Phenological and Environmental Factors’ Impact on Secondary Metabolites in Medicinal Plant Cotinus coggygria Scop.
Abstract
1. Introduction
2. Results
2.1. Total Content of Phenols (PC), Flavonoids (FC), Tannins (TC) and Triterpenoids (TTC)
2.2. LC–MS/MS and HPLC–UV–VIS–DAD Phytochemical Characterization
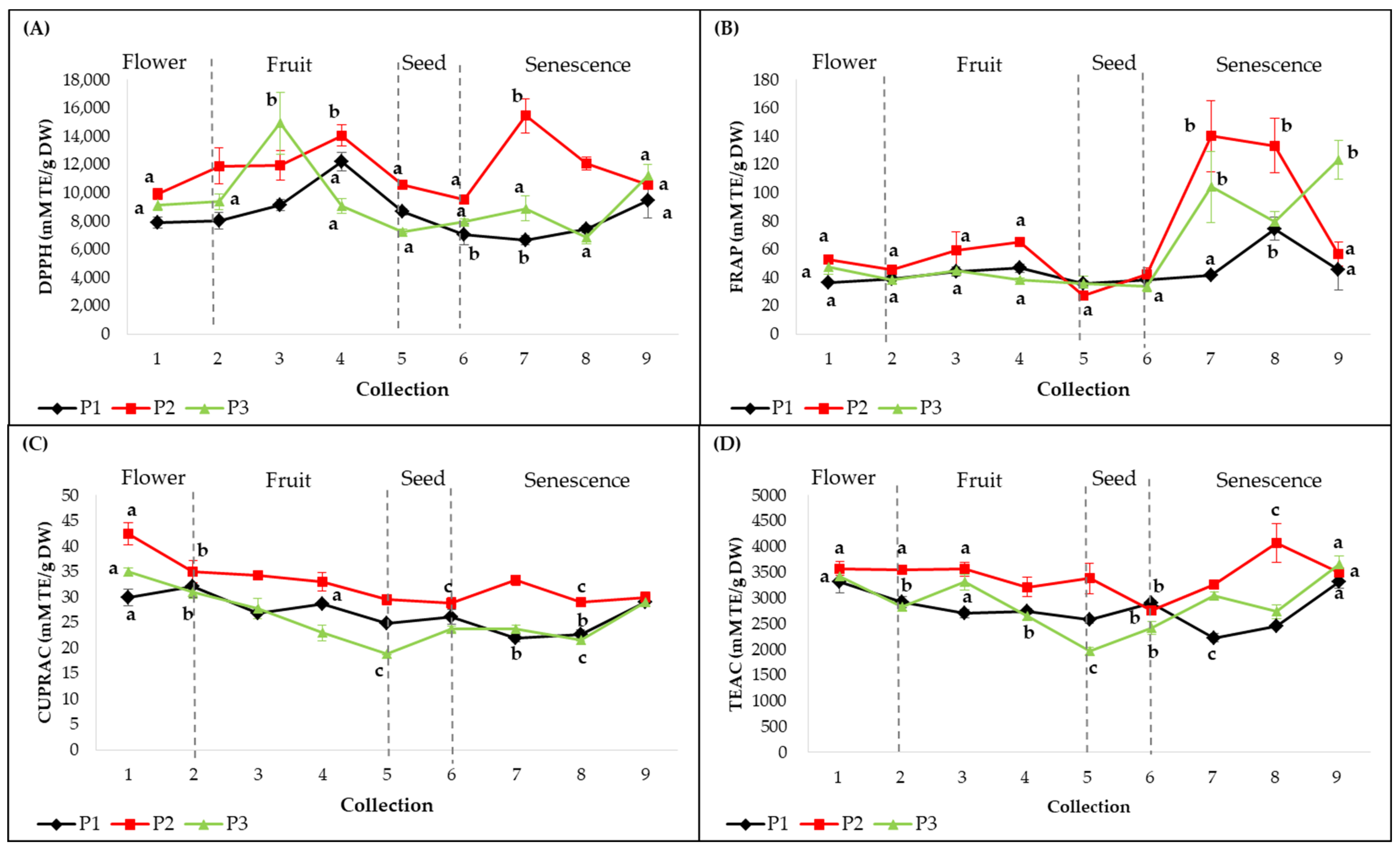
2.3. Antioxidant Activities (DPPH, FRAP, CUPRAC, TEAC) and Correlations with the Contents of Metabolites
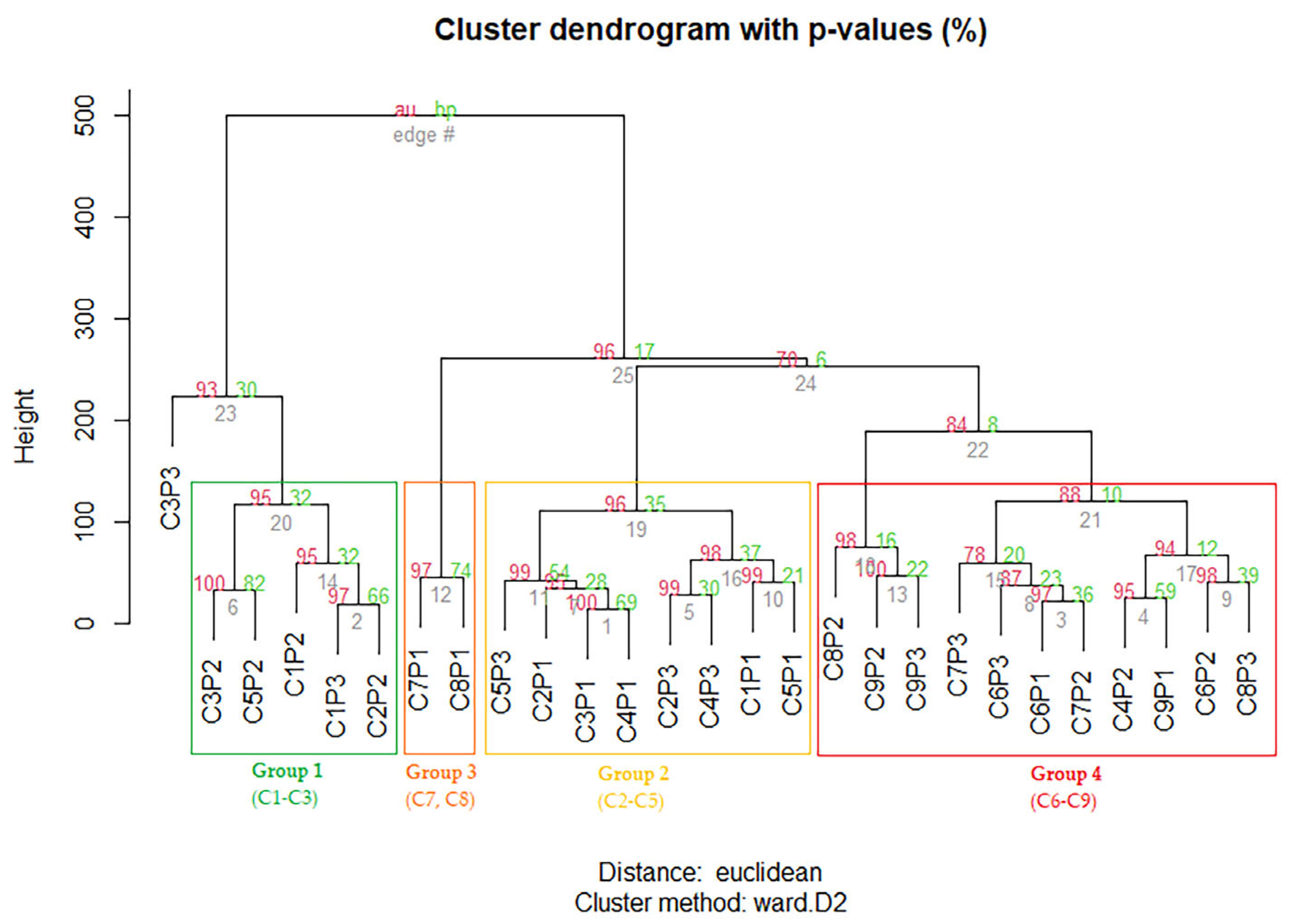
2.4. Dynamics of Secondary Metabolite Content Depending on the Phenological Phases
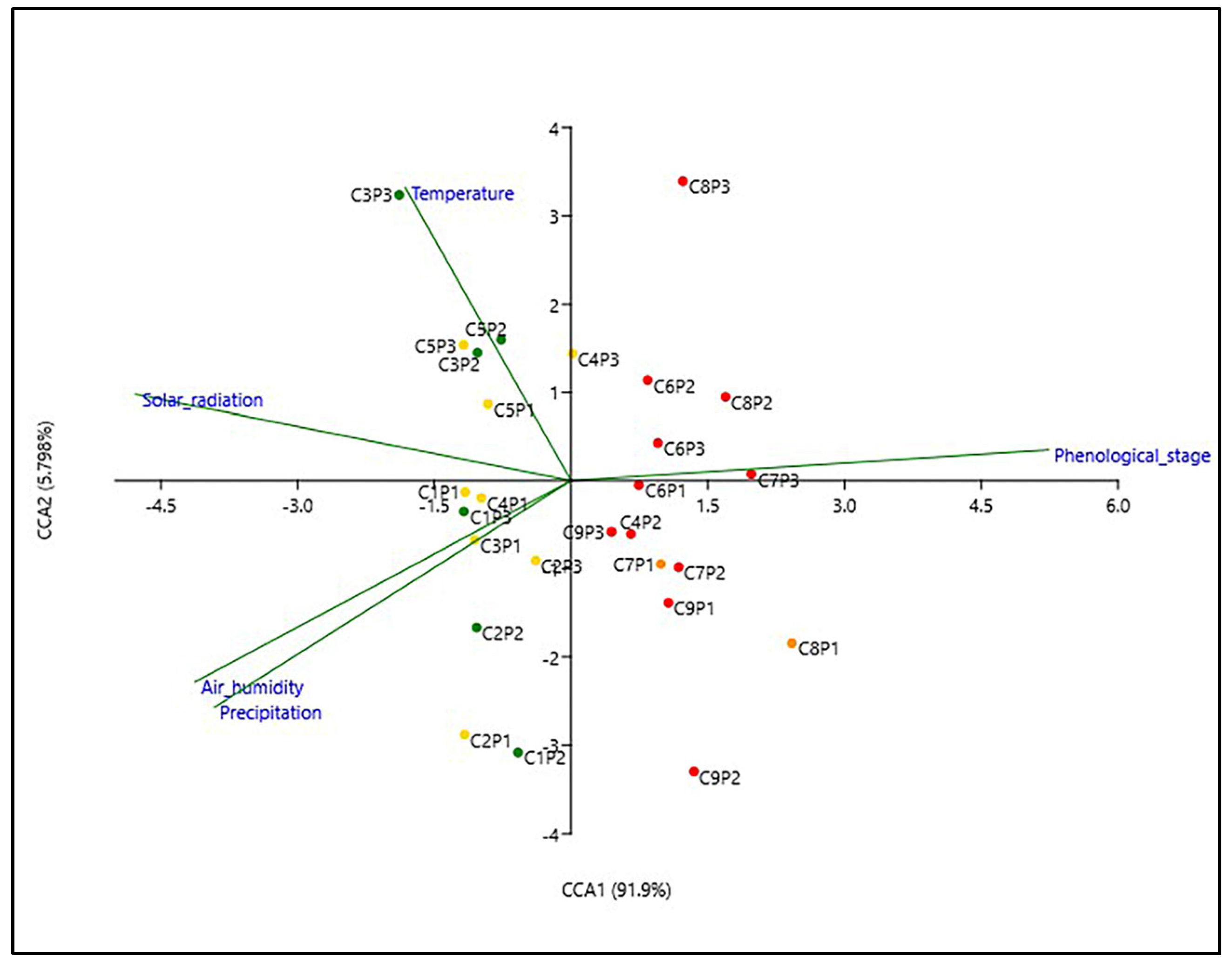
2.5. Variation of Secondary Metabolite Content under the Influence of the Environmental Factors
3. Discussion
4. Materials and Methods
4.1. Plant Material
| Phenological Stage | Collection | Month(s) |
|---|---|---|
| 1. Full leaf and inflorescence emergence | C1 | End of May |
| 2. Flowering | C2 | Mid-June |
| 3. Fruit development | C3 | End of June |
| 4. Fruit ripening | C4 | Mid-July |
| 5. Seed ripening | C5 | End of July |
| 6. Senescence (early) | C6 C7 | Mid-August End of August |
| 7. Senescence (advanced) | C8 C9 | Mid-September End of September |
4.2. Environmental Data
4.3. Chemicals
4.4. Extract Preparation
4.5. LC–MS/MS and HPLC–UV–VIS–DAD Analysis
4.6. Quantitative Determinations
4.6.1. Evaluation of Total Phenolic Content (PC)
4.6.2. Evaluation of Total Flavonoid Content (FC)
4.6.3. Evaluation of Total Triterpenoid Content (TTC)
4.6.4. Evaluation of Total Tannin Content (TC)
4.6.5. Evaluation of Antioxidant Activity through DPPH, FRAP, CUPRAC and TEAC Assays
4.6.6. Evaluation of Antioxidant Activity by HP–TLC
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An overview. In Plant Metabolites and Regulation Under Environmental Stress, 1st ed.; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Springer: Cham, Switzerland, 2018; pp. 153–167. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Frey, M.; Gómez-Zeledón, J.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacón (Smallanthus sonchifolius, Asteraceae). Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous influences on plant secondary metabolite levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges, 1st ed.; Ghorbanpour, M., Varman, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—A review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef]
- Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N.H. Discrimination of three pegaga (Centella) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1H nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2011, 60, 410–417. [Google Scholar] [CrossRef]
