A Decade after the Outbreak of Xylella fastidiosa subsp. pauca in Apulia (Southern Italy): Methodical Literature Analysis of Research Strategies
Abstract
1. Introduction
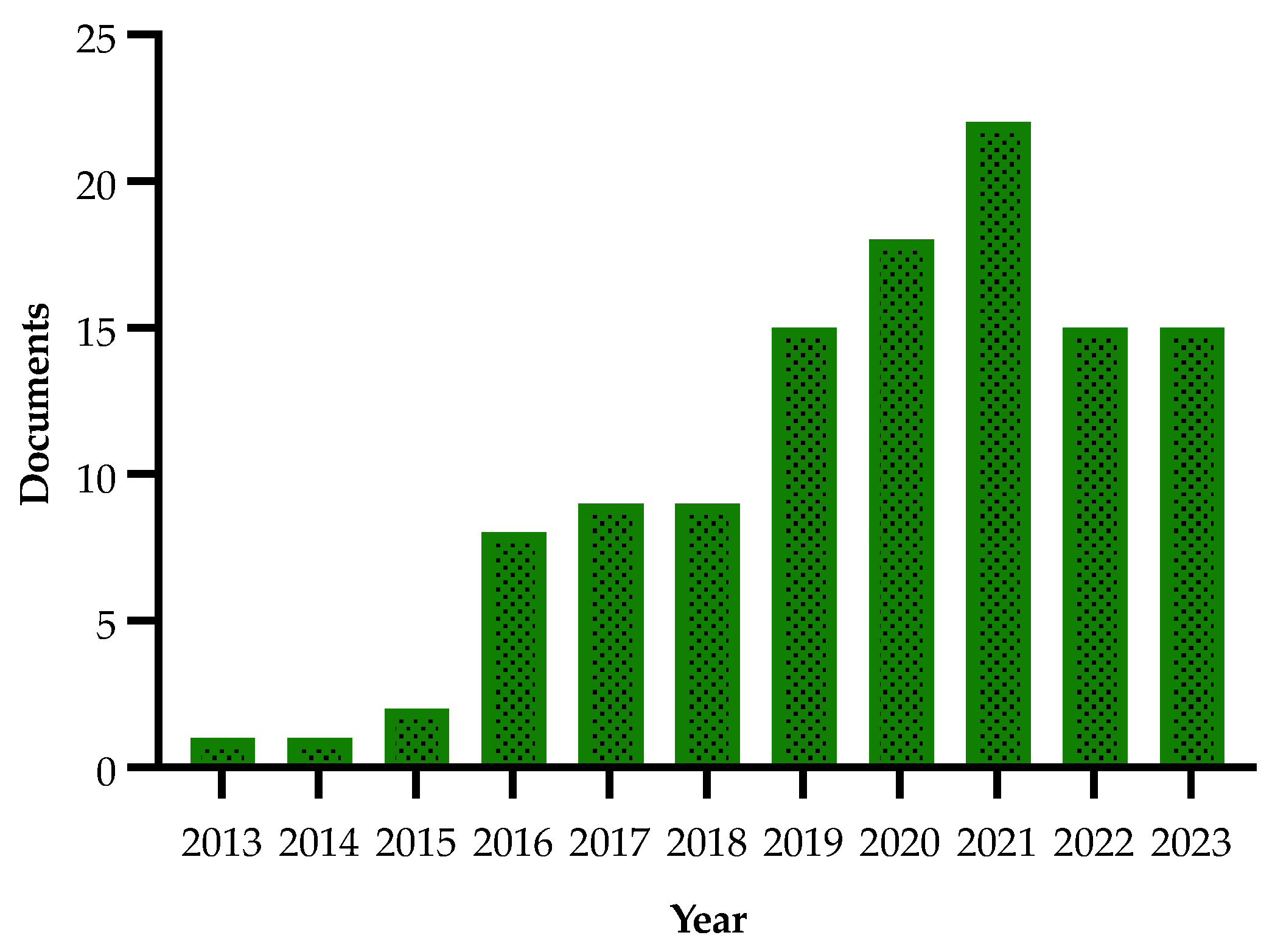
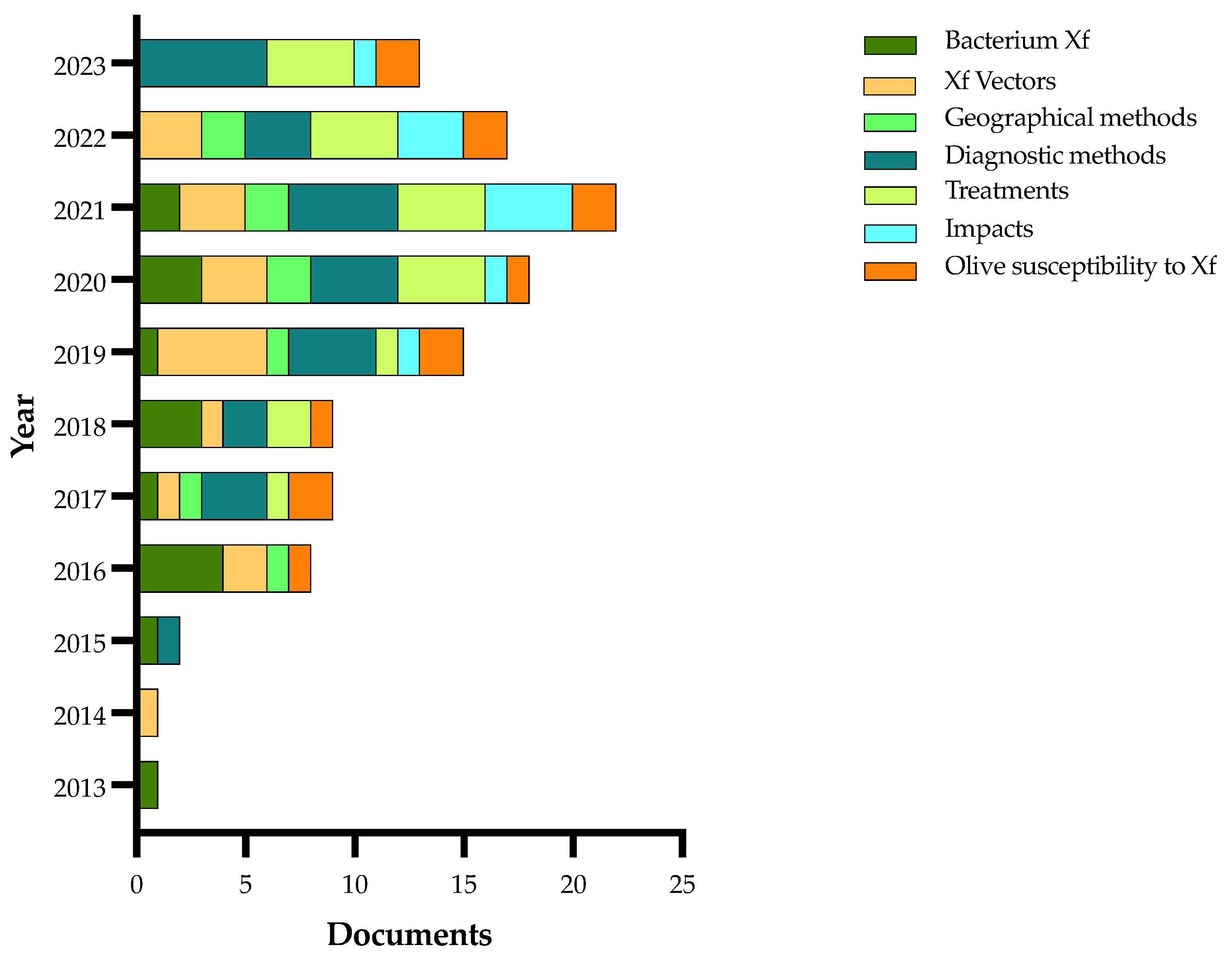
2. Results and Discussion
2.1. Xylella fastidiosa, the Bacterium
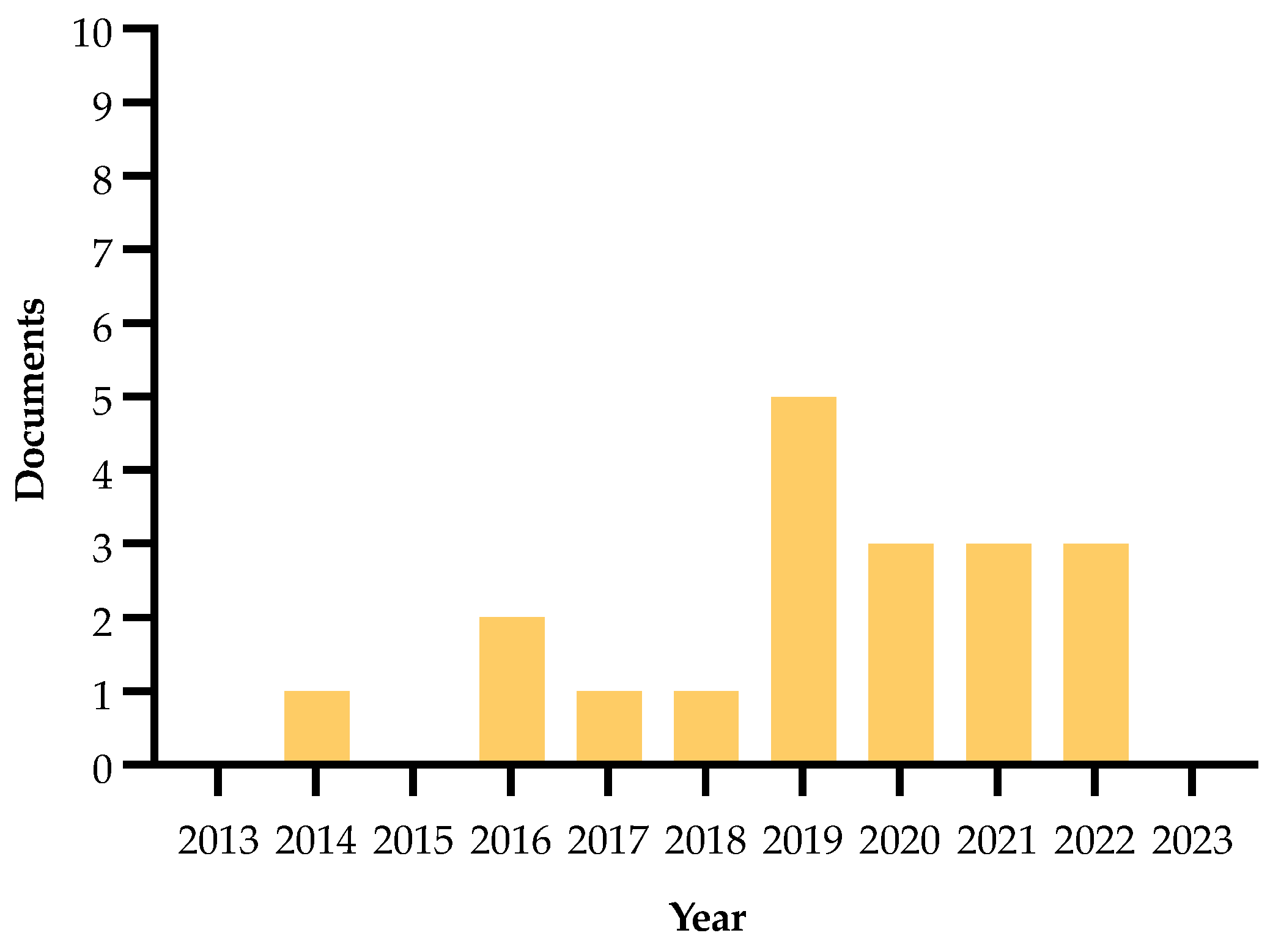
2.2. Xylella fastidiosa Vectors
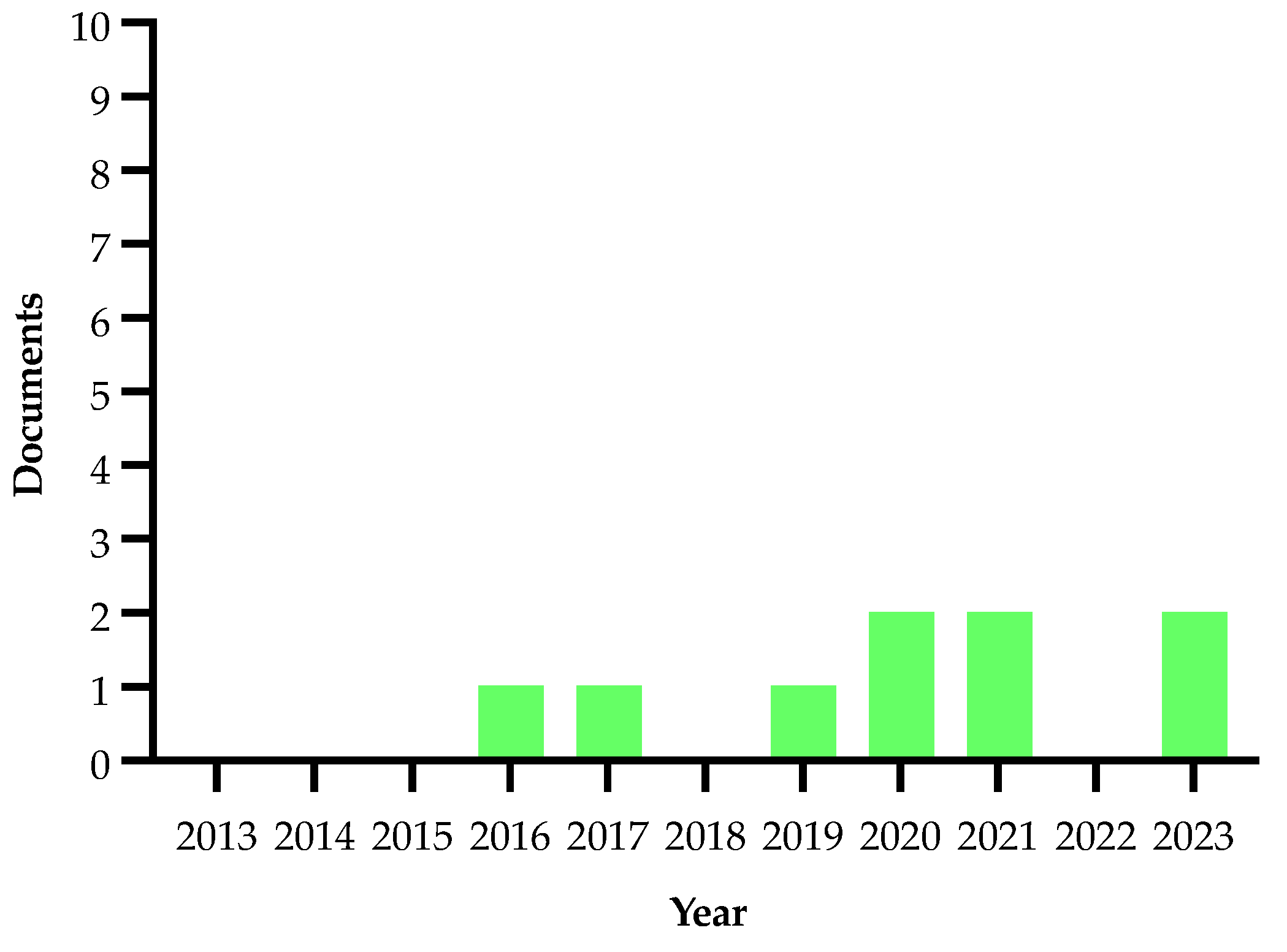
2.3. Geographical Methods
| Authors and Year | Study Area | Method | Main Findings |
|---|---|---|---|
| Bosso et al., 2016 [91] | Italian territory between latitudes 45° N and 36° N and longitudes 6° E and 18° E | Maxent ver. 3.3.3k to model the potential distribution of Xf in Italy | Species distribution models showed a high probability of Xf occurrence in the regions of Apulia, Calabria, Basilicata, Sicily, Sardinia and coastal areas of Campania, Lazio and southern Tuscany |
| White et al., 2017 [93] | Apulia | Gompertz equation | The model highlights the importance of non-olive hosts which increase the spread rate of the disease and may lead to an order of magnitude increase in risk |
| Bucci, 2019 [92] | Apulian olive orchards | Analysis of the infection monitoring data set available by the Apulian regional government | Yearly epidemic spread 1–15 km from olive trees labeled as infected |
| White et al., 2020 [94] | Apulian olive groves | Epidemiological model | Through a Bayesian method, tree desiccation was estimated to occur approximately 4.3 years after symptom appearance |
| Cendoya et al., 2020 [96] | Apulia | Bayesian hierarchical models through the integrated nested Laplace approximation (INLA) methodology | This substantial contribution of the spatial effect in the models might indicate that the current extent of Xf in the study regions arose from a single focus or from several foci, which had coalesced |
| Castrignanò et al., 2021 [95] | An olive grove located in Oria (province of Brindisi, Southern Italy) | Data fusion procedure, based on non-parametric multivariate geostatistics | Remote and proximal sensor data with visual inspections and plant diagnostic tests provided a probabilistic map of Xf infection risk |
| Kottelenberg et al., 2021 [97] | Apulian olive groves | Deterministic model (a negative exponential function, a logistic function or a CNE function) | The model indicates that the disease spread started, approximately, in 2008 and the estimated rate of movement of the disease was 10.0 km per year |
| Gilioli et al., 2023 [98] | Apulian olive groves | Eco-epidemiological Model (XEM) | The model obtained described the infection dynamics of Xf outbreaks and showed that the abundance of the vector is the key factor determining the spread rate of the pathogen |
| Bajocco et al., 2023 [99] | Apulian olive groves | Ecological niche model | The anthropogenic component significantly contributed to the epidemic, with the road system representing the main driver of diffusion and natural/seminatural areas hampering Xf spread at the landscape scale |
2.4. Diagnostic Methods
| Authors and Year | Methods | Aim of Diagnostic Tool | Main Findings |
|---|---|---|---|
| Yaseen et al., 2015 [102] | Real-time LAMP (real-time loop-mediated isothermal amplification) | Detecting on-site real-time Xf in host plants and insects | Real-time LAMP procedure displaying the advantages of an on-site detection method of easy handling, rapid execution and low cost |
| De Benedictis et al., 2017 [105] | Genotype identification and microscopy analysis | Identifying resistance mechanisms that can be exploited to prolong the productivity of olive orchards in the infected areas | They have shown that the interaction between Xf and O. europaea’s xylem vessels supports the indication of a secondary role for the pathogen in the occlusion process, where symptoms were correlated to a physiological response of the plant |
| Luvisi et al., 2017 [27] | Molecular analysis | Selecting markers to determine whether or not there is symptom progression | Differences in the induced responses of quinic acid among four olive cultivars (Cellina di Nardò, Ogliarola di Lecce, Frantoio and Leccino) suggest that they play defensive roles in olive tree response to Xf infection |
| Girelli et al., 2017 [117] | NMR spectroscopy | Metabonomics to explore complicated metabolic patterns linked with an organism’s response to physiological or pathological events | The changes in the metabolomic profiles obtained from Ogliarola salentina and Cellina di Nardò are reported upon the DENTAMET® treatments |
| Sabella et al., 2018 [107] | Molecular and phenolic analyses | Understanding the processes contributing to the putative Xf tolerance of olive trees cv. Leccino | Results suggest a critical role for lignin in Xf tolerance of cv. Leccino, since the quantification of lignin in healthy and infected branches of both cultivars showed a significant increase in total lignin in infected Leccino compared with that in the sensitive cultivar |
| Rey et al., 2018 [108] | Sensing technologies and field tests | Mapping the distribution of plant diseases for early detection of Xf in olive groves at plant to leaf levels | They developed a small low-cost field robot capable of inspecting the whole field continuously, capturing geolocated spectral information and the structure of the trees for later comparison with the in situ observations |
| Scortichini et al., 2019 [109] | Real-time PCR and inductively coupled plasma atomic emission spectroscopy (ICP-AES) | Management strategy aiming at reducing the spread of the bacterium through the study of the element content and availability in the soil and in the host leaves | They indicated that Xf. subsp. pauca infection causes a depletion of copper within olive leaves |
| Sabella et al., 2019 [103] | SEM-EDX analysis and molecular analysis | Evaluating cavitation susceptibility and activation of refilling mechanisms to restore hydraulic conductivity in olive plants subjected to Xf infection | They indicated that resulted gene expression patterns suggested that the infected plants of the cultivar Leccino strongly modulate the genes involved in embolism sensing and refilling |
| Manici et al., 2019 [110] | Molecular analysis (DGGE analysis) | Improving the decisional tools for selecting disease-decimated groves to be replaced with new olive trees | Multiple correlation and canonical correspondence analyses led to identification of a series of soil physical and fungal indicators, which were linearly correlated with the Xf-infected area |
| Vergine et al., 2019 [111] | Molecular and sequence analysis | Improving biological treatment of OQDS | The maintenance of a healthy microbiota with higher diversity and the presence of cultivar-specific microbes might support the resistance of “Leccino” to Xf |
| Castrignanò et al., 2020 [115] | Unmanned aerial vehicle (UAV) with a multispectral radiometer | Early detection of infection | The results encourage the application of UAV technology for the early detection of Xf infection |
| Di Nisio et al., 2020 [114] | Unmanned aerial vehicle (UAV) with multispectral imaging | Early detection of infection | Image processing of high-resolution visible and multispectral images acquired by a purposely equipped multirotor unmanned aerial vehicle (UAV) is proposed for fast detection of Xf symptoms in olive trees |
| Hornero et al., 2020 [116] | 3D radiative transfer modelling (3D-RTM) and Sentinel-2 satellite data | Early detection of infection | This study emphasizes the value of detecting anomalies in vegetation health by interpreting temporal variations in model retrievals |
| Poblete et al., 2020 [113] | Hyperspectral and thermal imagery | Early detection of infected hosts | Results of this study demonstrate that multispectral and thermal cameras can be used for large-scale monitoring of Xf-infected areas |
| Asteggiano et al., 2021 [121] | Liquid chromatography separation and high-resolution mass spectrometry detection | Identification of molecular markers to discriminate between healthy and infected olive tree leaves | Results obtained via multivariate analysis through an HPLC-ESI HRMS platform show a clear separation between healthy and infected samples |
| Faino et al., 2021 [120] | OXford Nanopore Technologies (ONT) MinION platform | Detection and identification of Xf from infected plant material | The results pave the way for novel opportunities for Nanopore sequencing as an effective surveillance tool for early detection of Xf |
| Jlilat et al., 2021 [118] | NMR spectroscopy and MS spectrometry with a non-targeted approach | Describing a set of metabolites playing a possible role as markers in the infections by Xf in olives | This study revealed that Xylella-infected plants were characterized by higher amounts of malic acid, formic acid, mannitol and sucrose than in Xf-non-infected ones |
| Riefolo et al., 2021 [123] | Real-time PCR, hyperspectral analysis and partial least square regression (PLSR) | Improving diagnostic assessment of plants and evaluation of their phytosanitary status | Using only spectral data, it is possible to discriminate the infected plants at a very early stage of infection, saving time and financial resources |
