Abstract
The Duboisia species, a group of plants native to Australia, have been historically valued for their pharmacological properties and have played a significant role in traditional medicine and pharmaceutical research. Persistent efforts are underway to enhance the efficacy of the active ingredient scopolamine, employing both conventional breeding methods and advanced biotechnology tools. The primary objective of this research was to establish a highly efficient method for isolating mesophyll protoplasts and facilitating their regeneration, thereby laying a robust foundation for the application of various advanced plant biotechnology tools in the pursuit of genetic enhancement. The mesophyll protoplast isolation process was developed for hybrid D. myoporoides × D. hopwoodii with careful optimisation of the following parameters: leaf strip size; incubation conditions; physical treatment; and enzyme concentration. The optimal parameters were combined in each individual step; the best enzyme concentration was determined to be 2% (w/v) cellulysin and 0.5% (w/v) macerase. Protoplast yield was found to be greatly affected by the enzyme concentrations. The isolated protoplasts were cultured at a density of 0.5 × 105 to best sustain the highest cell division (33.2%) and a microcalli induction frequency of 17.9%. After 40 days of culture in a modified KM8P medium at 25 °C in darkness, visible microcalli were transferred to a solidified Murashige and Skoog (MS) medium with 1 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D) for callus induction under a 16 h photoperiod. After 30 days of culture, compact organogenic calli were transferred into a solid MS medium with 6-benzylaminopurine (BA) alone or thidiazuron (TDZ) alone or in combination with BA or naphthalene acetic acid (NAA) for shoot regeneration. The maximum shoot regeneration frequency (63.3%) was observed in the medium with 1.5 mg L−1 TDZ alone. For the first time, a reliable protoplast isolation and regeneration system from mesophyll cells was established for Duboisia with high protoplast viability, successful microcalli formation, and intact plant regeneration. This innovation will significantly contribute towards the genetic enhancement of the Duboisia species.
1. Introduction
A plant cell devoid of a cell wall is termed protoplast and offers great potential for the genetic enhancement of many plant species via the fusion of such cells or by providing an easy platform for incorporating foreign DNA during genetic transformation [1]. Protoplasts also provide a one-of-a-kind possibility for medicinal species to be genetically enhanced. Bulk DNA transfer by protoplast fusion may result in novel germplasm with synergistic combinations of secondary metabolites or increased metabolites production [2]. Additionally, direct gene transfer through protoplasts is a rapid and efficient approach to introducing agronomically advantageous traits [3]. Recent scientific advances have made it possible to edit genes without chromosomal insertion by utilising clustered regularly interspaced short palindromic repeats (CRISPR)-mediated transient expression of RNPs and the regeneration of protoplasts [4]. This DNA-free genome editing system is ideal for generating foreign-DNA-free mutants delivering greater commercial value, unrestricted by current GMO legislations [5]. Despite the great potential and advantages of protoplast technology, regenerating plants from protoplasts is species-specific and remains the greatest obstacle to their widespread usage.
Establishing a reliable protoplast regeneration system is a prerequisite for the application of protoplast technologies, especially protoplast fusion and direct gene transfer [1]. Protoplast isolation is the first stage requiring an array of optimisations to achieve high-quality protoplasts with considerable yields. A protoplast can be isolated from different plant tissues and organs, such as leaf, suspension culture, hypocotyl, and cotyledon [6]. Protoplast isolation and regeneration success has been noted to be significantly influenced by both the choice of explant source and the specific genotype under consideration [7]. A suspension culture is widely used as a source material for protoplast isolation. However, the establishment of a suspension culture is time-consuming and associated with a high risk of somaclonal variation as well as the possibility of reducing the regeneration ability of cells [8]. Mesophyll cells are differentiated cells; thus, the direct use of mesophyll cells for protoplast isolation excludes many cycles of in vitro culture, reducing the risk of induced somaclonal variation [9]. Moreover, mesophyll protoplasts are the most commonly used protoplast for somatic hybridisation and efficient plant transformation, with simple and fast isolation procedures [10]. The vast majority of studies on mesophyll protoplast regeneration have been fruitful in herbaceous plants, such as rice [11], banana [12], and cabbage [13], whereas mesophyll protoplasts isolated from woody species have proven to be difficult to regenerate [14]. However, success in protoplast isolation and regeneration has been reported with several species, such as Passiflora edulis fv flavicarpa Degener. [15], Malus pumila [16], Morus indica [17], and Phellodendron amurense Rupr. [18].
The perennial shrub Duboisia (Solanaceae) is endemic to Australia [19]. This species contains abundant tropane alkaloids, scopolamine, and hyoscyamine, among other active ingredients [20]. Scopolamine, also identified as hyoscine, is the most commonly prescribed pharmaceutical for treating nausea, vomiting, motion sickness, and muscle spasms [21]. As the highest natural producer of scopolamine, Duboisia has been commercially cultivated for harvesting leaves for alkaloid extraction [22]. The growing demand for scopolamine exerts pressure on the Duboisia industry to provide consistent and sustainable raw scopolamine. The most sustainable way of increasing scopolamine production is through increasing the scopolamine content in leaves, which can be achieved through the development of new varieties via conventional breeding (taking over a decade to develop a variety) or through genetic manipulation with biotechnological tools. A simple and viable protoplast system would be highly beneficial to supporting future efforts in genetic enhancement to maximise scopolamine biosynthesis.
Duboisia protoplasts, with no exception, are recalcitrant to in vitro regeneration. Previous works on Duboisia protoplasts are only confined to cells from suspension cultures [23,24], where there is a high risk of induced somaclonal variation due to rapid cell division under high-hormone regimes. Mesophyll cells for protoplast preparation will alleviate the aforementioned issues; however, regenerating plants from mesophyll-derived protoplast is a formidable task. This study aimed to establish a reliable protoplast system using leaf tissues to avoid the high risk of mutation whilst achieving greater regeneration efficiency, building the foundation technology for future uses in Duboisia genetic enhancement approaches.
2. Results
2.1. Protoplast Isolation
2.1.1. Optimisation of Protoplast Digestion
Through a series of step-by-step experiments, a reliable protocol for the isolation of high-quantity and -quality protoplasts from Duboisia mesophyll tissues was established. The protocol includes several optimisation steps that were conducted in a progressive manner.
Factor 1–Leaf Strip Size
The first optimisation step focused on the size of the leaf strips used in the protoplast digestion process. Two different sizes, 0.5–1 mm and 2 mm (Figure 1a), were compared for protoplast release. Based on the microscopic observation, more protoplasts were observed in the leaf strips of size 0.5–1 mm compared to 2 mm (Figure 1b,c).
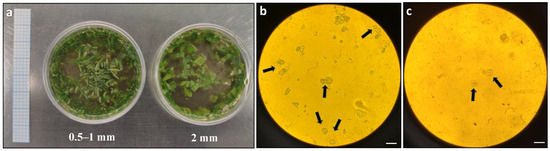
Figure 1.
Comparison of protoplast release from Duboisia leaves with different strip sizes: (a) different size of leaf strips in enzyme solution; (b) microscopic observation of protoplast release from 0.5–1 mm leaf strips; and (c) microscopic observation of protoplast release from 2 mm leaf strips. The arrows indicate the observed protoplasts under an Olympus BH2-RFCA light microscope with 20× magnification. Graph paper grid size = 2 × 2 mm; scale bars = 50 μm (b,c).
Factor 2–Physical Treatment
The next optimisation step involved testing the effects of physical treatment, specifically vacuum infiltration, on protoplast digestion. The results showed that vacuum infiltration was highly effective for protoplast digestion. In comparison to the control group without vacuum treatment, the vacuum-treated leaf strips released more protoplasts, which were more uniformly round and exhibited a better quality (Figure 2a). In contrast, the control group released fewer protoplasts, which exhibited irregular shapes and a lower overall quantity (Figure 2b).
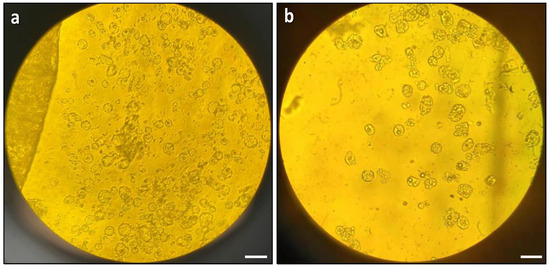
Figure 2.
Effects of vacuum infiltration on protoplast digestion: (a) microscopic observation of protoplast release with vacuum infiltration; and (b) microscopic observation of protoplast release without vacuum infiltration. The images were taken with an Olympus BH2-RFCA light microscope with 20× magnification. Scale bars = 50 μm.
Factor 3–Incubation Conditions
Shaking the leaf strips on an orbital shaker at 50 rpm during incubation severely damaged the protoplasts, resulting in membrane ruptures and irregular shapes (Figure 3b). In contrast, static incubation (no shaking) resulted in uniformly round and healthy protoplasts (Figure 3a) and was used for subsequent protoplast isolation experiments.