- Guo, X.R.; Yang, L.; Yu, J.H.; Tang, Z.H.; Zu, Y.G. Alkaloid variations in Catharanthus roseus seedlings treated by different temperatures in short term and long term. J. For. Res. 2007, 18, 313–315. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Diazgranados, M.; Da Costa, F.B. Biogeography shaped the metabolome of the genus Espeletia: A phytochemical perspective on an Andean adaptive radiation. Sci. Rep. 2017, 7, 8835. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Costa, F.B.D. Influence of abiotic environmental factors on the main constituents of the volatile oils of Tithonia diversifolia. Rev. Bras. Farmacogn. 2018, 28, 135–144. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Amrehn, E.; Frey, M.; Gómez-Zeledón, J.; Kaa, A.; Da Costa, F.B.; Spring, O. Metabolomic and Gene Expression Studies Reveal the Diversity, Distribution and Spatial Regulation of the Specialized Metabolism of Yacón (Smallanthus sonchifolius, Asteraceae). Int. J. Mol. Sci. 2020, 21, 4555. [Google Scholar] [CrossRef]
- Saldanha, L.L.; Allard, P.-M.; Afzan, A.; de Melo, F.P.d.S.R.; Marcourt, L.; Queiroz, E.F.; Vilegas, W.; Furlan, C.M.; Dokkedal, A.L.; Wolfender, J.-L. Metabolomics of Myrcia bella Populations in Brazilian Savanna Reveals Strong Influence of Environmental Factors on Its Specialized Metabolism. Molecules 2020, 25, 2954. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Diazgranados, M.; Da Costa, F.B. Effect of the Andean Geography and Climate on the Specialized Metabolism of Its Vegetation: The Subtribe Espeletiinae (Asteraceae) as a Case Example. Metabolites 2021, 11, 220. [Google Scholar] [CrossRef]
- Fuica Carrasco, C.A.; Toro-Núñez, Ó.; Lira-Noriega, A.; Pérez, A.J.; Hernández, V. Seasonality and Position with Respect to the Ecological Niche Centroid are Determinants of Metabolome Expression in Eucryphia cordifolia Cav. An Endemic Tree from the Southern South American Forests. SSRN Electron. J. 2022, 1–30. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family—A review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Săvulescu, T. Flora Republicii Populare Romîne; Editura Academiei Republicii Populare Romîne: București, Romania, 1958; Volume VI, pp. 214–218. [Google Scholar]
- Oprea, A. Lista Critică a Plantelor Vasculare din România; Editura Universității “Al. I. Cuza”: Iași, Romania, 2005; pp. 215–217. [Google Scholar]
- Antal, D.S.; Ardelean, F.; Jijie, R.; Pinzaru, I.; Soica, C.; Dehelean, C. Integrating Ethnobotany, Phytochemistry, and Pharmacology of Cotinus coggygria and Toxicodendron vernicifluum: What Predictions can be Made for the European Smoketree? Front. Pharmacol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Demirci, B.; Demirci, F.; Başer, K.H.C. Composition of the essential oil of Cotinus coggygria Scop. from Turkey. Flavour Fragr. J. 2003, 18, 43–44. [Google Scholar] [CrossRef]
- Wilson, B.; Chadburn, H. Cotinus coggygria. The IUCN Red List of Threatened Species; e.T202959A119996147; International Union for Conservation of Nature: Gland, Switzerland, 2018; pp. 1–8. [Google Scholar]
