Genome-Wide Association Analysis of Yield-Related Traits and Candidate Genes in Vegetable Soybean
Abstract
1. Introduction
2. Results
2.1. Phenotype Variations of Yield-Related Traits in Vegetable Soybean
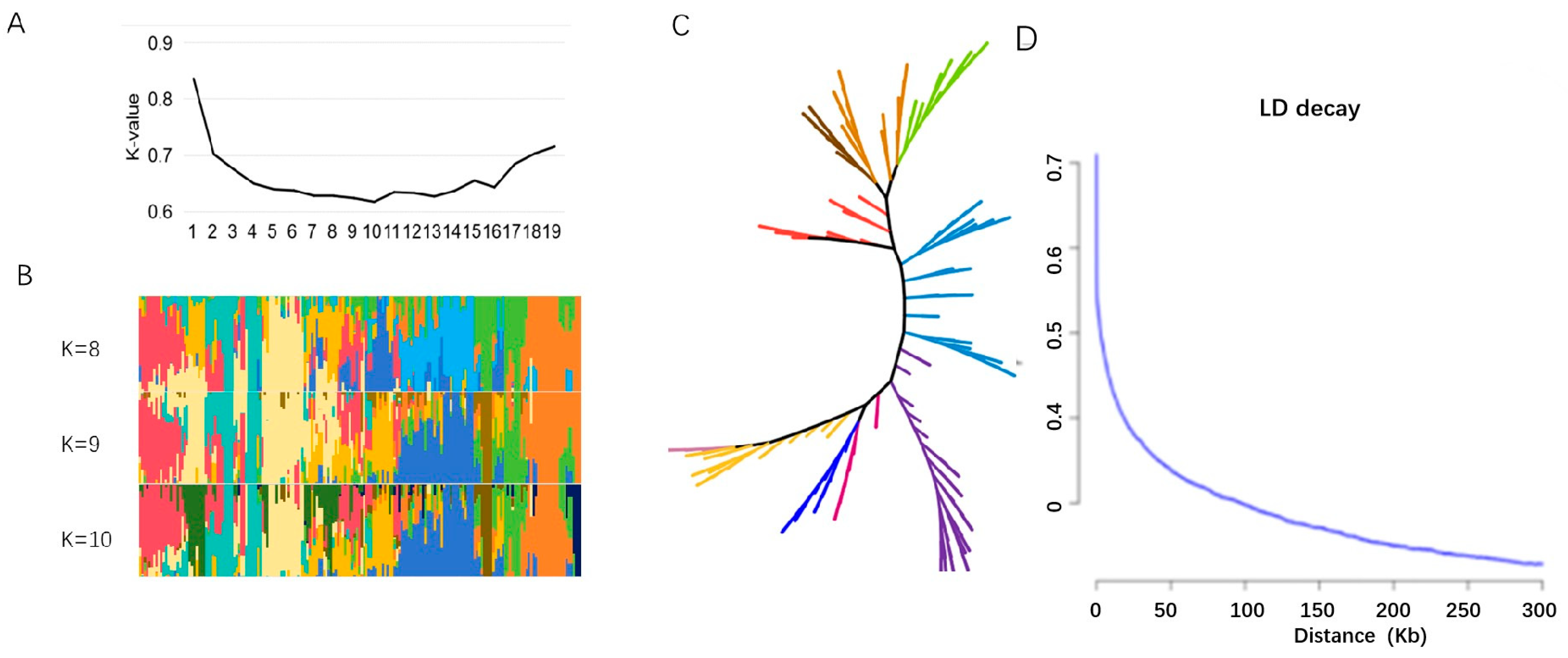
2.2. Population Structure Analysis of Vegetable Soybean
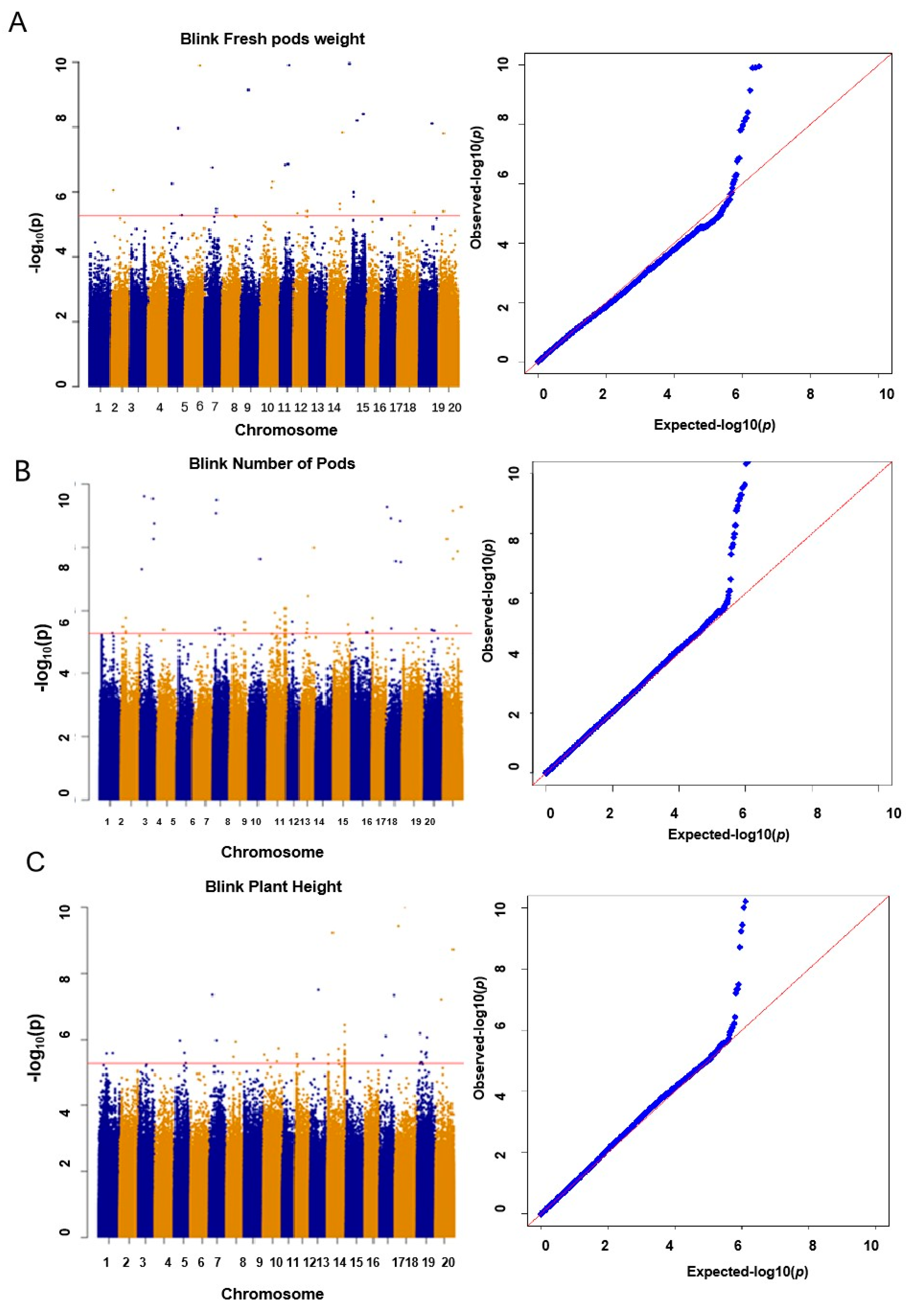
2.3. Yield-Related SNPs Were Identified in Vegetable Soybean via GWAS
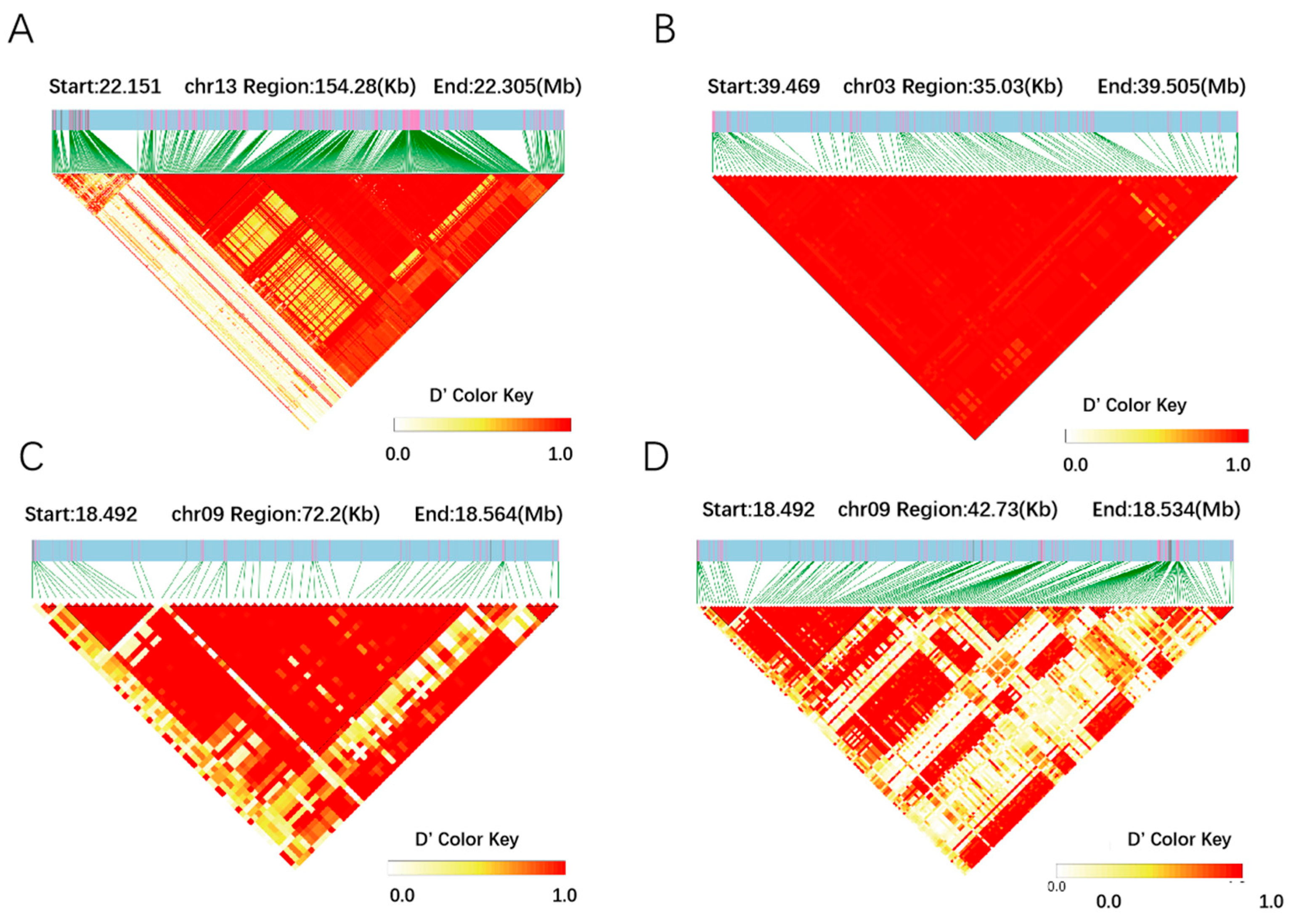
2.4. Candidate Gene Analysis of Yield-Related Traits in Vegetable Soybean
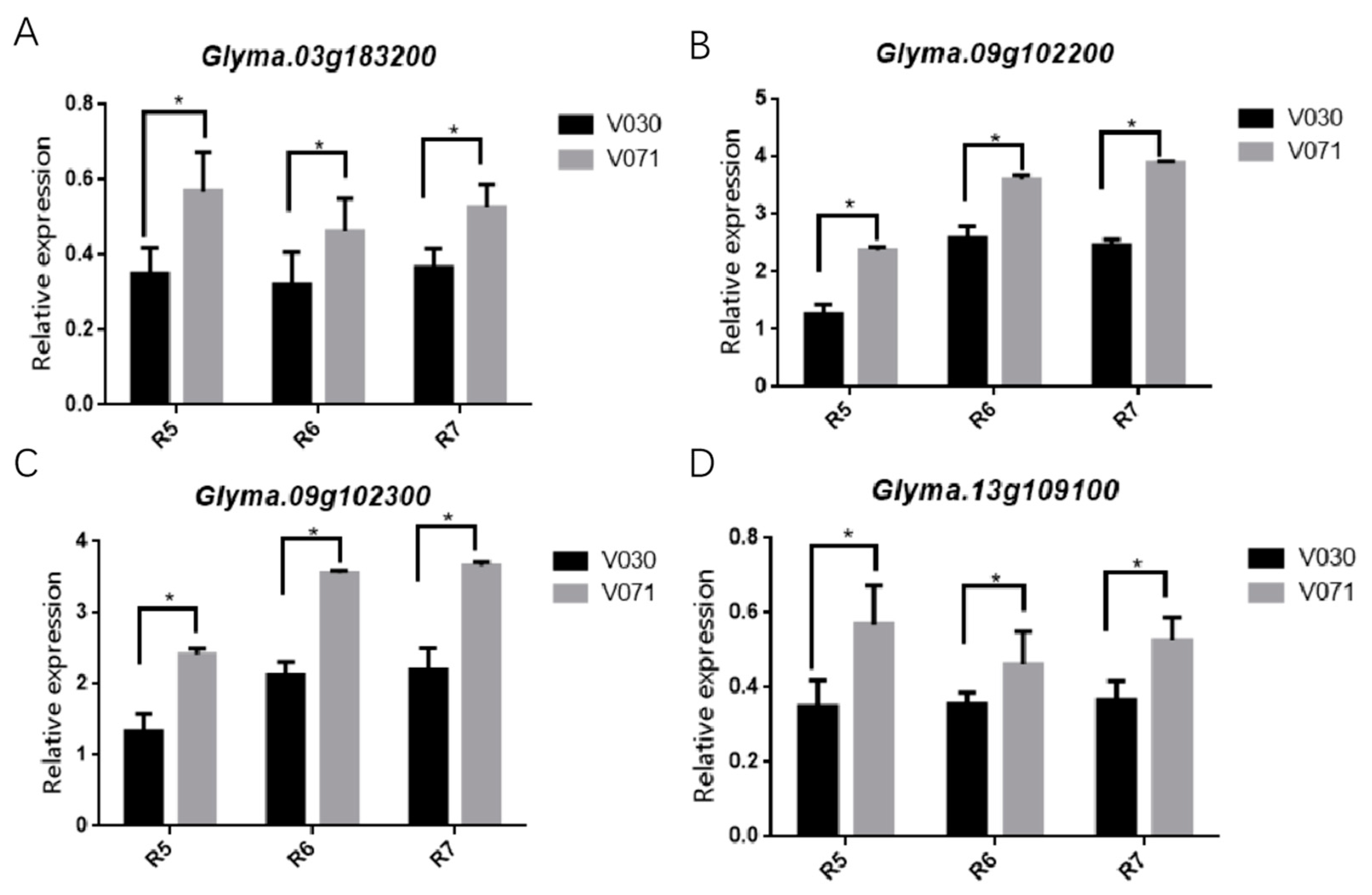
2.5. Different Expression Pattern of Candidate Genes in Pods and Stem
3. Discussion
4. Method
4.1. Plant Material and Field Experiments
4.2. Analysis of Vegetable Soybean Yield-Related Traits
4.3. Genotypic Data
4.4. Population Analysis
4.5. Genome-Wide Association Study and Candidate Gene Analysis
4.6. Real-Time Quantitative Reverse Transcription PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Niu, Y.; Zhang, G.; Feng, Z.; Bo, Y.; Lian, J.; Wang, B.; Gong, Y. Genome sequencing and population resequencing provide insights into the genetic basis of domestication and diversity of vegetable soybean. Hortic. Res. 2022, 9, uhab052. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; He, S.; Wang, C.; Lai, Z.; Lin, D.; Chen, S. A modified roger’s distance algorithm for mixed quantitative-qualitative phenotypes to establish a core collection for Taiwanese vegetable soybeans. Front. Plant Sci. 2021, 11, 612106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lord, N.; Kuhar, T.; Duncan, S.; Huang, H.; Ross, J.; Rideout, S.; Arancibia, R.; Reiter, M.; Li, S.; et al. VT Sweet: A vegetable soybean cultivar for commercial edamame production in the mid-Atlantic USA. J. Plant Regist. 2022, 16, 29–33. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, W.; Zhou, Y.; Ji, P.; Wan, Y.; Shi, S.; Yang, Z.; Gong, Y.; Mu, F.; Chen, S. Integrative analysis of metabolome and transcriptome reveals the improvements of seed quality in vegetable soybean (Glycine max (L.) Merr.). Phytochemistry 2022, 200, 113216. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.; Li, S.; Zhang, W.; Wang, Q.; Zhang, H.; Liu, X.; Cui, X.; Chen, X.; Tang, W.; et al. GWAS and identification of candidate genes associated with seed soluble sugar content in vegetable soybean. Agron. J. 2022, 12, 1470. [Google Scholar] [CrossRef]