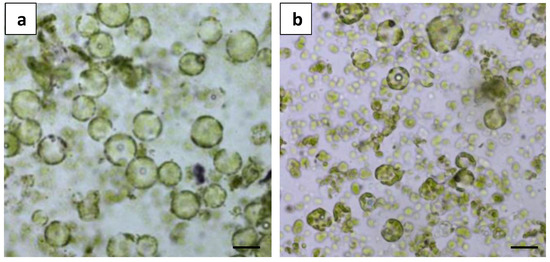
Figure 3.
Comparison of different incubation conditions on protoplast digestion: (a) microscopic observation of protoplast release from static incubation; and (b) microscopic observation of protoplast release from shake incubation at 50 rpm. Scale bars = 50 μm.
Factor 4–Digestion Duration
The duration of enzyme digestion emerged as a crucial factor affecting protoplast release. It was clear that a 16 h incubation period under dark condition was the optimal duration for digestion. The results demonstrated that protoplast release was not observed before 10 h of digestion, and only a few protoplasts with uniform round shapes and a healthy appearance were released at 10 h of digestion (Figure 4a). In contrast, a marked increase in protoplast release was observed at 16 h of digestion, with a substantial release of protoplasts which were uniformly round and healthy (Figure 4b).
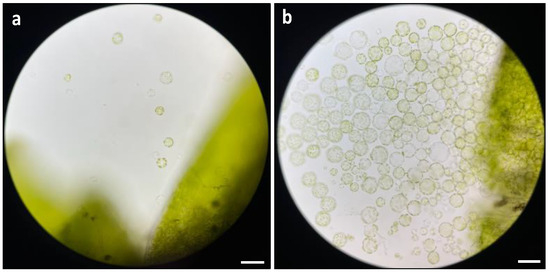
Figure 4.
Comparison of different duration on protoplast digestion: (a) microscopic observation of protoplast release at 10 h of digestion; and (b) microscopic observation of protoplast release at 16 h of digestion. The images were taken with a Zeiss Axio imager M2 fluorescence microscope with 20× magnification. Scale bars = 50 μm.
Factor 5–Enzyme Concentration
The final optimisation step for the protoplast isolation protocol involved determining the optimal enzyme concentration using the previously optimised parameters for leaf strip size, physical treatment, incubation condition, and digestion duration. Two different enzyme solutions containing varying concentrations of cellulysin and macerase were tested, and the released protoplasts were purified using gradient centrifugation (Figure 5a). Hemocytometry was used to determine the yield of purified protoplasts (Figure 5b), while viability was assessed using FDA staining (Figure 5c).
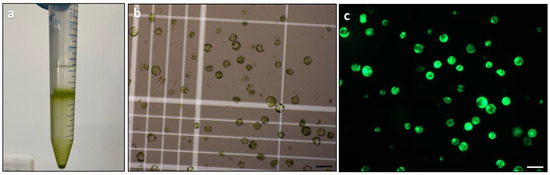
Figure 5.
Protoplast purification and assessment of yield and viability: (a) protoplast purification using gradient centrifugation; (b) yield assessment of protoplasts using a haemocytometer; and (c) viability assessment of protoplasts by FDA staining. Scale bars = 100 μm (b,c).
Protoplast yield was significantly affected by the concentration of cellulysin and macerase (Table 1). The combination of 1% (w/v) cellulysin and 0.25% (w/v) macerase could sustain no more than 1.9 × 105 cells per gram of fresh weight. Doubling the enzyme concentration significantly raised Duboisia protoplast yield to 8.9 × 105 cells per gram of fresh weight. As a result, a combination of 2% (w/v) cellulysin and 0.5% (w/v) macerase was determined as the optimum treatment and used for subsequent protoplast isolation procedures.

Table 1.
Effect of enzyme concentrations on Duboisia mesophyll protoplast isolation.
No statistical difference was observed in the protoplast viability between different enzyme treatments (Table 1). It was possible to improve protoplast viability to nearly 100% by reducing the enzyme digestion period. However, a shorter enzyme incubation period (10 h) dramatically diminished protoplast release (Figure 4a).
2.2. Plant Regeneration from Protoplasts
2.2.1. Selection of Culture System and Basal Medium
In the preliminary experiment aimed at selecting a suitable culture system, five culture systems, including a liquid culture, a droplets culture, a solid–liquid double layer system, an agar pool culture, and an alginate bead culture, were compared (Figure 6). However, no protoplast development could be observed in any of the tested culture systems. This posed the question of the suitability of the culture medium for survival.
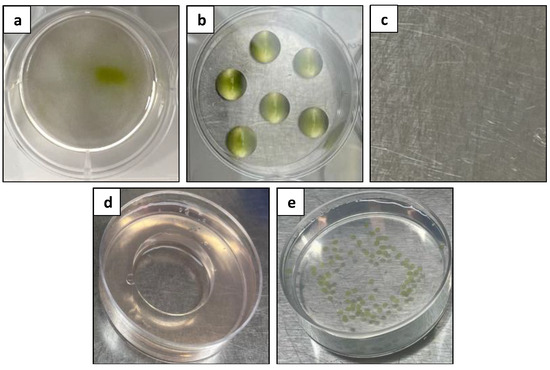
Figure 6.
Comparison of different protoplast culture systems: (a) liquid culture; (b) droplets culture; (c) solid–liquid double layer system; (d) agar pool culture; and (e) alginate bead culture.
The liquid culture was then used as a standardised system to evaluate the effects on microcalli formation with different basal media, specifically B5, WPM, MS-NH4, and KM8P media (Figure 7). After 11 days of culture, it was found that only the KM8P medium was able to support protoplast development, as evidenced by enlarged protoplasts and protoplast division (Figure 7d). In contrast, the protoplasts cultured in other media failed to develop (Figure 7a–c).
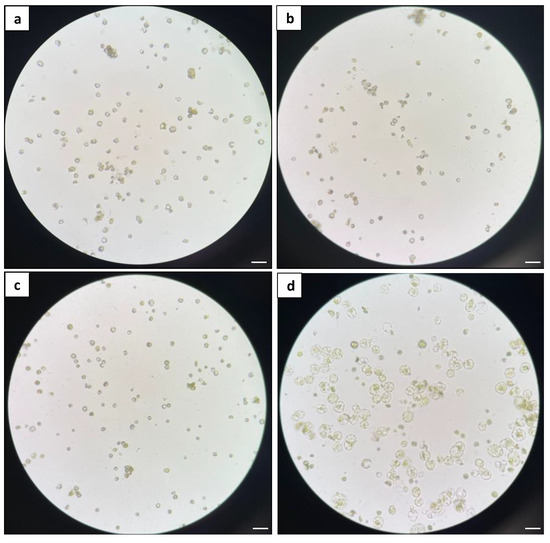
Figure 7.
Comparison of different basal media: (a) microscopic observation of protoplasts in B5 medium; (b) microscopic observation of protoplasts in MS medium; (c) microscopic observation of protoplasts in WPM; and (d) microscopic observation of protoplasts in KM8P medium. The images were taken with an Olympus CKX41 inverted microscope with 20× magnification. Scale bars = 50 μm.
2.2.2. Microcalli Induction and Proliferation
Following the selection of the liquid culture system and KM8P medium as the suitable culture system and basal medium for Duboisia mesophyll protoplast development, an evaluation of plating density and hormone combinations was conducted to enhance protoplast division and microcalli development.
The plating density had a significant effect on culturing Duboisia mesophyll protoplasts. Table 2 depicts the effects of three protoplast plating densities on cell division after 11 days and microcalli induction after 40 days. The lower plating at 0.5 × 105 resulted in the highest cell division efficiency at 33.2%, and a maximum of 17.9% microcalli induction frequency was observed in this case. A high protoplast density at 5 × 105 diminished cell division and microcalli formation frequency substantially. Even at the density of 105, nutritional competition resulting in necrosis and aberrant development was evident under a microscope when compared with the optimal density 0.5 × 105 (Figure 8), despite no statistical difference being recorded on cell division and microcalli induction frequency. Therefore, the optimum plating density was determined to be 0.5 × 105 and used for the subsequent regeneration process (Figure 9).

Table 2.
Effect of plating density on cell division and microcalli induction frequency of Duboisia mesophyll protoplast.
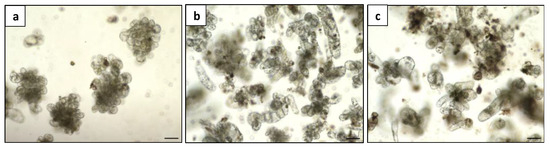
Figure 8.
Microcalli induction of Duboisia mesophyll protoplast at three densities: (a) 0.5 × 105; (b) 105; and (c) 5 × 105, bar = 100 μm.
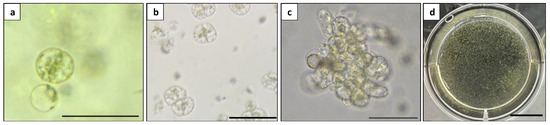
Figure 9.
Microcalli induction from Duboisia mesophyll protoplast: (a) first cell division; (b) second cell division; (c) multiple cells stage; and (d) visible microcalli. Scale bars = 100 μm (a–c) and = 1 cm (d).
