Combined Effect of Biological and Organic Fertilizers on Agrobiochemical Traits of Corn (Zea mays L.) under Wastewater Irrigation
Abstract
1. Introduction
2. Results
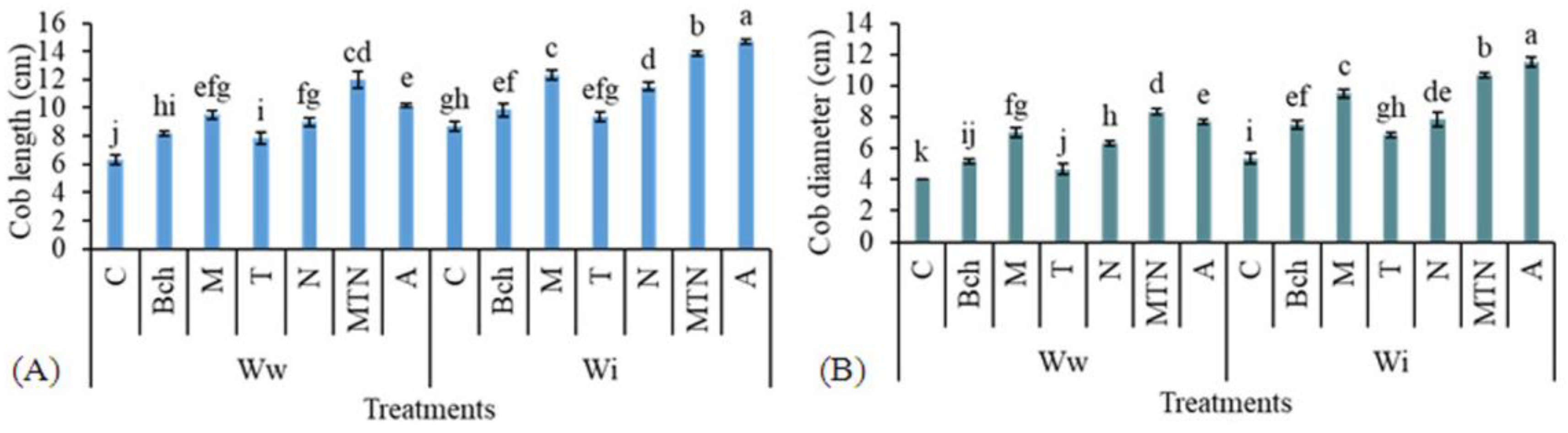
2.1. Cob Length
2.2. Cob Diameter
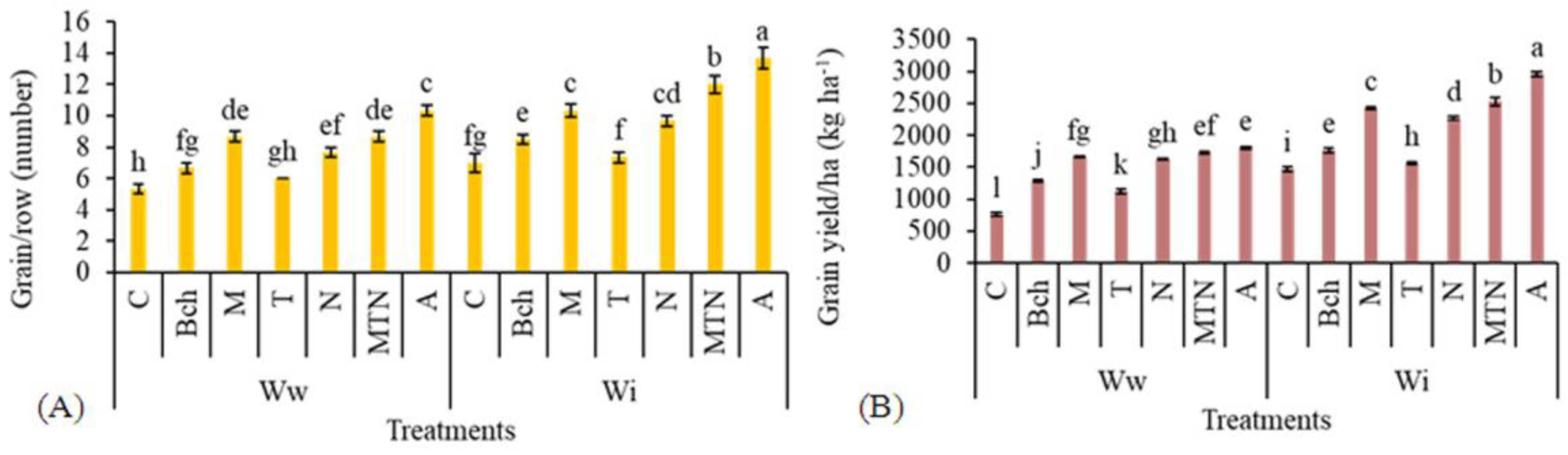
2.3. Number of Grains per Row
2.4. Grain Yield per ha
2.5. Antioxidant Indices
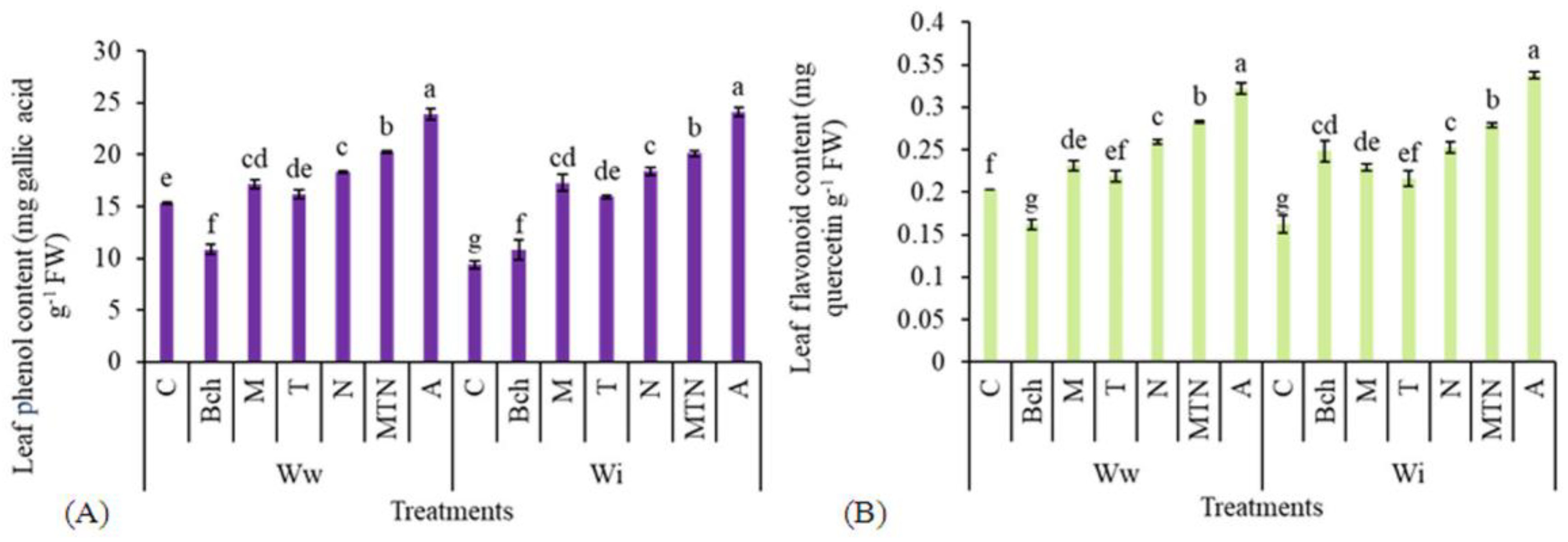
2.5.1. Leaf Phenols
2.5.2. Leaf Flavonoids
2.5.3. Polyphenolic Profile
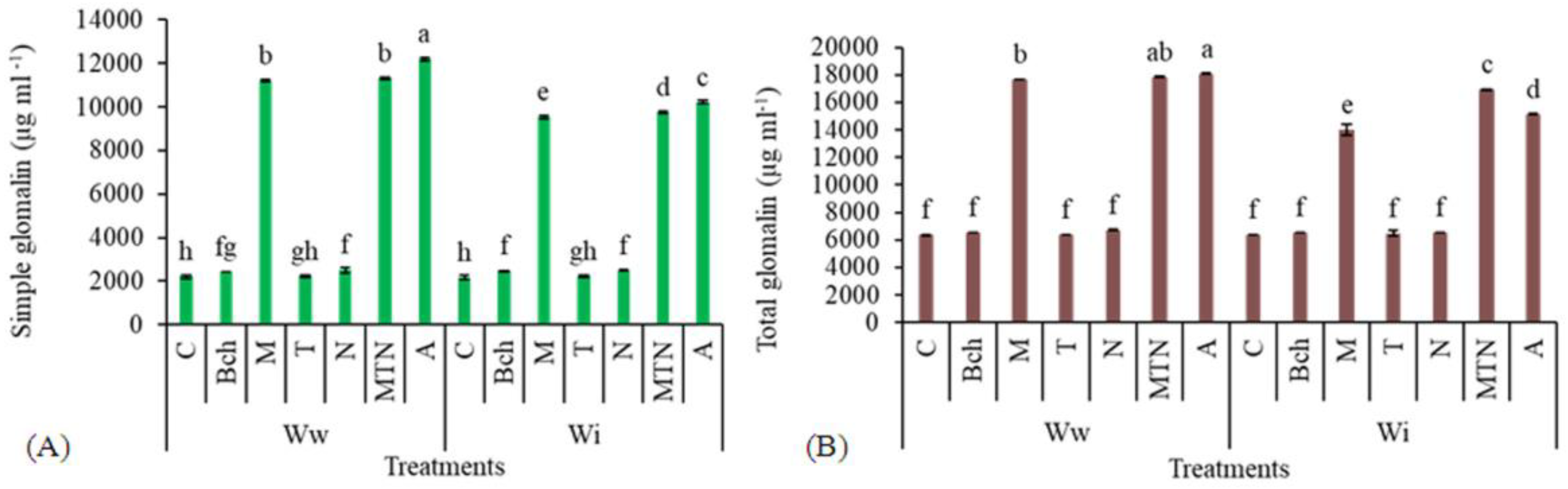
2.6. Simple Glomalin
2.7. Total Glomalin
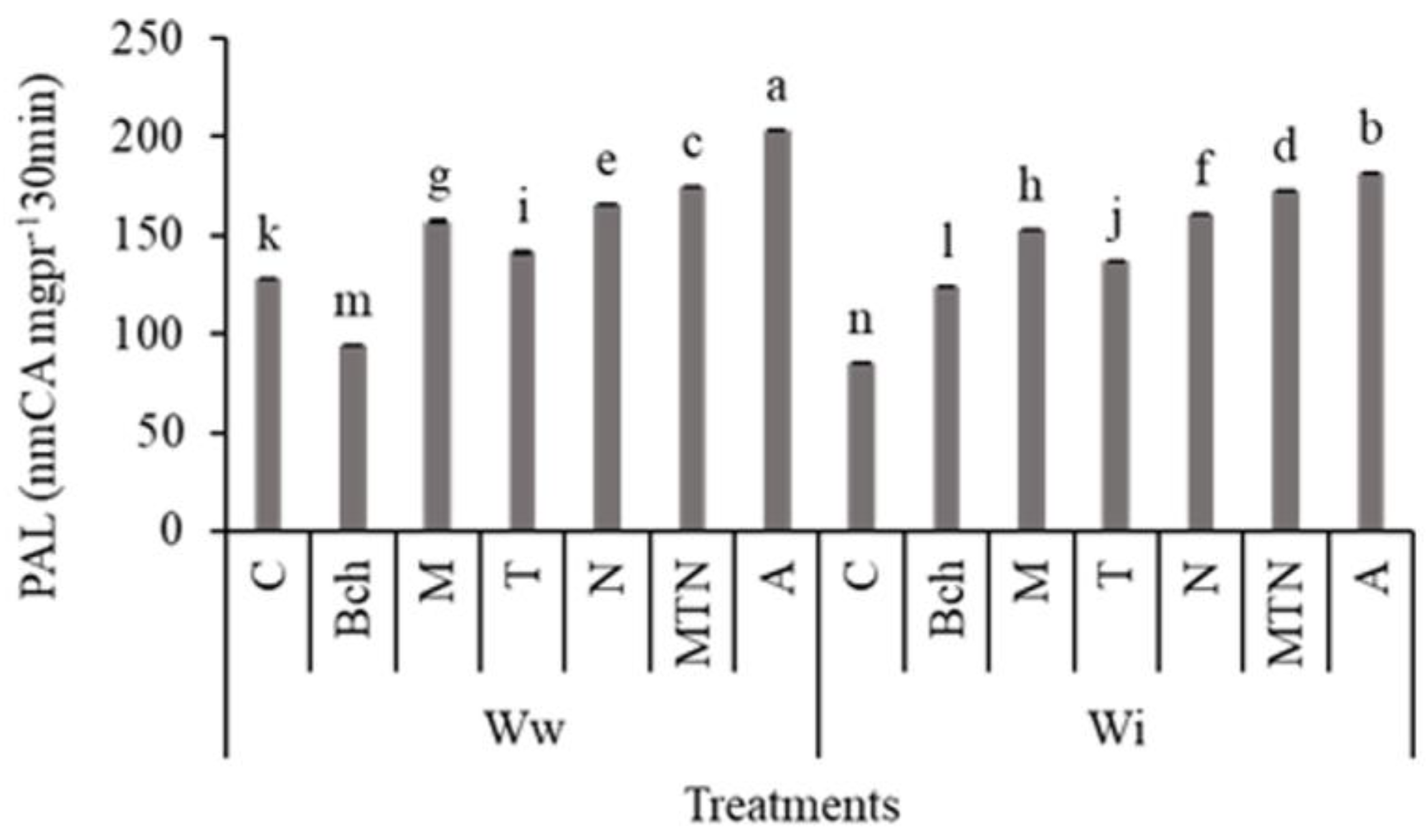
2.8. Phenylalanine Ammonia-Lyase (PAL)
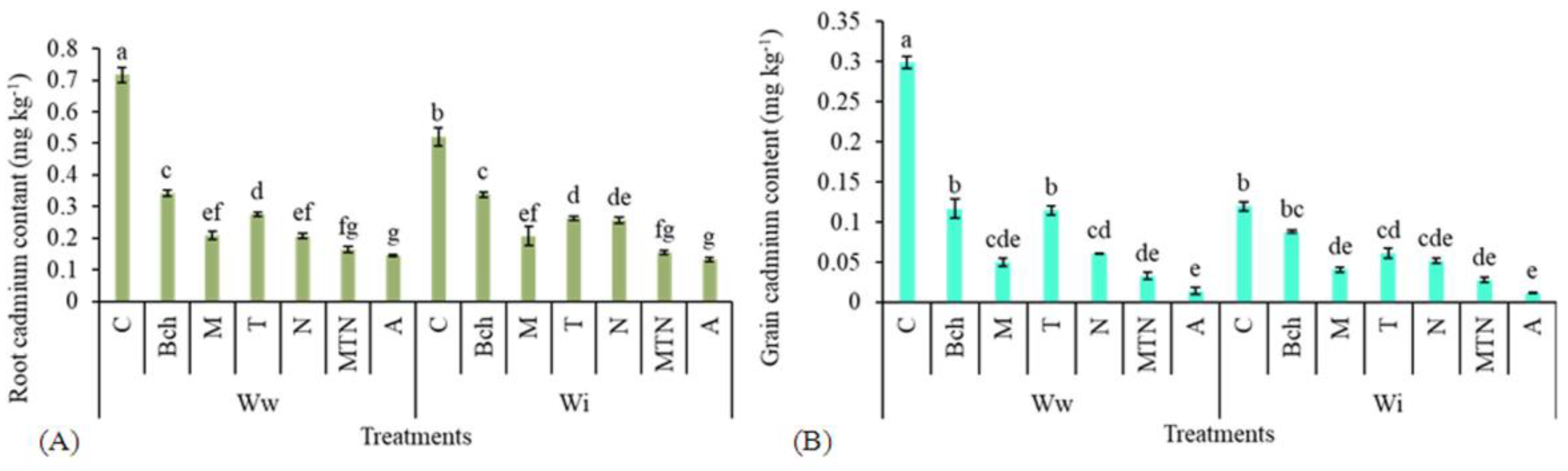
2.9. Cadmium Content in Root and Grain
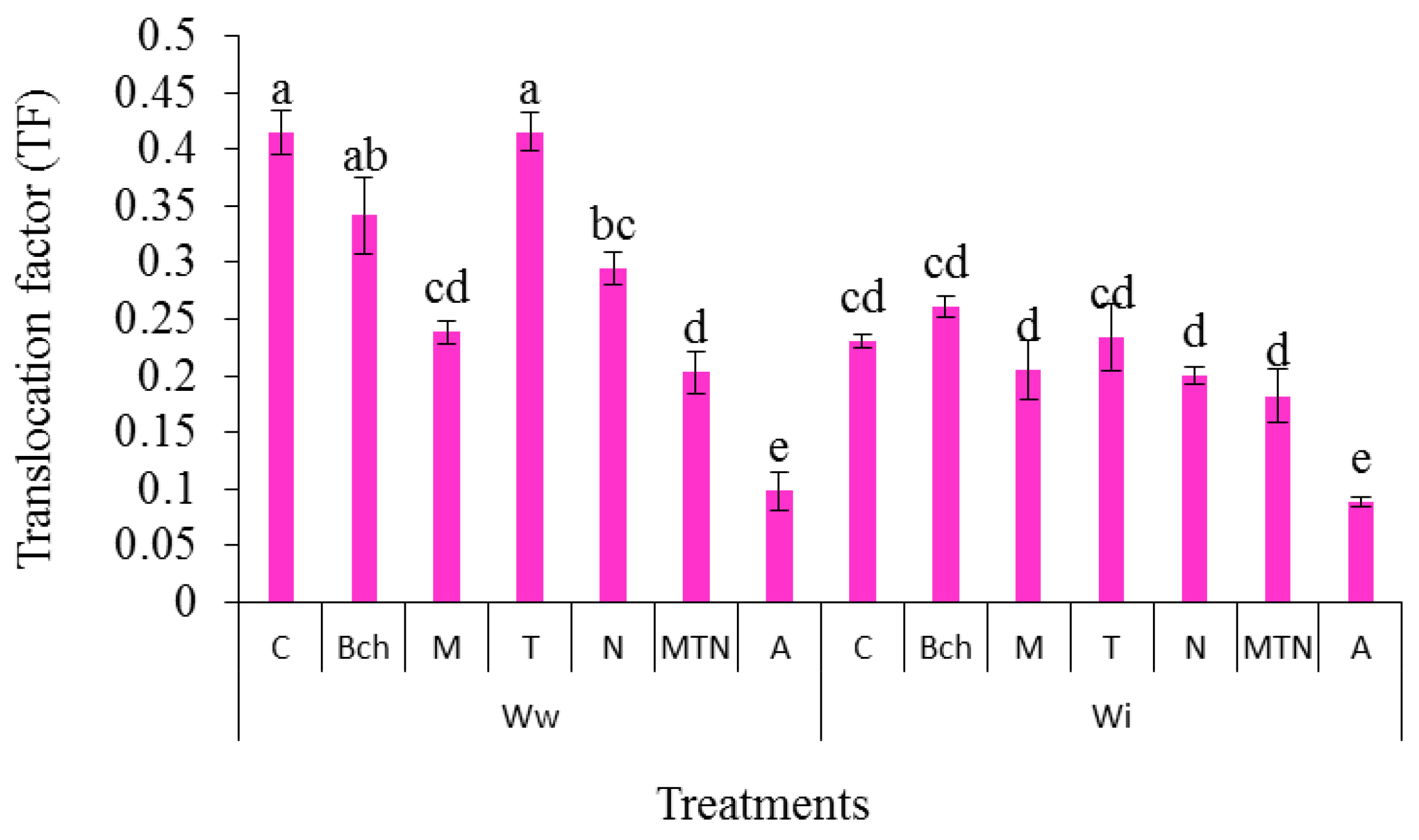
2.10. Translocation Factor (TF) from the Roots to the Grains
2.11. ANOVA of Morphological and Biochemical Traits
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Soil Preparation and Planting
4.3. Experimental Design
4.4. Measurement of Morphological Traits
4.5. Measurement of Total Phenol Content in Leaves
4.6. Measurement of Flavonoid Content in Leaves
4.7. Extraction of Polyphenols
4.8. Measurement of Glomalin
4.9. Measurement of Phenylalanine Ammonia-Lyase (PAL) Activity
4.10. Measurement of Cadmium Content
4.11. Translocation Factor (TF)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ajmal, M.; Ali, H.I.; Saeed, R.; Akhtar, A.; Tahir, M.; Mehboob, M.Z.; Ayub, A. Biofertilizer as an alternative for chemical fertilizers. Acad. J. Agric. Res. 2018, 6, 299–306. [Google Scholar]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef]
- Kumar, S.; Basavanneppa, M.A.; Koppalkar, B.G.; Umesh, M.R.; Gaddi, A.K. Precision nitrogen management in maize (Zea mays L.) through leaf colour chart tool in Tunga Bhadra Command area. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 43–46. [Google Scholar]
- Chandra, V. (Ed.) Technology, Adaptation, and Exports: How Some Developing Countries Got It Right; World Bank Publications: Washington, DC, USA, 2006. [Google Scholar]
- Parihar, C.M.; Kumar, B.; Jat, S.L.; Singh, A.K.; Jat, M.L.; Chaudhary, V.; Dass, S. Specialty corn for nutritional security and dietary diversification. In Biofortification of Food Crops; Singh, U., Praharaj, C., Singh, S., Singh, N., Eds.; Springer: New Delhi, India, 2018; pp. 387–398. [Google Scholar]
- Kumar, T.K.; Venkateswarlu, B. Baby corn (Zea mays L.) performance as vegetable-cum-fodder in intercropping with legume fodders under different planting patterns. Range Manag. Agrofor. 2013, 34, 137–141. [Google Scholar]
- Devi, S.; Poonia, P.K.; Kumar, V.; Tiwari, A.; Meena, R.K.; Kumar, U.; Gulnaz, A.; Al-Sadoon, M.K. Characterization of natural fiber extracted from corn (Zea mays L.) stalk waste for sustainable development. Sustainability. 2022, 14, 16605. [Google Scholar] [CrossRef]
