The Potential of Allelochemicals from Microalgae for Biopesticides
Abstract
1. Introduction
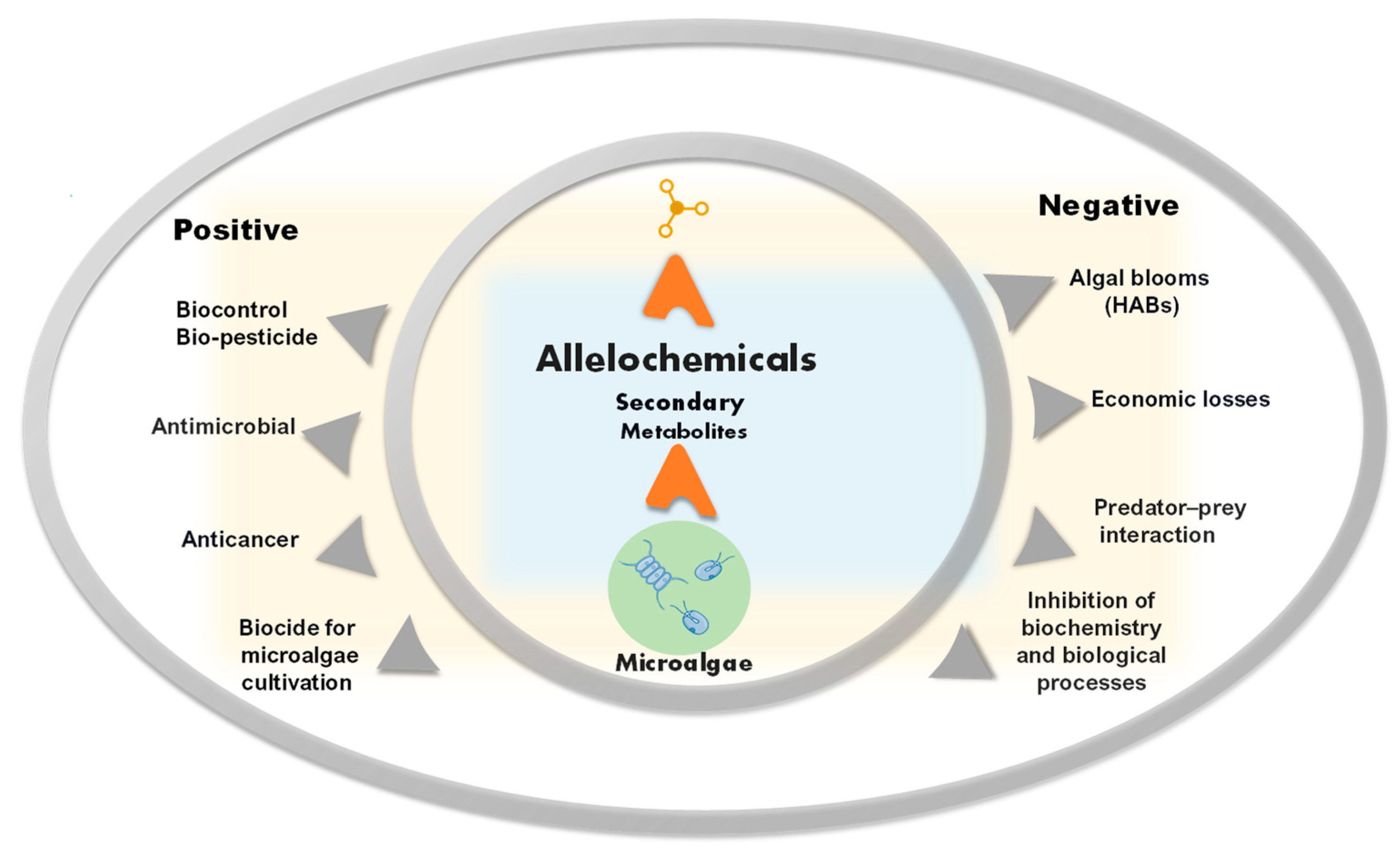
2. Allelopathy in Microalgae
3. Allelochemicals from Microalgae
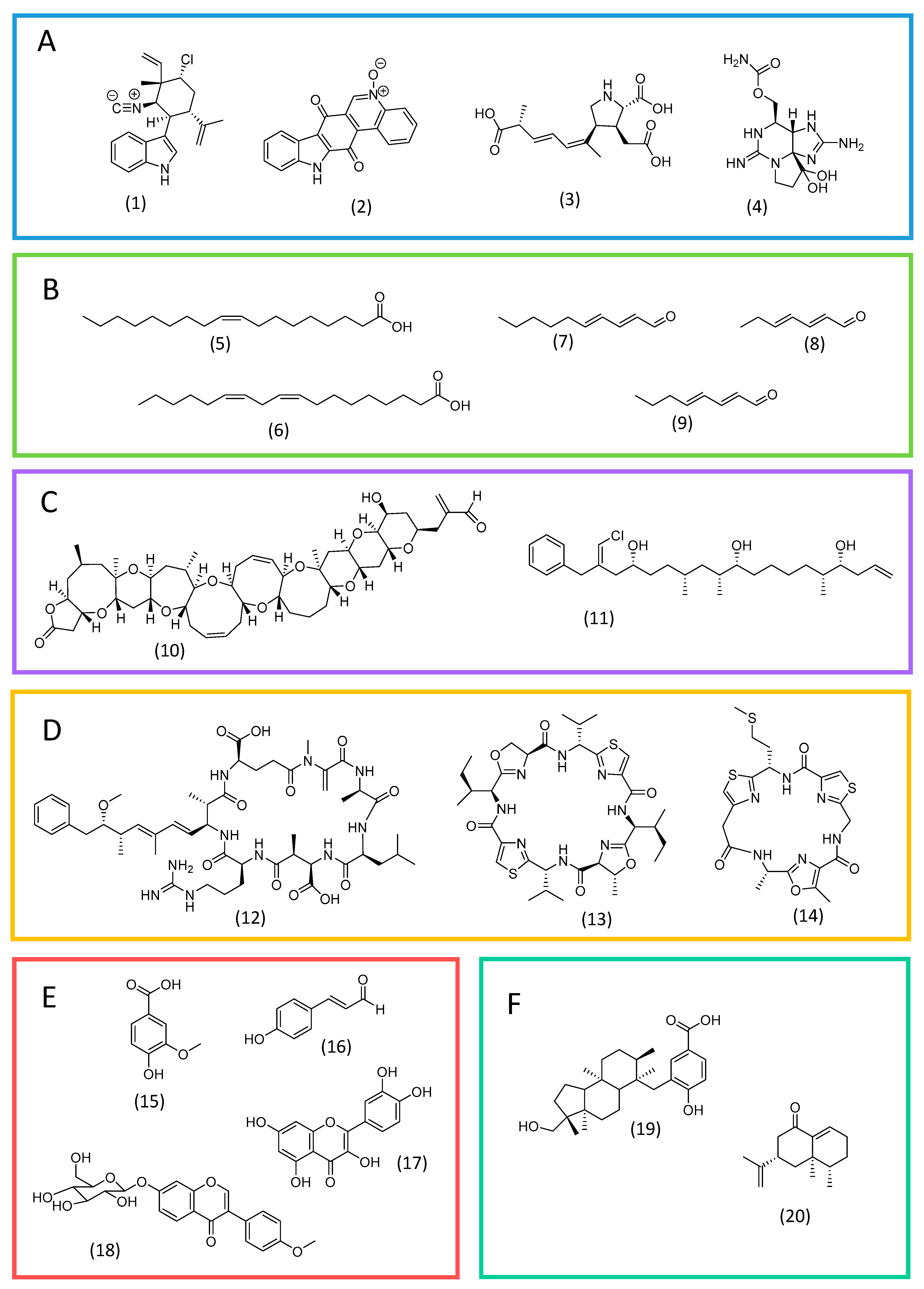
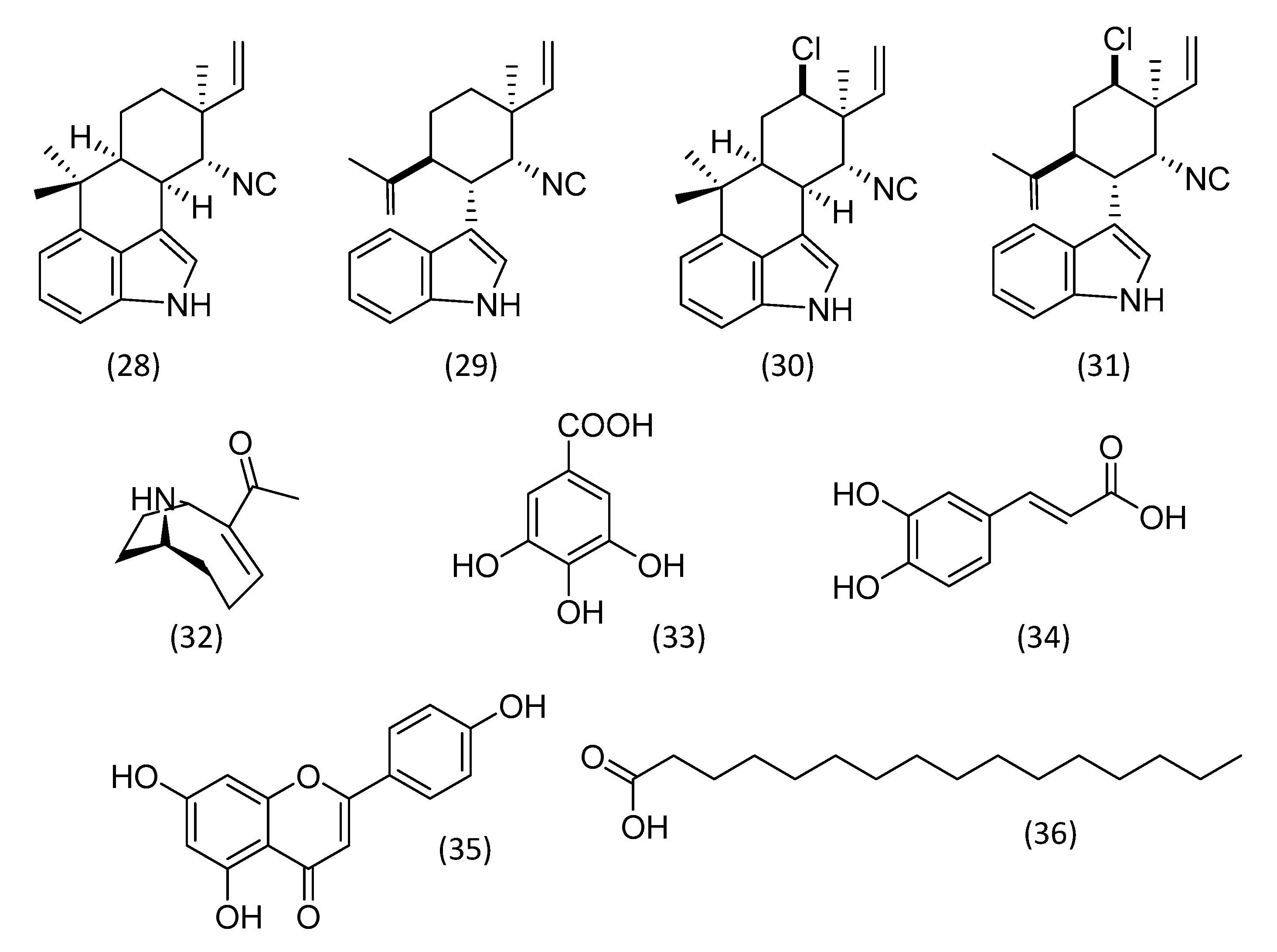
3.1. Alkaloids
3.2. Fatty acids and Derivatives
3.3. Polyketides
3.4. Peptides
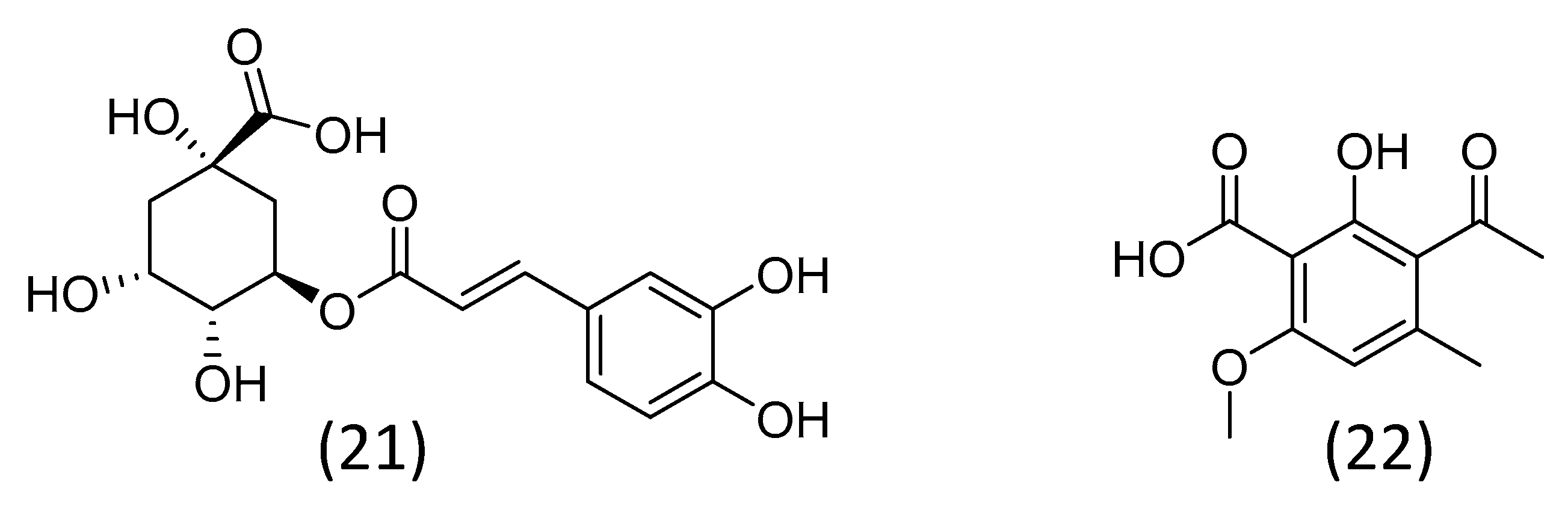
3.5. Phenolic Compounds
3.6. Terpenoids
3.7. Volatile Organic Compounds
4. Allelochemicals from Microalgae for Biopesticides
4.1. Allelochemicals against Phytopathogens
4.2. Allelochemicals as Herbicides
4.3. Allelochemicals as Insecticides
5. Safety and Toxicity of Allelochemicals and Synthetic Pesticides
6. Microalgae Allelochemicals: Gaps and Opportunities
7. Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Molisch, H. Der Einfluss einer Pflanze auf die Andere, Allelopathie. Nature 1938, 141, 493. [Google Scholar] [CrossRef]
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A review of allelopathy on microalgae. Microbiology 2019, 165, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Garcia, Q.S.; Barreto, L.C.; Pimenta, L.P.S.; Matheus, M.T.; Figueredo, C.C. Allelopathy: An overview from micro- to macroscopic organisms, from cells to environments, and the perspectives in a climate-changing world. Biologia 2017, 72, 113–129. [Google Scholar] [CrossRef]
- Inderjit; Dakshini, K.M.M. Algal allelopathy. Bot. Rev. 1994, 60, 182–196. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae Taxonomy and Breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 44–53. [Google Scholar]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.; Sánchez-Mirón, A.; García-Camacho, F.; López-Rosales, L.; Chisti, Y.; Molina-Grima, E. Bioactives from microalgal dinoflagellates. Biotechnol. Adv. 2012, 30, 1673–1684. [Google Scholar] [CrossRef]
- Stevens, P.F. Angiosperm Phylogeny and Diversification. In Encyclopedia of Evolutionary Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 78–83. ISBN 978-0-12-800426-5. [Google Scholar]
- Watson, S.B.; Whitton, B.A.; Higgins, S.N.; Paerl, H.W.; Brooks, B.W.; Wehr, J.D. Harmful Algal Blooms. In Freshwater Algae of North America; Elsevier: Amsterdam, The Netherlands, 2015; pp. 873–920. ISBN 978-0-12-385876-4. [Google Scholar]
- Mathieu, A. Advances in Bioengineering Study of Microalgae. J. Pet. Environ. Biotechnol. 2022, 13, 1000453. [Google Scholar]
- Mendes, L.B.B.; Vermelho, A.B. Allelopathy as a potential strategy to improve microalgae cultivation. Biotechnol. Biofuels 2013, 6, 152. [Google Scholar] [CrossRef]
- Leflaive, J.; Ten-Hage, L. Algal and cyanobacterial secondary metabolites in freshwaters: A comparison of allelopathic compounds and toxins. Freshw. Biol. 2007, 52, 199–214. [Google Scholar] [CrossRef]
- Shahbaz, A.; Hussain, N.; Saba, S.; Bilal, M. Actinomycetes, Cyanobacteria, and Fungi: A Rich Source of Bioactive Molecules. In Microbial Biomolecules; Elsevier: Amsterdam, The Netherlands, 2023; pp. 113–133. ISBN 978-0-323-99476-7. [Google Scholar]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L. Isolation, Structure Determination, and Biological Activity of Lyngbyabellin A from the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 611–615. [Google Scholar] [CrossRef]
- Legrand, C.; Rengefors, K.; Fistarol, G.O.; Granéli, E. Allelopathy in phytoplankton—Biochemical, ecological and evolutionary aspects. Phycologia 2003, 42, 406–419. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and Health Effects of Pesticide Residues. In Sustainable Agriculture Reviews 48; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2021; Volume 48, pp. 311–336. ISBN 978-3-030-54718-9. [Google Scholar]
