Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis of Nutmeg Essential Oil
2.2. LC-HRMS/MS Analysis of Nutmeg Residual Water, Spent, and Total Extracts
| No. | Compound | Class | TR (min) | HRMS | Exp. (m/z) | Calcd. (m/z) | Δ (ppm) | MF | HRMS/MS (m/z) | Ref. | NWE | NSE | NTE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Citric acid * | Organic acid | 2.7 | [M − H]− | 191.0199 | 191.0197 | −0.91 | C6H8O7 | 129.0198, 111.0100 | [25] | × | × | × |
| 2 | Quinic acid * | Organic acid | 5.9 | [M − H]− | 191.0566 | 191.0561 | −2.54 | C7H12O6 | 173.0431, 127.0395 | [25] | × | × | × |
| 3 | Dihydroxybenzoic acid | Phenolic acid | 13.1 | [M − H]− | 153.0198 | 153.0193 | −3.04 | C7H6O4 | 109.0284 | [26] | × | × | × |
| 4 | Hydroxybenzoic acid * | Phenolic acid | 15.3 | [M − H]− | 137.0239 | 137.0241 | 3.75 | C7H6O3 | 119.0125, 109.0205 | [26] | × | × | × |
| 5 | Catechin * | Flavonoid | 20.9 | [M − H]− | 289.0722 | 289.0718 | −1.51 | C15H14O6 | 271.0454, 245.0813, 227.0736, 203.0701, 179.0367, 151.0396, 125.0230, 109.0218 | [20] | × | × | × |
| 6 | Apigenin * | Flavonoid | 30.6 | [M − H]− | 269.0452 | 269.0455 | 1.28 | C15H10O5 | 241.0471, 227.0333, 201.0587, 185.0553, 169.0662, 133.0288 | [21] | × | × | × |
| 7 | Naringenin | Flavonoid | 33.8 | [M − H]− | 271.0623 | 271.0685 | −4.05 | C15H12O5 | 253.0523, 177.0182, 151.0038, 135.0265, 119.0512 | [21] | × | × | × |
| 8 | Fragransin C1/C2 | Lignan | 39.2 | [M − H]− | 373.1669 | 373.1657 | −3.31 | C21H26O6 | 355.1555, 327.1199, 263.1300, 249.1140, 245.1182, 227.1215, 179.0398, 135.0259, 123.0445, 109.0287 | [22] | - | × | × |
| 9 | 5-(6,7-Dimethoxy-3-methyl-5-propenyl-2,3-dihydro-benzofuran-2-yl)-3-methoxy-benzene-1,2-diol | Lignan | 43.1 | [M − H]− | 371.1491 | 371.1500 | 2.45 | C21H24O6 | 327.1592, 261.1130, 217.1224, 193.0860, 178.0623,163.0393 | [23] | - | × | × |
| 10 | Malabaricone C | Diarylnonanoid | 48.6 | [M − H]− | 357.1704 | 357.1707 | 0.97 | C21H26O5 | 313.1798, 289.1437, 247.1325, 135.0289, 109.0304 | [27] | × | × | × |
| 11 | Malabaricone B | Diarylnonanoid | 50.9 | [M − H]− | 341.1755 | 341.1758 | 0.10 | C21H26O4 | 323.1649, 273.1498, 231.1387, 135.0085, 109.0297 | [23] | × | × | × |
| 12 | Giganteone A | Diarylnonanoid | 51.8 | [M − H]− | 713.3323 | 713.3331 | 1.15 | C42H50O10 | 603.3014, 585.2901, 465.1956, 355.1580, 109.0296 | [23] | × | × | × |
| 13 | Malabaricone A | Diarylnonanoid | 53.6 | [M − H]− | 325.1803 | 325.1809 | 1.90 | C21H26O3 | 307.1730, 257.1552, 215.1394, 145.0365, 135.0087, 109.0289 | [23] | × | × | × |
| 14 | 1-(2,6-Dihydroxyphenyl)-9-[4-hydroxy-3-(p-menth-1-en-8-yloxy)phenyl]-1-nonanone | Diarylnonanoid | 54.7 | [M − H]− | 493.2989 | 493.2959 | −3.95 | C31H42O5 | 383.2594, 357.1668, 313.1813, 233.1184, 163.0420, 135.0160, 109.0289 | [24] | × | × | × |
2.3. Total Phenolic and Flavonoid Contents
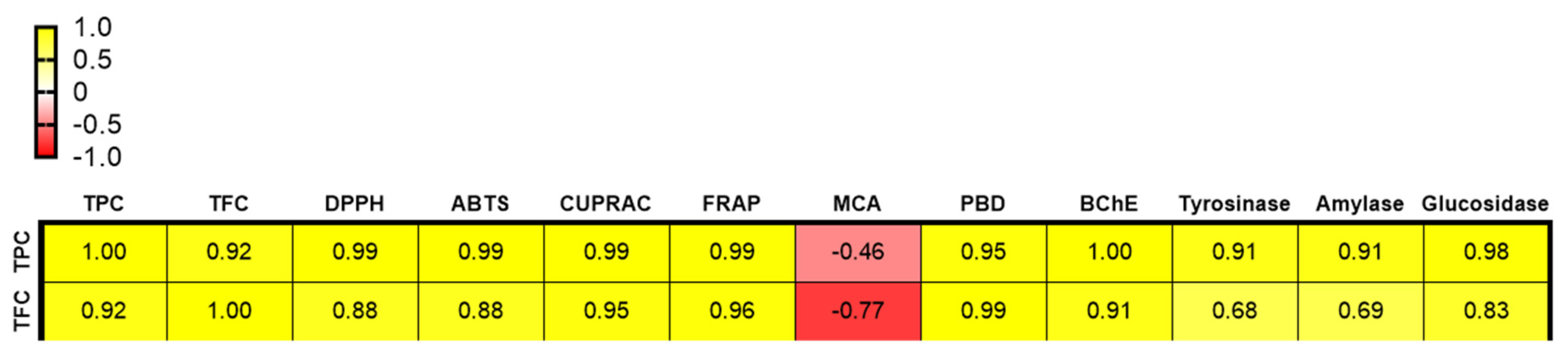
2.4. Antioxidant Activity
2.5. Enzyme Inhibition Activity
2.6. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.2.1. Isolation of Nutmeg Essential Oil
3.2.2. Obtaining Nutmeg Residual Water, Spent and Total Extracts
3.3. Phytochemical Screening
3.4. Antioxidant and Enzyme Inhibition Assays