| Pavan et al., 2021 [122] | Molecular analysis | Identifying genotypes putatively resistant to Xf | Some of the putatively resistant plants (Leccino) identified in this study might be exploited in cultivation or as parental clones of breeding programs |
| D’Onghia et al., 2022 [124] | Molecular and serological tests (qPCR, real-time LAMP, DAS-ELISA, DTBIA) | Early detection of infection | This study was conducted to compare molecular and serological tests for detection of Xf using stem xylem tissue as the most appropriate matrix for testing |
| Di Masi et al., 2022 [119] | High-performance liquid chromatography and quadrupole-time-of-flight high-resolution mass spectrometry (HPLCESI-Q-TOF-MS) | Discovering disease-associated biomarkers in plants | This study suggests there is a decrease in the defense capabilities of the host after Xf infection due to a significant dysregulation of some metabolites belonging to the flavonoid family |
| Montilon et al., 2022 [125] | Electron microscopy analysis | Early detection of infection | Their study suggests that exploitation of pit membranes is a key event in the infection process of Xf subsp. pauca ST53 in susceptible olive cultivars |
| Amoia et al., 2023 [129] | Isothermal amplification and colorimetric LAMP | Improving the monitoring and containment of the disease spread | A portable, sensitive and target-specific Xf field test was developed, which has a 40 min sample-to-answer time and does not require any DNA isolation procedure or laboratory equipment |
| Belmonte et al., 2023 [128] | Unmanned aerial vehicles (UAVs) | Implementing the automatic detection of infected plants in the early stages | The work has shown the potential of data from unmanned aerial vehicles in Xylella control, but many problems still need to be solved for the automatic detection of infected plants in the early stages |
| Blonda et al., 2023 [130] | High-resolution (HR) Sentinel-2 images and very-high-resolution (VHR) Pléiades images | Implementing the automatic detection of infected plants in the early stages | HR image data could be used to evaluate plant conditions at field level after treatments, while VHR imagery could be used to optimize treatment doses per cultivar |
| Greco et al., 2023 [127] | Molecular test (MLST) and phylogenetic analysis, FISH (fluorescence in situ hybridization) together with a CLSM analysis (confocal laser scanning microscope) | A diagnostic investigation for Xf is carried out on several small groups of chestnut trees in Salento, coupled with the observation of any visible symptoms | This work shows how knowledge of all host species, including the pauci-symptomatic and asymptomatic ones, and of the vectors present in a given area, is essential to make containment measures truly effective |
| Savoia et al., 2023 [131] | Molecular test (qPCR, genotyping) | Search for new genotypes that are tolerant or resistant to Xf | Identification of nine putatively resistant genotypes that represent the first panel of olive germplasm resources |
| Ciervo and Scortichini, 2023 [132] | Molecular and serological tests (PCR, ELISA) | Eliminate the rule requiring the uprooting of all host plants that surround one Xfp-positive tree in a radius of 50 m | They suggested that the rule requiring the uprooting of all host plants that surround one Xfp-positive tree in a radius of 50 m in the “containment” and “buffer” zones could be eliminated |
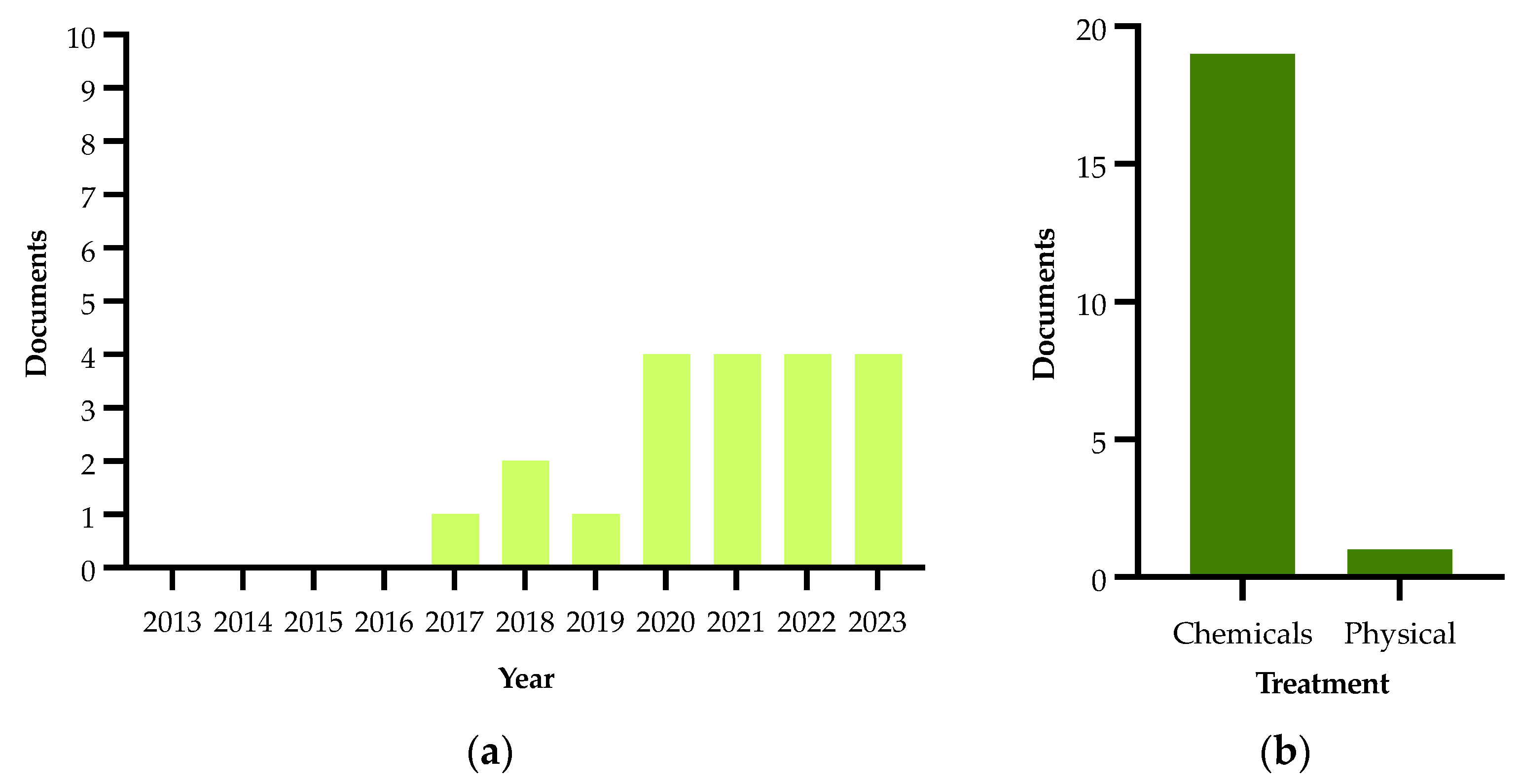
2.5. Treatments
| Authors and Year | Methods | Treatment Type | Main Findings |
|---|---|---|---|
| Girelli et al., 2017 [117] | NMR spectroscopy | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | In this study, the changes in the metabolomic profiles obtained from leaves of Ogliarola salentina and Cellina di Nardò are reported |
| Scortichini et al., 2018 [135] | Molecular analysis (quantitative real-time PCR), confocal laser scanning microscopy, fluorescent quantification and atomic emission spectroscopy | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | In this study, a 3-year field trial with Dentamet® in an olive orchard containing Cellina di Nardò and Ogliarola salentina olive trees was carried out. The data revealed a statistically significant reduction of Xf cell densities within the leaves of treated trees |
| Bleve et al., 2018 [137] | HPLC-DAD analysis and agar disk diffusion method | In vitro with agar disk diffusion method | In this study, in vitro antimicrobial activities of different classes of compounds against ST53 were evaluated |
| Girelli et al., 2019 [136] | NMR spectroscopy | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | A consistent increase in malic acid was observed for the Ogliarola salentina trees, whereas in the Cellina di Nardò trees, the treatments attenuate the metabolic response to the infection |
| Baldassarre et al., 2020 [142] | Toxicological study and antibacterial activity | In vitro characterization and analysis of Fosetyl-Al Nanocrystals | Results of in vitro assays (dosage and administration modality) suggest the possible use of this new nanoformulation in an integrated pest management strategy for an in-filed control of Xfp |
| Del Coco et al., 2020 [139] | Inductively coupled plasma atomic emission spectroscopy (ICP-AES) and molecular analysis (real-time PCR) | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | They observed that soil and leaf ionomic composition of olive farms growing in the pathogen-free areas north of the Salento and Potenza provinces is significantly different from that shown by the infected olive groves of the Salento areas |
| Liccardo et al., 2020 [141] | Numerical simulation | In vitro | They proposed an available natural enemy of Philaenus spumarius, i.e., Zelus renardii, for adult vector population and infection biocontrol, with a reduction in the pathogen incidence below 10% |
| Zicca et al., 2020 [140] | Molecular analysis | In vitro | The identification of antagonistic bacteria potentially deployable as biocontrol agents against Xf |
| Tatulli et al., 2021 [143] | In vitro antibacterial assays and molecular analysis (real-time PCR and qRT-PCR) | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | The compound showed in vitro bactericidal activity and inhibited the biofilm formation in representative strains of Xf on field, in the Xf-sensitive cultivars Ogliarola salentina and Cellina di Nardò or in the Xfp-resistant Leccino |
| Bruno et al., 2021 [145] | Molecular analysis (PCR and quantitative real-time PCR) | On field with active principle—NuovOlivo®, a natural detergent made from plants oils and extracts of multi botanical species plus sodium and calcium hydroxide and sulphur, activated with sodium bicarbonate | The leaves of treated plants showed a low total phenolic content and no cell membrane damage associated with lipid peroxidation and electrolyte leakage; therefore, NuovOlivo® works as a curative product limiting and/or stopping the destructive epidemic caused by this bacterium |
| Fausto et al., 2021 [146] | Gas chromatography–mass spectrometry | Trials were carried out, in vitro, in two olive groves, one organically and one conventionally managed (controls), successively both converted to sustainable management | Many of the compounds with increased levels under sustainable management have a well-known role as osmoprotectants or are involved in plant defense |
| Girelli et al., 2021 [144] | NMR spectroscopy | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | They highlighted a specificity in the metabolic response of the tolerant Leccino compared to susceptible cultivars |
| Scala et al., 2022 [147] | High-performance liquid chromatography–mass spectrometry–MS-based targeted lipidomics with supervised learning algorithms | On field with active principle—DENTAMET®, a CE-approved fertilizer containing zinc, copper and citric acid | They built classifiers using the relative differences in lipid species able to discriminate olive tree samples, infected and non-infected, belonging to different cultivars, and treated or untreated with DENTAMET® |
| Camposeo et al., 2022 [152] | Molecular analysis (quantitative real-time PCR) | On field with physical treatment—pruning | They set up a field trial to assess if pruning interventions could limit and/or recover Xf-infected trees by reducing the systemic spread of the bacterium but, no significant amelioration of the sanitary status of the infected olive trees was recorded |
| Ambrico et al., 2022 [148] | Emission spectroscopy and direct application of a surface dielectric barrier discharge (SDBD) plasma on bacteria cells and plasma-activated water (PAW) | In vitro to test the biocidal effect of the direct application of SDBD plasma on bacteria cells and PAW | They investigated the efficacy of the low-temperature plasma and plasma-activated water to kill bacterial cells and the results showed a high decontamination rate even for cells of Xf embedded in biofilms |