- Kiziloglu, F.M.; Turan, M.; Sahin, U.; Kuslu, Y.; Dursun, A. Effects of untreated and treated wastewater irrigation on some chemical properties of cauliflower (Brassica olerecea L. var. botrytis) and red cabbage (Brassica olerecea L. var. rubra) grown on calcareous soil in Turkey. Agric. Manag. Water 2008, 95, 716–724. [Google Scholar] [CrossRef]
- Papadopoulos, I. Wastewater Management for Agriculture Protection in the Near East Region; Technical Bulletin; FAO, Regional Office for the Near East: Cairo, Egypt, 1995. [Google Scholar]
- Mohammad, M.J.; Mazahreh, N. Changes in soil fertility parameters in response to irrigation of forage crops with secondary treated wastewater. Commun. Soil. Sci. Plant Anal. 2003, 34, 1281–1294. [Google Scholar] [CrossRef]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—A case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Khan, M.A.; Shaukat, S.S.; Khan, M.A. Economic benefits from irrigation of maize with treated effluent of waste stabilization ponds. Pak. J. Bot. 2008, 40, 1091–1098. [Google Scholar]
- Eissa, M.A.; Negim, O.E. Heavy metals uptake and translocation by lettuce and spinach grown on a metal-contaminated soil. J. Soil. Sci. Plant Nutr. 2018, 18, 1097–1107. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and plant development: An agony from seed to seed. Int. J. Mol. Sci. 2019, 20, 3971. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Alharbi, S.; Ali, E.F.; Ghoneim, A.M.; Hadi Al Fahd, M.; Wang, G.; Eissa, M.A. Effect of phosphorus-loaded biochar and nitrogen-fertilization on release kinetic of toxic heavy metals and tomato growth. Int. J. Phytorem. 2022, 24, 156. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; Ning, X.; Wall, D.; Murphy, J.D. Co-production of hydrochar, levulinic acid and value-added chemicals by microwave-assisted hydrothermal carbonization of seaweed. Chem. Eng. J. 2022, 441, 135915. [Google Scholar] [CrossRef]
- Rasam, S.; Talebkeikhah, F.; Talebkeikhah, M.; Salimi, A.; Moraveji, M.K. Physico-chemical properties prediction of hydrochar in macroalgae Sargassum horneri hydrothermal carbonisation. Int. J. Environ. Anal. Chem. 2021, 101, 2297–2318. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Iannazzo, D.; Len, T.; Balu, A.M.; Morabito, M.; Genovese, G.; Espro, C.; Bressi, V. Hydrochar from Sargassum muticum: A sustainable approach for high-capacity removal of Rhodamine B dye. RSC Sustain. 2023, 1, 1404–1415. [Google Scholar] [CrossRef]
- Silva, R.N.; Monteiro, V.N.; Steindorff, A.S.; Gomes, E.V.; Noronha, E.F.; Ulhoa, C.J. Trichoderma/pathogen/plant interaction in pre-harvest food security. Fungal Biol. 2019, 123, 565–583. [Google Scholar] [CrossRef]
- Banerjee, S.; Roy, P.; Nandi, S.; Roy, S. Advanced biotechnological strategies towards the development of crops with enhanced micronutrient content. Plant Growth Regul. 2023, 100, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Maldaner, J.; Steffen, G.P.K.; Missio, E.L.; Saldanha, C.W.; de Morais, R.M.; Nicoloso, F.T. Tolerance of Trichoderma isolates to increasing concentrations of heavy metals. Int. J. Environ. Stud. 2021, 78, 185–197. [Google Scholar] [CrossRef]
