A Genome-Wide Association Study of Protein, Oil, and Amino Acid Content in Wild Soybean (Glycine soja)
Abstract
1. Introduction
2. Results
2.1. Phenotypic Variation and Correlation Analysis
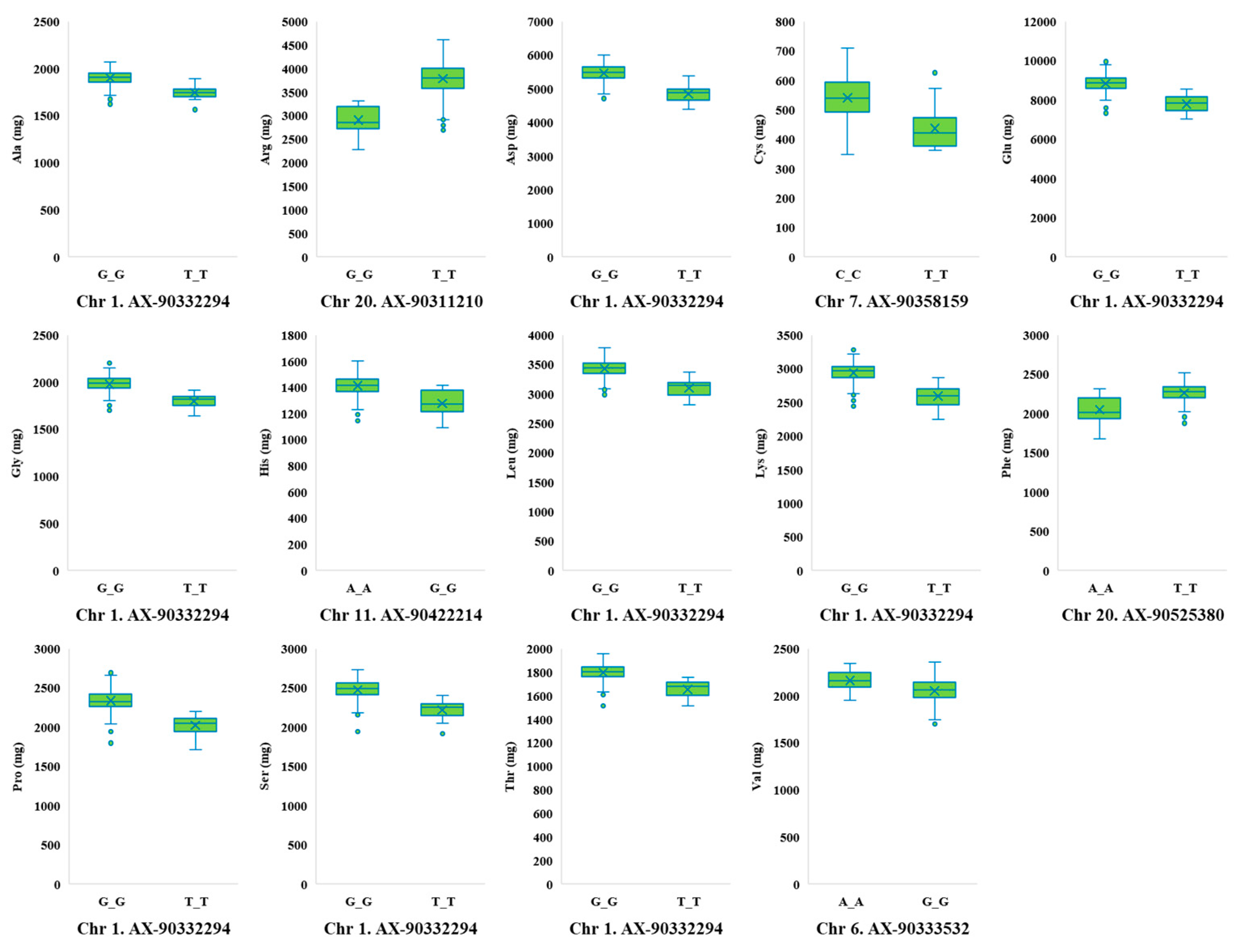
2.2. Genome-Wide Association Study for Protein, Oil, and Amino Acid Content
2.3. Candidate Genes for Trait-Associated SNP Markers
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Field Management
4.2. Analysis of Protein, Oil, and Amino Acid Content
4.3. DNA Extraction and SNP Genotyping
4.4. Genome-Wide Association Study and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panthee, D.R.; Pantalone, V.R.; Saxton, A.M.; West, D.R.; Sams, C.E. Genomic regions associated with amino acid composition in soybean. Mol. Breed. 2006, 17, 79–89. [Google Scholar] [CrossRef]
- Hyten, D.L.; Song, Q.; Zhu, Y.; Choi, I.-Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef]
- Kuroda, Y.; Tomooka, N.; Kaga, A.; Wanigadeva, S.M.S.W.; Vaughan, D.A. Genetic diversity of wild soybean (Glycine soja Sieb. et Zucc.) and Japanese cultivated soybeans [G. max (L.) Merr.] based on microsatellite (SSR) analysis and the selection of a core collection. Genet. Resour. Crop Evol. 2009, 56, 1045–1055. [Google Scholar] [CrossRef]
- Lee, J.-D.; Yu, J.-K.; Hwang, Y.-H.; Blake, S.; So, Y.-S.; Lee, G.-J.; Nguyen, H.T.; Shannon, J.G. Genetic diversity of wild soybean (Glycine soja Sieb. and Zucc.) accessions from South Korea and other countries. Crop Sci. 2008, 48, 606–616. [Google Scholar] [CrossRef]
- Liu, K. Chemistry and Nutritional Value of Soybean Components. In Soybeans: Chemistry, Technology, and Utilization; Springer: Berlin, Germany, 1997; pp. 25–113. [Google Scholar]