- Bahadirli, N.P. Essential oil content and composition of Cotinus coggygria Scop. from Hatay, Turkey. Int. J. Agric. For. Life Sci. 2020, 4, 111–114. Available online: http://dergipark.gov.tr/ijafls (accessed on 5 January 2023).
- Thapa, P.; Prakash, O.; Rawat, A.; Kumar, R.; Srivastava, R.M.; Rawat, D.S.; Pant, A.K. Essential Oil Composition, Antioxidant, Anti-inflammatory, Insect Antifeedant and Sprout Suppressant Activity in Essential Oil From Aerial Parts of Cotinus coggygria Scop. J. Essent. Oil Bear. Plants 2020, 23, 65–76. [Google Scholar] [CrossRef]
- Sukhikh, S.; Noskova, S.; Pungin, A.; Ivanova, S.; Skrypnik, L.; Chupakhin, E.; Babich, O. Study of the Biologically Active Properties of Medicinal Plant Cotinus coggygria. Plants 2021, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Asyakina, L.; Korobenkov, M.; Skrypnik, L.; Pungin, A.; Ivanova, S.; Larichev, T.; Larina, V.; Krol, O.; Ulrikh, E.; et al. Chemical Composition and Content of Biologically Active Substances Found in Cotinus coggygria, Dactylorhiza maculata, Platanthera chlorantha Growing in Various Territories. Plants 2021, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Sen, A.; Sancar, M.; Sekerler, T.; Akakin, D.; Bitis, L.; Uras, F.; Kultur, S.; Izzettin, F.V. Ethanol extract of Cotinus coggygria leaves accelerates wound healing process in diabetic rats. Pharm. Biol. 2016, 54, 2732–2736. [Google Scholar] [CrossRef] [PubMed]
- Marčetić, M.; Božić, D.; Milenković, M.; Malešević, N.; Radulović, S.; Kovačević, N. Antimicrobial, antioxidant and anti-inflammatory activity of young shoots of the smoke tree, Cotinus coggygria Scop. Phytother. Res. 2013, 27, 1658–1663. [Google Scholar] [CrossRef]
- Antal, D.S.; Ardelean, F. “Cotinus coggygria Scop,” in Romanian traditional medicine: Results of an ethnobotanical survey performed in the South-Western part of the country. In Natural Products as a Source of Compounds with Chemopreventive and Anti-inflammatory Activity. Abstracts of the International Workshop of the Romanian-French Bilateral Project PN II-CT-789/30.06.2014, 1st ed.; Antal, D.S., Ollivier, E., Eds.; Victor Babes: Timisoara, Romania, 2015; p. 22. ISBN 978-606-8456-67-6. [Google Scholar]
- Kültür, Ş. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Westenburg, H.E.; Lee, K.J.; Lee, S.K.; Fong, H.H.; van Breemen, R.B.; Pezzuto, J.M.; Kinghorn, A.D. Activity-Guided Isolation of Antioxidative Constituents of Cotinus coggygria. J. Nat. Prod. 2000, 63, 1696–1698. [Google Scholar] [CrossRef]
- Antal, D.S.; Schwaiger, S.; Ellmerer-Müller, E.P.; Stuppner, H. Cotinus coggygria wood: Novel flavanone dimer and development of an HPLC/UV/MS method for the simultaneous determination of fourteen phenolic constituents. Planta Med. 2010, 76, 1765–1772. [Google Scholar] [CrossRef]