- Mohammadian, E.; Ahari, A.B.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Abdenaceur, R.; Farida, B.; Mourad, D.; Rima, H.; Zahia, O.; Fatma, S.H. Effective biofertilizer Trichoderma spp. Isolates with enzymatic activity and metabolites enhancing plant growth. Int. Microbiol. 2022, 25, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Russo, V.; Manghisi, A.; Di Martino, A.; Morabito, M.; Genovese, G.; Trifilò, P. Screening on the Presence of Plant Growth Regulators in High Biomass Forming Seaweeds from the Ionian Sea (Mediterranean Sea). Sustainability. 2022, 14, 3914. [Google Scholar] [CrossRef]
- Rafi, M.; Febriany, S.; Wulandari, P.; Suparto, I.H.; Ridwan, T.; Rahayu, S.; Siswoyo, D.M. Total phenolics, flavonoids, and anthocyanin contents of six Vireya Rhododendron from Indonesia and evaluation of their antioxidant activities. J. Appl. Pharm. Sci. 2018, 8, 049–054. [Google Scholar]
- Garcia, C.; Blesso, C.N. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2011, 172, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current state of knowledge on the antioxidant effects and mechanisms of action of polyphenolic compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Schindler, F.V.; Mercer, E.J.; Rice, J.A. Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil. Biol. Biochem. 2007, 39, 320–329. [Google Scholar] [CrossRef]
- Atakan, A.; Özkaya, H.Ö. Arbuscular mycorrhizal fungi and glomalin. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 2371–2375. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Rahali, F.Z.; Msaada, K.; Ksouri, R.; Marzouk, B. Relation between salt tolerance and biochemical changes in cumin (Cuminum cyminum L.) seeds. J. Food Drug Anal. 2017, 25, 391–402. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, B.K. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef]
- Hemm, M.R.; Rider, S.D.; Ogas, J.; Murry, D.J.; Chapple, C. Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J. 2004, 38, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Tahsili, J.; Sharifi, M.; Safaie, N.; Esmaeilzadeh-Bahabadi, S.; Behmanesh, M. Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. J. Plant Interact. 2014, 9, 412–417. [Google Scholar] [CrossRef]
- Rangnekar, S.S.; Sahu, S.K.; Pandit, G.G.; Gaikwad, V.B. Study of uptake of Pb and Cd by three nutritionally important Indian vegetables grown in artificially contaminated soils of Mumbai, India. Int. Res. J. Environ. Sci. 2013, 2, 1–5. [Google Scholar]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of Earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Gupta, A.; Joia, J.; Sood, A.; Sood, R.; Sidhu, C.; Kaur, G. Microbes as potential tool for remediation of heavy metals: A review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar] [CrossRef]
- Mansour, S.A. Heavy metals of special concern to human health and environment, In Practical Food Safety: Contemporary Issues and Future Directions; Bhat, R., Gómez-López, V.M., Eds.; Wiley: New York, NY, USA, 2014; pp. 213–233. [Google Scholar]
