Potential of Novel Magnesium Nanomaterials to Manage Bacterial Spot Disease of Tomato in Greenhouse and Field Conditions
Abstract
1. Introduction
2. Results
2.1. Material Characterization
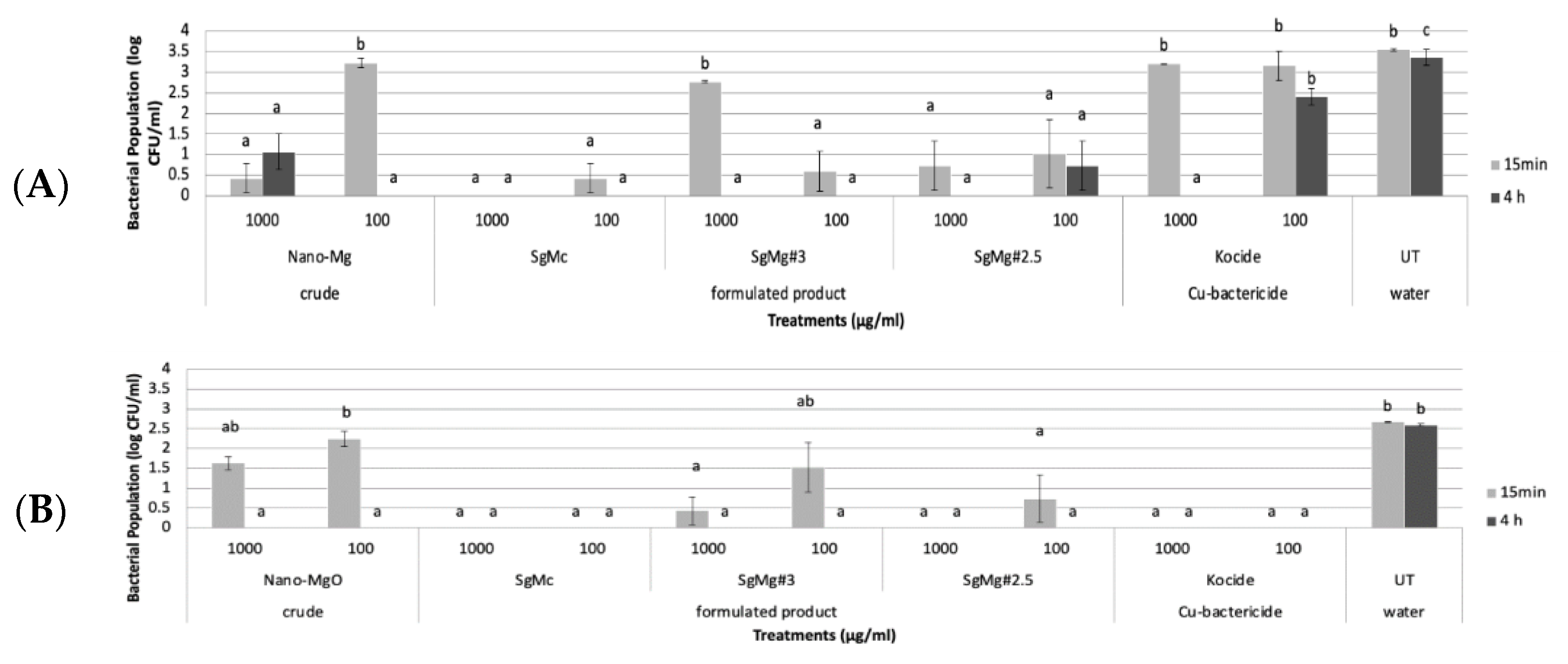
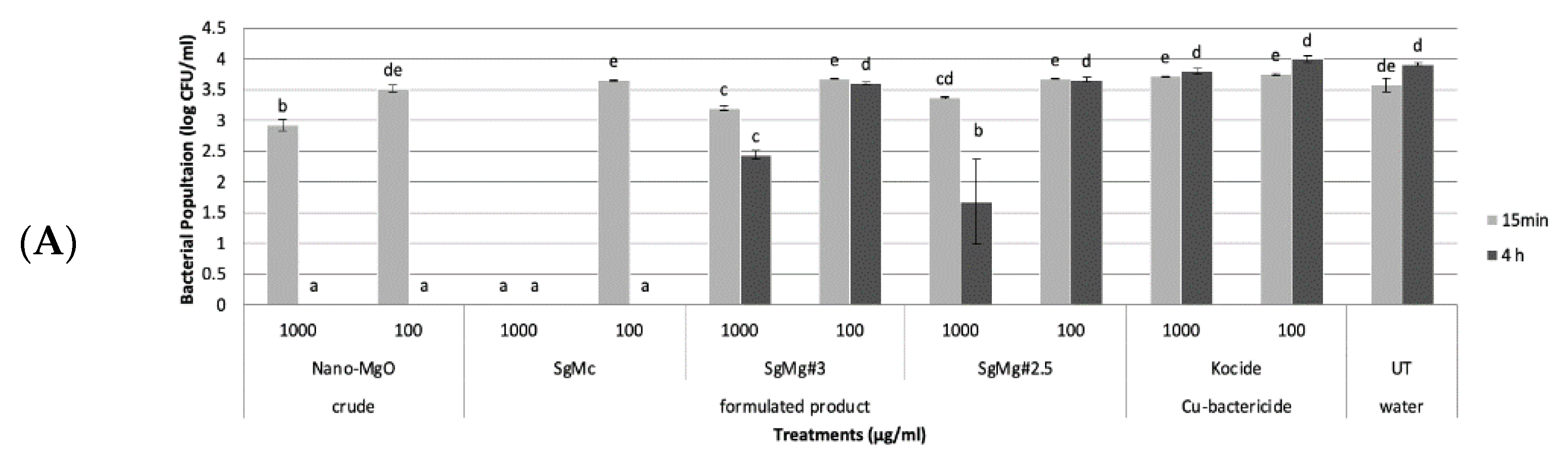
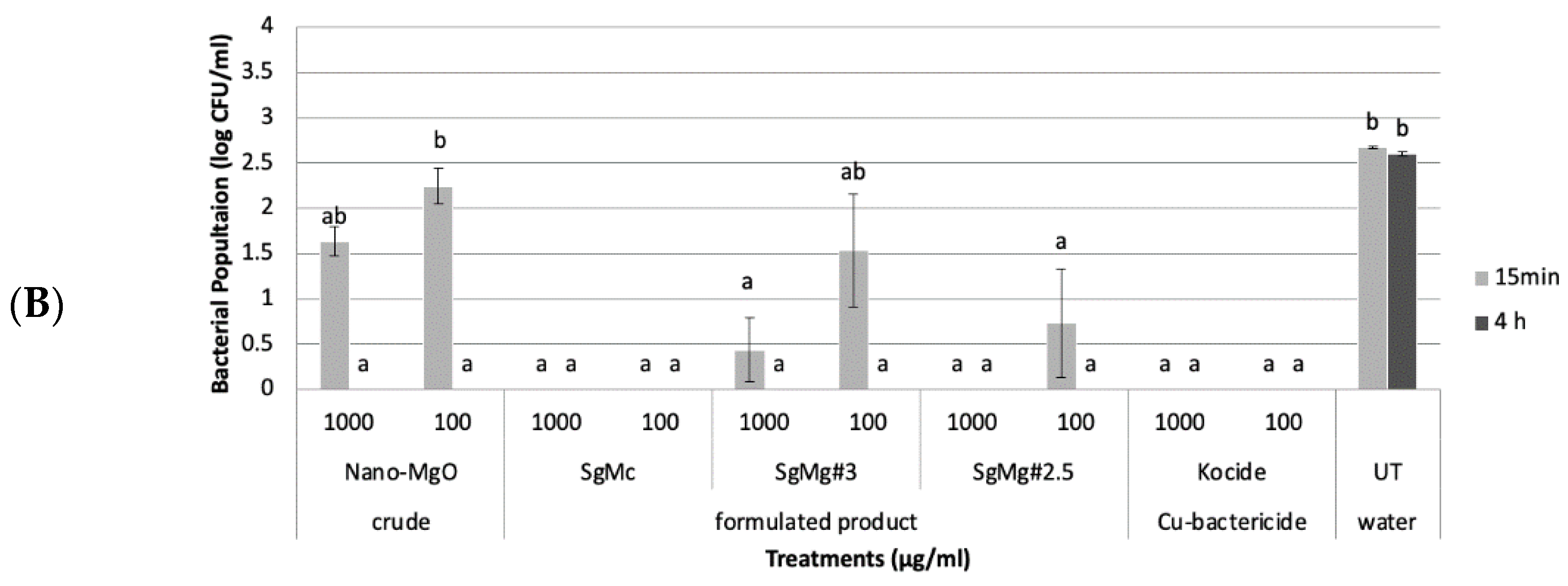
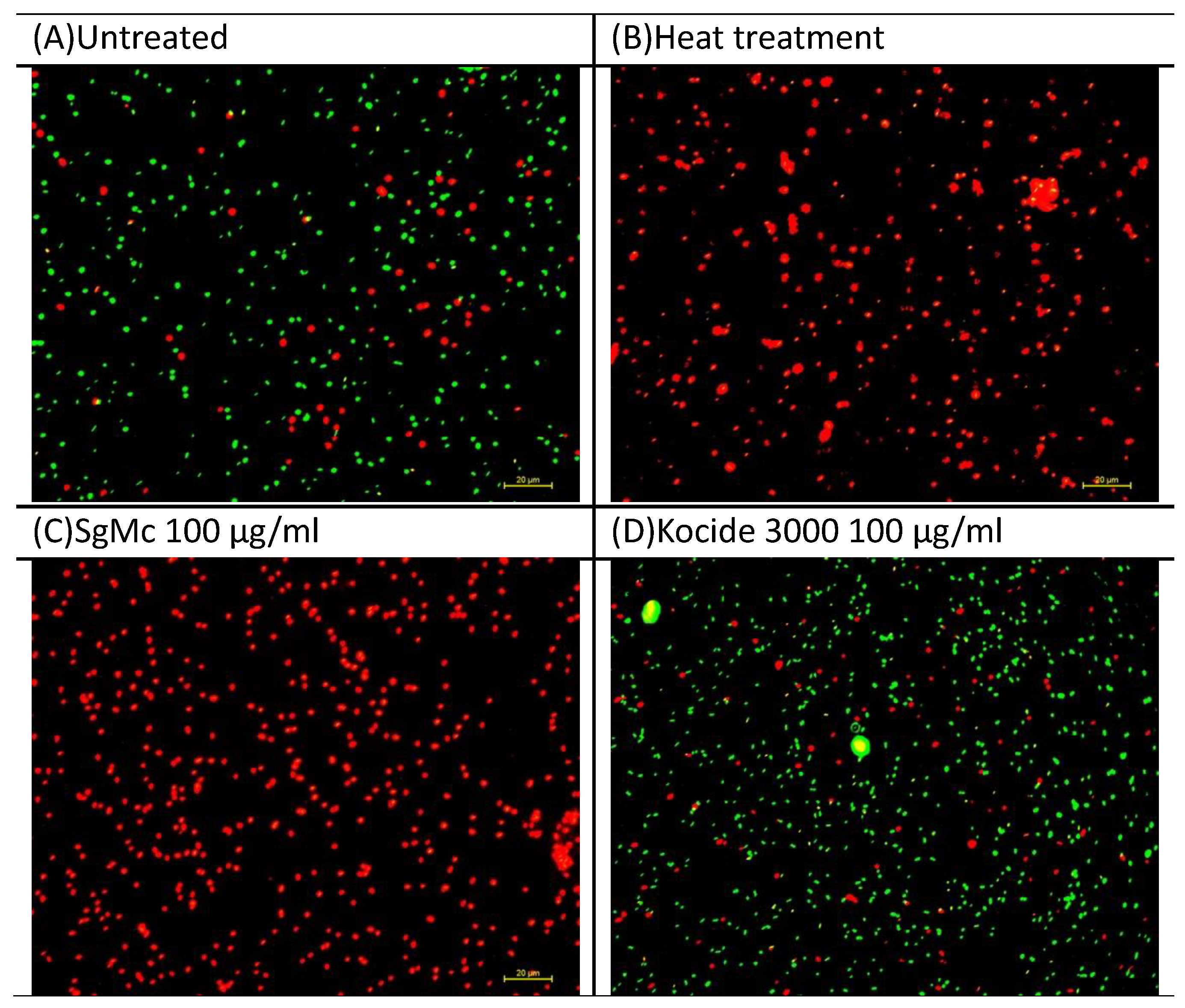
2.2. Antibacterial Activity of Formulated Mg Nanomaterials In Vitro
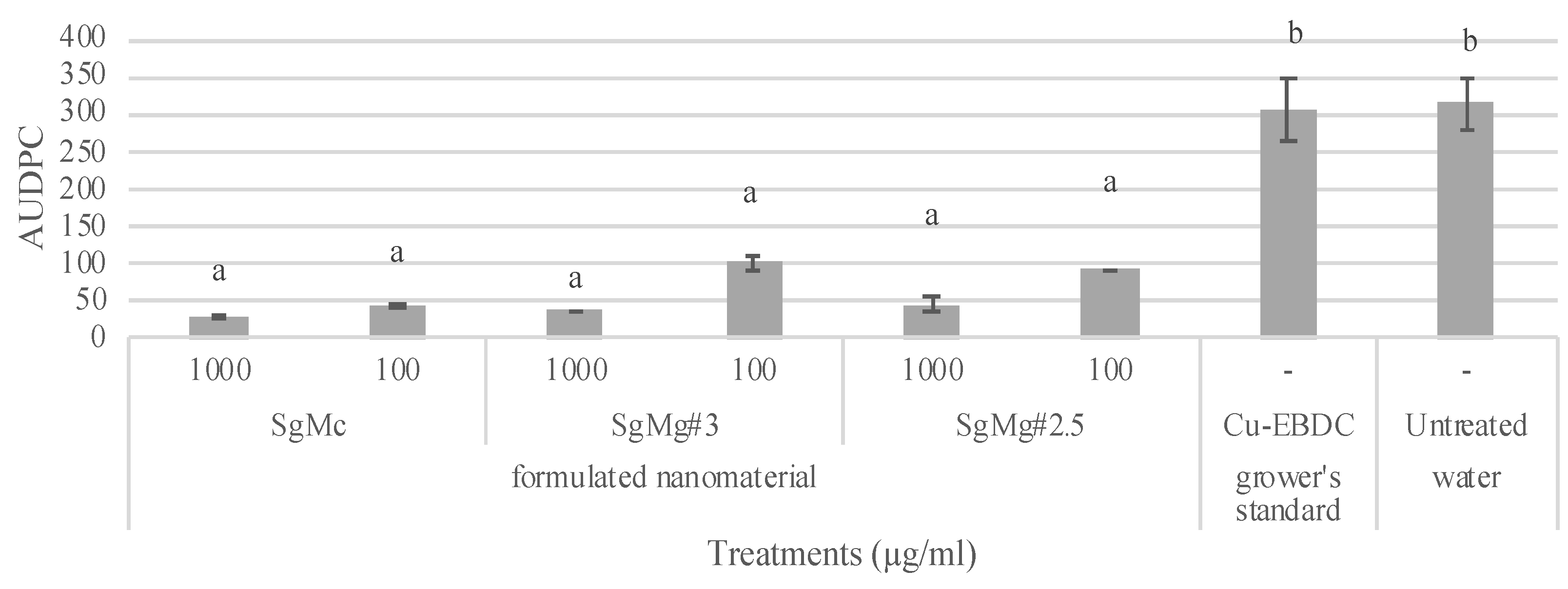
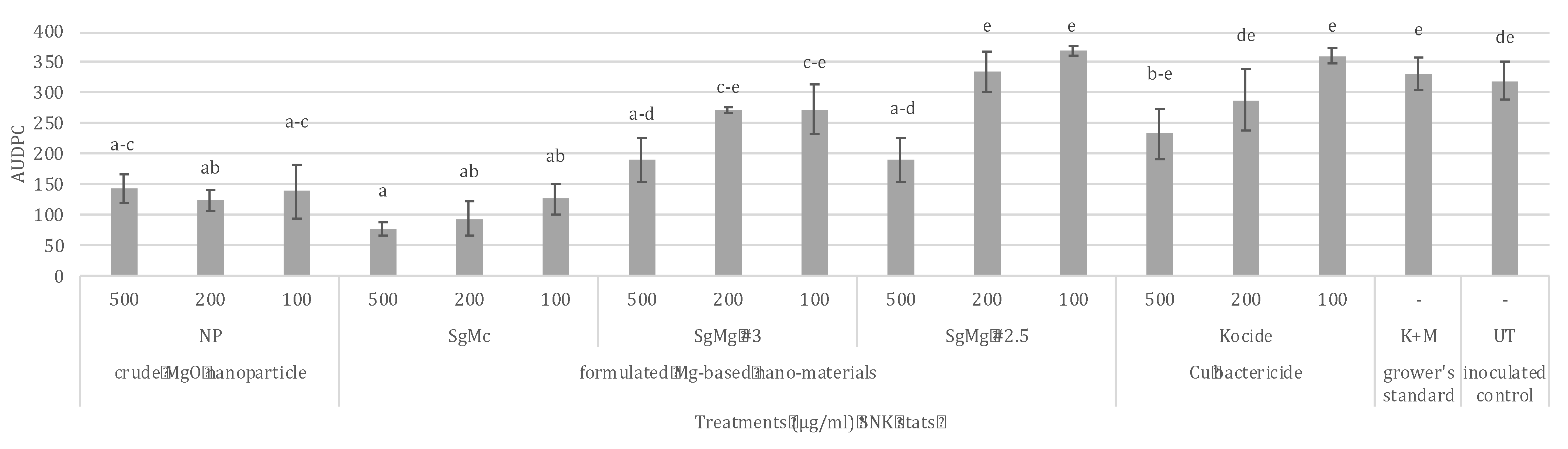
2.3. Comparison of the Efficacy of Formulated Mg Nanomaterials with Nano-MgO, Cu, and Cu-EBDC for the Management of Tomato Bacterial Spot in Greenhouse Conditions
3. Materials and Methods
3.1. Nanomaterial Formulation and Synthesis
3.2. Material Characterization
3.3. Bacterial Strain and Storage
3.4. In Vitro Assays Evaluating Direct Inhibition of the Growth of X. perforans
3.5. Viability Assay Evaluating Bactericidal Activity
3.6. Greenhouse Experiments Evaluating Efficacy of Mg-Formulated Nanomaterials against Bacterial Spot Disease of Tomato
3.7. Field Experiments Evaluating Efficacy of Mg-Formulated Nanomaterials against Bacterial Spot Disease of Tomato
3.8. Statistical Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.B.; Lacy, G.H.; Bouzar, H.; Stall, R.E.; Schaad, N.W. Reclassification of the xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 2004, 27, 755–762. [Google Scholar] [CrossRef]
- Gitaitis, R.; McCarter, S.; Jones, J. Disease control in tomato transplants produced in Georgia and Florida. Plant Dis. 1992, 76, 651–656. [Google Scholar] [CrossRef]