- Riyaz, M.; Ahmad Shah, R.; Sivasankaran, K. Pesticide Residues: Impacts on Fauna and the Environment. In Biodegradation Technology of Organic and Inorganic Pollutants; Ferreira Mendes, K., Nogueira de Sousa, R., Cabral Mielke, K., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83968-895-9. [Google Scholar]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Chaïb, S.; Pistevos, J.C.A.; Bertrand, C.; Bonnard, I. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res. 2021, 54, 102213. [Google Scholar] [CrossRef]
- Molisch, H.; Narwal, S.S. The Influence of One Plant on Another, Allelopathy; Published by Scientific Publishers (India) for International Allelopathy Foundation, Hisar: Jodhpur, India, 2001; ISBN 978-81-7233-285-3. [Google Scholar]
- Caruana, A.M.N.; Amzil, Z. Microalgae and Toxins. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 263–305. ISBN 978-0-12-811405-6. [Google Scholar]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Wiśniewska, K.; Konarzewska, Z.; Cieszyńska, A.; Felpeto, A.B.; Lewandowska, A.U.; Latała, A. The current state of knowledge on taxonomy, modulating factors, ecological roles, and mode of action of phytoplankton allelochemicals. Sci. Total. Environ. 2021, 773, 145681. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Li, W.; Wang, M.; Xiao, L. Interspecies competition between Scrippsiella acuminata and three marine diatoms: Growth inhibition and allelopathic effects. Aquat. Toxicol. 2021, 237, 105878. [Google Scholar] [CrossRef]
- Kumari, P.; Cna’ani, A.; Didi-Cohen, S.; Tzin, V.; Khozin-Goldberg, I. Nitrogen Deprivation-Induced Production of Volatile Organic Compounds in the Arachidonic-Acid-Accumulating Microalga Lobosphaera incisa Underpins Their Role as ROS Scavengers and Chemical Messengers. Front. Mar. Sci. 2020, 7, 410. [Google Scholar] [CrossRef]
- Sartori, R.B.; Siqueira, S.F.; Maroneze, M.M.; Fagundes, M.B.; Wagner, R.; Zepka, L.Q.; Jacob-Lopes, E. Microalgal secondary metabolites: Effect of climatic variables, seasons, and photocycles on the biogeneration of volatile organic compounds (VOCs). J. Appl. Phycol. 2021, 33, 1457–1472. [Google Scholar] [CrossRef]
- Grabski, K.; Tukaj, Z. Autoinduction activity of a conditioned medium obtained from high density cultures of the green alga Scenedesmus subspicatus. J. Appl. Phycol. 2007, 20, 323–330. [Google Scholar] [CrossRef]
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.-S.; Zimba, P.V.; do Carmo Bittencourt-Oliveira, M.; Gobler, C.J. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 2018, 74, 67–77. [Google Scholar] [CrossRef]
- Ali, S.S.; Mastropetros, S.G.; Schagerl, M.; Sakarika, M.; Elsamahy, T.; El-Sheekh, M.; Sun, J.; Kornaros, M. Recent advances in wastewater microalgae-based biofuels production: A state-of-the-art review. Energy Rep. 2022, 8, 13253–13280. [Google Scholar] [CrossRef]
- Casanova, L.M.; Mendes, L.B.B.; Corrêa, T.D.S.; da Silva, R.B.; Joao, R.R.; Macrae, A.; Vermelho, A.B. Development of Microalgae Biodiesel: Current Status and Perspectives. Microorganisms 2022, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An overview on microalgae as renewable resources for meeting sustainable development goals. J. Environ. Manag. 2022, 320, 115897. [Google Scholar] [CrossRef]
- Loftus, S.E.; Johnson, Z.I. Cross-study analysis of factors affecting algae cultivation in recycled medium for biofuel production. Algal Res. 2017, 24, 154–166. [Google Scholar] [CrossRef]
- Lu, Z.; Loftus, S.; Sha, J.; Wang, W.; Park, M.S.; Zhang, X.; Johnson, Z.I.; Hu, Q. Water reuse for sustainable microalgae cultivation: Current knowledge and future directions. Resour. Conserv. Recycl. 2020, 161, 104975. [Google Scholar] [CrossRef]