- Chen, Y.; Nelson, R. Genetic variation and relationships among cultivated, wild, and semiwild soybean. Crop Sci. 2004, 44, 316–325. [Google Scholar] [CrossRef]
- Kofsky, J.; Zhang, H.; Song, B.-H. The untapped genetic reservoir: The past, current, and future applications of the wild soybean (Glycine soja). Front. Plant Sci. 2018, 9, 949. [Google Scholar] [CrossRef]
- Leamy, L.J.; Leamy, L.J.; Zhang, H.; Li, C.; Chen, C.Y.; Song, B.-H. A genome-wide association study of seed composition traits in wild soybean (Glycine soja). BMC Genom. 2017, 18, 18. [Google Scholar] [CrossRef]
- Goldflus, F.; Ceccantini, M.; Santos, W. Amino acid content of soybean samples collected in different Brazilian states: Harvest 2003/2004. Braz. J. Poult. Sci. 2006, 8, 105–111. [Google Scholar] [CrossRef]
- La, T.; Large, E.; Taliercio, E.; Song, Q.; Gillman, J.D.; Xu, D.; Nguyen, H.T.; Shannon, G.; Scaboo, A. Characterization of select wild soybean accessions in the USDA germplasm collection for seed composition and agronomic traits. Crop Sci. 2019, 59, 233–251. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kang, S.-T. Identification of Quantitative Trait Loci (QTLs) Associated with Oil and Protein Contents in Soybean (Glycine max L.). J. Life Sci. 2004, 14, 453–458. [Google Scholar]
- Kim, H.; Kang, S.; Choung, M.; Jung, C.; Oh, K.; Baek, I.; Son, B. Simple sequence repeat markers linked to quantitative trait loci controlling seed weight, protein and oil contents in soybean. J. Life Sci. 2006, 16, 949–954. [Google Scholar]
