550 | 0.798 | 0.606 |
| Tryptophan | 0.588 | 0.993 | 0.802 | 0.608 | 0.853 | 0.769 |
| Tyrosine | 0.921 | 0.984 | 0.872 | 0.832 | 0.906 | 0.903 |
| Valine | nd | nd | nd | nd | 0.876 | 0.876 |
| Erythritol | 0.870 | 0.896 | 0.931 | 0.844 | 0.543 | 0.817 |
| myo-Inositol | 0.875 | 0.954 | 0.910 | 0.504 | 0.519 | 0.752 |
| Raffinose | 0.866 | 0.977 | 0.992 | 0.516 | 0.802 | 0.831 |
| Trehalose | 0.578 | 0.950 | 1.000 | 0.540 | 0.827 | 0.779 |
| Putrescine | nd | nd | nd | nd | 0.827 | 0.827 |
| Shikimic acid | 0.883 | 0.762 | 0.981 | 0.985 | 0.861 | 0.894 |
| Spermidine | 0.713 | 0.939 | 0.446 | 0.665 | 0.683 | 0.689 |
| Mean Value | 0.723 ± 0.021 b | 0.837 ± 0.022 a | 0.833 ± 0.027 a | 0.734 ± 0.025 b | 0.812 ± 0.020 a | 0.791 ± 0.014 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Páez, L.A.; Seleiman, M.F.; Alhammad, B.A.; Pineda-Rodríguez, Y.Y.; Pompelli, M.F.; Martins, A.O.; Dias-Pereira, J.; Araújo, W.L. Photosynthesis, Anatomy, and Metabolism as a Tool for Assessing Physiological Modulation in Five Native Species of the Brazilian Atlantic Forest. Plants 2024, 13, 1906. https://doi.org/10.3390/plants13141906
Rodríguez-Páez LA, Seleiman MF, Alhammad BA, Pineda-Rodríguez YY, Pompelli MF, Martins AO, Dias-Pereira J, Araújo WL. Photosynthesis, Anatomy, and Metabolism as a Tool for Assessing Physiological Modulation in Five Native Species of the Brazilian Atlantic Forest. Plants. 2024; 13(14):1906. https://doi.org/10.3390/plants13141906
Chicago/Turabian StyleRodríguez-Páez, Luis Alfonso, Mahmoud F. Seleiman, Bushra A. Alhammad, Yirlis Yadeth Pineda-Rodríguez, Marcelo F. Pompelli, Auxiliadora Oliveira Martins, Jaqueline Dias-Pereira, and Wagner L. Araújo. 2024. "Photosynthesis, Anatomy, and Metabolism as a Tool for Assessing Physiological Modulation in Five Native Species of the Brazilian Atlantic Forest" Plants 13, no. 14: 1906. https://doi.org/10.3390/plants13141906
APA StyleRodríguez-Páez, L. A., Seleiman, M. F., Alhammad, B. A., Pineda-Rodríguez, Y. Y., Pompelli, M. F., Martins, A. O., Dias-Pereira, J., & Araújo, W. L. (2024). Photosynthesis, Anatomy, and Metabolism as a Tool for Assessing Physiological Modulation in Five Native Species of the Brazilian Atlantic Forest. Plants, 13(14), 1906. https://doi.org/10.3390/plants13141906