- Nair, R.M.; Boddepalli, V.N.; Yan, M.R.; Kumar, V.; Gill, B.; Pan, R.S.; Wang, C.; Hartman, G.L.; Souza, S.E.R.; Somta, P. Global Status of Vegetable Soybean. Plants 2023, 12, 609. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, D.; Sitoe, H.M.; Yin, Z.; Hu, Z.; Zhang, G.; Yu, D. Genetic dissection of the relationship between plant architecture and yield component traits in soybean (Glycine max) by association analysis across multiple environments. Plant Breed 2015, 134, 564–572. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, H.; Chang, H.; Zhao, J.; Teng, W.; Qiu, L.; Li, W.; Han, Y. Genome wide association mapping and candidate gene analysis for hundred seed weight in soybean [Glycine max (L.) Merrill]. BMC Genom. 2019, 20, 648. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Zhu, L.; Bu, Y.; Wang, X.; Zhang, X.; Zhou, Y.; Wang, X.; Guo, N.; Qiu, L.; et al. Genome-wide association study of four yield-related traits at the R6 stage in soybean. BMC Genet. 2019, 20, 39. [Google Scholar] [CrossRef]
- Cao, Y.; Jia, S.; Chen, L.; Zeng, S.; Zhao, T.; Karikari, B. Identification of major genomic regions for soybean seed weight by genome-wide association study. Mol. Breed. 2022, 42, 38. [Google Scholar] [CrossRef]
- Ayalew, H.; Schapaugh, W.; Vuong, T.; Nguyen, H.T. Genome-wide association analysis identified consistent QTL for seed yield in a soybean diversity panel tested across multiple environments. Plant Genome 2022, 15, e20268. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, B.; Jing, Y.; Hu, X.; Guo, Y.; Chen, J.; Liu, Y.; Hao, J.; Li, W.X.; Ning, H. Detecting QTL and Candidate Genes for Plant Height in Soybean via Linkage Analysis and GWAS. Front. Plant Sci. 2022, 12, 803820. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Guo, C.; Sun, F.; Zhang, J.; Wang, Z.; Kong, J.; He, Q.; Sharmin, R.A.; Zhao, T. Genome-Wide Association Studies for Dynamic Plant Height and Number of Nodes on the Main Stem in Summer Sowing Soybeans. Front. Plant Sci. 2018, 20, 1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Fan, X.; Huang, W.; Yang, J.; Wen, Z.; Li, Y.; Guan, R.; Guo, Y.; Chang, R.; et al. Phenotypic characterization and genetic dissection of nine agronomic traits in Tokachi nagaha and its derived cultivars in soybean (Glycine max (L.) Merr.). Plant Sci. 2017, 256, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Boyse, J.F.; Song, Q.; Cregan, P.B.; Wang, D. Genomic consequences of selection and genome-wide association mapping in soybean. BMC Genom. 