- Diers, B.W.; Keim, P.; Fehr, W.R.; Shoemaker, R.C. RFLP analysis of soybean seed protein and oil content. Theor. Appl. Genet. 1992, 83, 608–612. [Google Scholar] [CrossRef]
- Chung, J.; Babka, H.L.; Graef, G.L.; Staswick, P.E.; Lee, D.J.; Cregan, P.B.; Shoemaker, R.C.; Specht, J.E. The seed protein, oil, and yield QTL on soybean linkage group I. Crop Sci. 2003, 43, 1053–1067. [Google Scholar] [CrossRef]
- Nichols, D.M.; Glover, K.D.; Carlson, S.R.; Specht, J.E.; Diers, B.W. Fine mapping of a seed protein QTL on soybean linkage group I and its correlated effects on agronomic traits. Crop Sci. 2006, 46, 834–839. [Google Scholar] [CrossRef]
- Rodrigues, J.I.d.S.; Miranda, F.D.d.; Ferreira, A.; Borges, L.L.; Ferreira, M.F.d.S.; Good-God, P.I.V.; Piovesan, N.D.; Barros, E.G.d.; Cruz, C.D.; Moreira, M.A. Mapping QTL for protein and oil content in soybean. Pesqui. Agropecuária Bras. 2010, 45, 472–480. [Google Scholar] [CrossRef]
- Sebolt, A.M.; Shoemaker, R.C.; Diers, B.W. Analysis of a quantitative trait locus allele from wild soybean that increases seed protein concentration in soybean. Crop Sci. 2000, 40, 1438–1444. [Google Scholar] [CrossRef]
- Warrington, C.V.; Abdel-Haleem, H.; Hyten, D.L.; Cregan, P.B.; Orf, J.H.; Killam, A.S.; Bajjalieh, N.; Li, Z.; Boerma, H.R. QTL for seed protein and amino acids in the Benning× Danbaekkong soybean population. Theor. Appl. Genet. 2015, 128, 839–850. [Google Scholar] [CrossRef]
- Kim, M.; Schultz, S.; Nelson, R.L.; Diers, B.W. Identification and fine mapping of a soybean seed protein QTL from PI 407788A on chromosome 15. Crop Sci. 2016, 56, 219–225. [Google Scholar] [CrossRef]
