Phytochemical Composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels Leaf and Stem Extracts
Abstract
1. Introduction
2. Results
2.1. Extract Yield
2.2. Preliminary Qualitative Phytochemical Screening
2.3. FTIR Spectroscopy
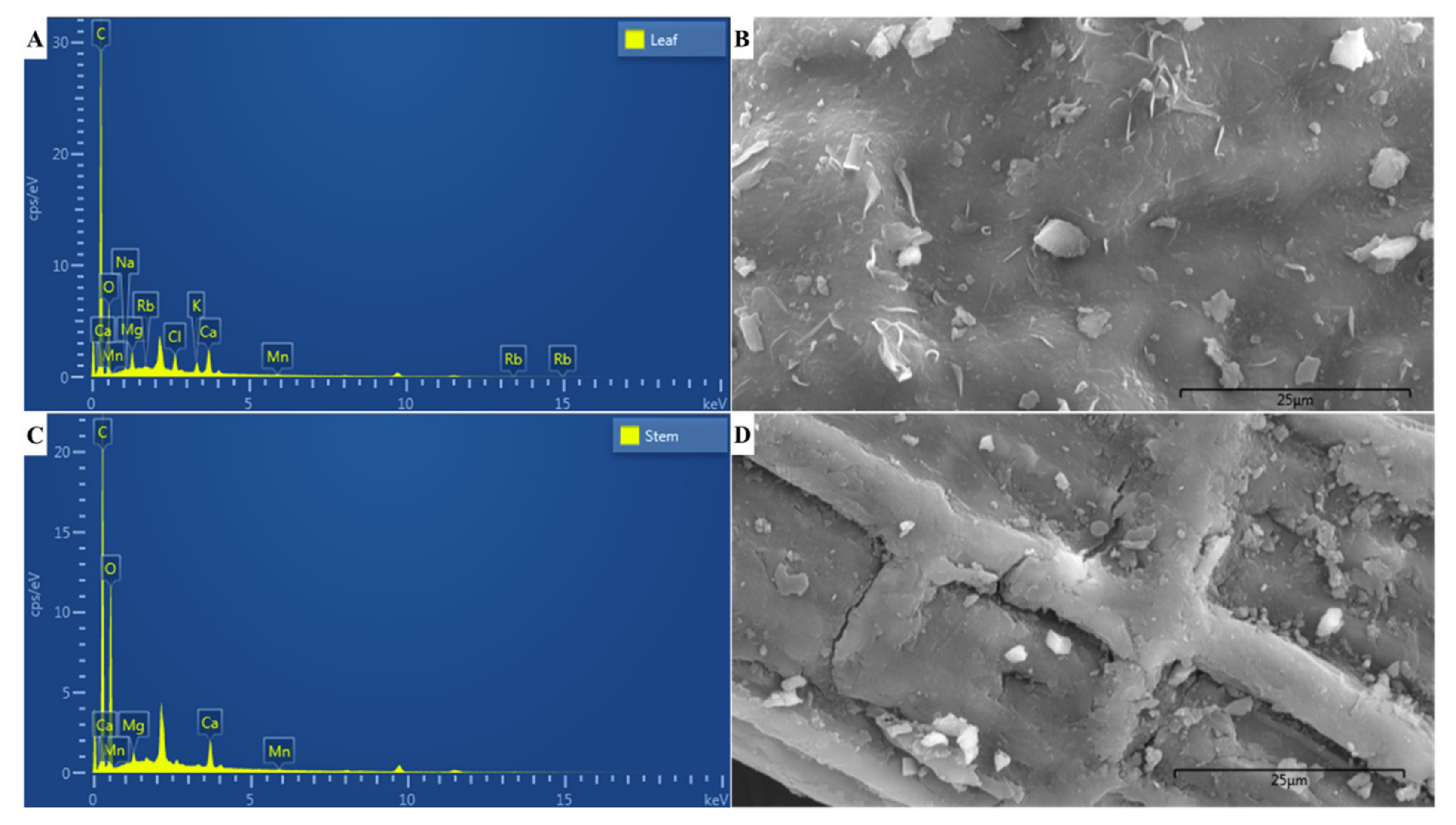
2.4. EDX Microanalyses
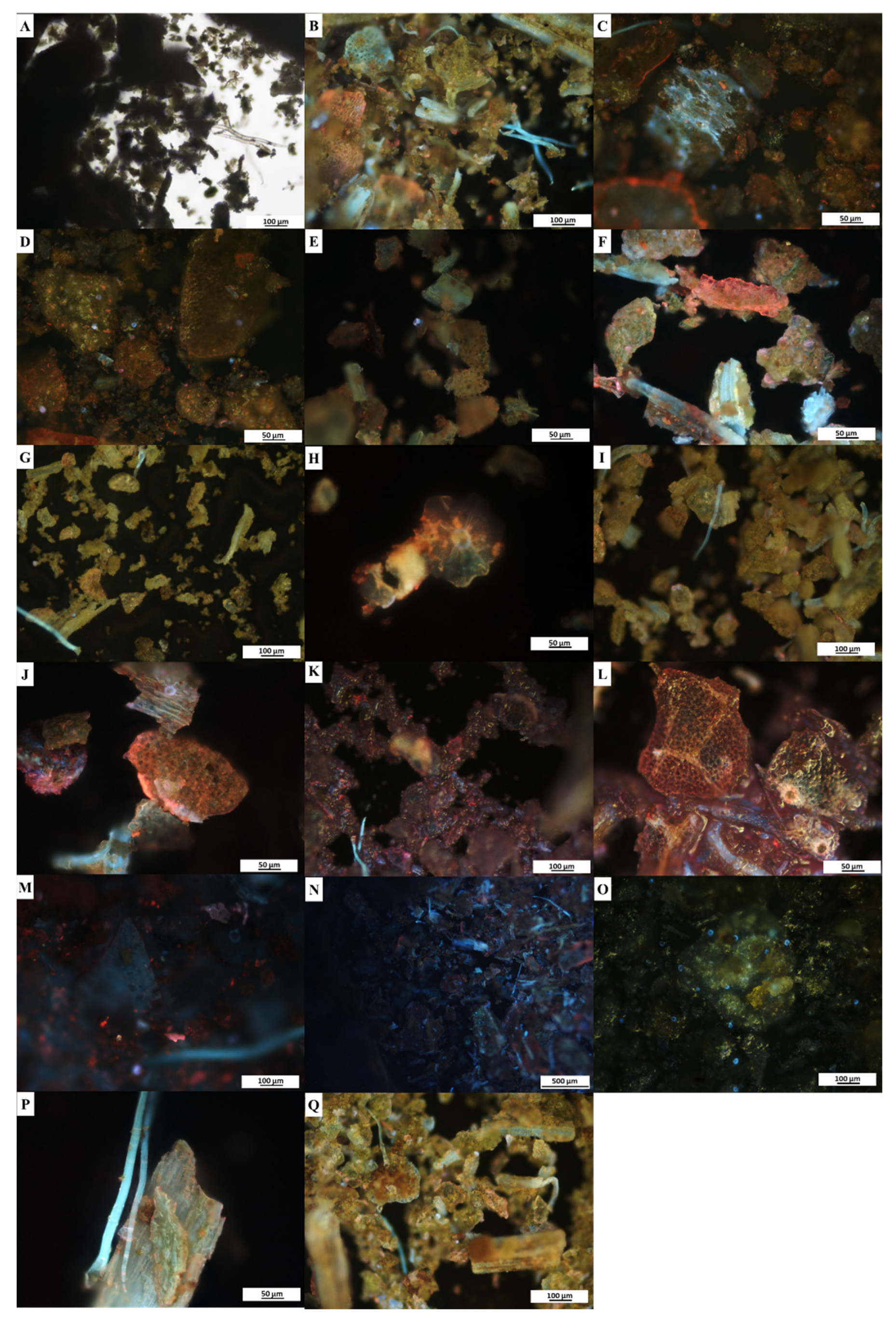
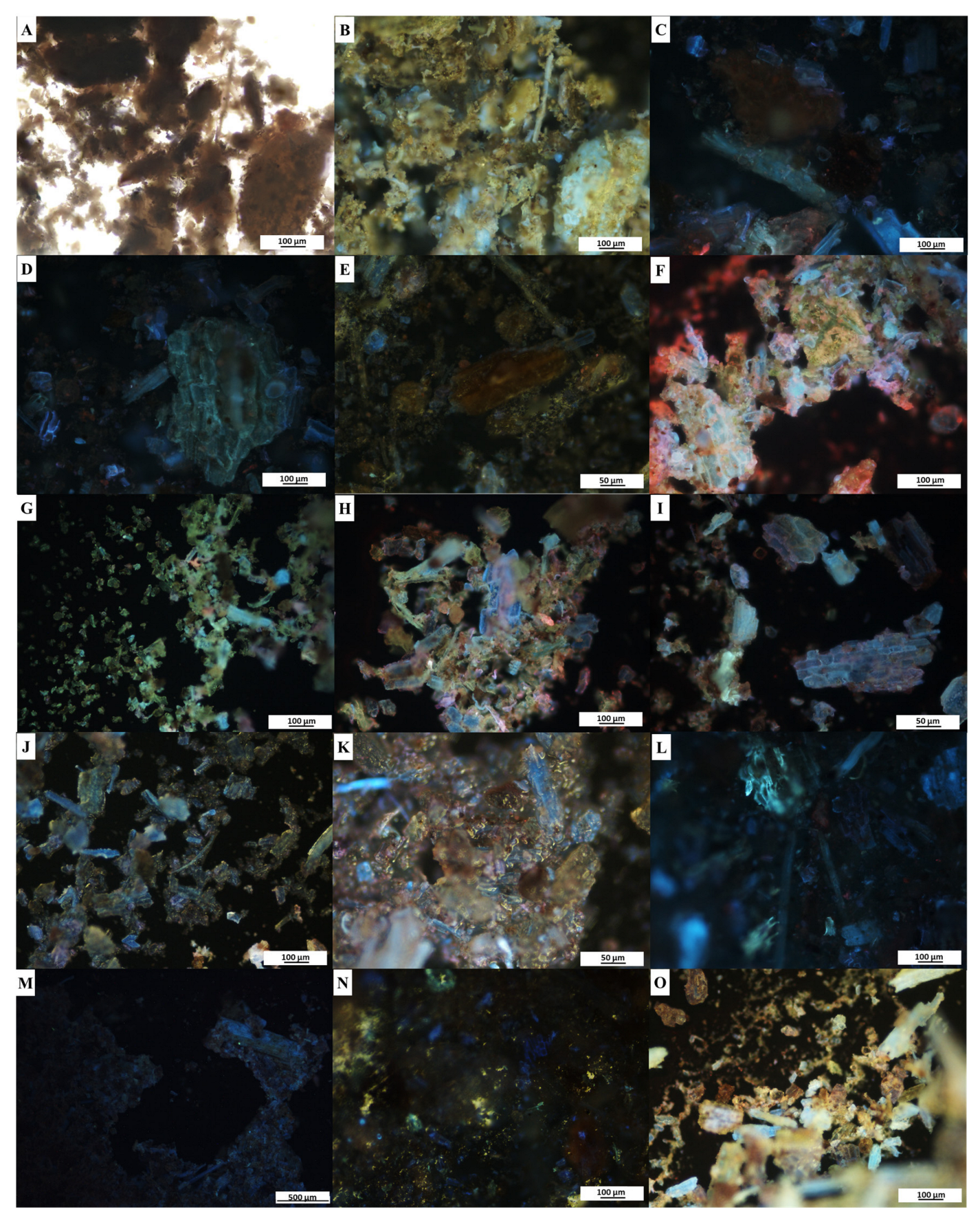
2.5. Fluorescence Microanalyses
3. Discussion
3.1. Yield and Phytochemical Composition of Leaf and Stem Extracts
3.2. Characterization of Powdered Samples and FTIR Spectroscopy
3.3. Elemental Composition
3.4. Fluorescence Microanalyses
4. Materials and Methods
4.1. Plant Material Collection and Extract Preparation
4.2. Qualitative Phytochemical Screening
4.3. Preparation of Powdered Samples
4.4. Fourier Transform Infrared (FTIR) Spectroscopy
4.5. Energy-Dispersive X-ray (EDX) Microanalyses
4.6. Fluorescence Microanalyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Agidew, M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Farooq, S.; Ngaini, Z. Natural and synthetic drugs as potential treatment for coronavirus disease 2019 (COVID-2019). Chem. Afr. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Kengne, I.C.; Feugap, L.D.T.; Njouendou, A.J.; Ngnokam, C.D.J.; Djamalladine, M.D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Tamokou, J.-D.-D. Antibacterial, antifungal and antioxidant activities of whole plant chemical constituents of Rumex abyssinicus. BMC Complement. Med. Ther. 2021, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.A. Trends and challenges of traditional medicine in Africa. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 115–123. [Google Scholar] [CrossRef]
- Moeti, M. African Traditional Medicine Day 2022. Available online: https://www.afro.who.int/regional-director/speeches-messages/african-traditional-medicine-day-2022 (accessed on 13 September 2022).