2015, 16, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Q.; Cregan, P.B.; Jiang, G.L. Genome-wide association study, genomic prediction and marker-assisted selection for seed weight in soybean (Glycine max). Theor. Appl. Genet. 2016, 129, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Assefa, T.; Otyama, P.I.; Brown, A.V.; Kalberer, S.R.; Kulkarni, R.S.; Cannon, S.B. Genome-wide associations and epistatic interactions for internode number, plant height, seed weight and seed yield in soybean. BMC Genom. 2019, 20, 527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, W.; Zhao, X.; Wang, J.; Liu, Z.; Han, Y.; Li, W. Genome-wide association mapping and candidate gene analysis for seed shape in soybean (Glycine max). Crop Pasture Sci. 2019, 70, 684–693. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, W.; Xue, D.; Li, X.; Zhou, Y.; Shen, J.; Feng, J.; Guo, N.; Qiu, L.; Xing, H.; et al. Genome-wide association studies of plant architecture-related traits and 100-seed weight in soybean landraces. BMC Genom. Data 2021, 6, 10. [Google Scholar] [CrossRef]
- Niu, M.; Tian, K.; Chen, Q.; Yang, C.; Zhang, M.; Sun, S.; Wang, X. A multi-trait GWAS-based genetic association network controlling soybean architecture and seed traits. Front. Plant Sci. 2024, 14, 1302359. [Google Scholar] [CrossRef]
- Copley, T.R.; Duceppe, M.O.; O’Donoughue, L.S. Identification of novel loci associated with maturity and yield traits in early maturity soybean plant introduction lines. BMC Genom. 2018, 19, 167. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Xu, W.; Zhang, H.; Liu, X.; Chen, X.; Chen, H. Genome-Wide Association Study and Identification of Candidate Genes Associated with Seed Number per Pod in Soybean. Int. J. Mol. Sci. 2024, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jin, Y.M.; Wu, T.; Hu, L.; Zhang, Y.; Jiang, W.; Du, X. OsDREB2B, an AP2/ERF transcription factor, negatively regulates plant height by conferring GA metabolism in rice. Front. Plant Sci. 2022, 13, 1007811. [Google Scholar] [CrossRef] [PubMed]