| Girelli et al., 2022 [149] | Agro-active endo-therapy and NMR spectroscopy | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | Metabolomics approach showed a significant decrease in both the disease biomarker with simultaneous increase in polyphenols and oleuropein in Ogliarola salentina and Cima di Melfi olive trees |
| Baldassarre et al., 2023 [150] | In vitro inhibition assay | In vitro | They reported the great antibacterial effect of thymol on Xf, suggesting the potential application of thymol-nanoparticles as effective biocides to control Xf infection |
| Hussain et al., 2023 [151] | Trunk injection and foliar spray treatments of infected olive trees and NMR spectroscopy | On field with active principle—Dentamet®, a CE-approved fertilizer containing zinc, copper and citric acid | They reported the medium-term effects of foliar spray and endotherapy treatments with different doses of Dentamet® in Xf-infected olive trees cvs Ogliarola salentina and Cellina di Nardò and the best results were found for monthly endotherapy treatments |
| Vizzarri et al., 2023 [153] | Endotherapeutic trial using a phenolic extract in comparison with a solution based on garlic powder and potassium phosphite | In vitro with a solution based on garlic powder and potassium phosphite | The use of phenolic extracts also gave better results in comparison to analogous treatments based on garlic powder and potassium phosphite |
| Orfei et al., 2023 [154] | In vitro antimicrobial activity and effect on bacterial biofilm formation and disruption | In vitro with electrochemically synthesized silver ultra nanoclusters ARGIRIUM-SUNCs® | ARGIRIUM-SUNC has strong antimicrobial activities against phytopathogenic bacteria and inhibits biofilm formation at low doses |
2.6. Impacts on the Environment, Man and Society
2.7. Olive Germplasm Susceptibility to Xf
2.8. Research Strategies Analysis
3. Methods
Data Collection and Data Elaboration
- -
- Authors;
- -
- Publication year;
- -
- Country of publication;
- -
- Research strategy;
- -
- Key conclusion.
- -
- Studies involving genetic and genomic features of Xylella fastidiosa subsp. Pauca;
- -
- Studies involving the biology and spread of the vector P. Spumarius;
- -
- Studies based on geographical and epidemiological methods;
- -
- Studies based on the use of diagnostic techniques;
- -
- Studies based on in vitro assays and treatments against infected plants;
- -
- Studies on Xylella fastidiosa subsp. Pauca impact on the environment and society;
- -
- Studies on olive germplasm susceptibility to Xylella fastidiosa subsp. pauca.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. One Health Joint Plan of Action (2022–2026): Working together for the Health of Humans, Animals, Plants and the Environment. Available online: https://www.who.int/publications/i/item/9789240059139 (accessed on 15 November 2023).
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). Plant Pathol. J. 2013, 95, 668. [Google Scholar]
- EFSA. Statement of EFSA on host plants, entry and spread pathways and risk reduction options for Xylella fastidiosa Wells et al. EFSA J. 2013, 11, 3468. [Google Scholar]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2015, 13, e3989. [Google Scholar] [CrossRef]
- EFSA. Update of a database of host plants of Xylella fastidiosa: 20 November 2015. EFSA J. 2016, 14, 4378. [Google Scholar]
- Elbeaino, T.; Valentini, F.; Abou Kubaa, R.; Moubarak, P.; Yaseen, T.; Digiaro, M. Multilocus sequence typing of Xylella fastidiosa isolated from olive affected by “olive quick decline syndrome” in Italy. Phytopathol. Mediterr. 2014, 53, 533–542. [Google Scholar]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Porcelli, F. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. J. Appl. Entomol. 2017, 141, 80–87. [Google Scholar] [CrossRef]
- Cornara, D.; Saponari, M.; Zeilinger, A.R.; de Stradis, A.; Boscia, D.; Loconsole, G.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Strona, G.; Carstens, C.J.; Beck, P. Network analysis reveal why Xylella fastidiosa will persist in Europe. Sci. Rep. 2017, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, D. Le Vegetazioni Erbacee e gli Arbusteti Italiani-Tipologie Fitosociologiche ed Ecologia, 3rd ed.; Aracne Editrice Internazionale: Roma, Italy, 2019; p. 336. [Google Scholar]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Jaques Miret, J.A.; Fejer Justesen, A.; MacLeod, A.; Magnusson, C.S.; Milonas, P.; et al. Effectiveness of in planta control measures for Xylella fastidiosa. EFSA J. 2019, 17, e05666. [Google Scholar]
- Manceau, C. Disease outbreaks caused by Xylella fastidiosa in Europe are due to multiple introductions. Plant Pathol. J. 2016, 98, 13. [Google Scholar]
- European Food Safety Authority (EFSA); Delbianco, A.; Gibin, D.; Pasinato, L.; Boscia, D.; Morelli, M. Update of the Xylella spp. host plant database–systematic literature search up to 30 June 2022. EFSA J. 2023, 21, e07726. [Google Scholar] [PubMed]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Parnell, S. Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, e05665. [Google Scholar]
- Scortichini, M.; Manetti, G.; Brunetti, A.; Lumia, V.; Sciarroni, L.; Pilotti, M. Xylella fastidiosa subsp. pauca, Neofusicoccum spp. and the decline of olive trees in Salento (Apulia, Italy): Comparison of symptoms, possible interactions, certainties and doubts. Plants 2023, 12, 3593. [Google Scholar] [CrossRef]
- Maggiotto, G.; Colangelo, G.; Milanese, M.; de Risi, A. Thermochemical Technologies for the Optimization of Olive Wood Biomass Energy Exploitation: A Review. Energies 2023, 16, 6772. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saponari, M.; Bosco, D. Bioecological traits of spittlebugs and their implications for the epidemiology and control of the Xylella fastidiosa epidemic in Apulia (Southern Italy). Phytopathology 2023, 113, 1647–1660. [Google Scholar] [CrossRef]
- Trkulja, V.; Tomić, A.; Iličić, R.; Nožinić, M.; Milovanović, T.P. Xylella fastidiosa in Europe: From the introduction to the current status. Plant Pathol. J. 2022, 38, 551. [Google Scholar] [CrossRef]
- Scortichini, M. The epidemiology and control of “olive quick decline syndrome” in Salento (Apulia, Italy). Agronomy 2022, 12, 2475. [Google Scholar] [CrossRef]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Mariconda, A.; Scali, E.; Bonomo, M.G.; Saturnino, C.; Longo, P.; Aquaro, S.; Sinicropi, M.S. Thidiazuron: New Trends and Future Perspectives to Fight Xylella fastidiosa in Olive Trees. Antibiotics 2022, 11, 947. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S.; et al. Xylella fastidiosa in Olive: A Review of Control Attempts and Current Management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Scortichini, M. The Multi-Millennial Olive Agroecosystem of Salento (Apulia, Italy) Threatened by Xylella Fastidiosa Subsp. Pauca: A Working Possibility of Restoration. Sustainability 2020, 12, 6700. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Colella, C. Problem setting and problem solving in the case of olive quick decline syndrome in Apulia, Italy: A sociological approach. Phytopathology 2019, 109, 187–199. [Google Scholar] [CrossRef]
- Cornara, D.; Garzo, E.; Morente, M.; Moreno, A.; Alba-Tercedor, J.; Fereres, A. EPG combined with micro-CT and video recording reveals new insights on the feeding behavior of Philaenus spumarius. PLoS ONE 2018, 13, e0199154. [Google Scholar] [CrossRef]
- Luvisi, A.; Aprile, A.; Sabella, E.; Vergine, M.; Nicoli, F.; Nutricati, E.; De Bellis, L. Xylella fastidiosa subsp. pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: Profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathol. Mediterr. 2017, 56, 259–273. [Google Scholar]
- Almeida, R.P.P. Xylella fastidiosa vector transmission biology. In Vector-Mediated Transmission of Plant Pathogens; American Phytopathological Society Press: Berkeley, CA, USA, 2016; pp. 165–174. [Google Scholar]
- Ciervo, M. The olive quick decline syndrome (OQDS) diffusion in Apulia region: An apparent contradiction according to the agricultural model. Belgeo 2016, 4. [Google Scholar] [CrossRef]
- Pautasso, M.; Petter, F.; Rortais, A.; Roy, A.S. Emerging risks to plant health: A European perspective. CABI Rev. 2015, 1–16. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: A R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, A.; LoPs, F.; Marchi, G.; Mugnai, L.; Surico, G. Has Xylella fastidiosa “chosen” olive trees to establish in the Mediterranean basin? Phytopathol. Mediterr. 2013, 52, 541–544. [Google Scholar]
- Sportelli, G.F. Malattia dell’Olivo nella baia di Gallipoli, mistero da risolvere. Terra E Vita 2013, 39, 25. [Google Scholar]
- Nigro, F.; Boscia, D.; Antelmi, I.; Ippolito, A. Fungal species associated with a severe decline of olive in southern Italy. Plant Pathol. J. 2013, 95, 688. [Google Scholar]
- EPPO Global Database. Distribution of Xylella Fastidiosa (XYLEFA). Available online: https://gd.eppo.int/taxon/XYLEFA/distribution (accessed on 12 April 2023).