The induced microcalli with a diameter of 0.1–0.5 mm (Figure 9d) were transferred to a solid MS medium supplemented with different hormone combinations to evaluate their effects on the proliferation of microcalli into calli. After 30 days of culture, the hormone combination 1 mg L−1 2,4-D + 0.5 mg L−1 BA supported the best microcalli proliferation at all three densities, producing a greater number of green and compact calli compared to the other hormone combinations. However, the calli produced from all the treatments exhibited a fuzzy surface texture, indicating anomalous cell proliferation (Figure 10).
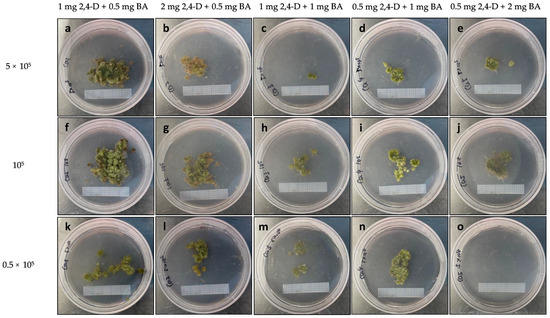
Figure 10.
Microcalli proliferation on MS medium with the indicated hormone combinations (mg L−1) at different plating densities: (a–e) 0.5 × 105; (f–j) 105; and (k–o) 5 × 105. Graph paper grid size = 2 × 2 mm.
A further investigation was carried out to examine the effects of BA on microcalli growth and address the fuzzy surface texture observed in the previous experiment. The microcalli induced at a plating density of 0.5 × 105 were cultured on a solid MS medium supplemented with 1 mg L−1 2,4-D with or without 0.5 mg L−1 BA (Figure 11). The deletion of 0.5 mg L−1 BA effectively eliminated the fuzzy surface texture from the induced calli, resulting in the production of green and healthy calli (Figure 11b).
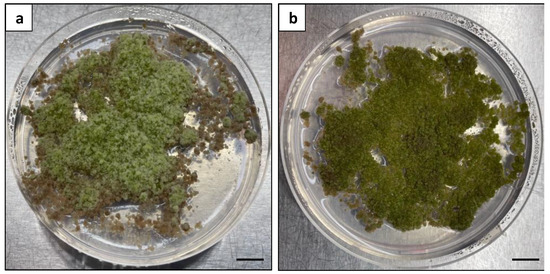
Figure 11.
Effects of BA on microcalli proliferation: (a) calli with a fuzzy surface texture produced from the MS medium with 1 mg L−1 2,4-D and 0.5 mg L−1 BA; (b) calli with a healthy green appearance produced from the MS medium with 1 mg L−1 2,4-D alone. Scale bars = 1 cm.
2.2.3. Plant Regeneration from Callus
To induce shoot regeneration, the healthy and compact calli were subcultured on an MS medium supplemented with various hormone combinations (Figure 12). After 6 weeks of culture, the calli treated with BA and free hormones exhibited a severe browning appearance, leading to mortality (Figure 12a–c). After 9 weeks of culture, shoot regeneration was observed on the calli treated with TDZ alone or in combination with BA or NAA. The shoot regeneration frequency was significantly influenced by the hormone treatments. The maximum shoot regeneration (63.3%) was observed on the calli treated with 1.5 mg L−1 TDZ alone (Figure 13a). Adding 1.5 mg L−1 BA or 0.1 mg L−1 NAA also stimulated shoot formation; however, significantly lower regeneration frequencies with delayed shoot growth were observed (Table 3). The use of free hormones medium and medium supplemented with BA alone or TDZ in combination with 0.5 mg L−1 NAA failed to induce shoots.
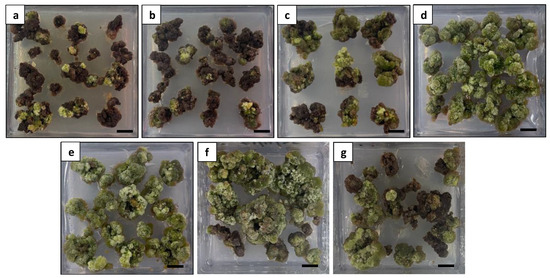
Figure 12.
Comparison of different hormone combinations on callus appearance at 6 weeks of culture: (a) calli treated with free hormones exhibiting a severe browning appearance; (b) calli treated with 2 mg L−1 BA exhibiting a severe browning appearance; (c) calli treated with 5 mg L−1 BA exhibiting a severe browning appearance; (d) calli treated with 1.5 mg L−1 TDZ exhibiting a green and healthy appearance; (e) calli treated with 1.5 mg L−1 TDZ and 1.5 mg L−1 BA exhibiting a green and healthy appearance; (f) calli treated with 1.5 mg L−1 TDZ and 0.1 mg L−1 NAA exhibiting an overall healthy appearance with limited browning; and (g) calli treated with 1.5 mg L−1 TDZ and 0.5 mg L−1 NAA exhibiting a moderate browning appearance. Scale bars = 1 cm.
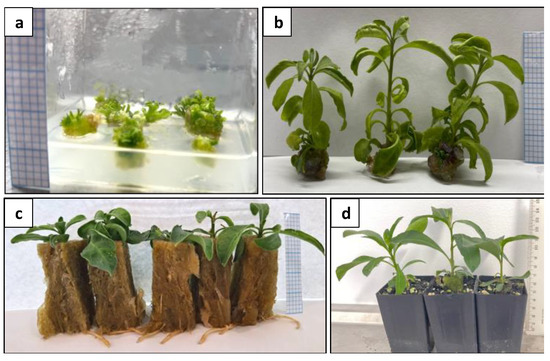
Figure 13.
Plant regeneration from protoplast-derived calli: (a) shoot induction from calli; (b) regenerated shoots; (c) rooted shoots; and (d) acclimatised plants. Graph paper grid size = 2 × 2 mm (a–c); Scale bar = 1 cm (d).

Table 3.
Effect of hormone type/concentration on shoot regeneration from mesophyll protoplast-derived calli of Duboisia.
After shoot regeneration, these shoots rooted at a rate of 100% (Figure 13c). No morphological difference was observed between the protoplast-derived plants and the donor plants at the time of intact plant regeneration.
2.3. Growth Evaluation of Protoplast-Derived Plants
The growth performance of the protoplast-derived plants and their donor plants was further compared (Figure 14) through the assessment of plant height, stem girth, leaf number, canopy, and branch number over 3 months.

Figure 14.
Comparison of growth performance between protoplast-derived plants and their donor plants. (a) protoplast-derived plants; and (b) donor plants.
2.3.1. Plant Height
No significant differences were observed in the data collected on plant height over the 12-week period between the protoplast-derived and the donor plants (Figure 15).
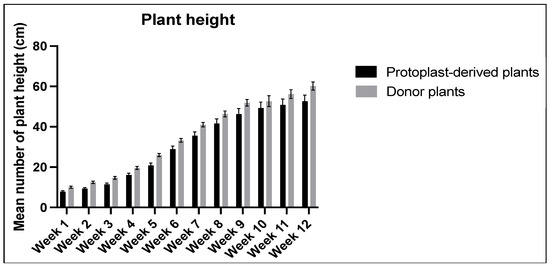
Figure 15.
Comparison of plant height between the protoplast-derived plants and the donor plants over 12 weeks. All the data are presented as the mean ± standard error of the mean (SEM), n = 10, duplicated samples.
2.3.2. Stem Girth
No statistically significant differences were observed in the stem girth between the protoplast-derived plants and the donor plants over the 12-week period (Figure 16).
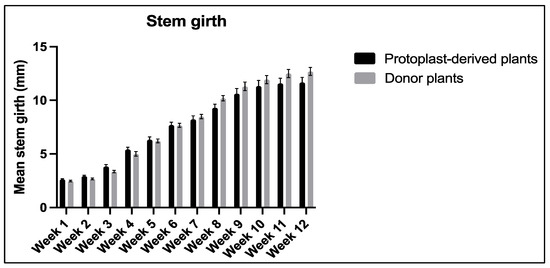
Figure 16.
Comparison of stem girth between the protoplast-derived plants and the donor plants over 12 weeks. All the data are presented as the mean ± standard error of the mean (SEM), n = 10, duplicated samples.
2.3.3. Number of Leaves and Canopy
Significant differences in the number of leaves and canopy were observed between the protoplast-derived plants and the donor plants. The data showed that, at week 6 and 7, the protoplast-derived plants produced significantly fewer leaves compared to the donor plants (Figure 17a). Similarly, from week 8 onwards, the canopy data indicated a significantly lower leaf yield in the protoplast-derived plants compared to the donor plants (Figure 17b).
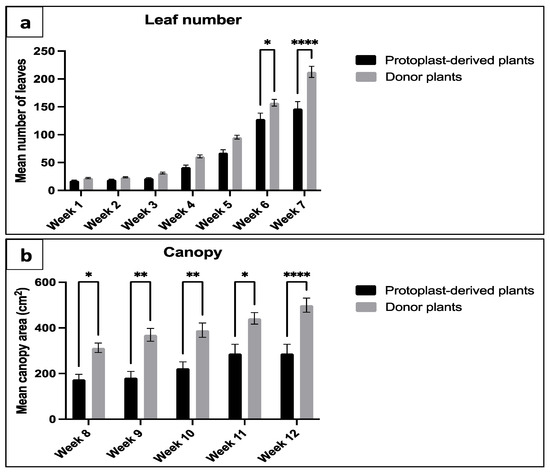
Figure 17.