3.5. Antimicrobial Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kuete, V. Myristica fragrans: A review. In Medicinal Spices and Vegetables from Africa; Kuete, V., Ed.; Academic Press: London, UK, 2017; pp. 497–512. [Google Scholar]
- Ha, M.T.; Vu, N.K.; Tran, T.H.; Kim, J.A.; Woo, M.H.; Min, B.S. Phytochemical and pharmacological properties of Myristica fragrans Houtt.: An updated review. Arch. Pharmacal Res. 2020, 43, 1067–1092. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A.; El-Alfy, A.T. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochem. Rev. 2016, 15, 1035–1056. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Simal-Gandara, J.; Murugan, M.; Dhanya, M.K.; Pandian, A. Nutmeg (Myristica fragrans Houtt.) essential oil: A review on its composition, biological, and pharmacological activities. Phytother. Res. 2022, 36, 2839–2851. [Google Scholar] [CrossRef]
- Periasamy, G.; Karim, A.; Gibrelibanos, M.; Gebremedhin, G. Nutmeg (Myristica fragrans Houtt.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 607–616. [Google Scholar]
- Schmidt, E. Production of Essential Oils. In Handbook of Essential Oils: Science, Technology, and Applications; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 127–164. [Google Scholar]
- Agustina, S.; Kholifah, L. Prospect of Essential Oil Industrial Waste as Energy Resources for Essential Oil Production Process. IOP Conf. Ser. Earth Environ. Sci. 2022, 1116, 012039. [Google Scholar] [CrossRef]
- Greff, B.; Lakatos, E.; Szigeti, J.; Varga, L. Co-composting with herbal wastes: Potential effects of essential oil residues on microbial pathogens during composting. Crit. Rev. Environ. Sci. Technol. 2021, 51, 457–511. [Google Scholar] [CrossRef]
- Gao, Y.; Ozel, M.Z.; Dugmore, T.; Sulaeman, A.; Matharu, A.S. A biorefinery strategy for spent industrial ginger waste. J. Hazard. Mater. 2021, 401, 123400. [Google Scholar] [CrossRef] [PubMed]
- Sijabat, P.S.; Siregar, Y. Study of distillation waste by clove for alternative fuel power plant: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 012081. [Google Scholar] [CrossRef]
- Bakar, M.S.A.; Ahmed, A.; Jeffery, D.M.; Hidayat, S.; Sukri, R.S.; Mahlia, T.M.I.; Jamil, F.; Khurrum, M.S.; Inayat, A.; Moogi, S. Pyrolysis of solid waste residues from Lemon Myrtle essential oils extraction for bio-oil production. Bioresour. Technol. 2020, 318, 123913. [Google Scholar] [CrossRef]
- Truzzi, E.; Chaouch, M.A.; Rossi, G.; Tagliazucchi, L.; Bertelli, D.; Benvenuti, S. Characterization and valorization of the agricultural waste obtained from Lavandula steam distillation for its reuse in the food and pharmaceutical fields. Molecules 2022, 27, 1613. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Al-Mahdy, D.A.; Wu, M.-L.; Zheng, X.-T.; Piao, X.-H.; Chen, A.-L.; Wang, S.-M.; Yang, Q.; Ge, Y.-W. LC-MS-based identification and antioxidant evaluation of small molecules from the cinnamon oil extraction waste. Food Chem. 2022, 366, 130576. [Google Scholar] [CrossRef]
- Turrini, F.; Beruto, M.; Mela, L.; Curir, P.; Triglia, G.; Boggia, R.; Zunin, P.; Monroy, F. Ultrasound-assisted extraction of lavender (Lavandula angustifolia Miller, cultivar Rosa) solid by-products remaining after the distillation of the essential oil. Appl. Sci. 2021, 11, 5495. [Google Scholar] [CrossRef]