- Ritchie, D.F. Bacterial spot of pepper and tomato. Plant Health Instructor. 2000. [Google Scholar] [CrossRef]
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015, 16, 907–920. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Pietrzak, U.; McPhail, D. Copper accumulation, distribution and fractionation in vineyard soils of Victoria, Australia. Geoderma 2004, 122, 151–166. [Google Scholar] [CrossRef]
- Obradovic, A.; Jones, J.; Momol, M.; Olson, S.; Jackson, L.; Balogh, B.; Guven, K.; Iriarte, F. Integration of biological control agents and systemic acquired resistance inducers against bacterial spot on tomato. Plant Dis. 2005, 89, 712–716. [Google Scholar] [CrossRef]
- Kucharek, T. Plant Pathology Fact Sheet: Bacterial Spot of Tomato and Pepper; Plant Pathology Department, Cooperative Extension Service; Online, publication BODY_VH007; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1994. [Google Scholar]
- Vallad, G.E. Integrated Management of Bacterial Spot on Tomato in Florida. EDIS 2019, 2019. [Google Scholar]
- Marco, G.M.; Stall, R.E. Control of Bacterial Spot of Pepper Initiated by Strains of Xanthomonas-Campestris Pv-Vesicatoria That Differ in Sensitivity to Copper. Plant Dis. 1983, 67, 779–781. [Google Scholar] [CrossRef]