- Fliege, C.E.; Ward, R.A.; Vogel, P.; Nguyen, H.; Quach, T.; Guo, M.; Viana, J.P.G.; dos Santos, L.B.; Specht, J.E.; Clemente, T.E.; et al. Fine mapping and cloning of the major seed protein quantitative trait loci on soybean chromosome 20. Plant J. 2022, 110, 114–128. [Google Scholar] [CrossRef]
- Lee, S.; Van, K.; Sung, M.; Nelson, R.; LaMantia, J.; McHale, L.K.; Mian, M.A.R. Genome-wide association study of seed protein, oil and amino acid contents in soybean from maturity groups I to IV. Theor. Appl. Genet. 2019, 132, 1639–1659. [Google Scholar] [CrossRef]
- Imsande, J. Selection of soybean mutants with increased concentrations of seed methionine and cysteine. Crop Sci. 2001, 41, 510–515. [Google Scholar] [CrossRef]
- Panthee, D.R.; Kwanyuen, P.; Sams, C.E.; West, D.R.; Saxton, A.M.; Pantalone, V.R. Quantitative trait loci for β-conglycinin (7S) and glycinin (11S) fractions of soybean storage protein. J. Am. Oil Chem. Soc. 2004, 81, 1005–1012. [Google Scholar] [CrossRef]
- Kim, W.J.; Kang, B.H.; Moon, C.Y.; Kang, S.; Shin, S.; Chowdhury, S.; Jeong, S.-C.; Choi, M.-S.; Park, S.-K.; Moon, J.-K.; et al. Genome-Wide Association Study for Agronomic Traits in Wild Soybean (Glycine soja). Agronomy 2023, 13, 739. [Google Scholar] [CrossRef]
- Chotekajorn, A.; Hashiguchi, T.; Hashiguchi, M.; Tanaka, H.; Akashi, R. Evaluation of seed amino acid content and its correlation network analysis in wild soybean (Glycine soja) germplasm in Japan. Plant Genet. Resour. 2021, 19, 35–43. [Google Scholar] [CrossRef]
- Ruta, V.; Longo, C.; Lepri, A.; De Angelis, V.; Occhigrossi, S.; Costantino, P.; Vittorioso, P. The DOF transcription factors in seed and seedling development. Plants 2020, 9, 218. [Google Scholar] [CrossRef]