- Wahba, H.E.; Abd Rabbu, H.S.; Ibrahim, M.E. Evaluation of essential oil isolated from dry coriander seeds and recycling of the plant waste under different storage conditions. Bull. Natl. Res. Cent. 2020, 44, 192. [Google Scholar] [CrossRef]
- Chouhan, K.B.S.; Tandey, R.; Sen, K.K.; Mehta, R.; Mandal, V. A unique model of gravity assisted solvent free microwave based extraction of essential oil from Mentha leaves ensuring biorefinery of leftover waste biomass for extraction of nutraceuticals: Towards cleaner and greener technology. J. Clean. Prod. 2019, 225, 587–598. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and antimicrobial activity of Mexican oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef]
- Dupuy, N.; Molinet, J.; Mehl, F.; Nanlohy, F.; Le Dréau, Y.; Kister, J. Chemometric analysis of mid infrared and gas chromatography data of Indonesian nutmeg essential oils. Ind. Crops Prod. 2013, 43, 596–601. [Google Scholar] [CrossRef]
- Kiarsi, Z.; Hojjati, M.; Behbahani, B.A.; Noshad, M. In vitro antimicrobial effects of Myristica fragrans essential oil on foodborne pathogens and its influence on beef quality during refrigerated storage. J. Food Saf. 2020, 40, e12782. [Google Scholar] [CrossRef]
- Ousji, O.; Sleno, L. Structural Elucidation of Novel Stable and Reactive Metabolites of Green Tea Catechins and Alkyl Gallates by LC-MS/MS. Antioxidants 2022, 11, 1635. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Hada, S.; Kawata, Y.; Tezuka, Y.; Kikuchi, T.; Namba, T. New 2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignans from the aril of Myristica fragrans. Chem. Pharm. Bull. 1987, 35, 3315–3322. [Google Scholar] [CrossRef]
- Pandey, R.; Mahar, R.; Hasanain, M.; Shukla, S.K.; Sarkar, J.; Rameshkumar, K.; Kumar, B. Rapid screening and quantitative determination of bioactive compounds from fruit extracts of Myristica species and their in vitro antiproliferative activity. Food Chem. 2016, 211, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Tao, H.-W.; Hao, X.; Gu, Q.-Q.; Zhu, W.-M. Cytotoxic and antioxidative phenolic compounds from the traditional Chinese medicinal plant, Myristica fragrans. Planta Med. 2009, 75, 1241–1245. [Google Scholar] [CrossRef]
- Luca, S.V.; Kulinowski, Ł.; Ciobanu, C.; Zengin, G.; Czerwińska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crops Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Luca, S.V.; Zengin, G.; Sinan, K.I.; Skalicka-Woźniak, K.; Trifan, A. Post-Distillation By-Products of Aromatic Plants from Lamiaceae Family as Rich Sources of Antioxidants and Enzyme Inhibitors. Antioxidants 2023, 12, 210. [Google Scholar] [CrossRef]
- Rastegari, A.; Manayi, A.; Rezakazemi, M.; Eftekhari, M.; Khanavi, M.; Akbarzadeh, T.; Saeedi, M. Phytochemical analysis and anticholinesterase activity of aril of Myristica fragrans Houtt. BMC Chem. 2022, 16, 106. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction kinetics of phenolic antioxidants from the hydro distillation residues of rosemary and effect of pretreatment and extraction parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef]