- Liao, Y.; Strayer-Scherer, A.; White, J.; De La Torre-Roche, R.; Ritchie, L.; Colee, J.; Vallad, G.; Freeman, J.; Jones, J.; Paret, M. Particle-size dependent bactericidal activity of magnesium oxide against Xanthomonas perforans and bacterial spot of tomato. Sci. Rep. 2019, 9, 18530. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Strayer-Scherer, A.; White, J.; Mukherjee, A.; De La Torre-Roche, R.; Ritchie, L.; Colee, J.; Vallad, G.; Freeman, J.; Jones, J. Nano-Magnesium oxide: A novel bactericide against copper-tolerant Xanthomonas perforans causing tomato bacterial spot. Phytopathology 2018, 109, 52–62. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Huang, Y.; Carvalho, R.; Choudhary, M.; Da Silva, S.; Colee, J.; Huerta, A.; Vallad, G.E.; Freeman, J.H.; Jones, J.B. Magnesium oxide nanomaterial, an alternative for commercial copper bactericides: Field-scale tomato bacterial spot disease management and total and bioavailable metal accumulation in soil. Environ. Sci. Technol. 2021, 55, 13561–13570. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Ishizu, N.; Itoh, M. Kinetic Analysis of the Bactericidal Action of Magnesium Oxide Powder Slurry against Escherichia coli. Biocontrol Sci. 2003, 8, 123–127. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Tso, C.-p.; Zhung, C.-m.; Shih, Y.-h.; Tseng, Y.-M.; Wu, S.-c.; Doong, R.-a. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Yaremko, Z.; Nikipanchuk, D.; Fedushinskaya, L.; Uspenskaya, I. Redispersion of highly disperse powder of titanium dioxide in aqueous medium. Colloid J. 2001, 63, 253–258. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Vermilyea, D.A. The dissolution of MgO and Mg(OH)2 in aqueous solutions. J. Electrochem. Soc. 1969, 116, 1179. [Google Scholar] [CrossRef]