- Ward, J.M.; Cufr, C.A.; Denzel, M.A.; Neff, M.M. The Dof transcription factor OBP3 modulates phytochrome and cryptochrome signaling in Arabidopsis. Plant Cell 2005, 17, 475–485. [Google Scholar] [CrossRef]
- Amable, R.A.; Obendorf, R.L. Soybean seed respiration during simulated preharvest deterioration. J. Exp. Bot. 1986, 37, 1364–1375. [Google Scholar] [CrossRef]
- Rizal, G.; Karki, S. Alcohol dehydrogenase (ADH) activity in soybean (Glycine max [L.] Merr.) under flooding stress. Electron. J. Plant Breed. 2011, 2, 50–57. [Google Scholar]
- Xu, X.P.; Liu, H.; Tian, L.; Dong, X.B.; Shen, S.H.; Qu, L.Q. Integrated and comparative proteomics of high-oil and high-protein soybean seeds. Food Chem. 2015, 172, 105–116. [Google Scholar] [CrossRef]
- Cao, J. Analysis of the prefoldin gene family in 14 plant species. Front. Plant Sci. 2016, 7, 317. [Google Scholar] [CrossRef]
- Nillegoda, N.B.; Kirstein, J.; Szlachcic, A.; Berynskyy, M.; Stank, A.; Stengel, F.; Arnsburg, K.; Gao, X.; Scior, A.; Aebersold, R.; et al. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 2015, 524, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Siegert, R.; Leroux, M.R.; Scheufler, C.; Hartl, F.; Moarefi, I. Structure of the molecular chaperone prefoldin: Unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell 2000, 103, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B. Engineering soybean for enhanced sulfur amino acid content. Crop Sci. 2005, 45, 454–461. [Google Scholar] [CrossRef]