- EPPO A1 List of Pests Recommended for Regulation as Quarantine Pests. 2023. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list (accessed on 18 June 2023).
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov.: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Bacteriol. 1987, 37, 136. [Google Scholar] [CrossRef]
- Cariddi, C.; Saponari, M.; Boscia, D.; De Stradis, A.; Loconsole, G.; Nigro, F.; Martelli, G.P. Isolation of a Xylella fastidiosa strain infecting olive and oleander in Apulia, Italy. Plant Pathol. J. 2014, 96, 1–5. [Google Scholar]
- Frisullo, S.; Camele, I.; Agosteo, G.E.; Boscia, D.; Martelli, G.P. Brief historical account of olive leaf scorch (“brusca”) in the Salento peninsula of Italy and state-of-the-art of the olive quick decline syndrome. Plant Pathol. J. 2014, 96, 441–449. [Google Scholar]
- Regione Puglia. Deliberazione della Giunta Regionale 29 ottobre 2013, n. 2023. Misure di Emergenza per la Prevenzione, il Controllo e la Eradicazione del Batterio da quarantena Xylella Fastidiosa associato al “Complesso del Disseccamento Rapido dell’Olivo”. Available online: http://www.emergenzaxylella.it/portal/portale_gestione_agricoltura/Documenti/normRegionale/PortalXylellaNormativaRegionaleIstanceWindow?IDNEWS=58&action=e&windowstate=normal&mode=view&ACTION_NEWS=DETAIL (accessed on 11 April 2024).
- Loconsole, G.; Potere, O.; Boscia, D.; Altamura, G.; Djelouah, K.; Elbeaino, T.; Frasheri, D.; Lorusso, D.; Palmisano, F.; Pollastro, P.; et al. Detection of Xylella fastidiosa in olive trees by molecular and serological methods. Plant Pathol. J. 2014, 96, 7–14. [Google Scholar]
- Nunney, L.; Ortiz, B.; Russell, S.A.; Ruiz Sanchez, R.; Stouthamer, R. The Complex Biogeography of the Plant Pathogen Xylella fastidiosa: Genetic Evidence of Intro ductions and Subspecific Introgression in Central America. PLoS ONE 2014, 9, e112463. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Chiumenti, M.; Saponari, M.; Donvito, G.; Italiano, A.; Loconsole, G.; Saldarelli, P. Draft genome sequence of the Xylella fastidiosa CoDiRO strain. Genome. Announc. 2015, 3, e01538-14. [Google Scholar] [CrossRef] [PubMed]
- Mang, S.M.; Frisullo, S.; Elshafie, H.S.; Camele, I. Diversity evaluation of Xylella fastidiosa from infected olive trees in Apulia (Southern Italy). Plant Pathol. J. 2016, 32, 102. [Google Scholar] [CrossRef]
- Francis, M.; Lin, H.; Cabrera-La Rosa, J.; Doddapaneni, H.; Civerelo, E.L. Genome-based PCR primers for specific and sensitive detection and quantification of Xylella fastidiosa. Eur. J. Plant Pathol. 2006, 115, 203–213. [Google Scholar] [CrossRef]
- Loconsole, G.; Saponari, M.; Boscia, D.; D’Attoma, G.; Morelli, M.; Martelli, G.P.; Almeida, R.P.P. Intercepted isolates of Xylella fastidiosa in Europe reveal novel genetic diversity. Eur. J. Plant Pathol. 2016, 146, 85–94. [Google Scholar] [CrossRef]
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2016, 144, 235–243. [Google Scholar] [CrossRef]
- Marcelletti, S.; Scortichini, M. Xylella fastidiosa CoDiRO strain associated with the olive quick decline syndrome in southern Italy belongs to a clonal complex of the subspecies pauca that evolved in Central America. Microbiology 2016, 162, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Cella, E.; Angeletti, S.; Fogolari, M.; Bazzardi, R.; De Gara, L.; Ciccozzi, M. Two different Xylella fastidiosa strains circulating in Italy: Phylogenetic and evolutionary analyses. J. Plant Interact. 2018, 13, 428–432. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Saponari, M.; Loconsole, G.; Boscia, D.; Savino, V.N.; Almeida, R.P.; Saldarelli, P. Genome-wide analysis provides evidence on the genetic relatedness of the emergent Xylella fastidiosa genotype in Italy to isolates from Central America. Phytopathol 2017, 107, 816–827. [Google Scholar] [CrossRef]
- Saponari, M.; Boscia, D.; Altamura, G.; Loconsole, G.; Zicca, S.; D’Attoma, G.; Morelli, M.; Palmisano, F.; Saponari, A.; Tavano, D.; et al. Isolation and pathogenicity of Xylella fastidiosa associated to the olive quick decline syndrome in southern Italy. Sci. Rep. 2017, 7, 17723. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Rahi, Y.J.; Taratufolo, M.C.; Tatì, M.; Turco, S.; Ciarroni, S.; Balestra, G.M. A new inclusive MLVA assay to investigate genetic variability of Xylella fastidiosa with a specific focus on the Apulian outbreak in Italy. Sci. Rep. 2020, 10, 10856. [Google Scholar] [CrossRef] [PubMed]
- D’Attoma, G.; Morelli, M.; De La Fuente, L.; Cobine, P.A.; Saponari, M.; de Souza, A.A.; Saldarelli, P. Phenotypic characterization and transformation attempts reveal peculiar traits of Xylella fastidiosa subspecies pauca strain De Donno. Microorganisms 2020, 8, 1832. [Google Scholar] [CrossRef]
- Van Sluys, M.A.; De Oliveira, M.C.; Monteiro-Vitorello, C.B.; Miyaki, C.Y.; Furlan, L.R.; Camargo, L.E.A.; Da Silva, A.C.R.; Moon, D.H.; Takita, M.A.; Lemos, E.G.M. Comparative analyses of the complete genome sequences of Pierce’s disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 2003, 185, 1018–1026. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Saponari, M.; Almeida, R.P.P.; Essakhi, S.; Boscia, D.; Loconsole, G.; Saldarelli, P. Complete genome sequence of the olive-infecting strain Xylella fastidiosa subsp. pauca De Donno. Genome. Announc. 2017, 5, 10.1128. [Google Scholar] [CrossRef]
- Ramazzotti, M.; Cimaglia, F.; Gallo, A.; Ranaldi, F.; Surico, G.; Mita, G.; Marchi, G. Insights on a founder effect: The case of Xylella fastidiosa in the Salento area of Apulia, Italy. Phytopathol. Mediterr. 2018, 57, 8–25. [Google Scholar]
- Scala, V.; Reverberi, M.; Salustri, M.; Pucci, N.; Modesti, V.; Lucchesi, S.; Loreti, S. Lipid profile of Xylella fastidiosa subsp. pauca associated with olive quick decline syndrome. Front. Microbiol. 2018, 14, 1839. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Cesari, G. An evaluation of monitoring surveys of the quarantine bacterium Xylella fastidiosa performed in containment and buffer areas of Apulia, southern Italy. Appl. Biosaf. 2019, 24, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Scala, V.; Pucci, N.; Salustri, M.; Modesti, V.; L’Aurora, A.; Scortichini, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; Loreti, S. Xylella fastidiosa subsp. pauca and olive produced lipids moderate the switch adhesive versus non-adhesive state and viceversa. PLoS ONE 2020, 15, e0233013. [Google Scholar] [CrossRef] [PubMed]
- Firrao, G.; Scortichini, M.; Pagliari, L. Orthology-based estimate of the contribution of horizontal gene transfer from distantly related bacteria to the intrasoecific diversity of Xylella fastidiosa. Pathogens 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Saponari, M.; Vanhove, M.; Castillo, A.I.; Giampetruzzi, A.; Loconsole, G.; Almeida, R.P. Introduction and adaptation of an emerging pathogen to olive trees in Italy. Microb. Genom. 2021, 7, 000735. [Google Scholar] [CrossRef]
- Frazier, N.W.; Freitag, J.H. Ten additional leafhopper vectors of the virus causing PierceÕs disease of grape. Phytopathology 1946, 36, 634–637. [Google Scholar]
- Hewitt, W.M.B.; Houston, B.R.; Frazier, N.W.; Freitag, J.H. Leafhopper transmission of the virus causing PierceÕs disease of grape and dwarf of alfalfa. Phytopathology 1946, 36, 117–128. [Google Scholar]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell, R.F.; Andersen, P.C. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Ann. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef]
- Hill, B.L.; Purcell, A.H. Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathology 1997, 87, 1197–1201. [Google Scholar] [CrossRef]
- Lahbib, N.; Picciotti, U.; Boukhris-Bouhachem, S.; Garganese, F.; Porcelli, F. Morphs of Philaenus species, candidate Xylella fastidiosa vectors. Bull. Insectology 2022, 75, 197–209. [Google Scholar]
- Weaver, C.R.; King, D.R. Meadow spittlebug, Philaenus leucophthalmus (L.). Ohio Res. Bull. Ohio Agric. Exp.Stn. 1954, 741, 1–99. [Google Scholar]
- Delong, M.D.; Severin, H.H.P. Spittle-insect vectors of Pierce’s disease virus I characters, distribution and food plants. Hilgardia 1950, 19, 339–356. [Google Scholar] [CrossRef]
- Moussa, I.E.; Mazzoni, V.; Valentini, F.; Yaseen, T.; Lorusso, D.; Speranza, S.; D’Onghia, A.M. Seasonal fluctuations of sap-feeding insect species infected by Xylella fastidiosa in Apulian olive groves of southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Bosco, D. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Marra, M.; Morente, M.; Garzo, E.; Moreno, A.; Saponari, M.; Fereres, A. Feeding behavior in relation to spittlebug transmission of Xylella fastidiosa. J. Pest Sci. 2020, 93, 1197–1213. [Google Scholar] [CrossRef]
- Patterson, I.J.; Massei, G.; Genov, P. The density of cicadas Cicada orni in Mediterranean coastal habitats. Ital. J. Zool. 1997, 64, 141–146. [Google Scholar] [CrossRef]
- Cornara, D.; Marra, M.; Tedone, B.; Cavalieri, V.; Porcelli, F.; Fereres, A.; Saponari, M. No evidence for cicadas’ implication in Xylella fastidiosa epidemiology. Entomol. Gen. 2019, 40, 125–132. [Google Scholar] [CrossRef]
- Fierro, A.; Liccardo, A.; Porcelli, F. A lattice model to manage the vector and the infection of the Xylella fastidiosa on olive trees. Sci. Rep. 2019, 9, 8723. [Google Scholar] [CrossRef]
- Whittaker, J.B.; Tribe, N.P. Predicting numbers of an insect (Neophilaenus lineatus: Homoptera) in a changing climate. J. Anim. Ecol. 1998, 67, 987–991. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Bosco, D. Plant selection and population trend of spittlebug immatures (Hemiptera: Aphrophoridae) in olive groves of the Apulia region of Italy. J. Econ. Entomol. 2019, 112, 67–74. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Bosco, D. Spittlebugs of Mediterranean olive groves: Host-plant exploitation throughout the year. Insects 2020, 11, 130. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Pegoraro, M.; Altamura, G.; Canuto, F.; Zicca, S.; Bosco, D. Temporal dynamics of the transmission of Xylella fastidiosa subsp. pauca by Philaenus spumarius to olive plants. Entomol. Gen. 2021, 1–17. [Google Scholar] [CrossRef]
- Cornara, D.; Panzarino, O.; Santoiemma, G.; Bodino, N.; Loverre, P.; Mastronardi, M.G.; Addante, R. Natural areas as reservoir of candidate vectors of Xylella fastidiosa. Bull. Insectol. 2021, 74, 173–180. [Google Scholar]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Simonetto, A.; Saladini, M.A.; Plazio, E.; Bosco, D. Dispersal of Philaenus spumarius (Hemiptera: Aphrophoridae), a vector of Xylella fastidiosa, in olive grove and meadow agroecosystems. Env. Entomol. 2021, 50, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, F.; Frem, M.; Fucilli, V.; Cardone, G.; Garofoli, P.F.; Geronimo, S.; Petrontino, A. Landscape and vegetation patterns zoning is a methodological tool for management costs implications due to Xylella fastidiosa invasion. Land 2022, 11, 1105. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Charles, V.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Ann. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Tripathi, K.A.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phytother. 2009, 1, 52–63. [Google Scholar]
- Ganassi, S.; Cascone, P.; Domenico, C.D.; Pistillo, M.; Formisano, G.; Giorgini, M.; Guerrieri, E. Electrophysiological and behavioural response of Philaenus spumarius to essential oils and aromatic plants. Sci. Rep. 2020, 10, 3114. [Google Scholar] [CrossRef]
- Cascone, P.; Quarto, R.; Iodice, L.; Cencetti, G.; Formisano, G.; Spiezia, G.; Guerrieri, E. Behaviouralresponse of the mainvector of Xylella fastidiosa towards olive VOCs. Entomol. Gen. 2022, 42, 35–44. [Google Scholar] [CrossRef]