- Thomas, P.K.; Hietala, D.C.; Cardinale, B.J. Tolerance to allelopathic inhibition by free fatty acids in five biofuel candidate microalgae strains. Bioresour. Technol. Rep. 2023, 21, 101321. [Google Scholar] [CrossRef]
- Qixin, L.; Xuan, F.; Zhiya, S.; Wenxin, S.; Shuo, W.; Ji, L. Enhanced wastewater treatment performance by understanding the interaction between algae and bacteria based on quorum sensing. Bioresour. Technol. 2022, 354, 127161. [Google Scholar] [CrossRef]
- Satake, M.; Honma, D.; Watanabe, R.; Oshima, Y. Alexandrolide, a diatom growth inhibitor isolated from the dinoflagellate Alexandrium catenella. Tetrahedron Lett. 2019, 60, 1341–1344. [Google Scholar] [CrossRef]
- Pouvreau, J.-B.; Housson, E.; Le Tallec, L.; Morançais, M.; Rincé, Y.; Fleurence, J.; Pondaven, P. Growth inhibition of several marine diatom species induced by the shading effect and allelopathic activity of marennine, a blue-green polyphenolic pigment of the diatom Haslea ostrearia (Gaillon/Bory) Simonsen. J. Exp. Mar. Biol. Ecol. 2007, 352, 212–225. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the post-genomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785. [Google Scholar] [CrossRef] [PubMed]
- Lichman, B.R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 2020, 38, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.T.; Rickards, R.W.; Rothschild, J.M.; Smith, G.D. Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin A from cyanobacteria of the genera Fischerella and Calothrix. J. Appl. Phycol. 2000, 12, 409–416. [Google Scholar] [CrossRef]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Wright, J.L.C. The Amnesic Shellfish Poisoning Mystery. Anal. Chem. 1989, 61, 1053A–1060A. [Google Scholar] [CrossRef]
- Takemoto, T.; Daigo, K. Constituents of Chondria Armata. Chem. Pharm. Bull. 1958, 6, 578–580. [Google Scholar] [CrossRef]
- Orr, R.J.S.; Stüken, A.; Murray, S.A.; Jakobsen, K.S. Evolution and Distribution of Saxitoxin Biosynthesis in Dinoflagellates. Mar. Drugs 2013, 11, 2814–2828. [Google Scholar] [CrossRef]
- Murray, S.A.; Mihali, T.K.; Neilan, B.A. Extraordinary Conservation, Gene Loss, and Positive Selection in the Evolution of an Ancient Neurotoxin. Mol. Biol. Evol. 2011, 28, 1173–1182. [Google Scholar] [CrossRef]
- Schantz, E.J.; Lynch, J.M.; Vayvada, G.; Matsumoto, K.; Rapoport, H. The Purification and Characterization of the Poison Produced by Gonyaulax catenella in Axenic Culture. Biochemistry 1966, 5, 1191–1195. [Google Scholar] [CrossRef]
- Jackim, E.; Gentile, J. Toxins of a Blue-Green Alga: Similarity to Saxitoxin. Science 1968, 162, 915–916. [Google Scholar] [CrossRef]
- Chiang, I.-Z.; Huang, W.-Y.; Wu, J.-T. Allelochemicals of Botryococcus braunii (Chloroficeae). J. Phycol. 2004, 40, 474–480. [Google Scholar] [CrossRef]
- DellaGreca, M.; Zarrelli, A.; Fergola, P.; Cerasuolo, M.; Pollio, A.; Pinto, G. Fatty Acids Released by Chlorella vulgaris and Their Role in Interference with Pseudokirchneriella subcapitata: Experiments and Modelling. J. Chem. Ecol. 2010, 36, 339–349. [Google Scholar] [CrossRef]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Cózar, A.; Morillo-García, S.; Ortega, M.J.; Li, Q.P.; Bartual, A. Macroecological patterns of the phytoplankton production of polyunsaturated aldehydes. Sci. Rep. 2018, 8, 12282. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some Chemical Properties of Maitotoxin, a Putative Calcium Channel Agonist Isolated from a Marine Dinoflagellate. J. Biochem. 1988, 104, 184–187. [Google Scholar] [CrossRef]