- Matić, S.; Stanić, S.; Bogojević, D.; Vidaković, M.; Grdović, N.; Dinić, S.; Solujić, S.; Mladenović, M.; Stanković, N.; Mihailović, M. Methanol extract from the stem of Cotinus coggygria Scop., and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2013, 755, 81–89. [Google Scholar] [CrossRef]
- Tanchev, S.S.; Timberlake, C.F. Anthocyanins in leaves of Cotinus coggygria. Phytochemistry 1969, 8, 2367–2369. [Google Scholar] [CrossRef]
- Iwashina, T. Detection and distribution of chrysanthemin and idaein in autumn leaves of plants by high performance liquid chromatography. Ann. Tsukuba Bot. Gard. 1996, 15, 1–8. Available online: https://cir.nii.ac.jp/crid/1520853835465945856 (accessed on 5 January 2023).
- Valianou, L.; Stathopoulou, K.; Karapanagiotis, I.; Magiatis, P.; Pavlidou, E.; Skaltsounis, A.-L.; Chryssoulakis, Y. Phytochemical analysis of young fustic (Cotinus coggygria heartwood) and identification of isolated colourants in historical textiles. Anal. Bioanal. Chem. 2009, 394, 871–882. [Google Scholar] [CrossRef]
- Novakovic, M.; Djordjevic, I.; Todorovic, N.; Trifunovic, S.; Andjelkovic, B.; Mandic, B.; Jadranin, M.; Vuckovic, I.; Vajs, V.; Milosavljevic, S.; et al. New aurone epoxide and auronolignan from the heartwood of Cotinus coggygria Scop. Nat. Prod. Res. 2018, 33, 2837–2844. [Google Scholar] [CrossRef]
- Rendeková, K.; Fialová, S.; Jánošová, L.; Mučaji, P.; Slobodníková, L. The Activity of Cotinus coggygria Scop. Leaves on Staphylococcus aureus Strains in Planktonic and Biofilm Growth Forms. Molecules 2016, 21, 50. [Google Scholar] [CrossRef]
- Hethelyi, I.; Domokos, J.; Lemberkovic, E.; Verzar-Petri, G. Analysis of the essential oil of Cotinus coggygria by means of mass spectrometry. Herba Hun 1986, 25, 135–148. [Google Scholar]
- Tsankova, E.T.; Dyulgerov, A.S.; Milenkov, B.K. Chemical composition of the Bulgarian sumac oil. J. Essent. Oil Res. 1993, 5, 205–207. [Google Scholar] [CrossRef]
- Tzakou, O.; Bazos, I.; Yannitsaros, A. Essential oils of leaves, inflorescences and infructescences of spontaneous Cotinus coggygria Scop. from Greece. Flavour Fragr. J. 2005, 20, 531–533. [Google Scholar] [CrossRef]
- Novaković, M.; Vučković, I.; Janaćković, P.; Soković, M.; Filipović, A.; Tešević, V.; Milosavljević, S. Chemical composition, antibacterial and antifungal activity of the essential oils of Cotinus coggygria from Serbia. J. Serb. Chem. Soc. 2007, 72, 1045–1051. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D. Chemical composition and antimicrobial activity of the essential oil of Cotinus coggygria Scoop. From Italy. J. Essent. Oil Bear. Plants 2014, 17, 366–370. [Google Scholar] [CrossRef]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Marriote, A.-M.; Ghedira, K.; Dijoux-Franca, M.-G.; Chekir Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. Vitro 2008, 22, 567–581. [Google Scholar] [CrossRef]