- Huang, Z.; Rajasekaran, P.; Ozcan, A.; Santra, S. Antimicrobial Magnesium Hydroxide Nanoparticles as an Alternative to Cu Biocide for Crop Protection. J. Agric. Food Chem. 2018, 66, 8679–8686. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, J.G.; Barratt, R. An improved grading system for measuring plant diseases. In Phytopathology; American Phytopathology Society: St. Paul, MN, USA, 1945; p. 655. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- McAvoy, T.; Freeman, J.H.; Rideout, S.L.; Olson, S.M.; Paret, M.L. Evaluation of grafting using hybrid rootstocks for management of bacterial wilt in field tomato production. HortScience 2012, 47, 621–625. [Google Scholar] [CrossRef]
- Olson, S.; Stall, W.; Vallad, G.; Webb, S.; Smith, S.; Simonne, E.; McAvoy, E.; Santos, B.; Ozores-Hampton, M. Tomato Production in Florida; University of Florida: Gainesville, FL, USA, 2012. [Google Scholar]
- Gavilan, H.; Kowalski, A.; Heinke, D.; Sugunan, A.; Sommertune, J.; Varon, M.; Bogart, L.K.; Posth, O.; Zeng, L.J.; Gonzalez-Alonso, D.; et al. Colloidal Flower-Shaped Iron Oxide Nanoparticles: Synthesis Strategies and Coatings. Part. Part. Syst. Charact. 2017, 34, 1700094. [Google Scholar] [CrossRef]
- Masoudipour, E.; Kashanian, S.; Azandaryani, A.H.; Omidfar, K.; Bazyar, E. Surfactant effects on the particle size, zeta potential, and stability of starch nanoparticles and their use in a pH-responsive manner. Cellulose 2017, 24, 4217–4234. [Google Scholar] [CrossRef]