Comparison of leaf number and canopy between the protoplast-derived plants and the donor plants over 12 weeks: (a) comparison of leaf number from week 1 to 7; and (b) comparison of canopy area from week 8 to 12. All the data are presented as the mean ± standard error of the mean (SEM), n = 10, duplicated samples. Significant differences between the data sets are indicated as follows: * p < 0.05; ** p < 0.01; and **** p < 0.0001.
2.3.4. Number of Branches
The data collected on the number of branches showed a significant difference between the protoplast-derived plants and the donor plants from week 5 to week 12. The protoplast-derived plants exhibited fewer branches compared to the donor plants during this period (Figure 18).
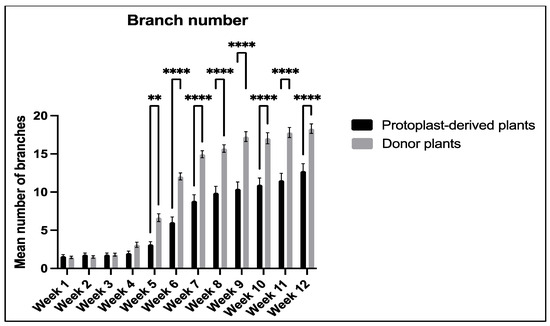
Figure 18.
Comparison of branch number between the protoplast-derived plants and the donor plants over 12 weeks. All the data are presented as the mean ± standard error of the mean (SEM), n = 10, duplicated samples. Significant differences between the data sets are indicated as follows: ** p < 0.01; and **** p < 0.0001.
3. Discussion
Protoplast technology has been deemed a significant and valuable technique for producing novel genotypes with desired features through protoplast fusion and genetic engineering. CRISPR/Cas9-mediated genome editing research is increasingly adopting protoplasts in the production of DNA-free mutants since this system has a greater potential for generating commercially feasible mutants with lower off-target effects than standard Agrobacterium-mediated transformation [25,26]. However, a protoplast-to-plant regeneration system is a prerequisite and remains challenging. A previous study successfully isolated and regenerated Duboisia protoplasts through a suspension culture system, but the fate of the isolated protoplasts did not move beyond transformation research [23]. In fact, genetic enhancement approaches generally require genetically stable materials, while the utilisation of suspension protoplasts carries the risk of somaclonal variation [27,28]. Mesophyll protoplasts, as a highly differentiated alternative, could limit this issue, but plant regeneration from mesophyll protoplasts is far more challenging. In this study, the first protocol of mesophyll protoplast isolation and regeneration for Duboisia has been established.
Several critical factors were optimised step-by-step for Duboisia mesophyll protoplast isolation, culture of protoplast, and intact plant regeneration. Starting from protoplast isolation, smaller leaf strips with a size of 0.5–1 mm resulted in a higher protoplast yield than the 2 mm leaf strips. This is likely due to the increased surface area to volume ratio of the smaller leaf strips, which may facilitate the diffusion of enzymes and aid in cell wall breakdown [29]. Relevant studies, such as the work by Liu et al. [30] and Kanai and Edwards [31], have also shown the importance of using a smaller leaf strip size for maximising protoplast yield in Ricinus communis L. and maize, respectively. Moreover, vacuum infiltration was found to be highly effective for Duboisia mesophyll protoplast isolation, resulting in more uniform and higher-quality protoplasts. The application of vacuum infiltration has been widely used to increase the efficiency and uniformity of protoplast isolation by aiding enzyme solution penetration into intercellular spaces, which is essential for the consistent release of intact protoplasts [32,33,34]. Shake incubation has been reported to be effective in speeding enzyme digestion, while a low shaking speed or static incubation can lead to longer incubation times and enzyme toxicity [33,35]. However, an excessive shaking speed may also lead to the disruption of chloroplast orientation and protoplast bursting [36]. In the present study, the shaking of leaf strips at 50 rpm during enzyme digestion resulted in the bursting of protoplasts, suggesting that shaking speed needs to be carefully optimised along with other factors, such as enzyme concentration and duration. A long duration (16 h) of static incubation, as demonstrated in this study, is a simple and effective method for achieving a sufficient yield of Duboisia mesophyll protoplasts. Exploring lower shaking speeds (below 50 rpm) could be beneficial to increasing protoplast yield; however, this may add-on further optimisation steps, with other digestion parameters. The balance of enzyme concentration and digestion duration is also essential for achieving high-quality protoplasts’ isolation. An excessive amount of enzymes can cause protoplast damage, whereas insufficient enzymes can result in incomplete digestion [37]. On the other hand, a prolonged digestion duration can lead to enzyme toxicity, while a shorter period can yield low-quality protoplasts [36]. For instance, Moon et al. [38] demonstrated that an 18 h enzyme incubation time yielded the highest extraction efficiency for potato mesophyll protoplasts, while a 5 h incubation led to low yields and a 24 h incubation resulted in decreased protoplast viability. The present study effectively demonstrated the balancing process for enzyme concentration and digestion duration through a series of progressive experiments: a final concentration of 2% (w/v) cellulysin and 0.5% (w/v) macerase with a 16 h digestion duration was found to be optimum for Duboisia mesophyll protoplast isolation.
An appropriate culture system and culture medium are essential for protoplast development, division, and regeneration. In contrast to the work by Kitamura et al. [23,24], which employed a B5 medium for Duboisia suspension culture protoplasts, the present investigation of mesophyll protoplasts found this medium to be ineffective. This highlights the notable influence of protoplast source on medium suitability. The present study also compared several more common media for protoplast culture and found that only the KM8P medium was able to support Duboisia protoplast development. This may be attributed to the presence of organic acids, casein hydrolysate and coconut water, which acted as necessary energy sources and provided vitamins, minerals, and sugars for protoplast development [6]. The superior performance of KM8P has been previously reported in the protoplast regeneration of Gossypium klotzschianum A. [39], Dendrobium [40], and Diospyros kaki L. [41]. Different culture systems can provide varying levels of nutrient availability and physical support [42], and the selection of a suitable system for successful protoplast development is species-specific and requires careful evaluation [43,44,45]. In this study, a liquid system along with the KMP8 medium was found to be a simple and effective method for protoplast development.
To further promote protoplast division and microcalli induction, plating density and hormone combination were also evaluated. It was found that protoplast culture and microcalli formation were negatively correlated with the tested plating densities. These data prove that protoplast culture densities were essential for cell division and microcalli induction in Duboisia. An excessive protoplast density in the culture medium depletes nutrients and causes phenol accumulation, resulting in a failure to maintain cell division and subsequent microcalli formation; this is well-demonstrated for the present study, as well as the works by Kang et al. [46] with Petunia hybrida, Avila-Peltroche et al. [47] with Hecatonema terminale, and Sangra et al. [36] with Medicago sativa. Moreover, the current findings revealed that protoplast plating density is highly dependent on the genotype and source of the protoplast at hand. Kitamura [23] reported that plating density at 5 × 105 is optimum for culturing Duboisia suspension protoplasts. In the present study, a density at 5 × 105 significantly reduced cell division and microcalli formation frequency for Duboisia mesophyll protoplasts. Furthermore, different combinations of 2,4-D and BA were evaluated on microcalli proliferation. In the literature, 2,4-D is recognised as a crucial plant growth regulator for microcalli development across various plant species, such as Brassica [48,49], Camellia oleifera [50], Zoysia japonica Steud. [51], and carrot [52]. Moreover, cytokinin or auxin are sometimes used in combination with 2,4-D to promote callus proliferation [17,53,54]. The present study demonstrated that the sole application of 1 mg L−1 2,4-D is effective for Duboisia microcalli proliferation, as additional BA resulted in excessive cell proliferation leading to a fuzzy surface texture on the calli.
Plant growth regulators (PGRs) or plant hormones were found to be crucial for the shoot regeneration of mesophyll protoplast-derived calli in Duboisia. While Kitamura et al. [23,24] reported that the application of 5 mg L−1 BA successfully triggered shoot regeneration for suspension protoplast-derived calli, it was found to be ineffective for the present study. This, again, suggests that the PGR requirement for shoot regeneration is dependent on the source of the protoplast. The highest shoot regeneration was observed in media containing 1.5 mg L−1 TDZ alone or, to a lesser degree, with extra 1.5 mg L−1 BA or 0.1 mg L−1 NAA but not in media devoid of PGR or with other hormone combinations. These results highlight the superior effect of TDZ for the shoot regeneration of mesophyll protoplast-derived calli, which was also reported for apple [16], mulberry [17], Tylophora indica [55], and field cress [56]. In plant tissue cultures, the synthetic cytokinin-like phytohormone TDZ has been demonstrated to be the most effective agent for inducing shoot formation [57,58]. It has been reported that the cytokinin/auxin ratio is an essential factor in shoot regeneration [59]. The in vitro application of TDZ modulates endogenous hormone levels, particularly the cytokinin/auxin ratio [60]. It has been proven that the action mechanism of this PGR is tightly associated with the biosynthesis and transport of endogenous auxin as well as the accumulation of cytokinin [61,62]. The application of TDZ alone triggered effective shoot regeneration for the Duboisia mesophyll protoplast-derived calli. This indicates that additional NAA or BA might imbalance endogenous hormone levels, thus reducing shoot regeneration frequency.