- Rahman, N.; Mahmood, K.; Kamilah, H.; Sulaiman, S.; Ibrahim, M.; Ariffin, F. Effects of blanching and pickling process on the alcohol acyltransferase (AAT) activity, myristicin content and quality parameters of pickled nutmeg (Myristica fragrans). J. Food Sci. Technol. 2021, 59, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Ooi, K.L. Antioxidant and anti food-borne bacterial activities of extracts from leaf and different fruit parts of Myristica fragrans Houtt. Food Control 2012, 25, 533–536. [Google Scholar] [CrossRef]
- Pashapoor, A.; Mashhadyrafie, S.; Mortazavi, P. The antioxidant potential and antihyperlipidemic activity of Myristica fragrans seed (nutmeg) extract in diabetic rats. J. Hum. Environ. Health Promot. 2020, 6, 91–96. [Google Scholar] [CrossRef]
- Waman, A.A.; Bohra, P.; Roy, T.K.; Shivashankara, K.S. Seed morphological and biochemical studies in certain wild nutmegs. Trees 2021, 35, 939–945. [Google Scholar] [CrossRef]
- Silva, A.S.; Nabavi, S.M. Antioxidants effects in health: Concluding remarks and future perspectives. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 851–858. [Google Scholar]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The key roles of ROS and RNS as a signaling molecule in plant–microbe interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Seneme, E.F.; Dos Santos, D.C.; Silva, E.M.R.; Franco, Y.E.M.; Longato, G.B. Pharmacological and therapeutic potential of myristicin: A literature review. Molecules 2021, 26, 5914. [Google Scholar] [CrossRef] [PubMed]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Okiki, P.A.; Nwobi, C.P.; Akpor, O.B.; Adewole, E.; Agbana, R.D. Assessment of Nutritional and Medicinal Properties of Nutmeg. Sci. Afr. 2023, 19, e01548. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Stanojevic, J.; Danilovic, B. Chemical composition, antioxidant and antimicrobial activity of nutmeg (Myristica fragrans Houtt.) seed essential oil. J. Essent. Oil-Bear. Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- Rashidian, G.; Shahin, K.; Elshopakey, G.E.; Mahboub, H.H.; Fahim, A.; Elabd, H.; Prokić, M.D.; Faggio, C. The dietary effects of nutmeg (Myristica fragrans) extract on growth, hematological parameters, immunity, antioxidant status, and disease resistance of common carp (Cyprinus carpio) against Aeromonas hydrophila. J. Mar. Sci. Eng. 2022, 10, 325. [Google Scholar] [CrossRef]
- Chen, S.-K.; Lin, H.-F.; Wang, X.; Yuan, Y.; Yin, J.-Y.; Song, X.-X. Comprehensive analysis in the nutritional composition, phenolic species and in vitro antioxidant activities of different pea cultivars. Food Chem. X 2023, 17, 100599. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Richane, A.; Rim, B.M.; Riadh, K.; Khaoula, A.; Nizar, M.; Hanen, B.I. Variability of phenolic compounds and antioxidant activities of ten Ceratonia siliqua L. provenances. Biochem. Syst. Ecol. 2022, 104, 104486. [Google Scholar] [CrossRef]