- de Oliveira Azevedo, A.; Campos, J.J.; de Souza, G.G.; de Carvalho Veloso, C.; Duarte, I.D.G.; Braga, F.C.; de Castro Perez, A. Antinociceptive and anti-inflammatory effects of myricetin 3-O-β-galactoside isolated from Davilla elliptica: Involvement of the nitrergic system. J. Nat. Med. 2015, 69, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Motlhatlego, K.E.; Abdalla, M.A.; Leonard, C.M.; Eloff, J.N.; McGaw, L.J. Inhibitory effect of Newtonia extracts and myricetin-3-o-rhamnoside (myricitrin) on bacterial biofilm formation. BMC Complement. Med. Ther. 2020, 20, 358. [Google Scholar] [CrossRef] [PubMed]
- Motlhatlego, K.E.; Mehrbod, P.; Fotouhi, F.; Abdalla, M.A.; Eloff, J.N.; McGaw, L.J. Anti-influenza A virus activity of two Newtonia species and the isolated compound myricetin-3-o-rhamnoside. BMC Complement. Med. Ther. 2021, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Al-Dosari, M.S.; Arbab, A.H.; Al-Rehaily, A.J.; Abdelwahid, M.A. Bioassay-guided isolation of anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharm. J. 2020, 28, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, S.E.; Ebrahimi, S.N.; Salehi, P.; Moridi Farimani, M.; Hamburger, M.; Jabbarzadeh, E. Wound healing potential of chlorogenic acid and Myricetin-3-O-β-Rhamnoside isolated from Parrotia persica. Molecules 2017, 22, 1501. [Google Scholar] [CrossRef]
- Whang, W.K.; Park, H.S.; Ham, I.; Oh, M.; Namkoong, H.; Kim, H.K.; Hwang, D.W.; Hur, S.Y.; Kim, T.E.; Park, Y.G.; et al. Methyl gallate and chemicals structurally related to methyl gallate protect human umbilical vein endothelial cells from oxidative stress. Exp. Mol. Med. 2005, 37, 343–352. [Google Scholar] [CrossRef]
- Crispo, J.A.; Piché, M.; Ansell, D.R.; Eibl, J.K.; Tai, I.T.; Kumar, A.; Ross, G.M.; Tai, T.C. Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. Biochem. Biophys. Res. Commun. 2010, 393, 773–778. [Google Scholar] [CrossRef]
- Chen, Y.; Onken, B.; Chen, H.; Xiao, S.; Liu, X.; Driscoll, M.; Cao, Y.; Huang, Q. Mechanism of longevity extension of Caenorhabditis elegans induced by pentagalloyl glucose isolated from eucalyptus leaves. J. Agric. Food Chem. 2014, 62, 3422–3431. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Tang, Y.; Lin, C.; Cao, Y.; Chen, Y. Mechanism of pentagalloyl glucose in alleviating fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 14110–14120. [Google Scholar] [CrossRef]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 10 January 2022).
- de Brito, E.S.; de Araújo, M.C.P.; Lin, L.Z.; Harnly, J. Determination of the flavonoid components of cashew apple (Anacardium occidentale) by LC-DAD-ESI/MS. Food Chem. 2007, 105, 1112–1118. [Google Scholar] [CrossRef]
- Shabana, M.M.; El Sayed, A.M.; Yousif, M.F.; El Sayed, A.M.; Sleem, A.A. Bioactive constituents from Harpephyllum caffrum Bernh. and Rhus coriaria L. Pharmacogn. Mag. 2011, 7, 298–306. [Google Scholar] [CrossRef]