- Picciotti, U.; Lahbib, N.; Sefa, V.; Porcelli, F.; Garganese, F. Aphrophoridae role in Xylella fastidiosa subsp. pauca ST53 invasion in southern Italy. Pathogens 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Boscia, D. Occurrence of Xylella fastidiosa in Apulia. International Symposium on the European Outbreak of Xylella fastidiosa in Olive. J. Plant Pathol. 2014, 96, S4.97–S4.104. [Google Scholar]
- Stokstad, E. Italy’s olives under siege: Blight alarms officials across Europe. Science 2015, 348, 620. [Google Scholar] [CrossRef] [PubMed]
- Bosso, L.; Russo, D.; Di Febbraro, M.; Cristinzio, G.; Zoina, A. Potential distribution of Xylella fastidiosa in Italy: A maximum entropy model. Phytopathol. Mediterr. 2016, 55, 62–72. [Google Scholar]
- Bucci, E.M. Effectiveness of the monitoring of X. fastidiosa subsp. pauca in the olive orchards of Southern Italy (Apulia). Rend. Lincei. 2019, 30, 681–688. [Google Scholar] [CrossRef]
- White, S.M.; Bullock, J.M.; Hooftman, D.A.; Chapman, D.S. Modelling the spread and control of Xylella fastidiosa in the early stages of invasion in Apulia, Italy. Biol. Invasions. 2017, 19, 1825–1837. [Google Scholar] [CrossRef]
- White, S.M.; Navas-Cortés, J.A.; Bullock, J.M.; Boscia, D.; Chapman, D.S. Estimating the epidemiology of emerging Xylella fastidiosa outbreaks in olives. Plant Pathol. 2020, 69, 1403–1413. [Google Scholar] [CrossRef]
- Castrignanò, A.; Belmonte, A.; Antelmi, I.; Quarto, R.; Quarto, F.; Shaddad, S.; Nigro, F. A geostatistical fusion approach using UAV data for probabilistic estimation of Xylella fastidiosa subsp. pauca infection in olive trees. Sci. Total Environ. 2021, 752, 141814. [Google Scholar] [CrossRef]
- Cendoya, M.; Martínez-Minaya, J.; Dalmau, V.; Ferrer, A.; Saponari, M.; Conesa, D.; Vicent, A. Spatial Bayesian modeling applied to the surveys of Xylella fastidiosa in Alicante (Spain) and Apulia (Italy). Front. Plant Sci. 2020, 11, 1204. [Google Scholar] [CrossRef]
- Kottelenberg, D.; Hemerik, L.; Saponari, M.; van der Werf, W. Shape and rate of movement of the invasion front of Xylella fastidiosa spp. pauca in Puglia. Sci. Rep. 2021, 11, 1061. [Google Scholar] [CrossRef]
- Gilioli, G.; Simonetto, A.; Colturato, M.; Bazarra, N.; Fernández, J.R.; Naso, M.G.; Saponari, M. An eco-epidemiological model supporting rational disease management of Xylella fastidiosa. An application to the outbreak in Apulia (Italy). Ecol. Model 2023, 476, 110226. [Google Scholar] [CrossRef]
- Bajocco, S.; Raparelli, E.; Bregaglio, S. Assessing the driving role of the anthropogenic landscape on the distribution of the Xylella fastidiosa-driven “olive quick decline syndrome” in Apulia (Italy). Sci. Total Environ. 2023, 896, 165231. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; Miglietta, P.P.; Lamastra, L.; Ficocelli, S.; Intini, F.; De Leo, F.; De Donno, A. Groundwater Nitrate Contamination and Agricultural Land Use: A Grey Water Footprint Perspective in Southern Apulia Region (Italy). Sci. Total Environ. 2018, 645, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic. Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Yaseen, T.; Drago, S.; Valentini, F.; Elbeaino, T.; Stampone, G.; Digiaro, M.; D’Onghia, A.M. On-site detection of Xylella fastidiosa in host plants and in” spy insects” using the real-time loop-mediated isothermal amplification method. Phytopathol. Mediterr. 2015, 54, 488–496. [Google Scholar]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P. Xylella fastidiosa: Insights into an emerging plant pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, M.; De Caroli, M.; Baccelli, I.; Marchi, G.; Bleve, G.; Gallo, A.; Di Sansebastiano, G.P. Vessel occlusion in three cultivars of Olea europaea naturally exposed to Xylella fastidiosa in open field. J. Phytopathol. 2017, 165, 589–594. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Morelli, M.; Saponari, M.; Loconsole, G.; Chiumenti, M.; Boscia, D.; Savino, V.; Martelli, G.P.; Saldarelli, P. Transcriptome profiling of two olive cultivars in response to infection by the CoDiRO strain of Xylella fastidiosa subsp. pauca. BMC Genom. 2016, 17, e475. [Google Scholar] [CrossRef]
- Sabella, E.; Luvisi, A.; Aprile, A.; Negro, C.; Vergine, M.; Nicolì, F.; De Bellis, L. Xylella fastidiosa induces differential expression of lignification related-genes and lignin accumulation in tolerant olive trees cv. Leccino. J. Plant Physiol. 2018, 220, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rey, B.; Aleixos, N.; Cubero, S.; Blasco, J. XF-ROVIM. A field robot to detect olive trees infected by Xylella fastidiosa using proximal sensing. Remote Sens. 2018, 11, 221. [Google Scholar] [CrossRef]
- Scortichini, M.; Migoni, D.; Angile, F.; Del Coco, L.; Girelli, C.R.; Zampella, L.; Fanizzi, F.P. Xylella fastidiosa subsp. pauca on olive in Salento (Southern Italy): Infected trees have low in planta micronutrient content. Phytopathol. Mediterr. 2019, 58, 39–48. [Google Scholar]
- Manici, L.M.; Castellini, M.; Caputo, F. Soil-inhabiting fungi can integrate soil physical indicators in multivariate analysis of Mediterranean agroecosystem dominated by old olive groves. Ecol. Indic. 2019, 106, 105490. [Google Scholar] [CrossRef]
- Vergine, M.; Meyer, J.B.; Cardinale, M.; Sabella, E.; Hartmann, M.; Cherubini, P.; Luvisi, A. The Xylella fastidiosa-resistant olive cultivar “Leccino” has stable endophytic microbiota during the olive quick decline syndrome (OQDS). Pathogens 2019, 9, 35. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernandez-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef]
- Poblete, T.; Camino, C.; Beck, P.S.A.; Hornero, A.; Kattenborn, T.; Saponari, M.; Boscia, D.; Navas-Cortes, J.A.; Zarco-Tejada, P.J. Detection of Xylella fastidiosa infection symptoms with airborne multispectral and thermal imagery: Assessing bandset reduction performance from hyperspectral analysis. J. Photogramm. Remote Sens. 2020, 162, 27–40. [Google Scholar] [CrossRef]
- Di Nisio, A.; Adamo, F.; Acciani, G.; Attivissimo, F. Fast detection of olive trees affected by Xylella fastidiosa from uavs using multispectral imaging. Sensors 2020, 20, 4915. [Google Scholar] [CrossRef] [PubMed]
- Castrignanò, A.; Belmonte, A.; Antelmi, I.; Quarto, R.; Quarto, F.; Shaddad, S.; Nigro, F. Semi-automatic method for early detection of xylella fastidiosa in olive trees using uav multispectral imagery and geostatistical-discriminant analysis. Remote Sens. 2020, 13, 14. [Google Scholar] [CrossRef]
- Hornero, A.; Hernandez-Clemente, R.; North, P.R.J.; Beck, P.S.A.; Boscia, D.; Navas-Cortes, J.A.; Zarco-Tejada, P.J. Monitoring the infection of Xylella fastidiosa infection in olive orchards using ground-based evaluations, airborne imaging spectroscopy and Sentinel-2 time series through 3D radiative transfer modelling. Remote Sens. Environ. 2020, 236, 111480. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Scortichini, M.; Petriccione, M.; Zampella, L.; Mastrobuoni, F.; Fanizzi, F.P. Xylella fastidiosa and olive quick decline syndrome (CoDiRO) in Salento (southern Italy): A chemometric 1 H NMR-based preliminary study on Ogliarola salentina and Cellina di Nardò cultivars. Chem. Biol. Technol. Agric. 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Jlilat, A.; Ragone, R.; Gualano, S.; Santoro, F.; Gallo, V.; Varvaro, L.; D’Onghia, A.M. A non-targeted metabolomics study on Xylella fastidiosa infected olive plants grown under controlled conditions. Sci. Rep. 2021, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Di Masi, S.; De Benedetto, G.E.; Malitesta, C.; Saponari, M.; Citti, C.; Cannazza, G.; Ciccarella, G. HPLC-MS/MS method applied to an untargeted metabolomics approach for the diagnosis of “olive quick decline syndrome”. Anal. Bioanal. Chem. 2022, 414, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Faino, L.; Scala, V.; Albanese, A.; Modesti, V.; Grottoli, A.; Pucci, N.; Doddi, A.; L’Aurora, A.; Tatulli, G.; Reverberi, M.; et al. Nanopore sequencing for the detection and identification of Xylella fastidiosa subspecies and sequence types from naturally infected plant material. Plant Pathol. 2021, 70, 1860–1870. [Google Scholar] [CrossRef]
- Asteggiano, A.; Franceschi, P.; Zorzi, M.; Aigotti, R.; Dal Bello, F.; Baldassarre, F.; Lops, F.; Carlucci, A.; Medana, C.; Ciccarella, G. HPLC-HRMS global metabolomics approach for the diagnosis of “Olive Quick Decline Syndrome” markers in olive trees leaves. Metabolites 2021, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Vergine, M.; Nicolì, F.; Sabella, E.; Aprile, A.; Negro, C.; Luvisi, A. Screening of olive biodiversity defines genotypes potentially resistant to Xylella fastidiosa. Front. Plant Sci. 2021, 12, 723879. [Google Scholar] [CrossRef]
- Riefolo, C.; Antelmi, I.; Castrignanò, A.; Ruggieri, S.; Galeone, C.; Belmonte, A.; Nigro, F. Assessment of the hyperspectral data analysis as a tool to diagnose Xylella fastidiosa in the asymptomatic leaves of olive plants. Plants 2021, 10, 683. [Google Scholar] [CrossRef]
- D’Onghia, A.M.; Santoro, F.; Minutillo, S.A.; Frasheri, D.; Gallo, M.; Gualano, S.; Valentini, F. Optimisation of sampling and testing for asymptomatic olive trees infected by Xylella fastidiosa in Apulia region, Italy. Phytopathol. Mediterr. 2022, 61(3), 439–449. [Google Scholar] [CrossRef]
- Montilon, V.; De Stradis, A.; Saponari, M.; Abou Kubaa, R.; Giampetruzzi, A.; D’Attoma, G.; Saldarelli, P. Xylella fastidiosa subsp. pauca ST53 exploits pit membranes of susceptible olive cultivars to spread systemically in the xylem. Plant Pathol. 2022, 72, 144–153. [Google Scholar] [CrossRef]
- Boscia, D.; Altamura, G.; Saponari, M.; Tavano, D.; Zicca, S.; Tanielli, M. Incidenza di Xylella in oliveti con disseccamento rapido. L’Informatore Agrar. 2017, 27, 47–50. [Google Scholar]
- Greco, D.; Sabella, E.; Carluccio, G.; Delle Donne, A.G.; De Bellis, L.; Luvisi, A. Xylella fastidiosa, Possible New Threat to Chestnut (Castanea sativa Mill.) in Italy. Horticulturae 2023, 9, 1315. [Google Scholar] [CrossRef]
- Belmonte, A.; Gadaleta, G.; Castrignanò, A. Use of Geostatistics for Multi-Scale Spatial Modeling of Xylella fastidiosa subsp. pauca (Xfp) Infection with Unmanned Aerial Vehicle Image. Remote Sens. 2023, 15, 656. [Google Scholar] [CrossRef]
- Amoia, S.S.; Loconsole, G.; Ligorio, A.; Pantazis, A.K.; Papadakis, G.; Gizeli, E.; Minafra, A. A Colorimetric LAMP Detection of Xylella fastidiosa in Crude Alkaline Sap of Olive Trees in Apulia as a Field-Based Tool for Disease Containment. Agriculture 2023, 13, 448. [Google Scholar] [CrossRef]
- Blonda, P.; Tarantino, C.; Scortichini, M.; Maggi, S.; Tarantino, M.; Adamo, M. Satellite monitoring of bio-fertilizer restoration in olive groves affected by Xylella fastidiosa subsp. pauca. Sci. Rep. 2023, 13, 5695. [Google Scholar] [CrossRef] [PubMed]
- Savoia, M.A.; Fanelli, V.; Miazzi, M.M.; Taranto, F.; Procino, S.; Susca, L.; Montilon, V.; Potere, O.; Nigro, F.; Montemurro, C. Apulian Autochthonous Olive Germplasm: A Promising Resource to Restore Cultivation in Xylella fastidiosa-Infected Areas. Agriculture 2023, 13, 1746. [Google Scholar] [CrossRef]
- Ciervo, M.; Scortichini, M. A decade of monitoring surveys for Xylella fastidiosa subsp. pauca in olive groves in Apulia (Italy) reveals a low incidence of the bacterium in the demarcated areas. J. Phytopathol. 2023, 172, e13272. [Google Scholar] [CrossRef]
- DENTAMET.: Il Fertilizzante per la Difesa Degli Ulivi dal Flagello Xylella. 2015. Available online: https://www.diachemitalia.it/prodotti/fertilizzanti-integrati/dentamet (accessed on 27 May 2023).