- Reyes, J.G.; Sánchez-Cárdenas, C.; Acevedo-Castillo, W.; Leyton, P.; López-González, I.; Felix, R.; Gandini, M.A.; Treviño, M.B.; Treviño, C.L. Maitotoxin: An Enigmatic Toxic Molecule with Useful Applications in the Biomedical Sciences. In Seafood and Freshwater Toxins; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 677–694. ISBN 978-0-429-09621-1. [Google Scholar]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and Derivative Brevetoxins: Historical Background, Multiplicity, and Effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef]
- McManus, K.M.; Kirk, R.D.; Via, C.W.; Lotti, J.S.; Roduit, A.F.; Teta, R.; Scarpato, S.; Mangoni, A.; Bertin, M.J. Isolation of Isotrichophycin C and Trichophycins G–I from a Collection of Trichodesmium thiebautii. J. Nat. Prod. 2020, 83, 2664–2671. [Google Scholar] [CrossRef]
- Bertin, M.J.; Wahome, P.G.; Zimba, P.V.; He, H.; Moeller, P.D.R. Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom. Mar. Drugs 2017, 15, 10. [Google Scholar] [CrossRef]
- Micallef, M.L.; D’Agostino, P.M.; Al-Sinawi, B.; Neilan, B.A.; Moffitt, M.C. Exploring cyanobacterial genomes for natural product biosynthesis pathways. Mar. Genom. 2014, 21, 1–12. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—Ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.-Y. Terpene Biosynthesis: Modularity Rules. Angew. Chem. Int. Ed. 2011, 51, 1124–1137. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel Extracellular Diterpenoids with Biological Activity from the Cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Höckelmann, C.; Becher, P.G.; von Reuß, S.H.; Jüttner, F. Sesquiterpenes of the Geosmin-Producing Cyanobacterium Calothrix PCC 7507 and their Toxicity to Invertebrates. Z. Naturforsch C. J. Biosci. 2009, 64, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Pozzer, A.C.; Gómez, P.A.; Weiss, J. Volatile organic compounds in aquatic ecosystems—Detection, origin, significance and applications. Sci. Total. Environ. 2022, 838, 156155. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Harper, J.C.; Manginell, R.P.; Moorman, M.W. Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity? Metabolites 2017, 7, 39. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, L.; Han, X.; Yang, Y. Research Advances in Allelopathy of Volatile Organic Compounds (VOCs) of Plants. Horticulturae 2021, 7, 278. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J. Environ. Sci. Health Part B 2019, 54, 366–375. [Google Scholar] [CrossRef]
- Berthon, J.-Y.; Michel, T.; Wauquier, A.; Joly, P.; Gerbore, J.; Filaire, E. Seaweed and microalgae as major actors of blue biotechnology to achieve plant stimulation and pest and pathogen biocontrol—A review of the latest advances and future prospects. J. Agric. Sci. 2021, 159, 523–534. [Google Scholar] [CrossRef]
- Pattanayak, S.; Das, S.; Biswal, G. Crop Disease Control through Application of Algal Biopesticides: Alternate Route Map in Organic Farming. In Biopesticides in Organic Farming; CRC Press: Boca Raton, FL, USA, 2021; pp. 123–126. ISBN 978-0-367-46017-4. [Google Scholar]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Bratchkova, A.; Alexander, D. Kroumov Microalgae as Producers of Biologically Active Compounds with Anti-bacterial, Antiviral, Antifungal, Antialgal, Antiprotozoal, Antiparasitic and Anticancer Activity. Acta Microbiol. Bulg. 2020, 36, 79–89. [Google Scholar]
- Stirk, W.A.; van Staden, J. Bioprospecting for bioactive compounds in microalgae: Antimicrobial compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N. Antibacterial, antifungal and antimycobacterial compounds from cyanobacteria. Biomed. Pharmacother. 2017, 90, 760–776. [Google Scholar] [CrossRef]