- Regazzoni, L.; Arlandini, E.; Garzon, D.; Santagati, N.A.; Beretta, G.; Facino, R.M. A rapid profiling of gallotannins and flavonoids of the aqueous extract of Rhus coriaria L. by flow injection analysis with high-resolution mass spectrometry assisted with database searching. J. Pharm. Biomed. Anal. 2013, 72, 202–207. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, O.; Carle, R.; Schweiggert, R.M. Identification of phenolic compounds in red and green pistachio (Pistacia vera L.) hulls (exo-and mesocarp) by HPLC-DAD-ESI-(HR)-MS n. J. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef]
- Buziashvili, I.S.; Komissarenko, N.F.; Kolesnikov, D.G. Polyphenolic compounds in the leaves of the Venetian sumac (Rhus coriaria) and sumac (Cotinus coggygria). Fenol’nye Soedin Fiziol. Svoistva Mater. Vses Simp Fenol’nym Soedin 1973, 2, 159–162. [Google Scholar]
- Savikin, K.; Zdunic, G.; Jankovic, T.; Stanojkovic, T.; Juranic, Z.; Menkovic, N. In vitro cytotoxic and antioxidative activity of Cornus mas and Cotinus coggygria. Nat. Prod. Res. 2009, 23, 1731–1739. [Google Scholar] [CrossRef]
- Özbek, H.; Yuca, H.; Dursunoğlu, B.; Gözcü, S.; Kazaz, C.; Güvenalp, Z. Secondary metabolites from Cotinus coggygria Scop. Planta Med. 2016, 82, P269. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Salmas, R.E.; Emerce, E.; Cankaya, I.I.T.; Yusufoglu, H.S.; Orhan, I.E. Evaluation of collagenase, elastase and tyrosinase inhibitory activities of Cotinus coggygria Scop. through in vitro and in silico approaches. S. Afr. J. Bot. 2020, 132, 277–288. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Hashoum, H.; Santonja, M.; Gauquelin, T.; Saatkamp, A.; Gavinet, J.; Greff, S.; Lecareux, C.; Bousquet-Mélou, A. Biotic interactions in a Mediterranean oak forest: Role of allelopathy along phenological development of woody species. Eur. J. Forest Res. 2017, 136, 699–710. [Google Scholar] [CrossRef]
- Gavinet, J.; Santonja, M.; Baldy, V.; Hashoum, H.; Peano, S.; Tchong, T.; Gros, R.; Greff, S.; Fernandez, C.; Bousquet-Mélou, A. Phenolics of the understory shrub Cotinus coggygria influence Mediterranean oak forests diversity and dynamics. Forest Ecol. Manag. 2019, 441, 262–270. [Google Scholar] [CrossRef]
- Kumar, V.; Suman, U.; Yadav, S.K. Flavonoid secondary metabolite: Biosynthesis and role in growth and development in plants. In Recent Trends and Techniques in Plant Metabolic Engineering; Yadav, S.K., Kumar, V., Singh, S.P., Eds.; Springer: Singapore, 2018; pp. 19–45. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsäläinen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A. Assessing the response of plant flavonoids to UV radiation: An overview of appropriate techniques. Phytochem. Rev. 2015, 14, 273–297. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.J.; Yang, C.R.; Zhang, Y.J. Phenolic antioxidants from Chinese toon (fresh young leaves and shoots of Toona sinensis). Food Chem. 2007, 101, 365–371. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc. Bot. Pol. 2012, 81, 117–122. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterisation of the flavonoids from Opuntia milpa alta skin. Czech J. Food Sci. 2010, 28, 108–116. [Google Scholar] [CrossRef]