- Ravelombola, W.; Qin, J.; Shi, A.; Song, Q.; Yuan, J.; Wang, F.; Chen, P.; Yan, L.; Feng, Y.; Zhao, T.; et al. Genome-wide association study and genomic selection for yield and related traits in soybean. PLoS ONE 2021, 16, e0255761. [Google Scholar] [CrossRef]
- Ojolo, S.P.; Cao, S.; Priyadarshani, S.V.G.N.; Li, W.; Yan, M.; Aslam, M.; Zhao, H.; Qin, Y. Regulation of Plant Growth and Development: A Review from a Chromatin Remodeling Perspective. Front. Plant Sci. 2018, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Jeong, H.J.; Baek, G.; Yang, J.; Peng, X.; Tun, W.; Kim, S.T.; An, G.; Cho, L.H. A VIN3-like Protein OsVIL1 Is Involved in Grain Yield and Biomass in Rice. Plants 2021, 11, 83. [Google Scholar] [CrossRef]
- Yang, J.; Cho, L.H.; Yoon, J.; Yoon, H.; Wai, A.H.; Hong, W.J.; Han, M.; Sakakibara, H.; Liang, W.; Jung, K.H.; et al. Chromatin interacting factor OsVIL2 increases biomass and rice grain yield. Plant Biotechnol. J. 2019, 17, 178–187. [Google Scholar] [CrossRef]
- Wang, T.; Jin, Y.; Deng, L.; Li, F.; Wang, Z.; Zhu, Y.; Wu, Y.; Qu, H.; Zhang, S.; Liu, Y.; et al. The transcription factor MYB110 regulates plant height, lodging resistance, and grain yield in rice. Plant Cell 2024, 36, 298–323. [Google Scholar] [CrossRef]
- Lin, C. Frozen edamame: Global market conditions. In Proceedings of the Second International Vegetable Soybean Conference, Tacoma, WA, USA, 10–12 August 2001; pp. 93–97. [Google Scholar]
- Zhang, Q.; Li., Y.; Chin, K.L.; Qi, Y. Vegetable soybean: Seed composition and production research. Ital. J. Agron. 2017, 12, 3. [Google Scholar] [CrossRef]
- Yano, K.; Yamamoto, E.; Aya, K.; Takeuchi, H.; Lo, P.C.; Hu, L.; Yamasaki, M.; Yoshida, S.; Kitano, H.; Hirano, K.; et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 2016, 48, 927. [Google Scholar] [CrossRef]
- Zeng, T.; Meng, Z.; Yue, R.; Lu, S.; Li, W.; Li, W.; Meng, H.; Sun, Q. Genome wide association analysis for yield related traits in maize. BMC Plant Biol. 2022, 21, 449. [Google Scholar] [CrossRef]
- Jiao, X.; Lyu, Y.; Wu, X.; Li, H.; Cheng, L.; Zhang, C.; Yuan, L.; Jiang, R.; Jiang, B.; Rengel, Z.; et al. Grain production versus resource and environmental costs: Towards increasing sustainability of nutrient use in China. J. Exp. Bot. 2016, 67, 4935–4949. [Google Scholar] [CrossRef]