- Nazir, R.; Khan, M.; Masab, M.; Rehman, H.U.; Rauf, N.U.; Shahab, S.; Ameer, N.; Sajed, M.; Ullah, M.; Rafeeq, M.; et al. Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam Kohat. J. Pharm. Sci. Res. 2015, 7, 89. [Google Scholar]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants. 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rathore, D. Role of transitory starch on growth, development and metal accumulation of Triticum aestivum cultivars grown under textile effluent fertilization. Environ. Sci. Pollut. Res. 2020, 27, 24201–24217. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Monteiro, C.; Moutinho-Pereira, J.; Correia, C.; Gonçalves, B.; Santos, C. Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol. Plant. 2013, 35, 1281–1289. [Google Scholar] [CrossRef]
- Swamy, K.N.; Anuradha, S.; Ramakrishna, B.; Siddulu, N.; Rao, S.S.R. Cadmium toxicity is diminished by 24- epibrassinolide in seedlings of Trigonella foenum—graecum L. Genet. Plant Physiol. 2011, 1, 163–175. [Google Scholar]
- Hassan, F.A.; Ali, H.M. Impact of irrigation with sewage effluent on the growth and wood properties of two forest tree seedlings. J. For. Prod. Ind. 2013, 2, 40–44. [Google Scholar]
- Mousa, M.A.; Abo-Elyousr, K.A.; Ibrahim, O.H.; Alshareef, N.O.; Eissa, M.A. Shrimp-waste-derived biochar induces metal toxicity tolerance of wastewater-irrigated quinoa (Chenopodium quinoa). Agric. 2022, 12, 1748. [Google Scholar] [CrossRef]
- Zen El-Dein, A.A.M.; El-Sorady, G.A.; Salama, N.G. Growth and productivity of maize in relation to preceding crops. Egyptian Acad. J. Biol. Sci. H. 2021, 12, 135–145. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Pichtel, J. Mycorrhizae: An Overview. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z.A., Akhtar, M.S., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–35. [Google Scholar]
- Nakmee, P.S.; Techapinyawat, S.; Ngamprasit, S. Comparative potentials of native arbuscular mycorrhizal fungi to improve nutrient uptake and biomass of Sorghum bicolor Linn. Agric. Nat. Resour. 2016, 50, 173–178. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic press: Cambridge, MA, USA, 2010. [Google Scholar]
- Albqmi, M.; Selim, S.S.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; Jaouni, S.K.A.; Hussein, S.; Warrad, M.; Sofy, M.R.; AbdElgawad, H. Interactive effect of arbuscular mycorrhizal fungi (AMF) and olive solid waste on wheat under arsenite toxicity. Plants 2023, 12, 1100. [Google Scholar] [CrossRef]
- Kanwal, S.; Bano, A.; Malik, R.N. Effects of arbuscular mycorrhizal fungi on wheat growth, physiology, nutrition and cadmium uptake under increasing cadmium stress. Int. J. Agron. Agric. Res. 2015, 7, 30–42. [Google Scholar]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytorem. 2019, 21, 663–671. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Heavy metal phytoremediation from a meta-analytical perspective. Environ. Pollut. 2007, 147, 231–237. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.; Abeed, A.H. Growth and biochemical changes in quail bush (Atriplex lentiformis (Torr.) S. Wats) under Cd stress. Environ. Sci. Pollut. Res. 2019, 26, 628–635. [Google Scholar] [CrossRef]