- Tepe, A.S.; Ozaslan, M. Anti-Alzheimer, anti-diabetic, skin-whitening, and antioxidant activities of the essential oil of Cinnamomum zeylanicum. Ind. Crops Prod. 2020, 145, 112069. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Enzyme inhibition: Mechanisms and scope. In Enzyme Inhibition and Bioapplications; Sharma, R.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–36. [Google Scholar]
- Lista, S.; Vergallo, A.; Teipel, S.J.; Lemercier, P.; Giorgi, F.S.; Gabelle, A.; Garaci, F.; Mercuri, N.B.; Babiloni, C.; Gaire, B.P. Determinants of approved acetylcholinesterase inhibitor response outcomes in Alzheimer’s disease: Relevance for precision medicine in neurodegenerative diseases. Ageing Res. Rev. 2022, 84, 101819. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yamafuji, C. Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids. J. Agric. Food Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig.(Apiaceae). Fitoterapia 2009, 80, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Cuong, T.D.; Hung, T.M.; Han, H.Y.; Sik Roh, H.; Seok, J.-H.; Lee, J.K.; Jeong, J.Y.; Choi, J.S.; Kim, J.A.; Min, B.S. Potent acetylcholinesterase inhibitory compounds from Myristica fragrans. Nat. Prod. Comm. 2014, 9, 499–502. [Google Scholar] [CrossRef]
- Dhingra, D.; Parle, M.; Kulkarni, S. Comparative brain cholinesterase-inhibiting activity of Glycyrrhiza glabra, Myristica fragrans, ascorbic acid, and metrifonate in mice. J. Med. Food 2006, 9, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Predicting tyrosinase inhibition by 3D QSAR pharmacophore models and designing potential tyrosinase inhibitors from Traditional Chinese medicine database. Phytomedicine 2018, 38, 145–157. [Google Scholar] [CrossRef]
- Jelenkovic, L.; Jovanovic, V.S.; Palic, I.; Mitic, V.; Radulovic, M. In vitro screening of α-amylase inhibition by selected terpenes from essential oils. Trop. J. Pharm. Res. 2014, 13, 1421–1428. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kono, R.; Kuse, M.; Yamashita, Y.; Ashida, H. Phenylpropanoids and neolignans isolated from Myristica fragrans enhance glucose uptake in myotubes. Food Funct. 2022, 13, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Al Kury, L.T.; Abdoh, A.; Ikbariah, K.; Sadek, B.; Mahgoub, M. In vitro and in vivo antidiabetic potential of monoterpenoids: An update. Molecules 2022, 27, 182. [Google Scholar] [CrossRef]
- Prabha, B.; Neethu, S.; Krishnan, S.L.; Sherin, D.; Madhukrishnan, M.; Ananthakrishnan, R.; Rameshkumar, K.; Manojkumar, T.; Jayamurthy, P.; Radhakrishnan, K. Antidiabetic potential of phytochemicals isolated from the stem bark of Myristica fatua Houtt. var. magnifica (Bedd.) Sinclair. Bioorg. Med. Chem. 2018, 26, 3461–3467. [Google Scholar] [CrossRef]
- Sivasothy, Y.; Leong, K.H.; Loo, K.Y.; Adbul Wahab, S.M.; Othman, M.A.; Awang, K. Giganteone A and malabaricone C as potential pharmacotherapy for diabetes mellitus. Nat. Prod. Res. 2022, 36, 1581–1586. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Perumal, S. Determination of antibacterial activity of essential oil of Myristica fragrans Houtt. using tetrazolium microplate assay and its cytotoxic activity against Vero cell line. Int. J. Pharmacol. 2012, 8, e6. [Google Scholar]
- EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID): Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]