- Jalali, A.; Dabaghian, F.; Akbrialiabad, H.; Foroughinia, F.; Zarshenas, M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother. Res. 2021, 35, 1925–1938. [Google Scholar] [CrossRef]
- Les, F.; Cásedas, G.; López, V. Bioactivity of medicinal plants and extracts. Biology 2021, 10, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R. Role of natural products against microorganisms. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1005. [Google Scholar]
- Muravnik, L. The Structural Peculiarities of the Leaf Glandular Trichomes: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Springer: Cham, Switzerland, 2021; pp. 63–97. [Google Scholar]
- Rademan, S.; Lall, N. Combretum molle, in Underexplored Medicinal Plants from Sub-Saharan Africa: Plants with Therapeutic Potential for Human Health, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2020; pp. 67–76. [Google Scholar]
- Van Wyk, B. Field Guide to Trees of Southern Africa; Penguin Random House: Midrand, South Africa, 2013. [Google Scholar]
- Burkill, H.M. The Useful Plants of West Tropical Africa; Royal Botanic Gardens: Kew, UK, 1985; Volume 1. [Google Scholar]
- Andefiki, U.; Ileigo, I.H.; Isah, A. In Vivo Activity of Fractions of Combretum molle R. and Haematological Profile of Trypanosoma Brucei Brucei Infected Mice. Am. J. Pharm. Pharmacol. 2017, 4, 15–20. [Google Scholar]
- Jordaan, M.; Van Wyk, A.E.B.; Maurin, O. A conspectus of Combretum (Combretaceae) in southern Africa, with taxonomic and nomenclatural notes on species and sections. Bothalia 2011, 41, 135–160. [Google Scholar] [CrossRef]
- Bein, E.; Jaber, A.; Birnie, A.; Tengnäs, B. Useful Trees and Shrubs in Eritrea; Regional Soil Conservation Unit, Rscu/Sida Nairobi Caton-Thompson, G., 1952: Kharga Oasis in prehistory; Athlone Press: London, UK, 1996. [Google Scholar]
- Ntshanka, N.M.; Ejidike, I.P.; Mthunzi, F.M.; Moloto, M.J.; Mubiayi, K.P. Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomed. Pharmacol. J. 2020, 13, 1683–1694. [Google Scholar] [CrossRef]
- Pegel, K.H.; Rogers, C.B. The characterisation of mollic acid 3β-D-xyloside and its genuine aglycone mollic acid, two novel 1α-hydroxycycloartenoids from Combretum molle. J. Chem. Soc. Perkin Trans. 1985, 1, 1711–1715. [Google Scholar] [CrossRef]
- Ponou, B.K.; Barboni, L.; Teponno, R.B.; Mbiantcha, M.; Nguelefack, T.B.; Park, H.-J.; Lee, K.-T.; Tapondjou, L.A. Polyhydroxyoleanane-type triterpenoids from Combretum molle and their anti-inflammatory activity. Phytochem. Lett. 2008, 1, 183–187. [Google Scholar] [CrossRef]
- Asres, K.; Bucar, F.; Knauder, E.; Yardley, V.; Kendrick, H.; Croft, S. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytother. Res. 2001, 15, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A. Cardiovascular effects of mollic acid glucoside, a 1α-hydroxycycloartenoid saponin extractive from Combretum molle R Br ex G Don (Combretaceae) leaf. Cardiovasc. J. Afr. 2008, 19, 128. [Google Scholar] [PubMed]
- Asres, K.; Bucar, F. Anti-HIV activity against immunodeficiency virus type 1 (HIV-I) and type II (HIV-II) of compounds isolated from the stem bark of Combretum molle. Ethiop. Med. J. 2005, 43, 15–20. [Google Scholar]
- Afreed Muhammed, N.; Ranjith, D.; Vinayakraj, M.; Rahman, M.; Sivan, V.; Sanis, J. Physical characteristics, extractive yield and qualitative phytochemical analysis of Flueggea leucopyrus Willd leaves. J. Med. Plants 2018, 6, 175–179. [Google Scholar]
- Chanda, S. Importance of pharmacognostic study of medicinal plants: An overview. J. Pharmacogn. Phytochem. 2014, 2, 69–73. [Google Scholar]
- Zaman, M.K.; Azzeme, A.M.; Ramli, S.N.; Shaharuddin, N.A.; Ahmad, S.; Abdullah, S.N.A. Solvent extraction and its effect on phytoch[1]emical yield and antioxidant capacity of woody medicinal plant, Polyalthia bullata. BioResources 2020, 15, 9555. [Google Scholar] [CrossRef]
- Hasmila, I.; Natsir, H.; Soekamto, N. Phytochemical analysis and antioxidant activity of soursop leaf extract (Annona muricata Linn.). J. Phys. Conf. Ser. 2019, 1341, 032027. [Google Scholar] [CrossRef]
- Simon, M.; Ajanusi, O.; Abubakar, M.; Idris, A.; Suleiman, M. The anthelmintic effect of aqueous methanol extract of Combretum molle (R. Br. x. G. Don) (Combretaceae) in lambs experimentally infected with Haemonchus contortus. Vet. Parasitol. 2012, 187, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Loneman, D.M.; Peddicord, L.; Al-Rashid, A.; Nikolau, B.J.; Lauter, N.; Yandeau-Nelson, M.D. A robust and efficient method for the extraction of plant extracellular surface lipids as applied to the analysis of silks and seedling leaves of maize. PLoS ONE 2017, 12, e0180850. [Google Scholar] [CrossRef]