- Hernández-Carlos, B.; Gamboa-Angulo, M.M. Metabolites from freshwater aquatic microalgae and fungi as potential natural pesticides. Phytochem. Rev. 2011, 10, 261–286. [Google Scholar] [CrossRef]
- Asthana, R.K.; Srivastava, A.; Singh, A.P.; Deepali; Singh, S.P.; Nath, G.; Srivastava, R.; Srivastava, B.S. Identification of an antimicrobial entity from the cyanobacterium Fischerella sp. isolated from bark of Azadirachta indica (Neem) tree. J. Appl. Phycol. 2006, 18, 33–39. [Google Scholar] [CrossRef]
- Kajiyama, S.-I.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an antifungal lipopeptide from the field-grown terrestrial blue-green alga Nostoc commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A Fatty Acid from the Diatom Phaeodactylum tricornutum is Antibacterial Against Diverse Bacteria Including Multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Senousy, H.H.; El-Sheekh, M.M.; Saber, A.A.; Khairy, H.M.; Said, H.A.; Alhoqail, W.A.; Abu-Elsaoud, A.M. Biochemical Analyses of Ten Cyanobacterial and Microalgal Strains Isolated from Egyptian Habitats, and Screening for Their Potential against Some Selected Phytopathogenic Fungal Strains. Agronomy 2022, 12, 1340. [Google Scholar] [CrossRef]
- Schmid, B.; Coelho, L.; Schulze, P.S.C.; Pereira, H.; Santos, T.; Maia, I.B.; Reis, M.; Varela, J. Antifungal properties of aqueous microalgal extracts. Bioresour. Technol. Rep. 2022, 18, 101096. [Google Scholar] [CrossRef]
- Almalki, M.A.; Khalifa, A.Y.Z.; Alkhamis, Y.A. In vitro Antibiosis of Chlorella vulgaris Extract against the Phytopathogen, Stenotrophomonas maltophilia. J. Pure Appl. Microbiol. 2022, 16, 630–637. [Google Scholar] [CrossRef]
- Ragaa, A.; Hamouda, M.S.M. El-Ansary Potential of Plant-Parasitic Nematode Control in Banana Plants by Micro-algae as a New Approach Towards Resistance. Egypt. J. Biol. Pest Control. 2017, 27, 165–172. [Google Scholar]
- Righini, H.; Francioso, O.; Quintana, A.M.; Roberti, R. Cyanobacteria: A Natural Source for Controlling Agricultural Plant Diseases Caused by Fungi and Oomycetes and Improving Plant Growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Pagnussatt, F.A.; Lemos, A.C.; Nicolli, C.P.; Del Ponte, E.M.; Badiale-Furlong, E. Nannochloropsis sp. and Spirulina sp. as a Source of Antifungal Compounds to Mitigate Contamination by Fusarium graminearum Species Complex. Curr. Microbiol. 2019, 76, 930–938. [Google Scholar] [CrossRef]
- Natarajan, C.; Prasanna, R.; Gupta, V.; Dureja, P.; Nain, L. Characterization of the fungicidal activity of Calothrix elenkinii using chemical methods and microscopy. Appl. Biochem. Microbiol. 2012, 48, 51–57. [Google Scholar] [CrossRef]
- Bang, K.-H.; Lee, D.-W.; Park, H.-M.; Rhee, Y.-H. Inhibition of Fungal Cell Wall Synthesizing Enzymes by trans-Cinnamaldehyde. Biosci. Biotechnol. Biochem. 2000, 64, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Chen, P.-F.; Chang, S.-T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-S.; Zhu, Y.-J.; Li, H.-L.; Zhuang, J.-X.; Zhang, C.-L.; Zhou, J.-J.; Li, W.-G.; Chen, Q.-X. Inhibitory Effects of Methyl trans-Cinnamate on Mushroom Tyrosinase and Its Antimicrobial Activities. J. Agric. Food Chem. 2009, 57, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Amborabé, B.-E.; Fleurat-Lessard, P.; Chollet, J.-F.; Roblin, G. Antifungal effects of salicylic acid and other benzoic acid derivatives towards Eutypa lata: Structure–activity relationship. Plant Physiol. Biochem. 2002, 40, 1051–1060. [Google Scholar] [CrossRef]