- Bashir, S.; Qayyum, M.A.; Husain, A.; Bakhsh, A.; Ahmed, N.; Hussain, M.B.; Elshikh, M.S.; Alwahibi, M.S.; Almunqedhi, B.M.; Hussain, R.; et al. Efficiency of different types of biochars to mitigate Cd stress and growth of sunflower (Helianthus; L. ) in wastewater irrigated agricultural soil. Saudi J. Biol. Sci. 2021, 28, 2453–2459. [Google Scholar]
- Afshari, H.; Esmaeeli, V.; Khazaee, M. Effects of various concentrations of trichoderma harzianum fungus on the phytochemical and antioxidative properties of cauliflower (Brassica oleracea. Convar. botrytis L.) in the soils contaminated with lead. J. Nutr. Fasting Health 2018, 6, 35–44. [Google Scholar]
- Štolfa, I.; Pfeiffer, T.Z.; Špoljarić, D.; Teklić, T.; Lončarić, Z. Heavy Metal-Induced Oxidative Stress in Plants: Response of the Antioxidative System. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 127–163. [Google Scholar]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Abeed, A.H.A.; Mahdy, R.E.; Alshehri, D.; Hammami, I.; Eissa, M.A.; Abdel Latef, A.A.H.; Mahmoud, G.A. Induction of resilience strategies against biochemical deteriorations prompted by severe cadmium stress in sunflower plant when Trichoderma and bacterial inoculation were used as biofertilizers. Front. Plant. Sci. 2022, 13, 1004173. [Google Scholar] [CrossRef]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L.). Ind. Crops Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Cuellar, M.; Baroni, V.; Pfaffen, V.; Griboff, J.; Ortiz, P.; Monferrán, M.V. Uptake and accumulation of Cr in edible parts of Eruca sativa from irrigation water. Effects on polyphenol profile and antioxidant capacity. Heliyon 2021, 7, e06086. [Google Scholar] [CrossRef] [PubMed]
- Gendy, A.S.; Said-Al Ahl, H.A.; Mahmoud, A.A.; Mohamed, H.F. Effect of nitrogen sources, bio-fertilizers and their interaction on the growth, seed yield and chemical composition of guar plants. Life Sci. J. 2013, 10, 389–402. [Google Scholar]
- Mahdavikia, H.; Rezaei-Chiyaneh, E.; Rahimi, A.; Mohammadkhani, N. Effects of fertilizer treatments on antioxidant activities and physiological traits of basil (Ocimum basilicum L.) under water limitation conditions. J. Med. Plants By-Prod. 2019, 8, 143–151. [Google Scholar]
- Karimi, G.; Pourakbar, L.; Siavash Moghaddam, S.; Rezaee Danesh, Y.; Popović-Djordjević, J. Effectiveness of fungal bacterial biofertilizers on agrobiochemical attributes of quinoa (Chenopodium quinoa willd.) under salinity stress. Int. J. Environ. Sci. Technol. 2022, 19, 11989–12002. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant. Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Lu, C.C.; Guo, N.; Yang, C.; Sun, H.B.; Cai, B.Y. Transcriptome and metabolite profiling reveals the effects of Funneliformis mosseae on the roots of continuously cropped soybeans. BMC Plant Biol. 2020, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Q.; Liu, G.; Xu, N.; Yang, Y.; Zeng, W.; Chen, A.; Wang, S. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Funct. Plant Biol. 2018, 46, 30–43. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil. Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil. Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Luo, X.; Chen, W.; Huang, Q. Arbuscular mycorrhizal-like fungi and glomalin-related soil protein drive the distributions of carbon and nitrogen in a large scale. J. Soils Sediments 2020, 20, 963–972. [Google Scholar] [CrossRef]
- Balík, J.; Sedlář, O.; Kulhánek, M.; Černý, J.; Smatanová, M.; Suran, P. Effect of organic fertilisers on glomalin content and soil organic matter quality. Plant, Soil. Environ. 2020, 66, 590–597. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wright, S.F.; Nichols, K.A.; Schmidt, W.F.; Torn, M.S. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil. 2001, 233, 167–177. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef] [PubMed]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-Mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ben Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Raja, B.L.; Ait-El-Mokhtar, M.; Mohamed, A.; Abderrahim, B.; Youssef, A.R.; Anas, R.; Khalid, O.; Said, W.; Abdelilah, M. Green compost combined with mycorrhizae and rhizobia: A strategy for improving alfalfa growth and yield under field conditions. Gesunde Pflanz. 2021, 73, 193–207. [Google Scholar]
- Ben-Laouane, R.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Niedźwiecki, J.; Grządziel, J.; Gawryjołek, K. Evaluation of changes in Glomalin-Related Soil Proteins (GRSP) content, microbial diversity and physical properties depending on the type of soil as the important biotic determinants of soil quality. Agronomy 2020, 10, 1279. [Google Scholar] [CrossRef]
- Vlček, V.; Pohanka, M. Glomalin—An interesting protein part of the soil organic matter. Soil. Water Res. 2020, 15, 67–74. [Google Scholar] [CrossRef]
- Šarapatka, B.; Alvarado-Solano, D.P.; Čižmár, D. Can glomalin content be used as an indicator for erosion damage to soil and related changes in organic matter characteristics and nutrients? Catena 2019, 181, 104078. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Farzand, A.; Heng, Z.; Khan, I.U.; Moosa, A.; Zubair, M.; Na, Y.; Ying, S.; Canming, T. Antagonistic potential of novel endophytic Bacillus strains and mediation of plant defense against Verticillium wilt in upland cotton. Plants 2020, 9, 1438. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Saxena, A.K.; Singh, J.S.; Singh, D.P. Impact of native ST-PGPR (Bacillus pumilus; EU927414) on PGP traits, antioxidants activities, wheat plant growth and yield under salinity. Clim. Chang. Environ. Sustain. 2019, 7, 157–168. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Li, B.B.; Fu, B.Y.S.; Li, X.X.; Yin, H.N.; Xi, Z.M. Brassinosteroids alleviate cadmium phytotoxicity by minimizing oxidative stress in grape seedlings: Toward regulating the ascorbate-glutathione cycle. Sci. Hortic. 2022, 299, 111002. [Google Scholar] [CrossRef]