- Ke, Z.C.; Zhu, Z.P.; Xu, Z.Y.; Fang, C.; Hu, S.Q. Response surface optimized extraction of total triterpene acids from Eriobotrya japonica (Thunb) Lindl (Loquat) leaf and evaluation of their in vitro antioxidant activities. Trop. J. Pharm. Res. 2014, 13, 787–792. [Google Scholar] [CrossRef]
- Makkar, H.P.; Blümmel, M.; Borowy, N.K.; Becker, K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol Composition and (Bio)Activity of Berberis Species and Wild Strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef]
- Ramírez-Briones, E.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Martínez-Gallardo, N.; Tiessen, A.; Molina-Torres, J.; Delano-Frier, J.P.; Zañudo-Hernández, J. Seasonal variation in non-structural carbohydrates, sucrolytic activity and secondary metabolites in deciduous and perennial Diospyros species sampled in Western Mexico. PLoS ONE 2017, 10, e0187235. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 7 July 2022).
- Hammer, Ø.; Harper, D.A.T.; Paul, D.R. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 8 July 2022).


| Peak No. | Rt (Min) | Tentative Identification | [M-H]− (m/z) | λmax (nm) | Formula | Major Fragments | References |
|---|---|---|---|---|---|---|---|
| 1 | 4.06 | Methyl gallate | 183 | 275 | C8H8O5 | 124, 168 | [40,59,61,62], PubChem |
| 2 | 7.09 | Methyl digallate I | 335 | 285 | C15H12O9 | 183 | [40,59,61,62] |
| 3 | 7.88 | Myricetin 3-O-galactoside | 479 | 267, 305, 365 | C21H20O13 | 316, 270 | [60,61,62], PubChem |
| 4 | 9.05 | Myricetin-3-O-rhamnoside (Myricitrin) | 463 | 265, 303, 356 | C21H20O12 | 316, 286, 107 | [40,58,60,61], PubChem |
| 5 | 9.39 | Methyl digallate II | 335 | 276 | C15H12O9 | 183 | [40,59,61,62] |
| 6 | 9.70 | Pentagalloyl glucose | 939 | 284 | C41H32O26 | 769, 787, 617, 261, 294, 429 | [40,60,61,62] |
| Compounds | Pn/Cn | Flowering Stages | Fruit and Seeds Stages | Senescence Stages | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | ||
| Myricetin- 3-O-galactoside | P1 | 0.1 ± 0.0 a | 0.1 ± 0.0 ab | 0.1 ± 0.0 a | 0.1 ± 0.0 b | 0.2 ± 0.0 b | 0.1 ± 0.0 ab | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.2 ± 0.0 c |
| P2 | 0.8 ± 0.0 a | 0.9 ± 0.0 ab | 1.1 ± 0.1 b | 0.9 ± 0.0 ab | 1.0 ± 0.0 ab | 1.0 ± 0.0 ab | 0.9 ± 0.0 a | 1.0 ± 0.0 ab | 0.8 ± 0.0 a | |
| P3 | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.4 ± 0.0 a | 0.4 ± 0.0 a | 0.3 ± 0.0 b | 0.4 ± 0.0 a | 0.6 ± 0.0 c | 0.4 ± 0.0 a | |
| Myricitrin | P1 | ND | 0.03 a | ND | ND | ND | ND | ND | 0.08 b | ND |