A growth evaluation of the protoplast-derived plants was conducted, comparing several key agronomic traits with their donor plants. The growth evaluation experiment revealed that the protoplast-derived plants showed no differences to the donor plants when plant height and stem girth were compared. However, the protoplast-derived plants had fewer leaves and branches. This could be attributed to the enzymatic digestion, mechanical disruption, and osmotic stress caused during the protoplast isolation process [63]. However, it is not possible to compare the results as no similar studies are found in the literature. In the research sphere of tissue culture, some reports identify TDZ as a strong hormone known for promoting cell division and regeneration. However, it has tendency to induce somaclonal variations, particularly when used at high concentrations [64]. In a separate study, the application of a high level of TDZ (9.09 mg L−1) had a carry-over effect resulting in decreased shoot numbers and shoot lengths of micro-propagated Cymbidium [65]. Despite the observed variations in the leaf number and branch number of the protoplast-derived plants, the developed mesophyll protoplast isolation and regeneration system utilised highly differentiated leaves as the source material, thereby imposing a lesser risk of somaclonal variation. Further studies employing molecular and biochemical approaches such as amplified fragment length polymorphism (AFLP) analysis and sequence repeats (SSR) analysis can be used to identify whether the branching and leaf number differences are related to the genetic stability of protoplast-derived plants.
4. Materials and Methods
4.1. Protoplast Isolation
4.1.1. Plant Materials and General Methods
A standardised method for protoplast isolation was developed with inputs from the related literature on several plant species [23,66,67]. Each digestion was carried out with 1 g of healthy and fully expanded leaves collected under aseptic conditions from in vitro cultures of Duboisia hybrid D. myoporoides × D. hopwoodii [68,69,70] with 10 mL of sterile enzyme solution. The enzyme solution was freshly prepared by dissolving digestion enzymes [Cellulysin® (Sigma-Aldrich, Bayswater, VIC, Australia), MaceraseTM (Sigma-Aldrich, Bayswater, VIC, Australia)] in Solution A (0.4 M mannitol, 10 mM CaCl2, 20 mM KCl, 0.1% (w/v) BSA, and 20 mM MES, with a pH of 5.7, stored at −20 °C and thawed before use). Different enzyme concentrations were used in specific experiments during the optimisation process; thus, the details are included in the respective experiments. The enzyme solution was preheated at 60 °C for 10 min to inactivate the protease enzymes. Meanwhile, the leaves were shred vertically into strips using a sharp blade, taking much care not to bruise the tissues. The leaf strips were then immediately transferred into a 30 mm sterile Petri dish and flooded with freshly prepared filter-sterilised 10 mL of enzyme solution (refer to the experiments for the exact enzyme concentrations). The Petri dish containing the leaf strips and the enzyme solution was incubated under dark conditions at room temperature to achieve substantial protoplast release.
All the liquid media, buffers, and solutions utilised in this study were filter-sterilised using a 0.22 μm filter (Milles®-GS, Sigma-Aldrich, Bayswater, VIC, Australia), unless stated otherwise.
4.1.2. Optimisation of Protoplast Digestion
The optimisation process considered several factors in the Duboisia mesophyll protoplast isolation process, including the following: leaf strip size, physical treatments, incubation conditions, digestion duration, and enzyme concentration. A series of progressive experiments were conducted in order to optimise one factor at a time to obtain a sufficient quantity of high-quality protoplasts required for further regeneration experiments. The outcomes of these experiments were visually assessed under a microscope; the slides for the microscope observations were prepared by randomly collecting three leaf strips from the digestion mixture. The image acquisition for the microscopic observations was taken consistently, capturing the area close to the edge of the selected leaf strip.
The optimisation procedure for an individual factor was not repeated; however, the optimal outcomes for each factor were amalgamated to form a final protocol and triplicated as independent experiments (Section 4.1.2, Factor 5) to ensure the robustness and reliability of the method.
Factor 1–Leaf Strip Size
Two different leaf strip sizes, 0.5–1 mm and 2 mm, were compared for protoplast digestion. The leaf strips were placed in a sterile Petri dish containing 10 mL of enzyme solution with 1% cellulysin and 0.25% macerase and cultured in darkness for 16 h at room temperature to observe protoplast release under a light microscope (Olympus BH2-RFCA, Notting Hill, VIC, Australia).
Factor 2–Physical Treatment
Following the optimisation of the leaf strip size, the effects of the physical treatment were evaluated. Specifically, vacuum infiltration was tested as a method to enhance protoplast release from the leaf tissue. The leaf strips were prepared as described previously and submerged in the enzyme solution. The aluminium foil-covered Petri dish containing the leaf strips and enzyme solution was then placed in a vacuum desiccator for vacuum infiltration at −40 kPa for 30 min. The Petri dish was subsequently incubated in darkness at room temperature for 16 h for the observation of protoplast release under a light microscope (Olympus BH2-RFCA).
Factor 3–Incubation Conditions
The next optimisation step was to evaluate the effects of incubation conditions on protoplast digestion. Similar to the previous steps, the leaf strips were prepared and treated with vacuum infiltration. Following this, the Petri dish was placed on an orbital shaker (Bioline, Narellan, NSW, Australia), shaking at 50 rpm, and incubated in darkness at room temperature for 16 h. Protoplast digestion was observed under a microscope (Zeiss Axio imager M2, North Ryde, NSW, Australia) to determine the optimal incubation conditions for protoplast release.
Factor 4–Digestion Duration
Subsequently, the optimisation was focused on the effects of digestion duration on protoplast release. After leaf preparation and vacuum infiltration, the leaf strips were incubated for different durations, including 3 h, 5 h, 10 h, and 16 h, at room temperature, in darkness, without shaking. Protoplast digestion was observed under a microscope (Zeiss Axio imager M2) after each time point to determine the optimal duration for protoplast release.
Factor 5–Enzyme Concentration
After these step-by-step optimisation processes, the optimal parameters determined were combined and used for the final optimisation step on enzyme concentration. The enzyme concentration was optimised by incubating the leaf strips in two enzyme solutions, one containing 1% cellulysin and 0.25% macerase and the other containing 2% cellulysin and 0.5% macerase. Data on protoplast yield and viability were recorded after purification. This experiment was triplicated as independent experiments to confirm the effectiveness of the developed protoplast digestion protocol.
4.1.3. Protoplast Collection and Purification
The methods for protoplast collection and purification were modified from Zhang et al. [71] and Hu et al. [42]. Specifically, 5 mL of washing buffer composed of 154 mM NaCl, 125 mM CaCl2, 5 mM KCl, and 2 mM MES at pH 5.7 was added into the Petri dish to stop enzyme digestion. Subsequently, the Petri dish was gently agitated for 2–3 min to release the protoplasts. The protoplast suspension was filtered through a 40 μm cell strainer (Sigma-Aldrich) placed on a 50 mL sterile falcon tube, while the remaining leaf strips were gathered in a corner of the Petri dish and gently squeezed using a sterile pipette tip to release more protoplasts. Another 5 mL of washing buffer was added into the Petri dish to rinse the squeezed leaf strips; then, the contents were filtered through the strainer. The resulting protoplast suspension was centrifuged at 100× g at 4 °C for 5 min; the supernatant was carefully removed using a serological pipette. The protoplast pellet was washed three times with a washing buffer by centrifugation, following the same parameters. The washed pellet was resuspended in 2 mL of washing buffer, carefully overlayed on 6 mL of 18% (w/v) sucrose solution in a 15 mL falcon tube, and centrifuged at 80× g at 4 °C for 10 min with a swinging bucket centrifuge (Multifuge X4 Pro, Thermo Fisher, Scoresby, VIC, Australia). After the gradient centrifugation, a band of purified protoplast was collected at the interface between the sucrose and the washing buffer. The purified protoplasts were washed again with the washing buffer to remove any remaining sucrose solution.
4.1.4. Protoplast Yield and Viability Test
A fluorescein diacetate (FDA) stock solution was prepared by dissolving 5 mg of FDA in 1 mL of acetone. An aliquot of 100 μL of protoplast suspension was mixed with 1 μL of FDA stock solution to make the final concentration of 0.01% in a 1.5 mL Enpendorf tube. The mixture was incubated at room temperature for 5 min in darkness. The protoplast yield and cell viability were determined using a haemocytometer under a fluorescence microscope (Zeiss Axio imager M2) at 420–490 nm. The viable cells stained by the FDA fluoresced of a yellow to green colour, while the dead cells remained unstained. Cell viability was calculated using the blow equation.