- Pochapski, D.J.; dos Santos, C.C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Gentry, S.T.; Kendra, S.F.; Bezpalko, M.W. Ostwald Ripening in Metallic Nanoparticles: Stochastic Kinetics. J. Phys. Chem. C 2011, 115, 12736–12741. [Google Scholar] [CrossRef]
- Sharma, G.; Park, S.C.; Bandi, R.; Ahn, J.; Alle, M.; Kim, J.C. Polyquaternium enhances the colloidal stability of chitosan-capped platinum nanoparticles and their antibacterial activity. Nanotechnology 2021, 32, 455603. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Infrared spectroscopy. In Introduction to Spectroscopy, 5th ed.; Cengage Learning: Boston, MA, USA, 2014; pp. 14–106. [Google Scholar]
- Chowdhury, A.H.; Bhanja, P.; Salam, N.; Bhaumik, A.; Islam, S.M. Magnesium oxide as an efficient catalyst for CO2 fixation and N-formylation reactions under ambient conditions. Mol. Catal. 2018, 450, 46–54. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Salem, J.K.; El-Nahhal, I.M.; Hammad, T.M.; Kuhn, S.; Abu Sharekh, S.; El-Askalani, M.; Hempelmann, R. Optical and fluorescence properties of MgO nanoparticles in micellar solution of hydroxyethyl laurdimonium chloride. Chem. Phys. Lett. 2015, 636, 26–30. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Hu, J.; Dang, B. Surface-modified MgO nanoparticle enhances the mechanical and direct-current electrical characteristics of polypropylene/polyolefin elastomer nanodielectrics. J. Appl. Polym. Sci. 2016, 132, 42863. [Google Scholar] [CrossRef]