| P2 | ND | 0.2 ± 0.1 a | ND | 0.08 | ND | ND | 0.2 ± 0.0 a | ND | 3.4 ± 0.1 b | |
| P3 | 0.07 | ND | ND | ND | ND | ND | ND | ND | ND | |
| Methyl gallate | P1 | 16.9 ± 0.3 a | 12.6 ± 0.4 b | 13.5 ± 0.5 ab | 7.3 ± 0.1 c | 12.6 ± 0.1 b | 10.6 ± 0.0 bc | 8.4 ± 0.0 bc | 11.9 ± 0.0 b | 14.1 ± 0.1 ab |
| P2 | 21.4 ± 0.4 a | 16.3 ± 0.5 b | 5.3 ± 0.2 cd | 15.8 ± 0.1 b | 13.5 ± 0.2 bc | 13.3 ± 0.3 bc | 14.3 ± 0.2 bc | 10.8 ± 0.2 c | 4.2 ± 0.2 d | |
| P3 | 12.3 ± 0.1 a | 12.0 ± 0.1 ab | 11.7 ± 0.1 b | 2.99 ± 0.0 c | 5.4 ± 0.1 bc | 5.1 ± 0.0 bc | 8.4 ± 0.1 b | 0.5 ± 0.0 d | 10.8 ± 0.0 ab | |
| Methyl digallate I | P1 | 3.2 ± 0.0 a | 3.5 ± 0.1 ab | 2.4 ± 0.1 ac | 6.3 ± 0.0 b | 2.7 ± 0.0 ac | 3.9 ± 0.1 bc | 2.6 ± 0.0 ac | 1.2 ± 0.0 c | 2.4 ± 0.0 ac |
| P2 | 2.4 ± 0.3 a | 5.0 ± 0.2 bc | 7.7 ± 0.5 c | 4.2 ± 0.0 ab | 4.4 ± 0.0 b | 3.9 ± 0.0 b | 2.9 ± 0.0 ab | 7.7 ± 0.0 c | 4.7 ± 0.0 b | |
| P3 | 4.9 ± 0.1 bc | 4.4 ± 0.5 bc | 5.5 ± 0.1 c | 4.5 ± 0.0 bc | 3.7 ± 0.0 b | 5.2 ± 0.2 bc | 3.4 ± 0.0 b | 1.0 ± 0.0 a | 4.1 ± 0.1 b | |
| Pentagalloyl glucose | P1 | 18.4 ± 0.1 a | 15.2 ± 0.7 ab | 14.8 ± 0.7 ab | 11.9 ± 0.0 bc | 14.6 ± 0.0 ab | 13.1 ± 0.0 b | 9.1 ± 0.0 c | 11.7 ± 0.0 bc | 15.3 ± 0.0 ab |
| P2 | 20.6 ± 2.7 a | 19.6 ± 1.0 a | 11.5 ± 0.9 bc | 18.0 ± 0.3 ab | 16.1 ± 0.3 b | 15.0 ± 0.2 bc | 14.9 ± 0.4 bc | 14.5 ± 0.1 bc | 7.8 ± 0.1 c | |
| P3 | 15.4 ± 0.1 a | 14.6 ± 0.3 ab | 15.8 ± 0.3 a | 7.2 ± 0.1 bc | 7.7 ± 0.0 bc | 8.7 ± 0.4 b | 10.1 ± 0.0 b | 1.9 ± 0.1 c | 13.8 ± 0.3 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciocan, A.-G.; Tecuceanu, V.; Enache-Preoteasa, C.; Mitoi, E.M.; Helepciuc, F.E.; Dimov, T.V.; Simon-Gruita, A.; Cogălniceanu, G.C. Phenological and Environmental Factors’ Impact on Secondary Metabolites in Medicinal Plant Cotinus coggygria Scop. Plants 2023, 12, 1762. https://doi.org/10.3390/plants12091762
Ciocan A-G, Tecuceanu V, Enache-Preoteasa C, Mitoi EM, Helepciuc FE, Dimov TV, Simon-Gruita A, Cogălniceanu GC. Phenological and Environmental Factors’ Impact on Secondary Metabolites in Medicinal Plant Cotinus coggygria Scop. Plants. 2023; 12(9):1762. https://doi.org/10.3390/plants12091762
Chicago/Turabian StyleCiocan, Alexandra-Gabriela, Victorița Tecuceanu, Cristian Enache-Preoteasa, Elena Monica Mitoi, Florența Elena Helepciuc, Tatiana Vassu Dimov, Alexandra Simon-Gruita, and Gina Carmen Cogălniceanu. 2023. "Phenological and Environmental Factors’ Impact on Secondary Metabolites in Medicinal Plant Cotinus coggygria Scop." Plants 12, no. 9: 1762. https://doi.org/10.3390/plants12091762
APA StyleCiocan, A.-G., Tecuceanu, V., Enache-Preoteasa, C., Mitoi, E. M., Helepciuc, F. E., Dimov, T. V., Simon-Gruita, A., & Cogălniceanu, G. C. (2023). Phenological and Environmental Factors’ Impact on Secondary Metabolites in Medicinal Plant Cotinus coggygria Scop. Plants, 12(9), 1762. https://doi.org/10.3390/plants12091762