Cell viability (%) = Viable cells/Sum of viable cells and dead cells × 100%
4.2. Plant Regeneration from Protoplasts
4.2.1. Selection of Culture System and Basal Medium
To select the optimum basal medium and culture system for the isolated Duboisia mesophyll protoplasts, a preliminary experiment was carried out to determine the best culture system. In this experiment, the choice of the Gamborg (B5) medium as the basal medium [72], a hormone combination of 1 mg L−1 2,4D and 1 mg L−1 zeatin, and a plating density of 5 × 105 cells mL−1 were based on previous reports of Duboisia suspension protoplast cultures [23]. Five different culture systems were evaluated using the same basal medium, hormones, and plating density for comparison. The five culture systems evaluated were adapted from previously published methods, including liquid culture [73], droplets culture [23], solid–liquid double layer system [42], agar pool culture [42], and alginate bead culture [74]. However, no protoplast development was observed in any of the tested systems, leading to fine optimisation experiments for selecting suitable basal media.
To compare different basal media, the protoplasts were cultured using a liquid culture system. The same plating density (5 × 105 cells mL−1) and hormone combination (1 mg L−1 2,4D and 1 mg L−1 zeatin) were used for all the media tested, viz., B5, MS medium without ammonium (MS-NH4), woody plant medium (WPM) [75], and KM8P medium [76] (comprising PhytoTech Labs Inc. Kao and Michayluk modified basal salts, 0.58 g L−1 L-glutamine, 0.25 g L−1 Casamino acids, 0.25 g L−1 D-ribose, 72g L−1 glucose, and 20 mL L−1 coconut water). An aliquot of 1 mL of purified protoplasts at a 5 × 105 cells mL−1 cell density was cultured in a 6-well plate (Sigma-Aldrich) and incubated at 25 °C in darkness. At 11 days of culture, protoplast development was assessed under an inverted microscope.
4.2.2. Microcalli Induction and Proliferation
After the selection of the liquid culture system and KM8P medium as the appropriate culture system and basal medium for Duboisia mesophyll protoplast development, further evaluation was conducted to enhance protoplast division and microcalli development. Two crucial factors—plating density and hormone combinations—were evaluated.
Initially, the plating density was evaluated to determine the optimum density for microcalli induction. The purified protoplasts were adjusted to different densities (0.5 × 105, 105 and 5 × 105 cells mL−1) in 1 mL of KM8P medium supplemented with 1 mg L−1 2,4D and 1 mg L−1 zeatin and cultured in a 6-well plate (Sigma-Aldrich) at 25 °C in darkness. To promote cell division and microcalli formation, the dilution with 0.5 mL of half osmotic (36 g L−1 glucose) KM8P medium started 11 days after the initial plating, at weekly intervals. At 11 days of culture, the cell division frequency was computed as the percentage of protoplasts undergoing at least one cell division. After 40 days of culture, the frequency of microcalli induction was calculated as the percentage of protoplasts inducing visible microcalli. All the data were obtained under an inverted microscope (Olympus CKX41), with five random microscopic fields observed to calculate the average frequency for each treatment.
Subsequently, for each of the three plating densities, the induced microcalli with 0.1–0.5 mm were transferred to a solid MS medium [77] to evaluate different hormone combinations on microcalli proliferation. The MS medium was supplemented with 2% (w/v) sucrose, 0.7% (w/v) agar, and various combinations of 2,4D and BA, specifically, 1 mg L−1 2,4-D + 0.5 mg L−1 BA, 2 mg L−1 2,4-D + 0.5 mg L−1 BA, 1 mg L−1 2,4-D + 1 mg L−1 BA, 0.5 mg L−1 2,4-D + 1 mg L−1 BA, and 0.5 mg L−1 2,4-D + 2 mg L−1 BA. The microcalli cultures were incubated at 25 °C with a 16 h/8 h (day/night) photoperiod (Lumilux cool daylight; OSRAM L 36W/865; PPFD 221.417 µmol m−2 s−1) for 30 days, after which the proliferated calli were visually assessed.
To investigate the impact of BA on microcalli proliferation and to address the observed anomalous cell proliferation leading to a fuzzy surface texture, an additional experiment was conducted. The microcalli were induced as previously mentioned with an initial plating density of 0.5 × 105. The microcalli with a size 0.1–0.5 mm were cultured on a solid MS medium supplemented with 2% (w/v) sucrose, 0.7% (w/v) agar, and 1 mg L−1 2,4-D, with or without 0.5 mg L−1 BA. After 30 days of culture under the same conditions described previously, the calli were evaluated visually to assess their proliferation and the impact of BA on microcalli growth.
4.2.3. Plant Regeneration from Calli
Compact and well-organised calli induced from the protoplasts were transferred to a regeneration medium for shoot induction. To evaluate the effects of different hormones on shoot regeneration, the calli were transferred to an MS medium supplemented with BA (2 and 5 mg L−1) alone or TDZ (1.5 mg L−1) alone or in combination with BA (1.5 mg L−1) or NAA (0.1 and 0.5 mg L−1). The calli were subcultured for 3-week intervals until shoot regeneration. Data on shoot regeneration frequency were recorded after 9 weeks of culture. The regenerated shoots were subsequently rooted according to the procedure by Xue et al. [68].
4.3. Growth Evaluation of Protoplast-Derived Plants
The rooted plants were acclimatised according to Xue et al. [68]. After acclimatisation, the plants were re-potted in individual 125 mm Anova pots ® with potting mixture UQ23 and maintained under open-field conditions at the University of Queensland for 3 months. The agronomy study of the protoplast-derived plants was carried out using a random block design, with two replicates consisting of ten protoplast-derived plants and ten donor plants. Data were collected each week on plant height, stem girth, number of leaves, and number of branches. To ensure accuracy in the data collection regarding leaf production, direct counting of the leaf number was limited to the initial 7 weeks. Subsequently, data on the canopy were obtained from the 8th week onwards as an indication of the leaf mass. The measurement of the canopy was taken by determining the widest distance from one branch tip to the opposite branch tip in two directions. The first measurement was taken at the point of maximum width of the canopy, while the second was taken perpendicularly to the first measurement, again, at the widest point. The canopy area was then calculated by multiplying the two measurements.
4.4. Data Analysis
All the data generated from this study were statistically analysed using the GraphPad Prism software (version 9.5.1, GraphPad Software Inc., San Diego, CA, USA). The data were analysed using independent samples t-test for two-group comparisons. For multiple group comparisons, an Anova test was conducted, and significant differences among the mean values were calculated using Tukey’s HSD test.
5. Conclusions
In this study, Duboisia mesophyll protoplast isolation as well as its plant regeneration protocols have been established for the first time. A number of factors—leaf strip size, physical treatments, incubation conditions, digestion duration, enzyme concentration, plating density, and plant growth regulator concentration and combination—were found to be very crucial for the success of Duboisia protoplast isolation and regeneration. The highest protoplast yield and viability could be achieved with the enzyme treatment of 2% (w/v) cellulysin and 0.5% (w/v) macerase, combined with a 30 min vacuum infiltration at −40 kPa, followed by a 16 h static incubation in dark conditions. The optimum plating density was determined to be 0.5 × 105, supporting the highest cell division (33.2%) and microcalli induction (17.9%) frequency in a KM8P medium using a liquid culture system. After calli induction in a solid MS medium supplemented with 1 mg L−1 2,4-D, the greatest shoot regeneration (63.3%) was observed with the medium containing 1.5 mg L−1 of TDZ alone. After this was followed by the rooting treatment, intact plant regeneration from Duboisia mesophyll protoplasts was achieved. The growth evaluation of the regenerated plants revealed no altered morphology, plant height, and stem girth compared to their donor plants; however, the protoplast-derived plants produced fewer leaves and branches. Despite these variations, the developed protoplast system, by employing highly differentiated mesophyll cells as the source material, imposed a lesser risk of somaclonal variation. This reliable mesophyll protoplast isolation and regeneration system could be further used as an effective tool for genetic manipulations including protoplast fusion and DNA-free genome editing.