- Eltaher, S.; Sallam, A.; Belamkar, V.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Poland, J.; Baenziger, P.S. Genetic Diversity and Population Structure of F3:6Nebraska Winter Wheat Genotypes Using Genotyping-By-Sequencing. Front. Genet. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Reif, J.C.; Hong, H.L.; Li, H.H.; Liu, Z.X.; Ma, Y.S.; Li, J.; Tian, Y.; Li, Y.F.; Li, W.B.; et al. Genome-wide association mapping of QTL underlying seed oil and protein contents of a diverse panel of soybean accessions. Plant Sci. 2018, 266, 95–101. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2021, 19, 702–716. [Google Scholar] [CrossRef]
- Qi, X.; Tang, W.; Li, W.; He, Z.; Xu, W.; Fan, Z.; Zhou, Y.; Wang, C.; Xu, Z.; Chen, J.; et al. Arabidopsis-Protein β Subunit AGB1 Negatively Regulates DNA Binding of MYB62, a Suppressor in the Gibberellin Pathway. Int. J. Mol. Sci. 2021, 22, 8270. [Google Scholar] [CrossRef]
- Li, S.; Cao, Y.; Wang, C.; Yan, C.; Sun, X.; Zhang, L.; Wang, W.; Song, S. Genome-wide association mapping for yield-related traits in soybean (Glycine max) under well-watered and drought-stressed conditions. Front. Plant Sci. 2023, 14, 1265574. [Google Scholar] [CrossRef] [PubMed]
- Sonah, H.; O’Donoughue, L.; Cober, E.; Rajcan, I.; Belzile, F. Identification of loci governing eight agronomic traits using a GBS-GWAS approach and validation by QTL mapping in soya bean. Plant Biotechnol. J. 2015, 13, 211–221. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, Y.; Zhao, Y.; Feng, C.; Zhang, Y.; Wang, F.; Li, X.; Gao, H.; Liu, W.; Jing, Y.; et al. Soybean F-Box-Like Protein GmFBL144 Interacts with Small Heat Shock Protein and Negatively Regulates Plant Drought Stress Tolerance. Front. Plant Sci. 2022, 13, 823529. [Google Scholar] [CrossRef]
- Bu, Q.; Lv, T.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H.; et al. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Y.; Luo, W.; Li, W.; Chen, N.; Zhang, D.; Chong, K. The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes, including leaf senescence, in rice. Plant Physiol. 2013, 163, 1673–1685. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, T.; Mao, Y.; Liu, Y.; Guo, S.; Wang, R.; Fang, Y.G.; He, L.; Zhao, B.; Bai, Q.; et al. The F-box protein MIO1/SLB1 regulates organ size and leaf movement in Medicago truncatula. J. Exp. Bot. 2021, 72, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, Y.; Xu, K.; Li, J. Regulatory networks of the F-box protein FBX206 and OVATE