- Devi, R.R.; Umlong, I.M.; Raul, P.K.; Das, B.; Banerjee, S.; Singh, L. Defluoridation of water using nano-magnesium oxide. J. Exp. Nanosci. 2014, 9, 512–524. [Google Scholar] [CrossRef]
- Nakamura, Y.; Okita, K.; Kudo, D.; Phuong, D.N.D.; Iwamoto, Y.; Yoshioka, Y.; Ariyoshi, W.; Yamasaki, R. Magnesium Hydroxide Nanoparticles Kill Exponentially Growing and Persister Escherichia coli Cells by Causing Physical Damage. Nanomaterials 2021, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Barnes, J.; Singleton, I. Investigation of potential reasons for bacterial survival on ‘ready-to-eat’ leafy produce during exposure to gaseous ozone. Postharvest Biol. Technol. 2016, 111, 185–190. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolra, R.; Poschenrieder, C. A Role for Zinc in Plant Defense Against Pathogens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M. Sustainable Management of Diseases in Horticulture: Conventional and New Options. Horticulturae 2022, 8, 517. [Google Scholar] [CrossRef]
- Schutte, G.C.; Kotze, C.; van Zyl, J.G.; Fourie, P.H. Assessment of retention and persistence of copper fungicides on orange fruit and leaves using fluorometry and copper residue analyses. Crop Prot. 2012, 42, 1–9. [Google Scholar] [CrossRef]
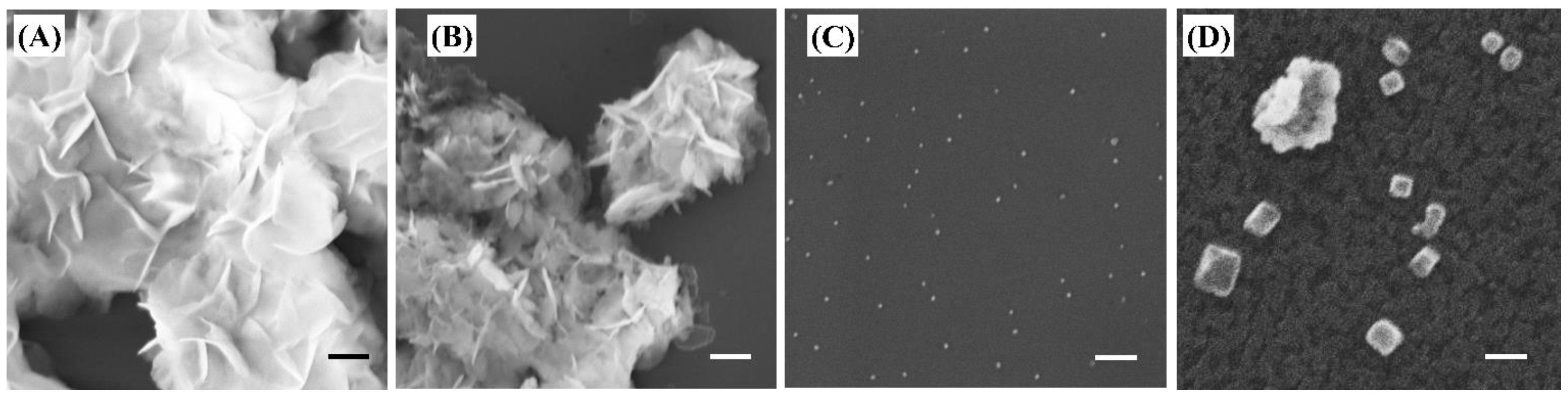
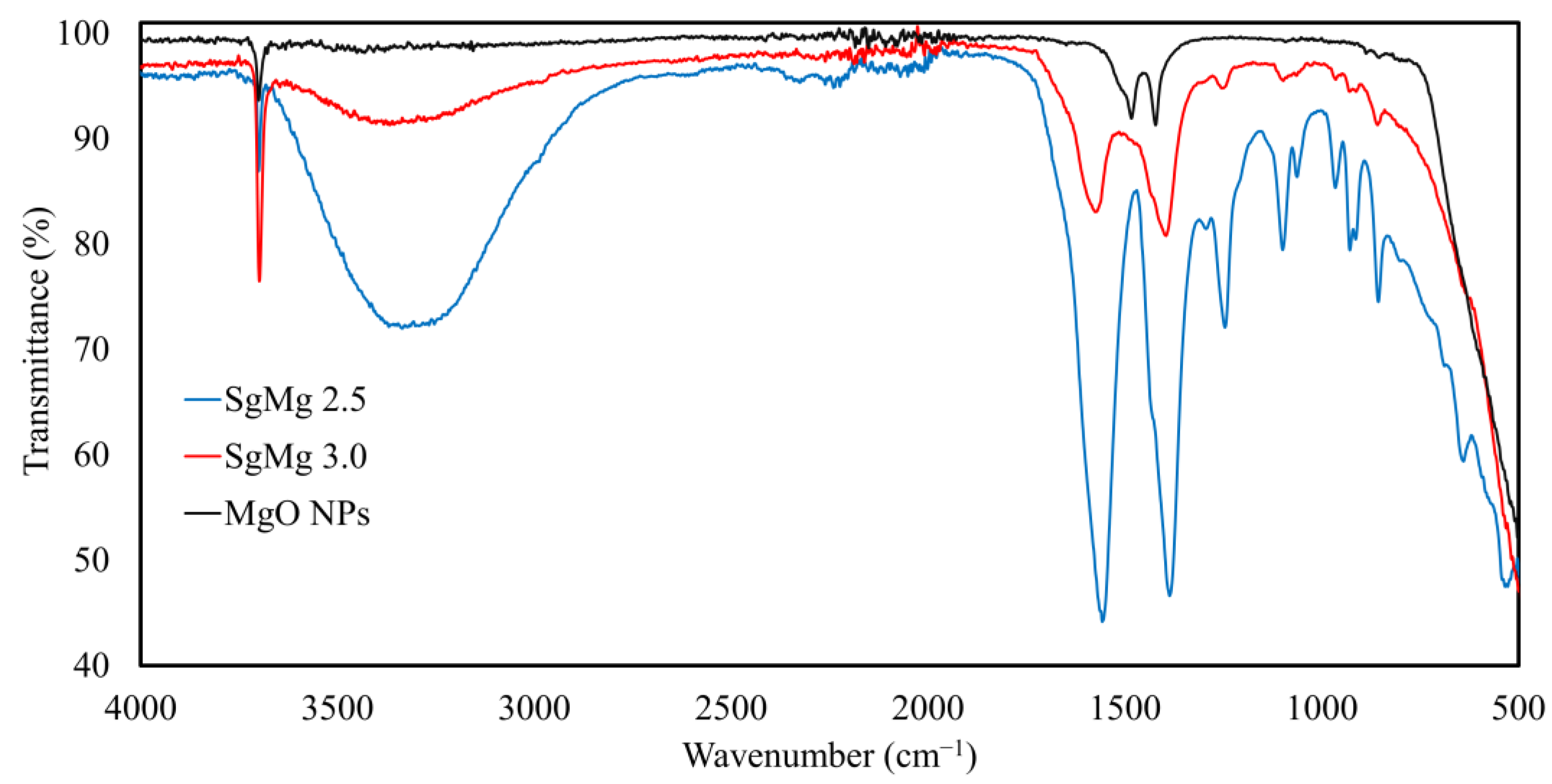
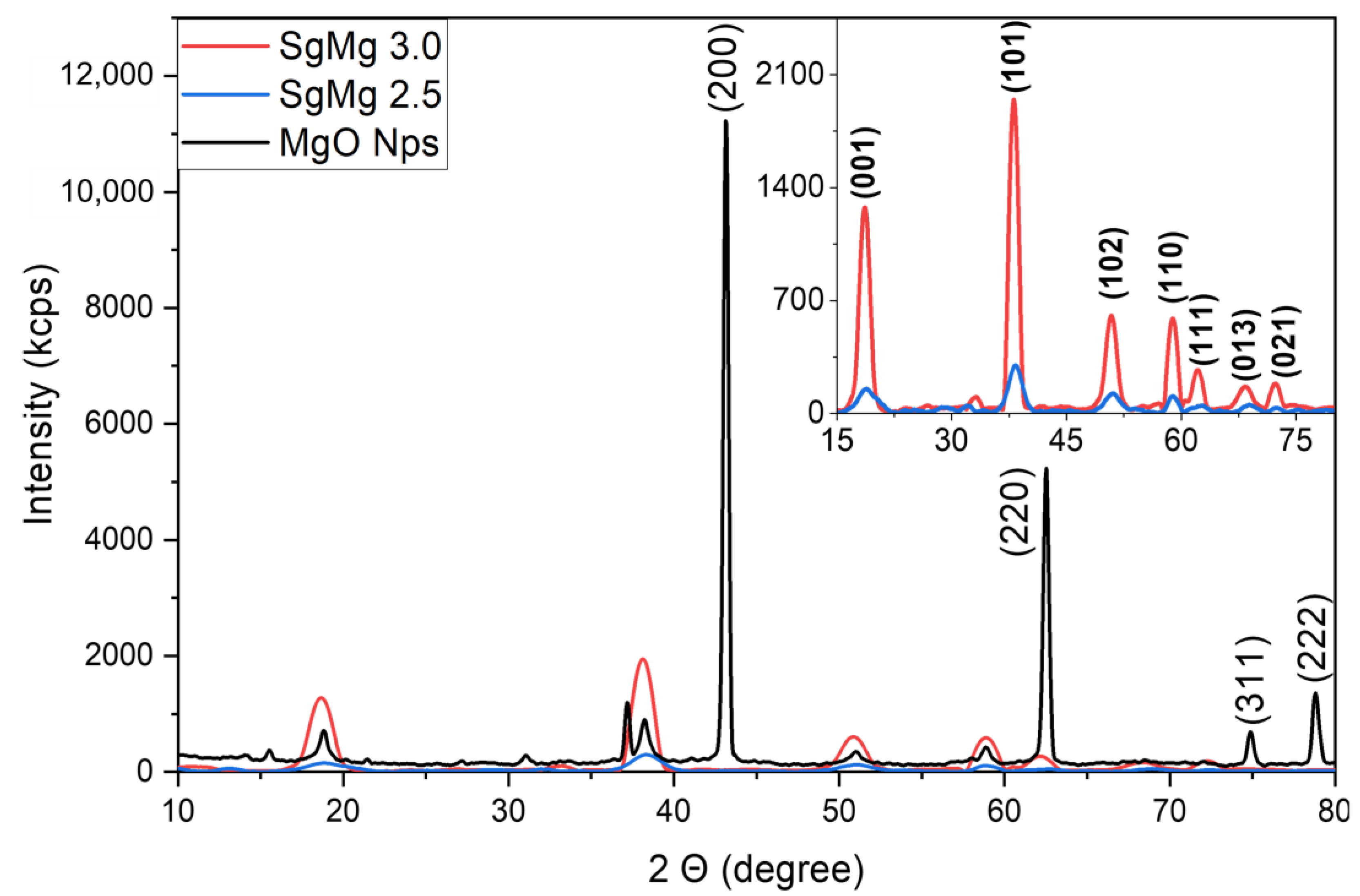
| Name | Magnesium to Citrate mol Ratio | Average Hydrodynamic Diameter | PDI | Zeta Potential (mv) | pH at 1000 µg/mL |