- Azadi, N.; Raiesi, F. Biochar alleviates metal toxicity and improves microbial community functions in a soil co-contaminated with cadmium and lead. Biochar 2021, 3, 485–498. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon. Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Gao, M.Y.; Chen, X.W.; Huang, W.X.; Wu, L.; Yu, Z.S.; Xiang, L.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Cell wall modification induced by an arbuscular mycorrhizal fungus enhanced cadmium fixation in rice root. J. Hazard. Mater. 2021, 416, 125894. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 2016, 26, 133–140. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Meng, S.; Wu, F.; Liu, T. Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii Rupr. at a moderate level of salinity. PLoS ONE 2020, 15, e0231497. [Google Scholar] [CrossRef]
- Nogueirol, R.C.; Monteiro, F.A.; de Souza Junior, J.C.; Azevedo, R.A. NO3−/NH4+ proportions affect cadmium bioaccumulation and tolerance of tomato. Environ. Sci. Pollut. Res. 2018, 25, 13916–13928. [Google Scholar] [CrossRef] [PubMed]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Alves, L.R.; Monteiro, C.C.; Carvalho, R.F.; Ribeiro, P.C.; Tezotto, T.; Azevedo, R.A.; Gratão, P.L. Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ. Exp. Bot. 2017, 134, 102–115. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, S.K.; Pathak, R.K.; Gupta, V. Accumulation of heavy metals in soil and paddy crop (Oryza sativa), irrigated with water of Ramgarh Lake, Gorakhpur, UP, India. Toxicol. Environ. Chem. 2011, 93, 462–473. [Google Scholar] [CrossRef]
- Khermandar, K.; Mahdavi, A. The study of cadmium uptake and accumulation in Acacia victoriae three months old seedlings. J. Water Soil Resour. Conserv. 2017, 6, 121–135. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. (Eds.) Part 3: Chemical methods. In Methods of Soil Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 14. [Google Scholar]
- Eaton, A.D.; Franson, M.A.H. Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Ribarova, F.; Atanassova, M.; Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Metal. 2005, 40, 255–260. [Google Scholar]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Turnau, K.; Ryszka, P.; Gianinazzi-Pearson, V.; Van Tuinen, D. Identification of arbuscular mycorrhizal fungi in soils and roots of plants colonizing zinc wastes in southern Poland. Mycorrhiza 2001, 10, 169–174. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Radhika, K.; Mathiyazhagan, S.; Bhaskaran, R.R.; Samiyappan, R.; Velazhahan, R. Induction of phenolics and defense-related enzymes in coconut (Cocos nucifera L.) roots treated with biocontrol agents. Braz. J. Plant Physiol. 2006, 18, 367–377. [Google Scholar] [CrossRef]
- Fayiga, A.Q.; Ma, L.Q. Using phosphate rock to immobilize metals in soils and increase arsenic uptake in Pteris vittata. Sci. Total Environ. 2006, 359, 17–25. [Google Scholar] [CrossRef]