- dos Santos Freitas, D.; Carlos, E.F.; Gil, M.r.C.S.d.S.; Vieira, L.G.E.; Alcantara, G.B. NMR-Based Metabolomic analysis of Huanglongbing-asymptomatic and-symptomatic citrus trees. J. Agric. Food Chem. 2015, 63, 7582–7588. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Chen, J.; De Caroli, M.; Dalessandro, G.; Pucci, N.; Modesti, V.; Loreti, S. A zinc, copper and citric acid biocomplex shows promise for control of Xylella fastidiosa subsp. pauca in olive trees in Apulia region (southern Italy). Phytopathol. Mediter. 2018, 57, 48–72. [Google Scholar]
- Girelli, C.R.; Angilè, F.; Del Coco, L.; Migoni, D.; Zampella, L.; Marcelletti, S.; Fanizzi, F.P. 1H-NMR metabolite fingerprinting analysis reveals a disease biomarker and a field treatment response in Xylella fastidiosa subsp. pauca-Infected Olive Trees. Plants 2019, 8, 115. [Google Scholar] [CrossRef]
- Bleve, G.; Gallo, A.; Altomare, C.; Vurro, M.; Maiorano, G.; Cardinali, A.; Mita, G. In vitro activity of antimicrobial compounds against Xylella fastidiosa, the causal agent of the olive quick decline syndrome in Apulia (Italy). FEMS Microbiol. Lett. 2018, 365, fnx281. [Google Scholar] [CrossRef]
- Bleve, G.; Marchi, G.; Ranaldi, F.; Gallo, A.; Cimaglia, F.; Logrieco, A.F.; Mita, G.; Ristori, J.; Surico, G. Molecular characteristics of a strain (Salento-1) of Xylella fastidiosaisolated in Apulia (Italy) from an olive plant with the quick decline syndrome. Phytopathol. Mediter. 2016, 55, 139–146. [Google Scholar]
- Del Coco, L.; Migoni, D.; Girelli, C.R.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Soil and leaf ionome heterogeneity in Xylella fastidiosa subsp. pauca-infected, non-infected and treated olive groves in Apulia, Italy. Plants 2020, 9, 760. [Google Scholar] [CrossRef]
- Zicca, S.; De Bellis, P.; Masiello, M.; Saponari, M.; Saldarelli, P.; Boscia, D.; Sisto, A. Antagonistic activity of olive endophytic bacteria and of Bacillus spp. strains against Xylella fastidiosa. Microbiol. Res. 2020, 236, 126467. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, A.; Fierro, A.; Garganese, F.; Picciotti, U.; Porcelli, F. A biological control model to manage the vector and the infection of Xylella fastidiosa on olive trees. PLoS ONE 2020, 15, e0232363. [Google Scholar] [CrossRef]
- Baldassarre, F.; Tatulli, G.; Vergaro, V.; Mariano, S.; Scala, V.; Nobile, C.; Pucci, N.; Dini, L.; Loreti, S.; Ciccarella, G. Sonication-assisted production of fosetyl-Al nanocrystals: Investigation of human toxicity and in vitro antibacterial efficacy against Xylella fastidiosa. Nanomaterials 2020, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Tatulli, G.; Modesti, V.; Pucci, N.; Scala, V.; L’Aurora, A.; Lucchesi, S.; Loreti, S. Further in vitro assessment and mid-term evaluation of control strategy of Xylella fastidiosa subsp. pauca in olive groves of Salento (Apulia, Italy). Pathogens 2021, 10, 85. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Angilè, F.; Scortichini, M.; Fanizzi, F.P. Olive cultivars susceptible or tolerant to Xylella fastidiosa subsp. pauca exhibit mid-term different metabolomes upon natural infection or a curative treatment. Plants 2021, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.L.; Cariddi, C.; Botrugno, L. Exploring a sustainable solution to control Xylella fastidiosa subsp. pauca on olive in the Salento Peninsula, Southern Italy. Crop Prot. 2021, 139, 105288. [Google Scholar] [CrossRef]
- Fausto, C.; Araniti, F.; Mininni, A.N.; Crecchio, C.; Scagliola, M.; Bleve, G.; Sofo, A. Differential olive grove management regulates the levels of primary metabolites in xylem sap. Plant Soil 2021, 460, 281–296. [Google Scholar] [CrossRef]
- Scala, V.; Salustri, M.; Loreti, S.; Pucci, N.; Cacciotti, A.; Tatulli, G.; Scortichini, M.; Reverberi, M. Mass spectrometry-based targeted lipidomics and supervised machine learning algorithms in detecting disease, cultivar and treatment biomarkers in Xylella fastidiosa subsp. pauca-infected olive trees. Front. Plant Sci. 2022, 13, 833245. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Zicca, S.; Ambrico, M.; Rotondo, P.R.; De Stradis, A.; Dilecce, G.; Saponari, M.; Boscia, D.; Saldarelli, P. Low temperature plasma strategies for Xylella fastidiosa inactivation. Appl. Sci. 2022, 12, 4711. [Google Scholar] [CrossRef]
- Girelli, C.R.; Hussain, M.; Verweire, D.; Oehl, M.C.; Massana-Codina, J.; Avendaño, M.S.; Fanizzi, F.P. Agro-active endo-therapy treated Xylella fastidiosa subsp. pauca-infected olive trees assessed by the first 1H-NMR-based metabolomic study. Sci. Rep. 2022, 12, 5973. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; Schiavi, D.; Ciarroni, S.; Tagliavento, V.; De Stradis, A.; Vergaro, V.; Ciccarella, G. Thymol-Nanoparticles as Effective Biocides against the Quarantine Pathogen Xylella fastidiosa. Nanomaterials 2023, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Girelli, C.R.; Verweire, D.; Oehl, M.C.; Avendaño, M.S.; Scortichini, M.; Fanizzi, F.P. 1H-NMR Metabolomics Study after Foliar and Endo-Therapy Treatments of Xylella fastidiosa subsp. pauca Infected Olive Trees: Medium Time Monitoring of Field Experiments. Plants 2023, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Camposeo, S.; Vivaldi, G.A.; Saponari, M. Attempts to Reduce the Systemic Spread of Xylella fastidiosa in Olive Trees by Pruning. Agronomy 2022, 12, 2917. [Google Scholar] [CrossRef]
- Vizzarri, V.; Ienco, A.; Benincasa, C.; Perri, E.; Pucci, N.; Cesari, E.; Novellis, C.; Rizzo, P.; Pellegrino, M.; Zaffina, F.; et al. Phenolic Extract from Olive Leaves as a Promising Endotherapeutic Treatment against Xylella fastidiosa in Naturally Infected Olea europaea (var. europaea) Trees. Biology 2023, 12, 1141. [Google Scholar] [CrossRef]
- Orfei, B.; Moretti, C.; Loreti, S.; Tatulli, G.; Onofri, A.; Scotti, L.; Aceto, A.; Buonaurio, R. Silver nanoclusters with Ag2+/3+ oxidative state are a new highly effective tool against phytopathogenic bacteria. Appl Microbiol Biotechnol 2023, 107, 4519–4531. [Google Scholar] [CrossRef] [PubMed]
- Ismea. Tendenze e Dinamiche Recenti. Olio d’oliva–Rapporto Settembre 2022; Ismea: Roma, Italy, 2022; p. 14. [Google Scholar]
- Ismea. Tendenze e Dinamiche Recenti. Olio d’oliva–Rapporto Settembre 2023; Ismea: Roma, Italy, 2023; p. 15. [Google Scholar]
- Schneider, K.; van der Werf, W.; Cendoya, M.; Mouritis, M.; Navas-Cortes, J.A.; Vincent, A.; Oude Lansink, A. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2022, 117, 9250–9259. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; van der Werf, W.; Lansink, A.O. Assessment of the environmental impacts of Xylella fastidiosa subsp. pauca in Puglia. Crop. Prot. 2021, 142, 105519. [Google Scholar] [CrossRef]
- Del Coco, L.; De Pascali, S.A.; Fanizzi, F.P. NMR-metabolomic study on monovarietal and blend Salento EVOOs including some from secular olive trees. Food Nutr. Sci. 2014, 5, 89–95. [Google Scholar]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; De Bellis, L. Phenolic profile and antioxidant activity of Italian monovarietal extravirgin olive oil. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef]
- Brunetti, M.; Capasso, V.; Montagna, M.; Venturino, E. A mathematical model for Xylella fastidiosa epidemics in the Mediterranean regions. Promoting good agronomic practices for their effective control. Ecol. Model 2020, 432, 109204. [Google Scholar] [CrossRef]
- Semeraro, T.; Gatto, E.; Buccolieri, R.; Vergine, M.; Gao, Z.; De Bellis, L.; Luvisi, A. Changes in olive urban forests infected by Xylella fastidiosa: Impact on microclimate and social health. Int. J. Environ. Res. Public Health 2019, 16, 2642. [Google Scholar] [CrossRef]
- Semeraro, T.; Buccolieri, R.; Vergine, M.; De Bellis, L.; Luvisi, A.; Emmanuel, R.; Marwan, N. Analysis of olive grove destruction by Xylella fastidiosa bacterium on the land surface temperature in Salento detected using satellite images. Forests 2021, 12, 1266. [Google Scholar] [CrossRef]
- Proietti, P.; Sdringola, P.; Brunori, A.; Ilarioni, L.; Nasini, L.; Regni, L.; Proietti, S. Assessment of carbon balance in intensive and extensive tree cultivation systems for oak, olive, poplar and walnut plantation. J. Clean Prod. 2016, 112, 2613–2624. [Google Scholar] [CrossRef]
- Petrosillo, I.; Marinelli, M.V.; Zurlini, G.; Valente, D. Cross scale spatial and temporal indicators for measuring the effects of landscape heterogeneity on pollination service. Ecol. Ind. 2022, 145, 109573. [Google Scholar] [CrossRef]
- Frem, M.; Fucilli, V.; Petrontino, A.; Acciani, C.; Bianchi, R.; Bozzo, F. Nursery Plant Production Models under Quarantine Pests’ Outbreak: Assessing the Environmental Implications and Economic Viability. Agronomy 2022, 12, 2964. [Google Scholar] [CrossRef]
- Alhajj Ali, S.; Vivaldi, G.A.; Garofalo, S.P.; Costanza, L.; Camposeo, S. Land Suitability Analysis of Six Fruit Tree Species Immune/Resistant to Xylella fastidiosa as Alternative Crops in Infected Olive-Growing Areas. Agronomy 2023, 13, 547. [Google Scholar] [CrossRef]
- Sardaro, R.; Fucilli, V.; Acciani, C.; Bozzo, F.; Petrontino, A.; Girone, S. Agro-biodiversity: An economic evaluation of benefits provided to regional community by the Apulian olive landraces. RIEDS 2016, 70, 12. [Google Scholar]
- Frem, M.; Santeramo, F.G.; Lamonaca, E.; El Moujabber, M.; Choueiri, E.; La Notte, P.; Fucilli, V. Landscape restoration due to Xylella fastidiosa invasion in Italy: Assessing the hypothetical public’s preferences. NeoBiota 2021, 66, 31–54. [Google Scholar] [CrossRef]
- Tipaldo, G.; Bruno, F.; Rocutto, S. Hands off the olive trees!: The epistemic war in the Xylella fastidiosa epidemic in Italy: A computer-assisted text analysis of usergenerated content on social media. In Cambio: Rivista sulle Trasformazioni Sociali, 1st ed.; Firenze University Press: Firenze, Italy, 2021; pp. 131–149. [Google Scholar]
- D’Attoma, G.; Morelli, M.; Saldarelli, P.; Saponari, M.; Giampetruzzi, A.; Boscia, D.; Savino, V.N. Ionomic differences between susceptible and resistant olive cultivars infected by Xylella fastidiosa in the outbreak area of Salento, Italy. Pathogens 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Baptista, P.; Morelli, M.; Cameirão, C.; Lino Neto, T.; Costa, D.; D’Attoma, G.; Kubaa, R.A.; Altamura, G.; Saponari, M.; et al. Differences in the endophytic microbiome of olive cultivars infected by Xylella fastidiosa across seasons. Pathogens 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: Revisiting a tale of plant–pathogen interaction. AoB Plants 2021, 13, plab027. [Google Scholar] [CrossRef] [PubMed]
- Surano, A.; Kubaa, R.A.; Nigro, F.; Altamura, G.; Losciale, P.; Saponari, M.; Saldarelli, P. Susceptible and resistant olive cultivars show differential physiological response to Xylella fastidiosa infection. Front. Plant Sci. 2022, 13, 968934. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.C.; White, S.M.; Fletcher, D.M.; Ruiz, S.A.; Rankin, K.E.; De Stradis, A.; Saponari, M.; Williams, K.A.; Petroselli, C.; Roose, T. The impact of xylem geometry on olive cultivar resistance to Xylella fastidiosa: An image-based study. Plant Pathol. 2023, 72, 521–535. [Google Scholar] [CrossRef]
- azzetta ufficiale della Repubblica Italiana n. 48 del 26/02/2021. Decreto legislativo n. 19 del 2 febbraio 2021. Norme per la protezione delle piante dagli organismi nocivi in attuazione dell’articolo 11 della legge 4 ottobre 2019, n. 117, per l’adeguamento della normativa nazionale alle disposizioni del regolamento (UE) 2016/2031 e del regolamento (UE) 2017/625. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2021-02-26&atto.codiceRedazionale=21G00021&elenco30giorni=false (accessed on 11 April 2024).
- Regione Puglia. Bollettino Ufficiale della Regione Puglia n. 139 del 27-12-2022. Deliberazione della Giunta Regionale, 12 dicembre 2022, n. 1866. Approvazione “Piano d’azione per contrastare la diffusione di Xylella fastidiosa (Well et al.) in Puglia”biennio 2023-2024. Available online: https://burp.regione.puglia.it/documents/20135/2000617/DEL_1866_2022.pdf/45c48e24-2356-789f-2048-85adabcadd72?version=1.0&t=1672138153490 (accessed on 11 April 2024).