- Berne, S.; Kovačič, L.; Sova, M.; Kraševec, N.; Gobec, S.; Križaj, I.; Komel, R. Benzoic acid derivatives with improved antifungal activity: Design, synthesis, structure–activity relationship (SAR) and CYP53 docking studies. Bioorganic Med. Chem. 2015, 23, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.A.; Ahmed, M.E.; El-Nagar, A. Benzoic Acid and Its Hydroxylated Derivatives Suppress Early Blight of Tomato (Alternaria solani) via the Induction of Salicylic Acid Biosynthesis and Enzymatic and Nonenzymatic Antioxidant Defense Machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- D’agostino, P.M.; Seel, C.J.; Ji, X.; Gulder, T.; Gulder, T.A.M. Biosynthesis of cyanobacterin, a paradigm for furanolide core structure assembly. Nat. Chem. Biol. 2022, 18, 652–658. [Google Scholar] [CrossRef]
- Berry, J. Marine and Freshwater Microalgae as a Potential Source of Novel Herbicides. In Herbicides and Environment; Kortekamp, A., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-476-4. [Google Scholar]
- Zhang, Y.; Whalen, J.K.; Sauvé, S. Phytotoxicity and bioconcentration of microcystins in agricultural plants: Meta-analysis and risk assessment. Environ. Pollut. 2020, 272, 115966. [Google Scholar] [CrossRef]
- Koodkaew, I.; Sunohara, Y.; Matsuyama, S.; Matsumoto, H. Isolation of ambiguine D isonitrile from Hapalosiphon sp. and characterization of its phytotoxic activity. Plant Growth Regul. 2012, 68, 141–150. [Google Scholar] [CrossRef]
- Biondi, N.; Piccardi, R.; Margheri, M.C.; Rodolfi, L.; Smith, G.D.; Tredici, M.R. Evaluation of Nostoc Strain ATCC 53789 as a Potential Source of Natural Pesticides. Appl. Environ. Microbiol. 2004, 70, 3313–3320. [Google Scholar] [CrossRef]
- Abdel-Baky, H.H.; Shallan, M.A.; Baroty, G.E.; El-Baz, F.K. Volatile Compounds of the Microalga Chlorella vulgaris and Their Phytotoxic Effect. Pak. J. Biol. Sci. 2001, 5, 61–65. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Perez, M.H.; Berry, G.; Noriega, F.G. Cyanobacterial Toxins as Allelochemicals with Potential Applications as Algaecides, Herbicides and Insecticides. Mar. Drugs 2008, 6, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Marten, G.G. Larvicidal algae. J. Am. Mosq. Control. Assoc. 2007, 23, 177–183. [Google Scholar] [CrossRef]
- Rankic, I.; Zelinka, R.; Ridoskova, A.; Gagic, M.; Pelcova, P.; Huska, D. Nano/microparticles in conjunction with microalgae extract as novel insecticides against Mealworm beetles, Tenebrio molitor. Sci. Rep. 2021, 11, 17125. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Jüttner, F. Insecticidal compounds of the biofilm-forming cyanobacterium Fischerella sp. (ATCC 43239). Environ. Toxicol. 2005, 20, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Keller, S.; Jung, G.; Süssmuth, R.; Jüttner, F. Insecticidal activity of 12-epi-hapalindole J isonitrile. Phytochemistry 2007, 68, 2493–2497. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, J.; Abdel-Hameed, A.; Sivonen, K.; Niemelä, S.I.; Carlberg, G. Toxicity of cyanobacteria to mosquito larvae—Screening of active compounds. Environ. Toxicol. Water Qual. 1993, 8, 63–71. [Google Scholar] [CrossRef]
- Delaney, J.; Wilkins, R. Toxicity of microcystin-LR, isolated from Microcystis aeruginosa, against various insect species. Toxicon 1995, 33, 771–778. [Google Scholar] [CrossRef]
- Saario, E.; Abdel-Hameed, A.; Kiviranta, J. Larvicidal microcystin toxins of cyanobacteria affect midgut epithelial cells of Aedes aegypti mosquitoes. Med. Veter. Èntomol. 1994, 8, 398–400. [Google Scholar] [CrossRef]
- Cook, W.; Beasley, V.; Dahlem, A.; Dellinger, J.; Harlin, K.; Carmichael, W. Comparison of effects of anatoxin-a(s) and paraoxon, physostigmine and pyridostigmine on mouse brain cholinesterase activity. Toxicon 1988, 26, 750–753. [Google Scholar] [CrossRef]