| Sources of Variation | df | Cob Length (cm) | Cob Diameter (cm) | Grains/Row (Number) | Grain Yield/ha (kg ha−1) | Leaf Phenols (mg g−1 FW 1) | Leaf Flavonoids (mg g−1 FW) | Simple Glomalin (µg ml−1) | Total Glomalin (µg ml−1) | PAL 2 (nmCA mgpr−1 30 min) | Root Cd Content (mg kg−1) | Grain Cd Content (mg kg−1) | TF 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Means of squares | |||||||||||||
| Replication | 2 | 1.79 ** | 1.79 ** | 1.68 * | 19,804.1 ** | 2.08 * | 0.0001 ns | 32,011.1 ns | 74,240 ns | 0.043 ns | 0.002 ns | 0.0002 ns | 0.001 ns |
| Irrigation (A) | 1 | 63.14 ** | 54.85 ** | 49.29 ** | 5,367,862.5 ** | 7.42 ** | 0.0004 ns | 5,833,314.1 ** | 12,126,324 ** | 573.76 ** | 0.00005 ns | 0.017 ** | 0.0007 ns |
| Fertilizers (B) | 6 | 24.14 ** | 21.27 ** | 25.99 ** | 1,271,598.6 ** | 121.05 ** | 0.015 ** | 12,051,206.1 ** | 176,962,602 ** | 6203.15 ** | 0.194 ** | 0.026 ** | 0.566 ** |
| A × B | 6 | 1.56 ** | 0.996 ** | 1.01 * | 84,981.94 ** | 7.44 ** | 0.002 ** | 13,131,508.5 ** | 3,727,531 ** | 727.51 ** | 0.021 ** | 0.006 ** | 0.198 ** |
| Experimental error | 26 | 0.21 | 0.98 | 0.39 | 1963.14 | 0.549 | 0.0001 | 100,982.6 | 41,855 | 0.045 | 0.001 | 0.0004 | 0.002 |
| CV 4 | 4.53 | 4.29 | 7.23 | 4.48 | 4.36 | 4.58 | 1.77 | 1.89 | 1.77 | 12.05 | 28.08 | 15.60 | |
| pH | EC 1 (ds/m) | Sand (g/kg) | Silt (g/kg) | Clay (g/kg) | Total N (g/kg) | Available P (mg/kg) | Available K (mg/kg) | OC 2 (g/kg) |
|---|---|---|---|---|---|---|---|---|
| 8.1 | 1.05 | 130 | 300 | 570 | 3.4 | 27 | 235 | 32 |
| pH | EC 1 (dS/m) | TDS 2 (mg/L) |
|---|---|---|
| 7.3 | 1.36 | 820 |
| N (g/kg) | P (g/kg) | K (g/kg) | EC 1 (ds/m) | OC 2 (g/kg) | pH | C/N |
|---|---|---|---|---|---|---|
| 8.6 | 3.1 | 3.9 | 0.74 | 550 | 8.15 | 57.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirzad, H.; Siavash Moghaddam, S.; Rahimi, A.; Rezapour, S.; Xiao, J.; Popović-Djordjević, J. Combined Effect of Biological and Organic Fertilizers on Agrobiochemical Traits of Corn (Zea mays L.) under Wastewater Irrigation. Plants 2024, 13, 1331. https://doi.org/10.3390/plants13101331
Shirzad H, Siavash Moghaddam S, Rahimi A, Rezapour S, Xiao J, Popović-Djordjević J. Combined Effect of Biological and Organic Fertilizers on Agrobiochemical Traits of Corn (Zea mays L.) under Wastewater Irrigation. Plants. 2024; 13(10):1331. https://doi.org/10.3390/plants13101331
Chicago/Turabian StyleShirzad, Hossein, Sina Siavash Moghaddam, Amir Rahimi, Salar Rezapour, Jianbo Xiao, and Jelena Popović-Djordjević. 2024. "Combined Effect of Biological and Organic Fertilizers on Agrobiochemical Traits of Corn (Zea mays L.) under Wastewater Irrigation" Plants 13, no. 10: 1331. https://doi.org/10.3390/plants13101331
APA StyleShirzad, H., Siavash Moghaddam, S., Rahimi, A., Rezapour, S., Xiao, J., & Popović-Djordjević, J. (2024). Combined Effect of Biological and Organic Fertilizers on Agrobiochemical Traits of Corn (Zea mays L.) under Wastewater Irrigation. Plants, 13(10), 1331. https://doi.org/10.3390/plants13101331