| No. | Compound | LRI a | (%) b |
|---|---|---|---|
| 1 | 3-Thujene | 927 | 2.33 ± 0.01 |
| 2 | α-pinene | 935 | 15.81 ± 0.26 |
| 3 | Camphene | 951 | 0.28 ± 0.01 |
| 4 | Sabinene | 974 | 21.71 ± 0.52 |
| 5 | β-Pinene | 979 | 12.70 ± 0.52 |
| 6 | β-Myrcene * | 989 | 2.04 ± 0.04 |
| 7 | α-Phellandrene | 1006 | 0.47 ± 0.01 |
| 8 | 3-Carene | 1009 | 0.77 ± 0.01 |
| 9 | α-Terpinene | 1018 | 1.42 ± 0.03 |
| 10 | p-Cymene | 1025 | 0.76 ± 0.02 |
| 11 | Limonene * | 1030 | 5.86 ± 0.09 |
| 12 | γ-Terpinene | 1060 | 2.24 ± 0.04 |
| 13 | cis-α-Terpineol | 1072 | 0.47 ± 0.01 |
| 14 | α-Terpinolene | 1086 | 0.99 ± 0.02 |
| 15 | p-Cymenene | 1090 | 0.04 ± 0.00 |
| 16 | Linalool * | 1098 | 0.33 ± 0.01 |
| 17 | trans-5-Caranol | 1101 | 0.44 ± 0.01 |
| 18 | cis-p-Menth-2-en-1-ol | 1126 | 0.24 ± 0.01 |
| 19 | trans-p-Menth-2-en-1-ol | 1144 | 0.14 ± 0.01 |
| 20 | Terpinen-4-ol | 1183 | 3.73 ± 0.08 |
| 21 | p-Cymen-8-ol | 1188 | 0.04 ± 0.00 |
| 22 | trans-α-Terpineol | 1196 | 0.61 ± 0.02 |
| 23 | Bornyl acetate | 1285 | 0.14 ± 0.01 |
| 24 | Safrole | 1291 | 2.55 ± 0.06 |
| 25 | Isopulegol acetate | 1296 | 0.13 ± 0.00 |
| 26 | Myrtanyl acetate | 1343 | 0.26 ± 0.01 |
| 27 | Eugenol | 1350 | 0.44 ± 0.02 |
| 28 | Geraniol acetate | 1374 | 0.16 ± 0.01 |
| 29 | Copaene | 1380 | 0.74 ± 0.03 |
| 30 | α-Cubenene | 1391 | 0.06 ± 0.00 |
| 31 | Methyleugenol | 1398 | 5.13 ± 0.12 |
| 32 | Caryophyllene * | 1427 | 0.23 ± 0.01 |
| 33 | (Z)-α-Bergamotene | 1436 | 0.15 ± 0.01 |
| 34 | Isoeugenol | 1449 | 0.53 ± 0.02 |
| 35 | Humulene * | 1464 | 0.04 ± 0.01 |
| 36 | Germacrene D | 1488 | 0.22 ± 0.01 |
| 37 | Methylisoeugenol | 1494 | 1.20 ± 0.03 |
| 38 | γ-Elemene | 1503 | 0.12 ± 0.01 |
| 39 | β-Bisabolene | 1510 | 0.10 ± 0.00 |
| 40 | Myristicin | 1524 | 13.39 ± 0.10 |
| 41 | α-Bisabolene | 1544 | 0.65 ± 0.02 |
| 42 | Methoxyeugenol | 1594 | 0.06 ± 0.02 |
| 43 | Guaiol | 1603 | 0.02 ± 0.02 |
| Hydrocarbon monoterpenes | 68.50 ± 0.60 | ||
| Oxygenated monoterpenes | 6.41 ± 0.13 | ||
| Hydrocarbon sesquiterpenes | 1.51 ± 0.04 | ||
| Oxygenated sesquiterpenes | 0.02 ± 0.02 | ||
| Aromatic compounds | 23.31 ± 0.31 | ||
| Total identified | 99.79 ± 0.09 |
| Sample | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg RE/g) |
|---|---|---|
| NWE | 10.02 ± 0.02 c | 2.12 ± 0.19 c |
| NSE | 63.31 ± 0.72 a | 8.31 ± 0.06 a |
| NTE | 57.42 ± 3.90 b | 5.33 ± 0.06 b |
| Sample | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Metal Chelating (mg EDTAE/g) | Phosphomolybdenum (mmol TE/g) |
|---|---|---|---|---|---|---|
| NEO | 28.61 ± 0.35 b | 60.20 ± 0.61 b | 113.74 ± 3.09 c | 105.28 ± 1.93 b | n.a. | 57.99 ± 0.19 a |
| NWE | 12.50 ± 0.56 c | 21.04 ± 0.41 c | 22.27 ± 0.29 d | 16.27 ± 0.11 d | 23.98 ± 0.31 a | 0.36 ± 0.01 d |
| NSE | 49.18 ± 0.13 a | 66.36 ± 0.04 a | 172.28 ± 2.66 a | 108.11 ± 3.18 a | 15.14 ± 1.48 b | 4.00 ± 0.20 b |
| NTE | 49.12 ± 0.17 a | 66.15 ± 0.17 a | 144.78 ± 4.36 b | 86.52 ± 0.94 c | 25.16 ± 1.92 a | 2.61 ± 0.05 c |
| Sample | Acetylcholinesterase (mg GALAE/g) | Butyrylcholinesterase (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|
| NEO | 4.04 ± 0.14 | 4.21 ± 0.02 b | 46.40 ± 2.39 b | 0.33 ± 0.01 b | 1.90 ± 0.07 a |