|---|---|---|---|---|---|
| MgO | 1.00 mol Mg:0.0 mol Citrate | 2586 | 1 | +11.4 | 10.20 |
| SgMg #3 | 1.00 mol Mg:0.42 mol Citrate | 2140 | 0.658 | −43.3 | 10.72 |
| SgMg #2.5 | 1.00 mol Mg:0.63 mol Citrate | 88.8 | 0.360 | −36.0 | 10.70 |
| SgMc | 1.00 mol Mg:0.50 | 279.8 | 0.476 | −16.9 | 10.69 |
| AUDPC y in Different Seasons w | |||
|---|---|---|---|
| Treatment | Rate (µg/mL) | Quincy, FL 2018 Spring | Treatment |
| Nano-MgO | 1000 | 1010.0 a | Nano-MgO |
| Nano-MgO | 100 | 791.9 a | Nano-MgO |
| SgMc | 1000 | 883.8 a | SgMc |
| SgMc | 100 | 1036.0 a | SgMc |
| Kocide 3000 | 2100 | 846.1 a | Kocide 3000 |
| Cu-EBDC x | 873.5 a | Cu-EBDC x | |
| Water (Untreated) | 822.0 a | 1520.3 a | |
| AUDPC y in Different Seasons w | |||
|---|---|---|---|
| Treatment | Rate (µg/mL) | Quincy, FL 2018 Fall | Wimauma, FL 2018 Fall |
| Nano-MgO | 1000 | 510.4 a | 585.9 ab |
| Nano-MgO | 100 | 502.1 a | 570.4 ab |
| SgMc | 1000 | 602.6 a | 437.3 a |
| SgMc | 100 | 604.4 a | 465.1 a |
| Kocide 3000 | 2100 | 592.1 a | 743.3 b |
| Cu-EBDC x | 519.4 a | 511.7 a | |
| Water (Untreated) | 596.8 a | 583.7 ab | |
| AUDPC y in Different Seasons w | |||
|---|---|---|---|
| Treatment | Rate (µg/mL) | Quincy, FL 2019 Spring | Wimauma, FL 2019 Spring |
| Nano-MgO | 1000 | 1424.6 ab | 650.3 a |
| Nano-MgO | 100 | 1119.6 a | 691.3 ab |
| SgMc | 1000 | 1274.5 ab | 632.1 a |
| SgMc | 100 | 1098.3 a | 903.9 bc |
| Kocide 3000 | 2100 | 1481.9 ab | 677.6 a |
| Cu-EBDC x | 1253.5 ab | 870.3 b | |