- Yusnawan, E. The effectiveness of polar and non polar fractions of Ageratum conyzoides l. to control peanut rust disease and phytochemical screenings of secondary metabolites. J. Trop. Plant Pests Dis. 2013, 13, 159–166. [Google Scholar] [CrossRef]
- Lichman, B.R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 2021, 38, 103–129. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-Derived Bioactives; Springer: Singapore, 2020; pp. 85–117. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Bantho, S.; Naidoo, Y.; Dewir, Y. The secretory scales of Combretum erythrophyllum (Combretaceae): Micromorphology, ultrastructure and histochemistry. S. Afr. J. Bot. 2020, 131, 104–117. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Chahil, G.S.; Gill, H.K.; Goyal, G. Food Chains and Webs: Interaction with Ecosystem. In Advances in Crop Environment Interaction; Springer: Singapore, 2018; pp. 405–424. [Google Scholar]
- Zeng, T.; Chen, Y.; Jian, Y.; Zhang, F.; Wu, R. Chemotaxonomic investigation of plant terpenoids with an established database (TeroMOL). New Phytol. 2022, 235, 662–673. [Google Scholar] [CrossRef]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H. Therapeutic and biomedical potentialities of terpenoids—A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Ascensão, L.; Pais, M. The leaf capitate trichomes of Leonotis leonurus: Histochemistry, ultrastructure and secretion. Ann. Bot. 1998, 81, 263–271. [Google Scholar] [CrossRef]
- Heinrich, G.; Pfeifhofer, H.; Stabentheiner, E.; Sawidis, T. Glandular hairs of Sigesbeckia jorullensis Kunth (Asteraceae): Morphology, histochemistry and composition of essential oil. Ann. Bot. 2002, 89, 459–469. [Google Scholar] [CrossRef]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1–7. [Google Scholar]
- Fyhrquist, P.; Mwasumbi, L.; Vuorela, P.; Vuorela, H.; Hiltunen, R.; Murphy, C.; Adlercreutz, H. Preliminary antiproliferative effects of some species of Terminalia, Combretum and Pteleopsis collected in Tanzania on some human cancer cell lines. Fitoterapia 2006, 77, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Baqami, N.M.; Khojah, E.; Mansour, A.M.; E. Al-Motaani, S.; A. Al-Salmi, F.; El-Megharbel, S.M. Possible antioxidant and antidiabetic effects of Combretum molle extract in a diabetes mellitus experimental model in male rats. Nat. Prod. Commun. 2021, 16, 1–10. [Google Scholar]
- Miaffo, D.; Wansi, S.L.; Ntchapda, F.; Kamanyi, A. Chronic oral safety study of the aqueous extract of Combretum molle twigs on biochemical, haematological and antioxidant parameters of Wistar rats. BMC Complement. Med. Ther. 2020, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Bantho, S.; Naidoo, Y.; Dewir, Y.H.; Bantho, A.; Murthy, H.N. Chemical Composition of Combretum erythrophyllum Leaf and Stem Bark Extracts. Horticulturae 2022, 8, 755. [Google Scholar] [CrossRef]
- Sousa, H.G.; Uchôa, V.T.; Cavalcanti, S.M.G.; de Almeida, P.M.; Chaves, M.H.; Lima Neto, J.D.S.; Nunes, P.H.M.; da Costa Júnior, J.S.; Rai, M.; Do Carmo, I.S. Phytochemical screening, phenolic and flavonoid contents, antioxidant and cytogenotoxicity activities of Combretum leprosum Mart. (Combretaceae). J. Toxicol. Environ. Health Part A 2021, 84, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Oloya, B.; Namukobe, J.; Ssengooba, W.; Afayoa, M.; Byamukama, R. Phytochemical screening, antimycobacterial activity and acute toxicity of crude extracts of selected medicinal plant species used locally in the treatment of tuberculosis in Uganda. Trop. Med. Health 2022, 50, 16. [Google Scholar] [CrossRef] [PubMed]
- Kulawe, D.; Muhammad, I.; Yuguda, U.A. Antibacterial effect of root fractions of Combretum molle (R. Br. Ex. G. Don) against selected pathogens. Bima J. Sci. Technol. 2020, 3, 78–85. [Google Scholar]
- Simon, M.; Nafarnda, W.; Obeta, S. Iridoid Glycosides Isolated from Combretum molle Stem Bark Aqueous Methanol Extract. Glob. Vet. 2012, 8, 237–243. [Google Scholar]
- Saidu, T.; Abdullahi, M. Phytochemical determinations and antibacterial activities of the leaf extracts of Combretum molle and Gossypium arboretum. Bayero J. Pure Appl. Sci. 2011, 4, 132–136. [Google Scholar] [CrossRef]