| NWE | n.a. | 2.81 ± 0.03 c | 16.16 ± 0.42 c | 0.16 ± 0.02 c | n.a. |
| NSE | n.a. | 4.78 ± 0.03 a | 47.74 ± 4.58 b | 0.35 ± 0.02 b | 1.69 ± 0.08 b |
| NTE | n.a. | 4.61 ± 0.05 a | 61.79 ± 2.39 a | 0.44 ± 0.00 a | 1.87 ± 0.01 a |
| Microorganism | NEO | NWE | NSE | NTE | Control | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | |
| Gram-positive bacteria | Vancomycin | ||||||||
| Bacillus cereus ATCC 10876 | 2000 | >2000 | >2000 | n.d. | 62.5 | 2000 | 250 | >2000 | 0.98 |
| Enterococcus faecalis ATCC 29212 | 2000 | >2000 | >2000 | n.d. | 500 | >2000 | 1000 | >2000 | 1.95 |
| Micrococcus luteus ATCC 10240 | 2000 | >2000 | >2000 | n.d. | 62.5 | 125 | 125 | 250 | 0.12 |
| Staphylococcus aureus ATCC 25923 | >2000 | >2000 | >2000 | n.d. | 125 | 250 | 250 | 250 | 0.98 |
| Staphylococcus aureus ATCC BAA-1707 * | >2000 | >2000 | >2000 | n.d. | 125 | 250 | 250 | 500 | 0.98 |
| Staphylococcus epidermidis ATCC 12228 | >2000 | >2000 | >2000 | n.d. | 500 | >2000 | 1000 | >2000 | 0.98 |
| Streptococcus pneumoniae ATCC 49619 | 1000 | 2000 | >2000 | n.d. | 62.5 | 1000 | 250 | 1000 | 0.24 |
| Streptococcus pyogenes ATCC 19615 | 2000 | >2000 | >2000 | n.d. | 250 | >2000 | 1000 | >2000 | 0.24 |
| Streptococcus mutans ATCC 25175 | 2000 | >2000 | >2000 | n.d. | 1000 | >2000 | >2000 | >2000 | 0.98 |
| Gram-negative bacteria | Ciprofloxacin | ||||||||
| Escherichia coli ATCC 25922 | >2000 | n.d. | >2000 | n.d. | 2000 | n.d. | >2000 | n.d. | 0.015 |
| Klebsiella pneumoniae ATCC 13883 | >2000 | n.d. | >2000 | n.d. | 2000 | n.d. | >2000 | n.d. | 0.122 |
| Proteus mirabilis ATCC 12453 | >2000 | n.d. | >2000 | n.d. | 2000 | n.d. | >2000 | n.d. | 0.030 |
| Pseudomonas aeruginosa ATCC 9027 | >2000 | n.d. | >2000 | n.d. | 2000 | n.d. | 2000 | n.d. | 0.488 |
| Salmonella Typhimurium ATCC 14028 | >2000 | n.d. | >2000 | n.d. | 2000 | n.d. | >2000 | n.d. | 0.061 |
| Yeasts | Nystatin | ||||||||
| Candida albicans ATCC 102231 | 2000 | >2000 | >2000 | >2000 | 1000 | >2000 | 2000 | >2000 | 0.24 |
| Candida glabrata ATCC 2091 | 1000 | >2000 | >2000 | >2000 | 2000 | >2000 | 2000 | >2000 | 0.48 |
| Candida parapsilosis ATCC 22019 | 250 | 2000 | 2000 | >2000 | 250 | >2000 | 500 | >2000 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifan, A.; Zengin, G.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Luca, S.V. Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products. Plants 2023, 12, 1741. https://doi.org/10.3390/plants12091741
Trifan A, Zengin G, Korona-Glowniak I, Skalicka-Woźniak K, Luca SV. Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products. Plants. 2023; 12(9):1741. https://doi.org/10.3390/plants12091741
Chicago/Turabian StyleTrifan, Adriana, Gokhan Zengin, Izabela Korona-Glowniak, Krystyna Skalicka-Woźniak, and Simon Vlad Luca. 2023. "Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products" Plants 12, no. 9: 1741. https://doi.org/10.3390/plants12091741
APA StyleTrifan, A., Zengin, G., Korona-Glowniak, I., Skalicka-Woźniak, K., & Luca, S. V. (2023). Essential Oils and Sustainability: In Vitro Bioactivity Screening of Myristica fragrans Houtt. Post-Distillation By-Products. Plants, 12(9), 1741. https://doi.org/10.3390/plants12091741