- dos Santos, D.S.; Rosa, M.E.; Zanatta, A.P.; Oliveira, R.S.; de Almeida, C.G.M.; Leal, A.P.; Sanz, M.; Fernandes, K.A.; de Souza, V.Q.; de Assis, D.R.; et al. Neurotoxic effects of sublethal concentrations of cyanobacterial extract containing anatoxin-a(s) on Nauphoeta cinerea cockroaches. Ecotoxicol. Environ. Saf. 2018, 171, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.E.; Mohafrash, S.M.M.; Fallatah, S.A.; El-Sayed, A.E.-K.B.; Mossa, A.-T.H. Eco-friendly larvicide of Amphora coffeaeformis and Scenedesmus obliquus microalgae extracts against Culex pipiens. J. Appl. Phycol. 2021, 33, 2683–2693. [Google Scholar] [CrossRef]
- Dolma, S.K.; Singh, P.P.; Reddy, S.G.E. Insecticidal and Enzyme Inhibition Activities of Leaf/Bark Extracts, Fractions, Seed Oil and Isolated Compounds from Triadica sebifera (L.) Small against Aphis craccivora Koch. Molecules 2022, 27, 1967. [Google Scholar] [CrossRef] [PubMed]
- Punia, A.; Chauhan, N.S.; Singh, D.; Kesavan, A.K.; Kaur, S.; Sohal, S.K. Effect of gallic acid on the larvae of Spodoptera litura and its parasitoid Bracon hebetor. Sci. Rep. 2021, 11, 531. [Google Scholar] [CrossRef]
- Joshi, R.S.; Wagh, T.P.; Sharma, N.; Mulani, F.A.; Sonavane, U.; Thulasiram, H.V.; Joshi, R.; Gupta, V.S.; Giri, A.P. Way toward “Dietary Pesticides”: Molecular Investigation of Insecticidal Action of Caffeic Acid against Helicoverpa armigera. J. Agric. Food Chem. 2014, 62, 10847–10854. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Shen, J.; Zhou, Y.; Wei, Z.-P.; Gao, J.-M. Insecticidal Constituents from Buddlej aalbiflora Hemsl. Nat. Prod. Res. 2017, 31, 1446–1449. [Google Scholar] [CrossRef]
- Saber, A.A.; Moussa, S.; Abdel-Rahim, E.F.M.; Cantonati, M. Insecticidal Prospects of Algal and Cyanobacterial Extracts against the Cotton Leaf Worm Spodoptera littoralis. Vie Milieu Life Environ. 2018, 68, 199–212. [Google Scholar]
- Sigamani, S.; Chinnasamy, R.; Dharmaraj, R.K.; Ramamurthy, D.; Devarajan, N.; Narayanasamy, M.; Natarajan, H. Larvicidal potency of the extracts from Chlorella sp. against Aedes aegypti. Biocatal. Agric. Biotechnol. 2020, 27, 101663. [Google Scholar] [CrossRef]
- Rahuman, A.A.; Gopalakrishnan, G.; Ghouse, B.S.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- Aguilar-Marcelino, L.; Pineda-Alegría, J.A.; Salinas-Sánchez, D.O.; Hernández-Velázquez, V.M.; Silva-Aguayo, G.I.; Navarro-Tito, N.; Sotelo-Leyva, C. In Vitro Insecticidal Effect of Commercial Fatty Acids, β-Sitosterol, and Rutin against the Sugarcane Aphid, Melanaphis sacchari Zehntner (Hemiptera: Aphididae). J. Food Prot. 2022, 85, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Jang, M.J.; Kim, J.-R.; Kadarkarai, M.; Ahn, Y.-J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasites Vectors 2015, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Ghatrehsamani, S.; Jha, G.; Dutta, W.; Molaei, F.; Nazrul, F.; Fortin, M.; Bansal, S.; Debangshi, U.; Neupane, J. Artificial Intelligence Tools and Techniques to Combat Herbicide Resistant Weeds—A Review. Sustainability 2023, 15, 1843. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Zhang, A.; Luo, W.; Sun, J.; Xiao, H.; Liu, W. Distribution and uptake pathways of organochlorine pesticides in greenhouse and conventional vegetables. Sci. Total. Environ. 2015, 505, 1142–1147. [Google Scholar] [CrossRef]
- Balmer, J.E.; Morris, A.D.; Hung, H.; Jantunen, L.; Vorkamp, K.; Rigét, F.; Evans, M.; Houde, M.; Muir, D.C. Levels and trends of current-use pesticides (CUPs) in the arctic: An updated review, 2010–2018. Emerg. Contam. 2019, 5, 70–88. [Google Scholar] [CrossRef]
- Liu, L.; Pohnert, G.; Wei, D. Extracellular Metabolites from Industrial Microalgae and Their Biotechnological Potential. Mar. Drugs 2016, 14, 191. [Google Scholar] [CrossRef]