- Koevi, K.-K.A.; Millogo, V.; Fokou, J.B.H.; Sarr, A.; Ouedraogo, G.A.; Bassene, E. Phytochemical analysis and antioxidant activities of Combretum molle and Pericopsis laxiflora. Int. J. Biol. Chem. Sci. 2015, 9, 2423–2431. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud Univ. -Sci. 2020, 32, 643–647. [Google Scholar] [CrossRef]
- Naidoo, C.M.; Naidoo, Y.; Dewir, Y.H.; Singh, M.; Daniels, A.N.; El-Ramady, H. In Vitro investigation of the antioxidant and cytotoxic potential of Tabernaemontana ventricosa hochst. Ex A. DC. leaf, stem, and latex extracts. Horticulturae 2022, 8, 91. [Google Scholar] [CrossRef]
- Burman, S.; Bhattacharya, K.; Mukherjee, D.; Chandra, G. Antibacterial efficacy of leaf extracts of Combretum album Pers. against some pathogenic bacteria. BMC Complement. Altern. Med. 2018, 18, 213. [Google Scholar] [CrossRef]
- Sall, C.; Ndoye, S.F.; Dioum, M.D.; Seck, I.; Gueye, R.S.; Faye, B.; Thiam, C.O.; Seck, M.; Gueye, P.M.; Fall, D. Phytochemical Screening, Evaluation of Antioxidant and Anti-sickling Activities of Two Polar Extracts of Combretum glutinosum Leaves. Perr. ex DC. Br. J. Appl. Sci. Technol. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Z.; Ping, G. Macroscopic identification of Chinese medicinal materials: Traditional experiences and modern understanding. J. Ethnopharmacol. 2011, 134, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of micro-fourier transform infrared spectroscopy (FTIR) in the geological sciences—A review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; IntechOpen: London, UK, 2019; Volume 1. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Bhuyan, D.J.; Basu, A. Phenolic compounds potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Waste; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735. [Google Scholar] [CrossRef]
- Vanitha, A.; Kalimuthu, K.; Chinnadurai, V.; Nisha, K.J. Phytochemical screening, FTIR and GC-MS analysis of aqueous extract of Caralluma bicolor–An endangered plant. Asian J. Pharm. Sci. 2019, 5, 1122–1130. [Google Scholar]
- Asres, K.; Mazumder, A.; Bucar, F. Antibacterial and antifungal activities of extracts of Combretum molle. Ethiop. Med. J. 2006, 44, 269. [Google Scholar]
- Neriyana, P.S.; Alva, V.D. A green approach: Evaluation of Combretum indicum (CI) leaf extract as an eco-friendly corrosion inhibitor for mild steel in 1M HCl. Chem. Afr. 2020, 3, 1087–1098. [Google Scholar] [CrossRef]
- Bush, R.; McInerney, F. Variation in n-Alkane Distributions of Modern Plants: Questioning Applications of n-Alkanes in Chemotaxonomy and Paleoecology; Fall Meeting Abstracts; American Geophysical Union (AGU): Washington, DC, USA, 2010; p. PP21C-1704. [Google Scholar]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef]
- de María, P.D.; van Gemert, R.W.; Straathof, A.J.; Hanefeld, U. Biosynthesis of ethers: Unusual or common natural events? Nat. Prod. Rep. 2010, 27, 370–392. [Google Scholar] [CrossRef]
- Fuhrmann, E.; Talbiersky, J. Synthesis of alkyl aryl ethers by catalytic Williamson ether synthesis with weak alkylation agents. Org. Process Res. Dev. 2005, 9, 206–211. [Google Scholar] [CrossRef]
- Kpemissi, M.; Eklu-Gadegbeku, K.; Veerapur, V.P.; Potârniche, A.-V.; Adi, K.; Vijayakumar, S.; Banakar, S.M.; Thimmaiah, N.; Metowogo, K.; Aklikokou, K. Antioxidant and nephroprotection activities of Combretum micranthum: A phytochemical, in-vitro and ex-vivo studies. Heliyon 2019, 5, e01365. [Google Scholar] [CrossRef] [PubMed]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, R.J.; Mgedle, N.; Mavumengwana, V.; Oluwafemi, O.S. Biosynthesis of Smaller-Sized Platinum Nanoparticles Using the Leaf Extract of Combretum erythrophyllum and its Antibacterial Activities. Antibiotics 2021, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Tsay, Y.-F. Transport systems of mineral elements in plants: Transporters, regulation and utilization. Plant Cell Physiol. 2021, 62, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; Choudhary, P.; Parameswari, E.; Alhariri, A.; Samota, M.K.; Damale, R.D. Evaluation of nutritional, phytochemical, and mineral composition of selected medicinal plants for therapeutic uses from cold desert of Western Himalaya. Plants 2021, 10, 1429. [Google Scholar]
- Lovkova, M.Y.; Buzuk, G.; Sokolova, S.; Kliment’eva, N. Chemical features of medicinal plants. Appl. Biochem. Microbiol. 2001, 37, 229–237. [Google Scholar] [CrossRef]