family proteins modulate brassinosteroid pathway to regulate grain size and yield in rice. J. Exp. Bot. 2024, 75, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Han, J.H.; Park, Y.I.; Colas des Francs-Small, C.; Small, I.; Kang, H. The mitochondrial pentatricopeptide repeat protein PPR19 is involved in the stabilization of NADH dehydrogenase 1 transcripts and is crucial for mitochondrial function and Arabidopsis thaliana development. New Physiol. 2017, 215, 202–216. [Google Scholar] [CrossRef]
- Cai, M.; Li, S.; Sun, F.; Sun, Q.; Zhao, H.; Ren, X.; Zhao, Y.; Tan, B.C.; Zhang, Z.; Qiu, F. Emp10 encodes a mitochondrial PPR protein that affects the cis-splicing of nad2 intron 1 and seed development in maize. Plant J. 2017, 91, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhang, H.; Sun, G.; Zhang, W.; Qiu, L. Evolution and association analysis of GmCYP78A10 gene with seed size/weight and pod number in soybean. Mol. Biol. Rep. 2015, 42, 489–496. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, Q.; Ren, Y.; Lan, J.; Miao, R.; Feng, M.; Wang, X.; Liu, X.; Zhang, S.; Pan, T.; et al. A CYP78As-small grain4-coat protein complex II pathway promotes grain size in rice. Plant Cell 2023, 35, 4325–4346. [Google Scholar] [CrossRef]
- Zhao, B.; Dai, A.; Wei, H.; Yang, S.; Wang, B.; Jiang, N.; Feng, X. Arabidopsis KLU homologue GmCYP78A72 regulates seed size in soybean. Plant Mol. Biol. 2016, 90, 33–47. [Google Scholar] [CrossRef]
- Guo, L.; Ma, M.; Wu, L.; Zhou, M.; Li, M.; Wu, B.; Li, L.; Liu, X.; Jing, R.; Chen, W.; et al. Modified expression of TaCYP78A5 enhances grain weight with yield potential by accumulating auxin in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2022, 20, 168–182. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Arunachalam, C.; Malla, K.B.; Kahla, A.; Perochon, A.; Jia, J.; Thapa, G.; Doohan, F.M. A wheat cytochrome P450 enhances both resistance to deoxynivalenol and grain yield. PLoS ONE 2018, 3, e0204992. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, Y.; Zhu, J.; Miao, J.; Liu, J.; Liu, Y.; Yi, C.; Yang, Z.; Gong, Z.; Liang, G. GNS4, a novel allele of DWARF11, regulates grain number and grain size in a high-yield rice variety. Rice 2017, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, Y.; Cai, J.; Liu, C.; Zhu, H.; Jiang, R.; Zhong, Y.; Zhang, G.; Tan, B.; Liu, G.; et al. Substitution mapping of QTLs controlling seed dormancy using single segment substitution lines derived from multiple cultivated rice donors in seven cropping seasons. Theor. Appl. Genet. 2017, 130, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [PubMed]