| Water (Untreated) | 1657.1 bc | 1033.2 bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.-Y.; Pereira, J.; Huang, Z.; Fan, Q.; Santra, S.; White, J.C.; De La Torre-Roche, R.; Da Silva, S.; Vallad, G.E.; Freeman, J.H.; et al. Potential of Novel Magnesium Nanomaterials to Manage Bacterial Spot Disease of Tomato in Greenhouse and Field Conditions. Plants 2023, 12, 1832. https://doi.org/10.3390/plants12091832
Liao Y-Y, Pereira J, Huang Z, Fan Q, Santra S, White JC, De La Torre-Roche R, Da Silva S, Vallad GE, Freeman JH, et al. Potential of Novel Magnesium Nanomaterials to Manage Bacterial Spot Disease of Tomato in Greenhouse and Field Conditions. Plants. 2023; 12(9):1832. https://doi.org/10.3390/plants12091832
Chicago/Turabian StyleLiao, Ying-Yu, Jorge Pereira, Ziyang Huang, Qiurong Fan, Swadeshmukul Santra, Jason C. White, Roberto De La Torre-Roche, Susannah Da Silva, Gary E. Vallad, Joshua H. Freeman, and et al. 2023. "Potential of Novel Magnesium Nanomaterials to Manage Bacterial Spot Disease of Tomato in Greenhouse and Field Conditions" Plants 12, no. 9: 1832. https://doi.org/10.3390/plants12091832
APA StyleLiao, Y.-Y., Pereira, J., Huang, Z., Fan, Q., Santra, S., White, J. C., De La Torre-Roche, R., Da Silva, S., Vallad, G. E., Freeman, J. H., Jones, J. B., & Paret, M. L. (2023). Potential of Novel Magnesium Nanomaterials to Manage Bacterial Spot Disease of Tomato in Greenhouse and Field Conditions. Plants, 12(9), 1832. https://doi.org/10.3390/plants12091832