Author Contributions
Conceptualization, J.C.A.H.-B.; conducted experiments, Y.X., Z.H. and Z.Z.; wrote and revised the manuscript Y.X. and J.C.A.H.-B.; supervision and funding acquisition, J.C.A.H.-B. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by India Glycol Limited through a collaborative research agreement with The University of Queensland. Y.X. is supported through the Research Training Program scholarship (Ph.D.) by the University of Queensland.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. Plant Protoplast Technology: Current Status. Acta Physiol. Plant. 2005, 27, 117–130. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Somatic Cell Fusion: Relevance to Medicinal Plants. In Development of Plant-Based Medicines: Conservation, Efficacy and Safety; Saxena, P.K., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 167–181. ISBN 978-94-015-9779-1. [Google Scholar]
- Saul, M.W.; Potrykus, I. Direct Gene Transfer to Protoplasts: Fate of the Transferred Genes. Dev. Genet. 1990, 11, 176–181. [Google Scholar] [CrossRef]
- Metje-Sprink, J.; Menz, J.; Modrzejewski, D.; Sprink, T. DNA-Free Genome Editing: Past, Present and Future. Front. Plant Sci. 2019, 9, 1957. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Puchta, H. Knocking out Consumer Concerns and Regulator’s Rules: Efficient Use of CRISPR/Cas Ribonucleoprotein Complexes for Genome Editing in Cereals. Genome Biol. 2017, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.M.; Bargmann, B.O.R. Protoplast Regeneration and Its Use in New Plant Breeding Technologies. Front. Genome Ed. 2021, 3, 734951. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, R.; Harrison, D.K.; Williams, R.R. Intraspecific Somatic Hybridization of Mango (Mangifera indica L.) through Protoplast Fusion. JAH 2011, 13, 101–107. [Google Scholar] [CrossRef]
- Lynch, P.T.; Benson, E.E. Cryopreservation: A Method for Maintaining Plant Regeneration Capability of Rice Cell Suspension Cultures. In Rice Genetics II; Rice Genetics Collection; World Scientific Publishing Company: Singapore, 2008; Volume 2, pp. 321–332. ISBN 978-981-281-866-9. [Google Scholar] [CrossRef]
- Kim, J.B.; Bergervoet, J.E.M.; Raemakers, C.J.J.M.; Jacobsen, E.; Visser, R.G.F. Isolation of Protoplasts, and Culture and Regeneration into Plants in Alstroemeria. Vitr. Cell. Dev. Biol. Plant 2005, 41, 505–510. [Google Scholar] [CrossRef]
- Sheen, J. Signal Transduction in Maize and Arabidopsis Mesophyll Protoplasts. Plant Physiol. 2001, 127, 1466–1475. [Google Scholar] [CrossRef]
- Gupta, H.S.; Pattanayak, A. Plant Regeneration from Mesophyll Protoplasts of Rice (Oryza sativa L.). Nat. Biotechnol. 1993, 11, 90–94. [Google Scholar] [CrossRef]
- Partovi, R.; Farahani, F.; Sheidai, M.; Satari, T.N. Isolation of Protoplasts Banana (Musa Acuminate Colla) Cvs. Dwarf Cavendish and Valery and Research Morphological and Cytogenetic Their Plantlets Regenerated. Cytologia 2017, 82, 395–401. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, S.; Lin, Y. Plant Regeneration from Mesophyll Protoplast Culture of Cabbage (Brassica oleracea Var ‘Capitata’). Theoret. Appl. Genet. 1985, 71, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Advances in the Protoplast Culture of Woody Plants. In Micropropagation of Woody Plants; Ahuja, M.R., Ed.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 1993; pp. 67–91. ISBN 978-94-015-8116-5. [Google Scholar] [CrossRef]
- d’Utra Vaz, F.B.; dos Santos, A.V.P.; Manders, G.; Cocking, E.C.; Davey, M.R.; Power, J.B. Plant Regeneration from Leaf Mesophyll Protoplasts of the Tropical Woody Plant, Passionfruit (Passiflora edulis Fv Flavicarpa Degener.): The Importance of the Antibiotic Cefotaxime in the Culture Medium. Plant Cell Rep. 1993, 12, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wallin, A.; Johansson, L. Plant Regeneration from Leaf Mesophyll Protoplasts of in vitro Cultured Shoots of a Columnar Apple. J. Plant Physiol. 1990, 135, 565–570. [Google Scholar] [CrossRef]
- Umate, P.; Rao, K.V.; Kiranmayee, K.; Sree, T.J.; Sadanandam, A. Plant Regeneration of Mulberry (Morus indica) from Mesophyll-Derived Protoplasts. Plant Cell Tiss Organ Cult. 2005, 82, 289–293. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Yokota, S.; Ishiguri, F.; Yoshizawa, N. Plant Regeneration from Mesophyll Protoplasts of a Medicinal Plant, Phellodendron amurense Rupr. Vitr. Cell. Dev. Biol. Plant 2006, 42, 502–507. [Google Scholar] [CrossRef]
- Barnard, C. The Duboisias of Australia. Econ. Bot. 1952, 6, 3–17. [Google Scholar] [CrossRef]
- Besher, S.; Al-Ammouri, Y.; Murshed, R. Production of Tropan Alkaloids in the in vitro and Callus Cultures of Hyoscyamus aureus and Their Genetic Stability Assessment Using ISSR Markers. Physiol. Mol. Biol. Plants 2014, 20, 343–349. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Ding, R.; Chai, Y.; Bonfill, M.; Moyano, E.; Oksman-Caldentey, K.-M.; Xu, T.; Pi, Y.; Wang, Z.; Zhang, H. Engineering Tropane Biosynthetic Pathway in Hyoscyamus niger Hairy Root Cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 6786–6791. [Google Scholar] [CrossRef]
- Foley, P. Duboisia Myoporoides: The Medical Career of a Native Australian Plant. Hist. Rec. Aust. Sci. 2006, 17, 31–69. [Google Scholar] [CrossRef]
- Kitamura, Y. Regeneration of Plants from Protoplasts of Duboisia. In Plant Protoplasts and Genetic Engineering IV; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1993; pp. 18–31. ISBN 978-3-642-78037-0. [Google Scholar] [CrossRef]
- Kitamura, Y.; Morikawa, T.; Miura, H. Isolation and Culture of Protoplasts from Cell Suspension Cultures of Duboisia myoporoides with Subsequent Plant Regeneration. Plant Sci. 1989, 60, 245–250. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Park, S.; Yoon, J.; Park, A.Y.; Choe, S. DNA-Free Genome Editing via Ribonucleoprotein (RNP) Delivery of CRISPR/Cas in Lettuce. In Plant Genome Editing with CRISPR Systems: Methods and Protocols; Qi, Y., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 337–354. ISBN 978-1-4939-8991-1. [Google Scholar]
- Oksman-Caldentey, K.-M.; Strauss, A. Somaclonal Variation of Scopolamine Content in Protoplast-Derived Cell Culture Clones of Hyoscyamus muticus. Planta Methods 1986, 52, 6–12. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B. Somaclonal Variation in Coffea Arabica: Effects of Genotype and Embryogenic Cell Suspension Age on Frequency and Phenotype of Variants. Tree Physiol. 2003, 23, 419–426. [Google Scholar] [CrossRef]
- Wu, F.-H.; Shen, S.-C.; Lee, L.-Y.; Lee, S.-H.; Chan, M.-T.; Lin, C.-S. Tape-Arabidopsis Sandwich—A Simpler Arabidopsis Protoplast Isolation Method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Y.; Tang, J.; Chen, J.; Chen, M. Efficient Mesophyll Protoplast Isolation and Development of a Transient Expression System for Castor-Oil Plant (Ricinus communis L.). Biol. Futur. 2019, 70, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Edwards, G.E. Separation of Mesophyll Protoplasts and Bundle Sheath Cells from Maize Leaves for Photosynthetic Studies. Plant Physiol. 1973, 51, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome Editing in Rice and Wheat Using the CRISPR/Cas System. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef]
- Cheng, N.; Nakata, P.A. Development of a Rapid and Efficient Protoplast Isolation and Transfection Method for Chickpea (Cicer arietinum). MethodsX 2020, 7, 101025. [Google Scholar] [CrossRef]
- Nanjareddy, K.; Arthikala, M.-K.; Blanco, L.; Arellano, E.S.; Lara, M. Protoplast Isolation, Transient Transformation of Leaf Mesophyll Protoplasts and Improved Agrobacterium-Mediated Leaf Disc Infiltration of Phaseolus vulgaris: Tools for Rapid Gene Expression Analysis. BMC Biotechnol. 2016, 16, 53. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Lee, H.-Y.; Kim, S.W.; Noh, Y.-S.; Seo, P.J. Optimization of Protoplast Regeneration in the Model Plant Arabidopsis thaliana. Plant Methods 2021, 17, 21. [Google Scholar] [CrossRef]