- Hickman, D.T.; Rasmussen, A.; Ritz, K.; Birkett, M.A.; Neve, P. Review: Allelochemicals as multi-kingdom plant defence compounds: Towards an integrated approach. Pest Manag. Sci. 2020, 77, 1121–1131. [Google Scholar] [CrossRef]
- Qureshi, H.; Anwar, T.; Ali, Q.; Haider, M.Z.; Habib, N.; Fatima, S.; Waseem, M.; Bibi, Y.; Arshad, M.; Adkins, S.W. Isolation of natural herbicidal compound from Lantana camara. Int. J. Environ. Anal. Chem. 2019, 101, 631–638. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides—Current Research and Case Studies in Use; Price, A., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1112-2. [Google Scholar]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Ran, Y.; Lai, W.; Ran, Z.; Xu, J.; Zhou, C.; Yan, X. Characterization of Steryl Glycosides in Marine Microalgae by Gas Chromatography-Triple Quadrupole Mass Spectrometry (GC-QQQ-MS): Steryl Glycosides in Microalgae Analyzed by GC-QQQ-MS. J. Sci. Food Agric. 2018, 98, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Cichoński, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the Role of Allelochemicals in Plant Defence. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 19–54. ISBN 978-0-12-801431-8. [Google Scholar]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive Peptides Produced by Cyanobacteria of the Genus Nostoc: A Review. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef]
- Mendes, L.B.B.; Viegas, C.V.; Joao, R.R.; da Silva, R.B. Microalgae Production: A Sustainable Alternative for a Low-carbon Economy Transition. Open Microalgae Biotechnol. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Grand View Research GVR Report CoverAstaxanthin Market Size, Share & Trends Report Astaxanthin Market Size, Share & Trends Analysis Report by Product (Dried Algae Meal or Biomass, Oil, Softgel, Liquid), by Source (Natural, Synthetic), by Application, by Region, and Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/global-astaxanthin-market (accessed on 1 March 2023).
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Algal biorefinery: An integrated approach for sustainable biodiesel production. Biomass Bioenergy 2019, 131, 105398. [Google Scholar] [CrossRef]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Martin, S.S.; Agurto, C.; Freitas, M.A.V.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Biotechnological potential of Phaeodactylum tricornutum for biorefinery processes. Fuel 2020, 268, 117357. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, M.J.M.F.; van Heck, R.G.A.; Lam, C.M.C.; Scaife, M.A.; dos Santos, V.A.P.M.; Smith, A.G.; Schaap, P.J. Green genes: Bioinformatics and systems-biology innovations drive algal biotechnology. Trends Biotechnol. 2014, 32, 617–626. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, L.M.; Macrae, A.; de Souza, J.E.; Neves Junior, A.; Vermelho, A.B. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants 2023, 12, 1896. https://doi.org/10.3390/plants12091896
Casanova LM, Macrae A, de Souza JE, Neves Junior A, Vermelho AB. The Potential of Allelochemicals from Microalgae for Biopesticides. Plants. 2023; 12(9):1896. https://doi.org/10.3390/plants12091896
Chicago/Turabian StyleCasanova, Livia Marques, Andrew Macrae, Jacqueline Elis de Souza, Athayde Neves Junior, and Alane Beatriz Vermelho. 2023. "The Potential of Allelochemicals from Microalgae for Biopesticides" Plants 12, no. 9: 1896. https://doi.org/10.3390/plants12091896
APA StyleCasanova, L. M., Macrae, A., de Souza, J. E., Neves Junior, A., & Vermelho, A. B. (2023). The Potential of Allelochemicals from Microalgae for Biopesticides. Plants, 12(9), 1896. https://doi.org/10.3390/plants12091896