- Sonah, H.; Bastien, M.; Iquira, E.; Tardivel, A.; Légaré, G.; Boyle, B.; Normandeau, É.; Laroche, J.; Larose, S.; Jean, M.; et al. An improved genotyping by sequencing (GBS) approach offering increased versatility and efficiency of SNP discovery and genotyping. PLoS ONE 2013, 8, e54603. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. Structure Harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package) Version 3.6; Department of Genome Sciences, University of Washington: Seattle, WA, USA, 2005. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Sabeti, P.C.; Reich, D.E.; Higgins, J.M.; Levine, H.Z.; Richter, D.J.; Schaffner, S.F.; Gabriel, S.B.; Platko, J.V.; Ptterson, N.J.; Lander, E.S. Detecting recent positive selection in the human genome from haplotype structure. Nature 2002, 419, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, S.S.; Xu, J.Y.; He, W.M.; Yang, T.L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, X.; Zhou, Y.; Summers, R.M.; Zhang, Z. BLINK: A package for the next level of genome-wide association studies with both individuals and markers in the millions. Gigascience 2019, 8, giy154. [Google Scholar] [CrossRef] [PubMed]
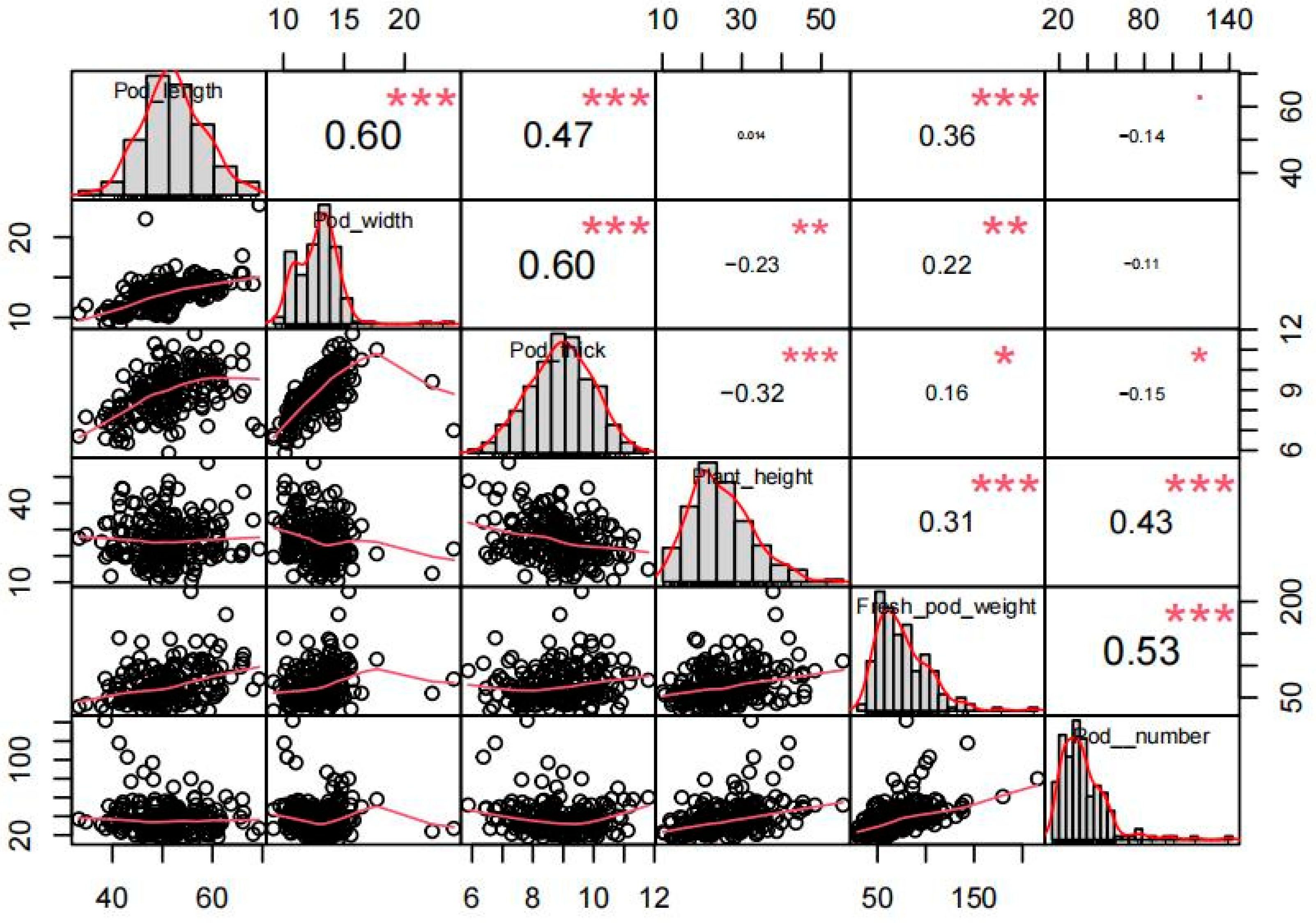

| Trait | Max | Min | Mean | SD | CV (%) |
|---|---|---|---|---|---|
| Pod length (um) | 69.33 | 33.30 | 50.90 | 6.73 | 13.22% |
| Pod width (um) | 24.04 | 9.19 | 12.97 | 1.90 | 14.65% |
| Pod thickness (um) | 11.80 | 5.87 | 8.84 | 1.13 | 12.75% |
| Plant height (cm) | 55.45 | 10.10 | 26.18 | 8.40 | 32.08% |
| Pods number | 80.00 | 5.00 | 9.00 | 2.00 | 19.41% |
| Fresh pod weight (g) | 215.67 | 29.30 | 70.34 | 28.78 | 40.91% |
| Traits | Chr | SNP | −log10(P) | Candidate | Position (bp) | Gene Annotation | Known QTL/Ref. |
|---|---|---|---|---|---|---|---|
| Fresh pod weight | 09 | ss18491673 | 3 × 10−5 | Glyma.09G102300 | 18,200,141–18,203,467 | F-box protein | Seed weight/30-5 [21] |
| Fresh pod weight | 09 | ss18491673 | 3 × 10−5 | Glyma.09G101100 | 18,049,793–18,052,768 | WAT1-related protein | Seed weight/30-5 [21] |