- Sangra, A.; Shahin, L.; Dhir, S.K. Optimization of Isolation and Culture of Protoplasts in Alfalfa (Medicago sativa) Cultivar Regen-SY. Am. J. Plant Sci. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Ren, R.; Gao, J.; Lu, C.; Wei, Y.; Jin, J.; Wong, S.-M.; Zhu, G.; Yang, F. Highly Efficient Protoplast Isolation and Transient Expression System for Functional Characterization of Flowering Related Genes in Cymbidium Orchids. Int. J. Mol. Sci. 2020, 21, 2264. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-B.; Park, J.-S.; Park, S.-J.; Lee, H.-J.; Cho, H.-S.; Min, S.-R.; Park, Y.-I.; Jeon, J.-H.; Kim, H.-S. A More Accessible, Time-Saving, and Efficient Method for In Vitro Plant Regeneration from Potato Protoplasts. Plants 2021, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, X.; Huang, C.; Nie, Y.; Guo, X. Factors Influencing in vitro Regeneration from Protoplasts of Wild Cotton (G. Klotzschianum A) and RAPD Analysis of Regenerated Plantlets. Plant Growth Regul. 2005, 46, 79–86. [Google Scholar] [CrossRef]
- Khentry, Y.; Paradornuvat, A.; Tantiwiwat, S.; Phansiri, S.; Thaveechai, N. Protoplast Isolation and Culture of Dendrobium Sonia “Bom 17”. Agric. Nat. Resour. 2006, 40, 361–369. [Google Scholar]
- Tao, R.; Tamura, M.; Yonemori, K.; Sugiura, A. Plant Regeneration from Callus Protoplasts of Adult Japanese Persimmon (Diospyros kaki L.). Plant Sci. 1991, 79, 119–125. [Google Scholar] [CrossRef]
- Hu, X.; Yin, Y.; He, T. Plant Regeneration from Protoplasts of Gentiana Macrophylla Pall. Using Agar-Pool Culture. Plant Cell Tiss Organ Cult. 2015, 121, 345–351. [Google Scholar] [CrossRef]
- Masani, M.Y.A.; Noll, G.; Parveez, G.K.A.; Sambanthamurthi, R.; Prüfer, D. Regeneration of Viable Oil Palm Plants from Protoplasts by Optimizing Media Components, Growth Regulators and Cultivation Procedures. Plant Sci. 2013, 210, 118–127. [Google Scholar] [CrossRef]
- Borgato, L.; Pisani, F.; Furini, A. Plant Regeneration from Leaf Protoplasts of Solanum virginianum L. (Solanaceae). Plant Cell Tiss Organ Cult. 2007, 88, 247–252. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. An Alginate-Layer Technique for Culture of Brassica oleracea L. Protoplasts. Vitr. Cell. Dev. Biol. Plant 2012, 48, 265–273. [Google Scholar] [CrossRef]
- Kang, H.H.; Naing, A.H.; Kim, C.K. Protoplast Isolation and Shoot Regeneration from Protoplast-Derived Callus of Petunia hybrida Cv. Mirage Rose. Biology 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Avila-Peltroche, J.; Won, B.Y.; Cho, T.O. Protoplast Isolation and Regeneration from Hecatonema terminale (Ectocarpales, Phaeophyceae) Using a Simple Mixture of Commercial Enzymes. J. Appl. Phycol. 2019, 31, 1873–1881. [Google Scholar] [CrossRef]
- Fransz, P.F.; Leunissen, E.H.M.; Colijn-Hooymans, C.M. 2,4-Dichlorophenoxyacetic Acid Affects Mode and Frequency of Regeneration from Hypocotyl Protoplasts of Brassica oleracea. Protoplasma 1993, 176, 125–132. [Google Scholar] [CrossRef]
- Hussain, M.; Li, H.; Badri Anarjan, M.; Lee, S. Development of a General Protoplast-Mediated Regeneration Protocol for Brassica: Cabbage and Cauliflower as Examples. Hortic. Environ. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Li, S.-F.; Ye, T.-W.; Xu, X.; Yuan, D.-Y.; Xiao, S.-X. Callus Induction, Suspension Culture and Protoplast Isolation in Camellia oleifera. Sci. Hortic. 2021, 286, 110193. [Google Scholar] [CrossRef]
- Inokuma, C.; Sugiura, K.; Cho, C.; Okawara, R.; Kaneko, S. Plant Regeneration from Protoplasts of Japanese Lawngrass. Plant Cell Rep. 1996, 15, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Grzebelus, E.; Szklarczyk, M.; Baranski, R. An Improved Protocol for Plant Regeneration from Leaf- and Hypocotyl-Derived Protoplasts of Carrot. Plant Cell Tiss Organ Cult. 2012, 109, 101–109. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Wang, H.-L.; Ma, Y.; Kang, Y.-Q. Regeneration of Haploid Plants from Isolated Microspores of Asparagus (Asparagus officinalis L.). Plant Cell Rep. 1994, 13, 637–640. [Google Scholar] [CrossRef]
- Assani, A.; Chabane, D.; Foroughi-Wehr, B.; Wenzel, G. An Improved Protocol for Microcallus Production and Whole Plant Regeneration from Recalcitrant Banana Protoplasts (Musa Spp.). Plant Cell Tiss Organ Cult. 2006, 85, 257–264. [Google Scholar] [CrossRef]
- Thomas, T.D. Isolation, Callus Formation and Plantlet Regeneration from Mesophyll Protoplasts of Tylophora indica (Burm. f.) Merrill: An Important Medicinal Plant. Vitr. Cell. Dev. Biol. Plant 2009, 45, 591. [Google Scholar] [CrossRef]
- Sandgrind, S.; Li, X.; Ivarson, E.; Ahlman, A.; Zhu, L.-H. Establishment of an Efficient Protoplast Regeneration and Transfection Protocol for Field Cress (Lepidium campestre). Front. Genome Ed. 2021, 3, 757540. [Google Scholar] [CrossRef] [PubMed]
- Deepa, A.V.; Anju, M.; Dennis Thomas, T. The Applications of TDZ in Medicinal Plant Tissue Culture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 297–316. [Google Scholar] [CrossRef]
- Novikova, T.I.; Zaytseva, Y.G. TDZ-Induced Morphogenesis Pathways in Woody Plant Culture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 61–94. ISBN 978-981-10-8004-3. [Google Scholar] [CrossRef]
- Skoog, F.; Miller, C.O. Chemical Regulation of Growth and Organ Formation in Plant Tissues Cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar] [PubMed]
- Mok, M.C.; Mok, D.W.S.; Turner, J.E.; Mujer, C.V. Biological and Biochemical Effects of Cytokinin-Active Phenylurea Derivatives in Tissue Culture Systems. HortScience 1987, 22, 1194–1197. [Google Scholar] [CrossRef]
- Thomas, J.C.; Katterman, F.R. Cytokinin Activity Induced by Thidiazuron. Plant Physiol. 1986, 81, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Nisler, J. TDZ: Mode of Action, Use and Potential in Agriculture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Ahmad, N., Faisal, M., Eds.; Springer: Singapore, 2018; pp. 37–59. ISBN 978-981-10-8004-3. [Google Scholar] [CrossRef]
- Larkin, P.J.; Brettell, R.; Ryan, S.; Scowcroft, W. Protoplasts and Variation from Culture. In Protoplasts 1983: Lecture Proceedings; Potrykus, I., Harms, C.T., Hinnen, A., Hütter, R., King, P.J., Shillito, R.D., Eds.; EXS 46: Experientia Supplementum; Birkhäuser: Basel, Switzerland, 1983; pp. 51–56. ISBN 978-3-0348-6776-4. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-Induced Abnormalities in Plant Tissue Cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.R.; Sajeev, S.; Pattanayak, A.; Deka, B.C. TDZ Induced Micropropagation in Cymbidium giganteum Wall. Ex Lindl. and Assessment of Genetic Variation in the Regenerated Plants. Plant Growth Regul. 2012, 68, 435–445. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, R.; Xu, X.; Yang, Z.; Zhou, Y.; Deng, R.; Xu, X.; Yang, Z. Isolation of Mesophyll Protoplasts from Tea (Camellia sinensis) and Localization Analysis of Enzymes Involved in the Biosynthesis of Specialized Metabolites. Beverage Plant Res. 2021, 1, 2. [Google Scholar] [CrossRef]
- Svozil, J.; Gruissem, W.; Baerenfaller, K. Meselect—A Rapid and Effective Method for the Separation of the Main Leaf Tissue Types. Front. Plant Sci. 2016, 7, 1701. [Google Scholar] [CrossRef]
- Xue, Y.; Hiti-Bandaralage, J.C.A.; Jambuthenne, D.T.; Zhao, Z.; Mitter, N. Micropropagation of Duboisia Species via Shoot Tip Meristem. Horticulturae 2023, 9, 1313. [Google Scholar] [CrossRef]
- Xue, Y.; Hiti-Bandaralage, J.C.A.; Mitter, N. Micropropagation of Duboisia Species: A Review on Current Status. Agronomy 2023, 13, 797. [Google Scholar] [CrossRef]
- Mason, P.; Hiti-Bandaralage, J.; Xue, Y.; Mitter, N.; Henry, R. Advancing the Duboisia Industry for Sustainable Alkaloids. In Proceedings of the TropAg 2022, Brisbane, Australia, 31 October–2 November 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Duan, S.; Ao, Y.; Dai, J.; Liu, J.; Wang, P.; Li, Y.; Liu, B.; Feng, D.; et al. A Highly Efficient Rice Green Tissue Protoplast System for Transient Gene Expression and Studying Light/Chloroplast-Related Processes. Plant Methods 2011, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient Requirements of Suspension Cultures of Soybean Root Cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.D.; Vyvadilová, M.; Klíma, M.; Bechyně, M. A Simple Procedure for Mesophyll Protoplast Culture and Plant Regeneration in Brassica oleracea L. and Brassica napus L. Czech J. Genet. Plant Breed. 2006, 42, 103–110. [Google Scholar] [CrossRef]
- Tricoli, D.M.; Hein, M.B.; Carnes, M.G. Culture of Soybean Mesophyll Protoplasts in Alginate Beads. Plant Cell Rep. 1986, 5, 334–337. [Google Scholar] [CrossRef]
- McCown, B.H. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture for Woody Plant Species. Hort. Sci. 1981, 16, 453. [Google Scholar]
- Kao, K.N.; Michayluk, M.R. Nutritional Requirements for Growth of Vicia hajastana Cells and Protoplasts at a Very Low Population Density in Liquid Media. Planta 1975, 126, 105–110. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).