- European Commission. Commission implementing regulation (EU) 2020/1201 of 14 August 2020, as regards measures to prevent the introduction into and the spread within the Union of Xylella fastidiosa (Wells et al.). Off. J. Eur. Union 2020, 269, 2–39. [Google Scholar]


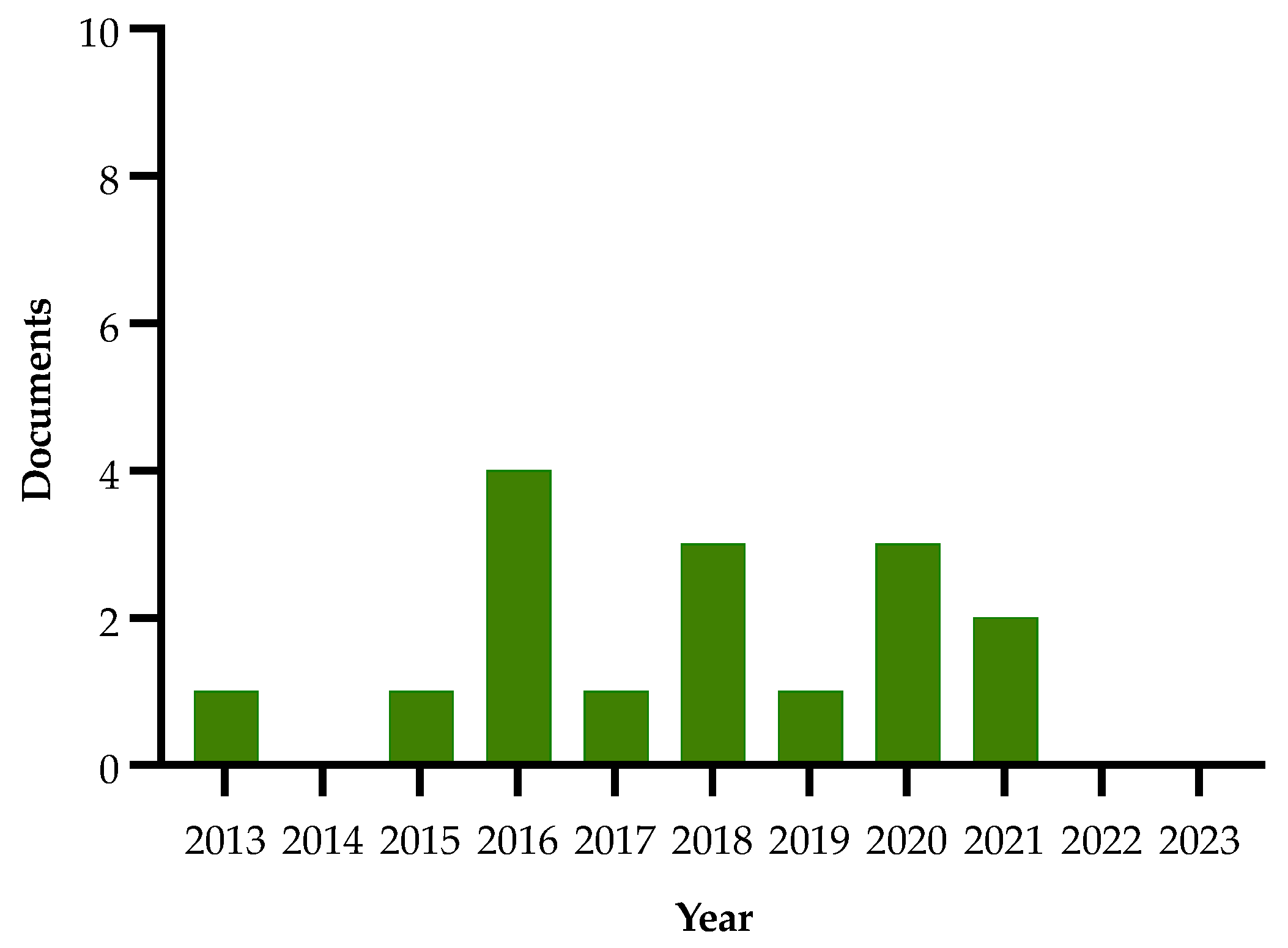
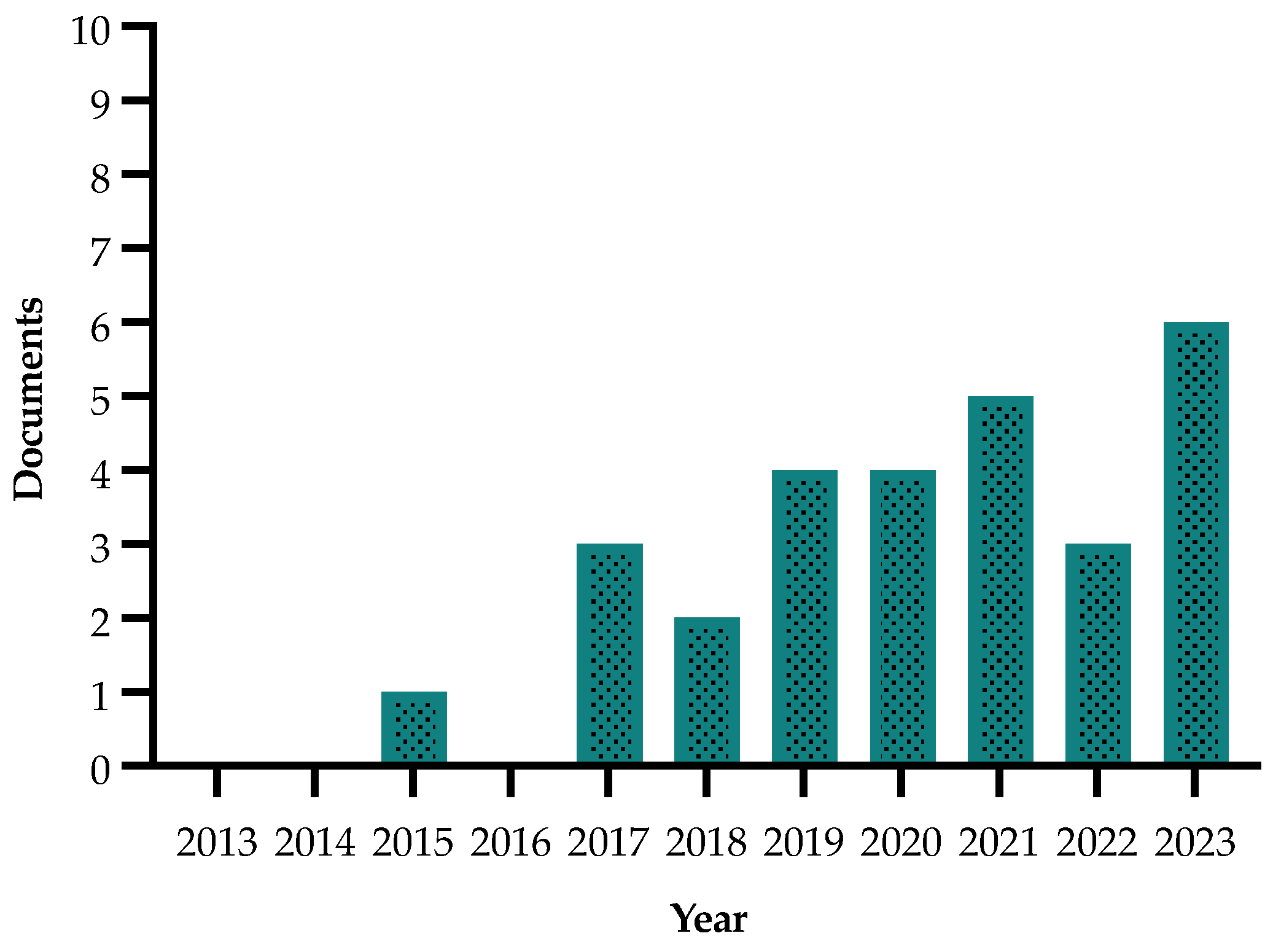
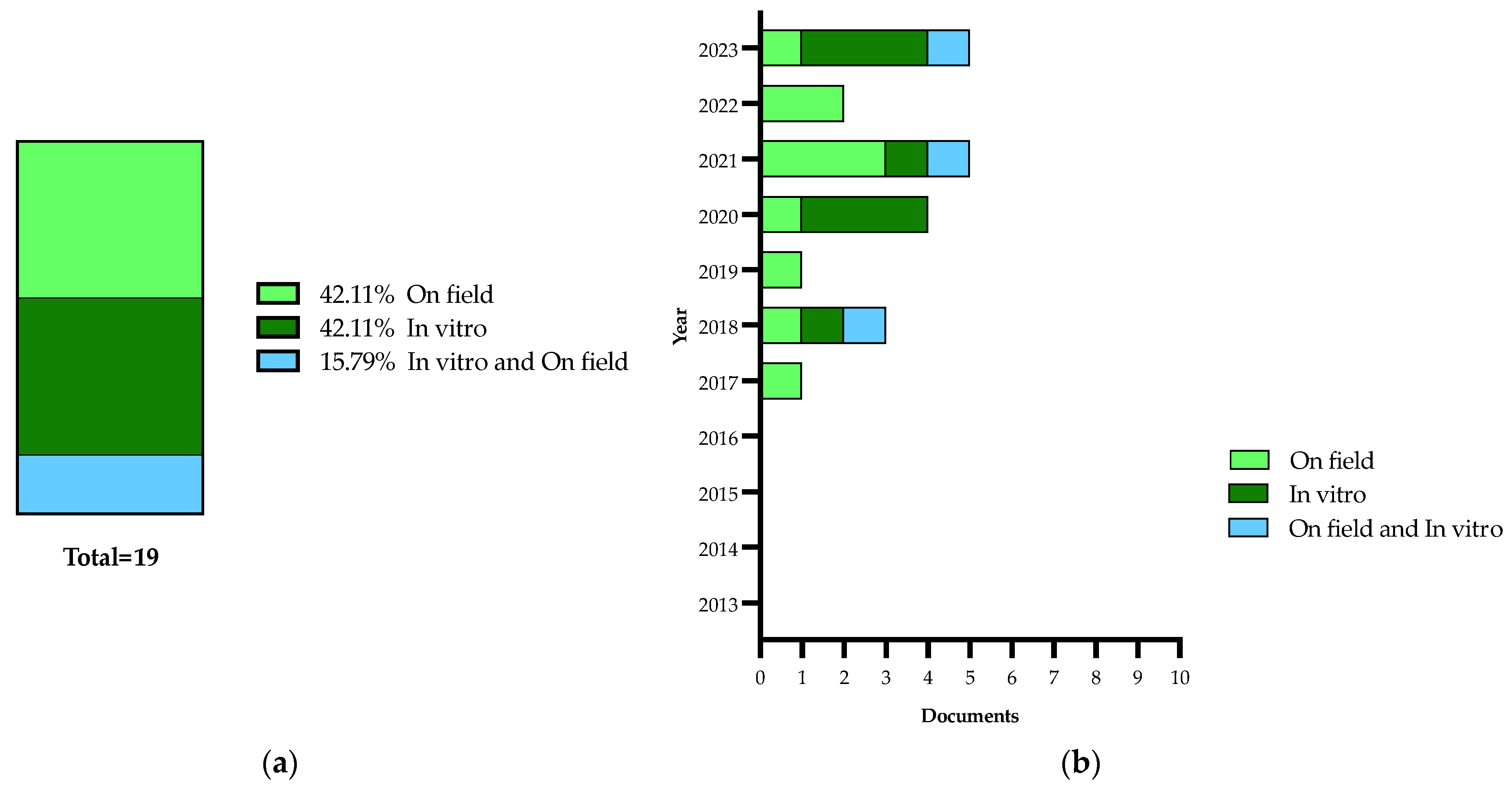


| Authors and Year | Method | Main Findings |
|---|---|---|
| Carlucci et al., 2013 [32] | Definition of phytosanitary emergency | Xf in olive plants in the Mediterranean area |
| Giampetruzzi et al., 2015 [43] | Genomic DNA investigation | They determined the draft genome sequence of the Xf CoDiRO strain; it is associated with olive quick decline syndrome (OQDS) and characterized by extensive scorching and desiccation of leaves and twigs |
| Mang et al., 2016 [44] | PCR assays | Nucleotide variation present on gyrB gene allowed separation of Xf subsp. pauca from the other subspecies multiplex and fastidiosa. The Xf strain from Apulia region was included in the subspecies pauca based on three gene phylogenetic analyses |
| Loconsole et al., 2016 [46] | MLST and phylogenetic analyses | They reported new foci as well as host plant species positive with Xf, including cherry, myrtle leaf and rosemary; all were found to be infected with the same sequence type of this bacterium (ST53 or CoDiRO strain) |
| Marcelletti and Scortichini, 2016 [48] | Genome-wide approach | This study strongly supports the possibility of the introduction of Xf into southern Italy through coffee plants grown in Central America |
| Martelli et al., 2016 [47] | Definition of phytosanitary emergency | The bacterium was isolated in culture and identified as a genotype of Xf subsp. pauca, molecularly identical to an isolate from Costa Rica. Philaenus spumarius (meadow spittlebug), a froghopper quite common in the Salento area where it thrives on olives, was identified as the main vector |
| Saponari et al., 2017 [51] | PCR assays | Needle-inoculation experiments under different environmental conditions proved that the Salentinian isolate De Donno belonging to the subspecies pauca is able to multiply and systemically invade artificially inoculated hosts, reproducing symptoms observed in the field |
| Cella et al., 2018 [49] | Phylogenetic and evolutionary analysis | Xf strains belonging to Xf subsp. pauca and subsp. sandyi were reported to infect olive trees and coffee plants, respectively. The phylogeographic analysis also revealed and confirmed these two different ways of provenience |
| Ramazzotti et al., 2018 [56] | VNTR, RAPD and rep-PCR (ERIC and BOX motifs) analyses | Genome-wide indices ANIm and dDDH indicated that the three isolates of Xf from Salento (Apulia, Italy), namely Salento-1, Salento-2 and De Donno, whose complete genome sequence has recently been released, share a very recent common ancestor |
| Scala et al., 2018 [57] | LC-TOF and LC-MS/MS techniques | Different lipid compounds present a clear distribution pattern within the infected plant tissues compared to the uninfected ones |
| Scortichini and Cesari, 2019 [58] | Serological and molecular techniques | The cultivars “Nociara”, “Cima di Melfi” and “Cellina di Nardò” showed the highest occurrence of decline symptoms |
| D’Attoma et al., 2020 [53] | In vitro behavior of the strain and compare its relevant biological features with those of the strain Temecula1 | The study showed that the strain De Donno did not show fringe on the agar plates, produced larger amounts of biofilm and had a more aggregative behavior than the strain Temecula1 |
| Mazzaglia et al., 2020 [52] | Molecular techniques (a multiple locus VNTR analysis assay) | A total of 37 TR loci were amplified on the genomic DNAs of the Apulian strains from representatives of Xf subspecies and directly on DNA extracted from infected plants |
| Scala et al., 2020 [59] | LC-MS/MS and multiple reaction monitoring (MRM) methods | This study provides novel insights on OQDS lipid hallmarks and on molecules that might modulate biofilm phase in Xf subsp. pauca |
| Firrao et al., 2021 [60] | BLASTP analysis | The results of the pan-genome analyses stressed the additional relevance of environmental DNA in shaping their genomes |
| Sicard et al., 2021 [61] | Genomic and phylogenetic analysis | They first showed that the outbreak in Apulia is due to a single introduction from Central America that was estimated to have occurred in 2008 |
| Authors and Year | Method | Insects Tested | Main Findings |
|---|---|---|---|
| Saponari et al., 2014 [7] | PCR assays | Ps and El | Ps identified as a vector of Xf infecting olive trees in the Salento Peninsula |
| Moussa et al., 2016 [69] | PCR assays | Ps, Nc and El | Ps was the dominant species with the highest adult abundance in summer months |
| Cornara et al., 2016 [8] | Transmission tests and PCR assays | Ps | The number of PCR-positive Ps on each plant was positively correlated with the plant infection status. These data show that field-collected Ps have high rates of Xf infection and are competent vectors |
| Cornara et al., 2017 [9] | Real-time PCR | Ps and Nc | Data demonstrated that Ps acquired and transmitted Xf from several host plant species in the field, with the highest acquisition rate from olive, polygala and acacia |
| Cornara et al., 2018 [26] | EPG-assisted characterization of P. spumarius female feeding behavior | Ps | Ps feeding behavior can be described by five main distinct waveforms including pathway, xylem contact/pre-ingestion, xylem sap ingestion, resting, interruption within the xylem phase |
| Bodino et al., 2019 [70] | Field surveys | Ps, Nc and Aphrophora alni | Data on the life cycle of spittlebugs within an olive agroecosystem with Ps adults being abundant on the herbaceous cover and olive trees in late spring, then dispersing to wild woody hosts during the summer and returning to the olive groves in autumn |
| Fierro et al., 2019 [75] | Lattice model proposal with numerical simulation and field surveys | Ps, Pi and Nc | A lattice model was constructed to simulate the bacterium/vector/tree infection interplay under different control actions, in order to explain to what extent the infection can be mitigated even in un-favorable conditions |
| Cavalieri et al., 2019 [71] | Quantitative PCR assays | Pi Drosopolous and Remane, Nc and Latilica tunetana (Matsumura) (Issidae) | The vector-mediated transmission experiments conducted over a two-year period showed that, besides Ps, two additional spittlebug species are competent vectors of the strain of Xf subsp. pauca ST53 |
| Dongiovanni et al., 2019 [77] | Field surveys (randomized plant sampling and quadrats sampling) | Ps and Nc | The botanic families presenting the highest number of plants infested by Ps nymphs were Asteraceae, Fabaceae and Apiaceae, peaking in early April |
| Cornara et al., 2019 [74] | Field surveys, quantitative PCR assays and EPG procedures | Ps | First insights into the transmission dynamics of the bacterium Xf by Ps: acquisition occurs at a very low rate during the first minutes when the insect is ingesting the xylem sap |
| Bodino et al., 2020 [78] | Field surveys | Ps, Nc and Aphrophora alni (L.) | Ps was the predominant species in Apulia olive groves, however principal alternative woody hosts are Quercus spp. and Pistacia spp. |
| Ganassi et al., 2020 [86] | Field surveys and electroantennographic recording (EAG) | Ps | The electrophysiological and behavioral responses of adult Ps towards some essential oils and related plants were reported |
| Cornara et al., 2020 [72] | Transmission tests and quantitative PCR assays | Platypedia minor, Cicada orni | Data suggest that the cicada species have no, or a negligible, role in the natural spread of Xf |
| Bodino et al., 2021 [79] | Field surveys (MRR experiments) | Ps | The dispersal of Ps is limited to some hundreds of meters throughout the whole year, although it can be influenced to a great extent by the structure of the agroecosystem (olive groves and meadows) |
| Cornara et al., 2021 [80] | Field surveys | Nc, Ps and Pi Drosopoulos et Remane | Reported data on the presence and abundance of spittlebugs during the year in four different habitats interspersed with cultivated orchards within a natural area in Apulia |
| Bodino et al., 2021 [81] | Quantitative PCR assays | Ps | Ps is a competent Xf vector to olives throughout its adult life; bacterial load in the vector foregut increases during the first 2–3 weeks after acquisition |
| Lahbib et al., 2022 [66] | Field surveys and light microscope and SEM observations | Philaenus species | The study revealed the true phenology of Philaenus species individuals, allowing for the classification of ambiguous individuals |