- Kohzadi, S.; Shahmoradi, B.; Ghaderi, E.; Loqmani, H.; Maleki, A. Concentration, source, and potential human health risk of heavy metals in the commonly consumed medicinal plants. Biol. Trace Elem. Res. 2019, 187, 41–50. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 89–98. [Google Scholar] [CrossRef]
- Mtunzi, F.; Singo, T.; Pholosi, A.; Mzinyane, N.; Modise, J.; Sipamla, A. Investigation of the nutritive value and mineral elements of Combretum molle leaves. Pak. J. Nutr. 2012, 11, 176. [Google Scholar] [CrossRef]
- Aliyu, A.; Musa, A.; Oshanimi, J.; Ibrahim, H.; Oyewale, A. Phytochemical analyses and mineral elements composition of some medicinal plants of Northern Nigeria. Niger. J. Pharm. Sci. 2008, 7, 119–125. [Google Scholar]
- Anitha, R.; Sandhiya, T. Occurrence of calcium oxalate crystals in the leaves of medicinal plants. Int. J. Pharmacogn. 2014, 1, 389–393. [Google Scholar]
- Ekeke, C.; Agbagwa, I.O. Ergastic substances (calcium oxalate crystals) in the leaf of Combretum Loefl. (Combretaceae) species in Nigeria. Am. J. Plant Sci. 2014, 2014, 47704. [Google Scholar]
- Chen, W.; He, Z.L.; Yang, X.E.; Mishra, S.; Stoffella, P.J. Chlorine nutrition of higher plants: Progress and perspectives. J. Plant Nutr. 2010, 33, 943–952. [Google Scholar] [CrossRef]
- Amtmann, A.; Rubio, F. Potassium in Plants; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological Essence of Magnesium in Plants and Its Widespread Deficiency in the Farming System of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Yalcin, B.; Turan, S.; Saracoglu, I.A.; Karadeniz, S.; Yalcin, I.E.; Demir, G. Investigation of heavy metal level and mineral nutrient status in widely used medicinal plants’ leaves in Turkey: Insights into health implications. Biol. Trace Elem. Res. 2018, 182, 387–406. [Google Scholar] [CrossRef]
- Nkuba, L.L.; Mohammed, N.K. Heavy metals and essential elements in selected medicinal plants commonly used for medicine in Tanzania. Chem. Sci. Int. J. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Asuk, A.A.; Agiang, M.A.; Dasofunjo, K.; Willie, A.J. The biomedical significance of the phytochemical, proximate and mineral compositions of the leaf, stem bark and root of Jatropha curcas. Asian Pac. J. Trop. Biomed. 2015, 5, 650–657. [Google Scholar] [CrossRef]
- Anke, M.; Angelow, L.; Müller, R.; Anke, S. Recent progress in exploring the essentiality of the ultratrace element rubidium to the nutrition of animals and man. Biomed. Res. Trace Elem. 2005, 16, 203–207. [Google Scholar]
- Kosla, T.; Skibniewska, E.; Debski, B.; Urbanska-Slomka, G. Rubidium in the trophic chain soil-plants-animals. Trace Elem. Electrolytes 2002, 19, 171–176. [Google Scholar]
- Ujowundu, C.; Okafor, O.; Agha, N.; Nwaogu, L.; Igwe, K.; Igwe, C. Phytochemical and chemical composition of Combretum zenkeri leaves. J. Med. Plants Res. 2010, 4, 965–968. [Google Scholar]
- Pandavadra, M.; Chanda, S. Development of quality control parameters for the standardization of Limonia acidissima L. leaf and stem. Asian Pac. J. Trop. Med. 2014, 7, S244–S248. [Google Scholar] [CrossRef] [PubMed]
- Zalke, A.S.; Duraiswamy, B.; Gandagule, U.B. Pharmacognostical studies of leaves of Combretum albidum G. Don. Anc. Sci. Life 2013, 32, 187. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis, 1st ed.; Chapman and Hall Ltd.: London, UK, 1973. [Google Scholar]
- Trease, G.; Evans, W. Pharmacognosy, 13th ed.; Bailliere Tindall Ltd.: London, UK, 1989. [Google Scholar]
- Sofowora, A. Research on medicinal plants and traditional medicine in Africa. J. Altern. Complement. Med. 1996, 2, 365–372. [Google Scholar] [CrossRef] [PubMed]

| Solvent | Extract Yield (%) | |
|---|---|---|
| Leaf | Stem | |
| Hexane | 7.00 | 4.30 |
| Chloroform | 7.10 | 6.50 |
| Methanol | 12.90 | 11.30 |
| Phytocompound | Test | Extract | |||||
|---|---|---|---|---|---|---|---|
| Leaf | Stem | ||||||
| H | C | M | H | C | M | ||
| Alkaloids | Wagner’s test | + | + | ++ | + | + | ++ |
| Mayer’s test | + | + | + | + | + | + | |
| Flavonoids | Lead acetate | ++ | ++ | ++ | ++ | ++ | ++ |
| Alkaline reagent test | + | + | + | + | + | + | |
| Acid hydrolysis test | + | + | + | + | + | + | |
| Phenolic compounds | Ferric chloride test | + | + | + | + | + | + |
| Lead acetate test | + | + | + | + | + | + | |
| Polyphenols | Ferric chloride test | + | + | + | + | + | + |
| Terpenoids | Salkowski’s test | + | + | + | + | + | + |
| Liebermann–Burchard test | + | + | + | + | + | + | |
| Tannins | Ferric chloride test | + | + | + | + | + | + |