| Fresh pod weight | 09 | ss18491673 | 3 × 10−5 | Glyma.09G101200 | 18,059,406–18,066,433 | Transcriptional regulator SNIP1 | Seed weight/30-5 [21] |
| Fresh pod weight | 09 | ss18491673 | 3 × 10−5 | Glyma.09G101300 | 18,077,704–18,089,366 | Solute carrier family 35, member C2 (SLC35C2) | Seed weight/30-5 [21] |
| Fresh pod weight | 09 | ss18491673 | 3 × 10−5 | Glyma.09G102200 | 18,535,131–18,535,371 | CYP72A154 | Seed weight/30-5 [21] |
| Fresh pod weight | 10 | ss36769025 | 5.73 × 10−5 | Glyma.10G135500 | 36,601,462–36,604,173 | F-box domain | [20] |
| Pod number | 03 | ss39469452 | 1.94 × 10−15 | Glyma.03G182100 | 40,584,252–40,584,617 | Small auxin-up RNA | [22] |
| Pod number | 03 | ss39469452 | 1.94 × 10−15 | Glyma.03G183200 | 40,675,941–40,676,324 | Auxin-responsive family protein | [22] |
| Plant height | 13 | ss22150035 | 3.17 × 10−8 | Glyma.13G107400 | 21,210,684–21,224,769 | Myosin-11-RELATED protein | [24,25] |
| Plant height | 13 | ss22150035 | 3.17 × 10−8 | Glyma.13G109100 | 21,330,744–21,332,237 | MYB-related transcription factors | [24,25,26] |
| Plant height | 07 | ss8350061 | 4.49 × 10−8 | Glyma.07G089000 | 8,151,468–8,158,252 | Vernalization-insensitive protein3 | [23,27,28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wu, G.; Wu, F.; Zhou, X.; Zhou, Y.; Xu, K.; Li, Y.; Zhang, W.; Zhao, K.; Jing, Y.; et al. Genome-Wide Association Analysis of Yield-Related Traits and Candidate Genes in Vegetable Soybean. Plants 2024, 13, 1442. https://doi.org/10.3390/plants13111442
Gao H, Wu G, Wu F, Zhou X, Zhou Y, Xu K, Li Y, Zhang W, Zhao K, Jing Y, et al. Genome-Wide Association Analysis of Yield-Related Traits and Candidate Genes in Vegetable Soybean. Plants. 2024; 13(11):1442. https://doi.org/10.3390/plants13111442
Chicago/Turabian StyleGao, Hongtao, Guanji Wu, Feifei Wu, Xunjun Zhou, Yonggang Zhou, Keheng Xu, Yaxin Li, Wenping Zhang, Kuan Zhao, Yan Jing, and et al. 2024. "Genome-Wide Association Analysis of Yield-Related Traits and Candidate Genes in Vegetable Soybean" Plants 13, no. 11: 1442. https://doi.org/10.3390/plants13111442
APA StyleGao, H., Wu, G., Wu, F., Zhou, X., Zhou, Y., Xu, K., Li, Y., Zhang, W., Zhao, K., Jing, Y., Feng, C., Wang, N., & Li, H. (2024). Genome-Wide Association Analysis of Yield-Related Traits and Candidate Genes in Vegetable Soybean. Plants, 13(11), 1442. https://doi.org/10.3390/plants13111442