| Bozzo et al., 2022 [82] | Spatial pattern clustering methodological approach | Ps | Spatial variation and territorial differentiation may differ from zone to zone in the same invaded area |
| Cascone et al., 2022 [87] | Field surveys and olfactometer bioassay | Ps | The response of Ps towards olive varieties was sex-dependent: males were totally unresponsive whilst females were attracted by Ogliarola, Rotondella and Frantoio |
| Authors and Year | Methods | Impact | Main Findings |
|---|---|---|---|
| Semeraro et al., 2019 [162] | Methodological analysis using the environmental impact assessment (EIA) and the geographic information system software QGIS (qgis.org, accessed on 20 May 2019) | Impact of changes in olive urban forests affected by Xf on ecosystem services | The study revealed that direct effects on ecosystem services are principally linked with regulation functions and cultural services |
| Brunetti et al., 2020 [161] | Mathematical model and numerical simulations | Improvement of control strategies within the integrated pest management framework | Implemented mathematical model suggests that a removal of a suitable amount of weed biomass (reservoir of Xf) from olive orchards and surrounding areas was the most efficient strategy to control the spread of OQDS |
| Semeraro et al., 2021 [163] | Landsat data and moderate resolution imaging spectroradiometer (MODIS) images (https://lpdaac.usgs.gov/dataset_discovery/modis/modis_products_table, accessed on 12 October 2020). | Impact of destruction of olive groves by Xf on local climate change | This study analyzed how the destruction of olive groves by Xf affects local climate change |
| Frem et al., 2021 [166] | Choice experiment | Impact on the provision of olive landscape services which yield changes in components of public’s well-being | The study revealed that for the local citizens interviewed, the most appreciated olive landscape services are cultural heritage and aesthetic values |
| Ali et al., 2021 [158] | Environmental risk assessment | Short- and long-term impacts of this disease and the control measures against it on ecosystem services | The study provided the first assessment of the wider environmental impacts of Xf subsp. pauca |
| Tipaldo et al., 2021 [170] | Computer-assisted text analysis | Impact on social media | They find that discourses on Xf are strongly polarized and structured around two conflicts: «expertise vs. politics» on one hand and «scientific vs. alternative» solutions on the other |
| Petrosillo et al., 2022 [165] | Multiscale spatial and temporal analysis | Planning of landscape functionality recovery | The multitemporal analysis has allowed the authors to show the evident change in the landscape functioning in the provinces interested by the infection of Xf from 2013 to 2021 |
| Frem et al., 2022 [169] | Environmental implications assessment (LCA) | Impact assessment | This research provided a useful decision support, through a study to assess the environmental implications and economic viability of novel and sustainable ornamental production versus the conventional production options |
| Schneider et al., 2022 [157] | Climatic suitability map, economic model | Impact assessment | They developed a spatially bio-economic model to compute potential future economic impact of the Xfp strain. For Italy, the potential economic impact over 50 y ranges from 1.9 to 5.2 billion Euros for the economic worst-case scenario |
| Alhajj Ali et al., 2023 [167] | Land suitability analysis and GIS | Practical containment measures against the diffusion of Xfp | Their results can help in the selection of the right immune/resistant tree species for replanting in Xfp-infected zones |
| Authors and Year | Methods | Olive Cultivars Tested | Main Findings |
|---|---|---|---|
| Giampetruzzi et al., 2016 [106] | Molecular analysis | Ogliarola salentina and Leccino | Xfp elicits a different transcriptome response in the two cultivars, which determines a lower pathogen concentration in cv. Leccino |
| De Benedictis et al., 2017 [105] | Genotype identification and microscopy analysis | Ogliarola salentina, Cellina di Nardò and Leccino | Occlusions were caused by tyloses and, as observed in Leccino plants, they are not a marker of tolerance/resistance to the disease |
| Luvisi et al., 2017 [27] | Molecular analysis | Cellina di Nardò, Ogliarola salentina, Frantoio and Leccino | Differences in the induced responses of phenolic compounds (hydroxytyrosol glucoside and quinic acid) among cultivars suggest that they play defensive roles in olive tree response to Xf infection |
| Sabella et al., 2018 [107] | Molecular and phenolic analyses | Cellina di Nardò and Leccino | Results suggest a critical role for lignin in X. fastidiosa tolerance of cv. Leccino |
| D’Attoma et al., 2019 [171] | Inductively coupled plasma optical emission spectroscopy (ICP-OES) | Ogliarola salentina and Leccino | Their analyses showed that the ionome differences in the two varieties, particularly a higher concentration of calcium (Ca) and Mn levels in the Leccino cultivar, contribute to protection against disease caused by X. fastidiosa infection |
| Sabella et al., 2019 [103] | SEM-EDX analysis and molecular analysis | Cellina di Nardò and Leccino | Leccino cv was anatomically less susceptible to cavitation and it may also be able to activate more efficient refilling mechanisms, restoring vessel hydraulic conductivity |
| Giampetruzzi et al., 2020 [172] | Molecular and metagenome shotgun sequencing (WMSS) | Kalamata and FS17 | The progression of the infections detected in both cultivars suggested that Xf tends to occupy the whole ecological niche suppressing the diversity of the endophytic microbiome, but this trend was mitigated in the resistant cultivar FS17 |
| Pavan et al., 2021 [122] | Molecular analysis | Nocellara Messinese, Frantoio, Bella di Spagna, Pendolino, Cellina di Nardò, Ogliarola Salentina and Leccino | Their results indicated the possibility to characterize resistance to Xf in cultivars genetically related to putatively resistant plants |
| Petit et al., 2021 [173] | Characterization and measurements of functional xylem anatomy | Cellina di Nardò and Leccino | The higher air embolism vulnerability of the larger vessels in Cellina di Nardò possibly facilitates Xf infection compared to Leccino |
| Surano et al., 2022 [174] | Measurements of stomatal conductance and stem water potential | Cellina di Nardò, Leccino and FS17 | In this study, both resistant olive cultivars showed lower water stress upon Xfp infections, compared to the susceptible one |
| Montilon et al., 2022 [125] | Electron microscopy analysis | Ogliarola salentina, Cellina di Nardò and Leccino | Their results suggested that Xfp ST53 exploited the pit membranes of the susceptible cultivar Cellina di Nardò to spread systemically. In Leccino, occluded vessels were mainly filled by callose-like granules that tightly entrapped XfDD cells |
| Savoia et al., 2023 [131] | Molecular test (qPCR, genotyping) | Leccino and Cellina di Nardò | They identified nine putatively resistant genotypes that represent the first panel of olive germplasm resources |
| Walker et al., 2023 [175] | Molecular analysis and X-ray computed tomography | Ogliarola, Koroneiki, Leccino and FS17 | Their results showed susceptible cultivars, having a greater proportion of larger vessels, are more vulnerable to air embolisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serio, F.; Imbriani, G.; Girelli, C.R.; Miglietta, P.P.; Scortichini, M.; Fanizzi, F.P. A Decade after the Outbreak of Xylella fastidiosa subsp. pauca in Apulia (Southern Italy): Methodical Literature Analysis of Research Strategies. Plants 2024, 13, 1433. https://doi.org/10.3390/plants13111433
Serio F, Imbriani G, Girelli CR, Miglietta PP, Scortichini M, Fanizzi FP. A Decade after the Outbreak of Xylella fastidiosa subsp. pauca in Apulia (Southern Italy): Methodical Literature Analysis of Research Strategies. Plants. 2024; 13(11):1433. https://doi.org/10.3390/plants13111433
Chicago/Turabian StyleSerio, Francesca, Giovanni Imbriani, Chiara Roberta Girelli, Pier Paolo Miglietta, Marco Scortichini, and Francesco Paolo Fanizzi. 2024. "A Decade after the Outbreak of Xylella fastidiosa subsp. pauca in Apulia (Southern Italy): Methodical Literature Analysis of Research Strategies" Plants 13, no. 11: 1433. https://doi.org/10.3390/plants13111433
APA StyleSerio, F., Imbriani, G., Girelli, C. R., Miglietta, P. P., Scortichini, M., & Fanizzi, F. P. (2024). A Decade after the Outbreak of Xylella fastidiosa subsp. pauca in Apulia (Southern Italy): Methodical Literature Analysis of Research Strategies. Plants, 13(11), 1433. https://doi.org/10.3390/plants13111433