| Lead acetate test | + | + | + | + | + | + | |
| Gelatin test | + | + | + | + | + | + | |
| Coumarin | Sodium hydroxide test | + | + | + | + | + | + |
| Saponin | Foam test | + | + | + | + | + | + |
| Olive oil test | – | – | + | – | – | + | |
| Phytosterols | Salkowski’s test | + | + | + | + | + | + |
| Liebermann–Burchard test | + | + | + | + | + | + | |
| Gums and mucilage | Precipitation test | + | – | – | + | – | – |
| Ruthenium red test | + | + | + | + | + | + | |
| Resins | Acetone test | – | – | – | – | – | – |
| Glycosides | Keller–Killani test | – | – | – | – | – | – |
| Carbohydrates | Molisch’s test | + | + | + | + | + | + |
| Fehling’s test | + | + | + | + | + | + | |
| Amino acids and proteins | Ninhydrin test | + | + | + | + | + | + |
| Biuret test | + | + | + | + | + | + | |
| Lipids and fixed oils | Spot test | – | – | ++ | – | – | ++ |
| Sample | Peak Absorption Frequency (cm−1) | Intensity | Functional Group | Compound Class | Type of Vibration |
|---|---|---|---|---|---|
| Leaf | 3283.18 | strong, broad | O-H | alcohol | stretching |
| 2917.81 | medium | C-H | alkane | stretching | |
| 1617.72 | medium | N-H | primary amine | bending | |
| 1318.83 | medium | O-H | phenol | bending | |
| 1233.97 | strong | C-O | alkyl aryl ether | stretching | |
| 1032.32 | medium | C-N | primary amine | stretching | |
| 521.38 | strong | C-I | alkyl halide (halo compound) | stretching | |
| Stem | 3318.91 | strong, broad | O-H | alcohol | stretching |
| 1619.25 | medium | N-H | primary amine | bending | |
| 1317.13 | medium | O-H | phenol | bending | |
| 1032.68 | medium | C-N | primary amine | stretching | |
| 780.86 | strong | C-Cl | alkyl halide (halo compound) | stretching | |
| 516.39 | strong | C-I | alkyl halide (halo compound) | stretching |
| Element | Composition (%) | |
|---|---|---|
| Leaf | Stem | |
| C | 68.44 | 54.92 |
| O | 26.72 | 42.86 |
| Na | 0.13 | – |
| Mg | 0.93 | 0.43 |
| Cl | 0.96 | – |
| K | 0.71 | – |
| Ca | 1.87 | 1.70 |
| Mn | 0.12 | 0.09 |
| Rb | 0.10 | – |
| Treatment | Sample | |||
|---|---|---|---|---|
| Leaf | Stem | |||
| Brightfield | UV Light | Brightfield | UV Light | |
| Powder only (control) | Green | Multicoloured fluorescence | Brown | Green and light blue fluorescence |
| Powder and distilled water | Green | Green with blue and red fluorescence | Brown | Orange with blue and purple fluorescence |
| Powder and hexane | Green | Green with pink and blue fluorescence | Brown | Green with blue and gold fluorescence |
| Powder and chloroform | Green | Blue, pink, green and purple fluorescence | Brown | Multicoloured fluorescence |
| Powder and methanol | Green | Green with blue and orange fluorescence | Brown | Green, blue and purple fluorescence |
| Powder and ethanol | Green | Green with multicoloured fluorescence | Brown | Multicoloured fluorescence |
| Powder and acetic acid | Green | Pink, purple and orange fluorescence | Brown | Brown with blue and purple fluorescence |
| Powder and sodium hydroxide | Green | Blue and red fluorescence | Brown | Blue and turquoise fluorescence |
| Powder and sulphuric acid | Green | Blue, purple and pink fluorescence | Brown | Blue fluorescence |
| Powder and hydrochloric acid | Green | Green with yellow and blue fluorescence | Brown | Green with yellow and blue fluorescence |
| Powder and acetone | Green | Gold with blue and orange fluorescence | Brown | Gold with blue and orange fluorescence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parusnath, M.; Naidoo, Y.; Singh, M.; Rihan, H.; Dewir, Y.H. Phytochemical Composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels Leaf and Stem Extracts. Plants 2023, 12, 1702. https://doi.org/10.3390/plants12081702
Parusnath M, Naidoo Y, Singh M, Rihan H, Dewir YH. Phytochemical Composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels Leaf and Stem Extracts. Plants. 2023; 12(8):1702. https://doi.org/10.3390/plants12081702
Chicago/Turabian StyleParusnath, Myuri, Yougasphree Naidoo, Moganavelli Singh, Hail Rihan, and Yaser Hassan Dewir. 2023. "Phytochemical Composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels Leaf and Stem Extracts" Plants 12, no. 8: 1702. https://doi.org/10.3390/plants12081702
APA StyleParusnath, M., Naidoo, Y., Singh, M., Rihan, H., & Dewir, Y. H. (2023). Phytochemical Composition of Combretum molle (R. Br. ex G. Don.) Engl. & Diels Leaf and Stem Extracts. Plants, 12(8), 1702. https://doi.org/10.3390/plants12081702