- de la Torre, F.; El-Azaz, J.; Avila, C.; Cánovas, F. Deciphering the role of aspartate and prephenate aminotransferase activities in plastid nitrogen metabolism. Plant Physiol. 2014, 164, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kang, B.H.; Moon, C.Y.; Kang, S.; Shin, S.; Chowdhury, S.; Choi, M.-S.; Park, S.-K.; Moon, J.-K.; Ha, B.-K. Quantitative Trait Loci (QTL) Analysis of Seed Protein and Oil Content in Wild Soybean (Glycine soja). Int. J. Mol. Sci. 2023, 24, 4077. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-G.; Jeong, N.; Kim, J.H.; Lee, K.; Kim, K.H.; Pirani, A.; Ha, B.-K.; Kang, S.-T.; Park, B.-S.; Moon, J.-K.; et al. Development, validation and genetic analysis of a large soybean SNP genotyping array. Plant J. 2015, 81, 625–636. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 0.7. 6. Comput. Softw. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 19 January 2023).
- Browning, B.L.; Browning, S.R. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am. J. Hum. Genet. 2009, 84, 210–223. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Y.; Hou, L.; Ma, J.; Chen, C.; Ai, H.; Huang, L.; Ren, J. Genome-wide detection of genetic markers associated with growth and fatness in four pig populations using four approaches. Genet. Sel. Evol. 2017, 49, 21. [Google Scholar] [CrossRef]
- Kim, B.; Dai, X.; Zhang, W.; Zhuang, Z.; Sanchez, D.L.; Lübberstedt, T.; Kang, Y.; Udvardi, M.K.; Beavis, W.D.; Xu, S.; et al. GWASpro: A high-performance genome-wide association analysis server. Bioinformatics 2019, 35, 2512–2514. [Google Scholar] [CrossRef]
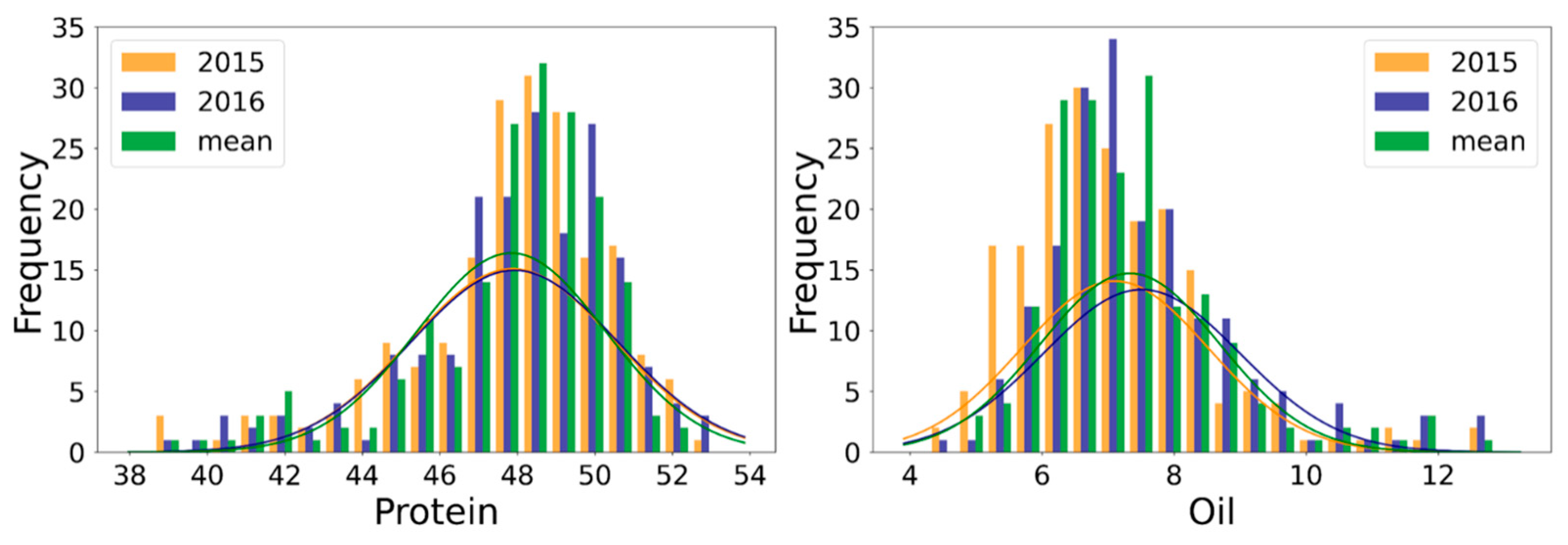
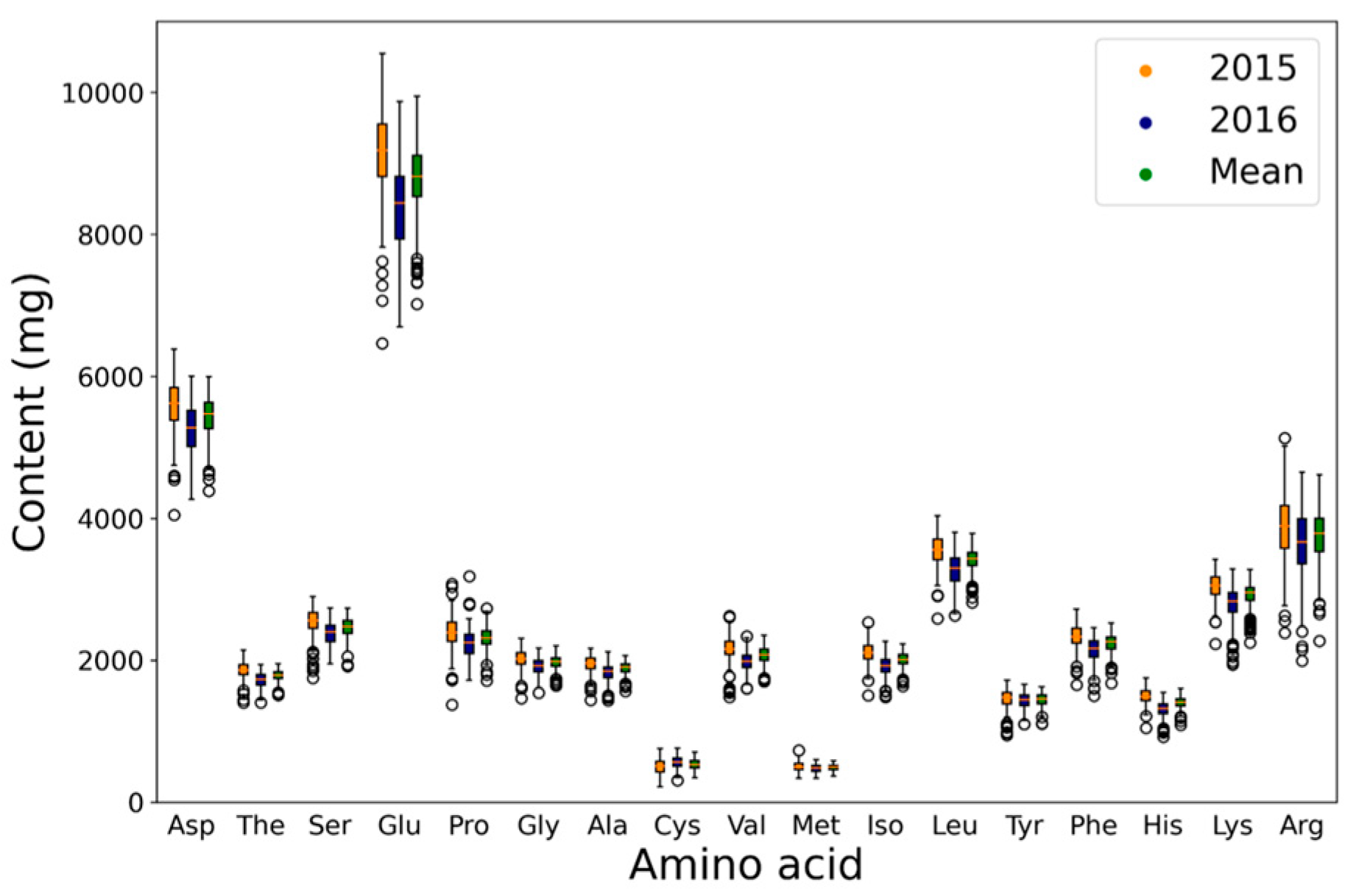

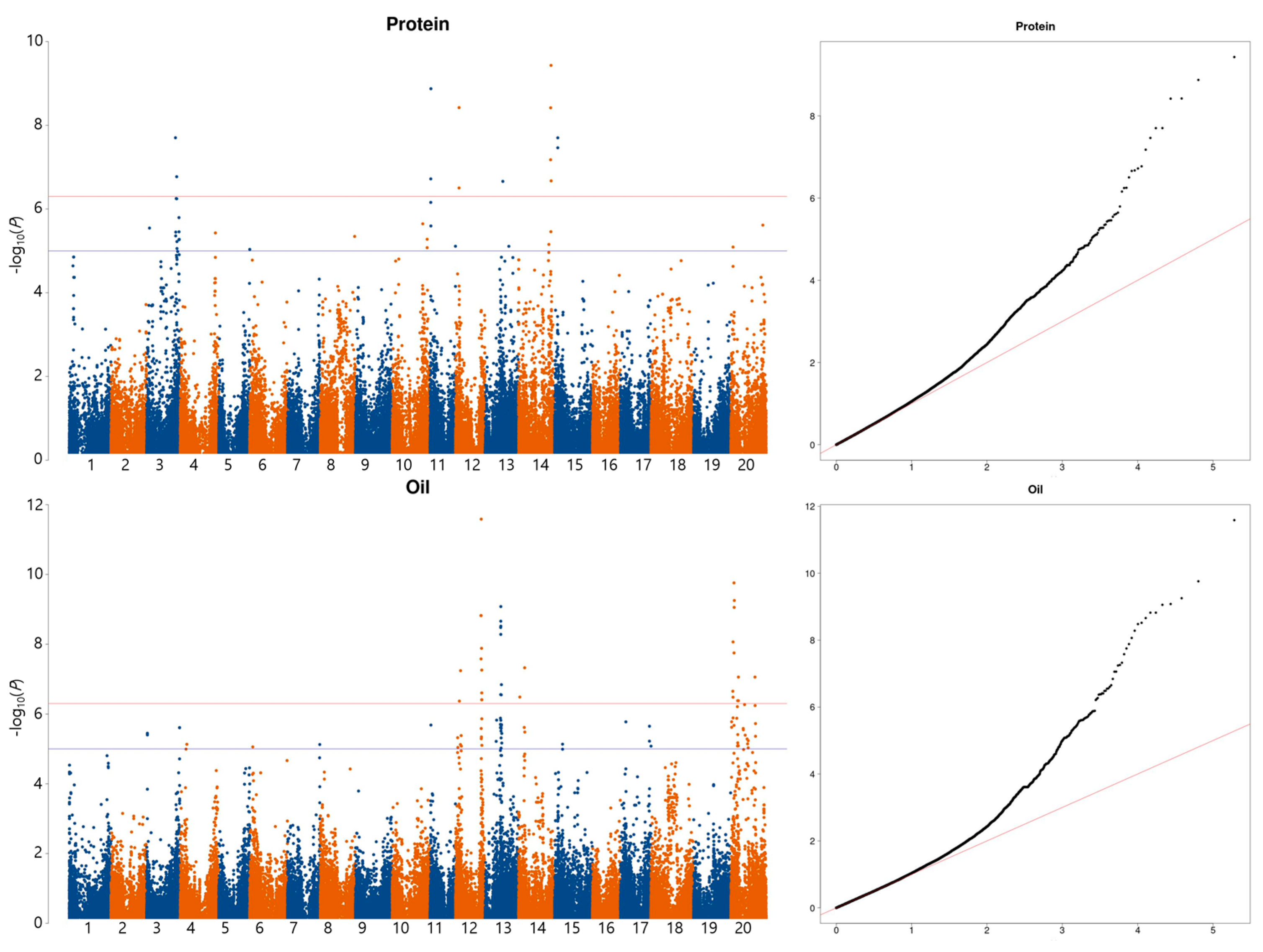
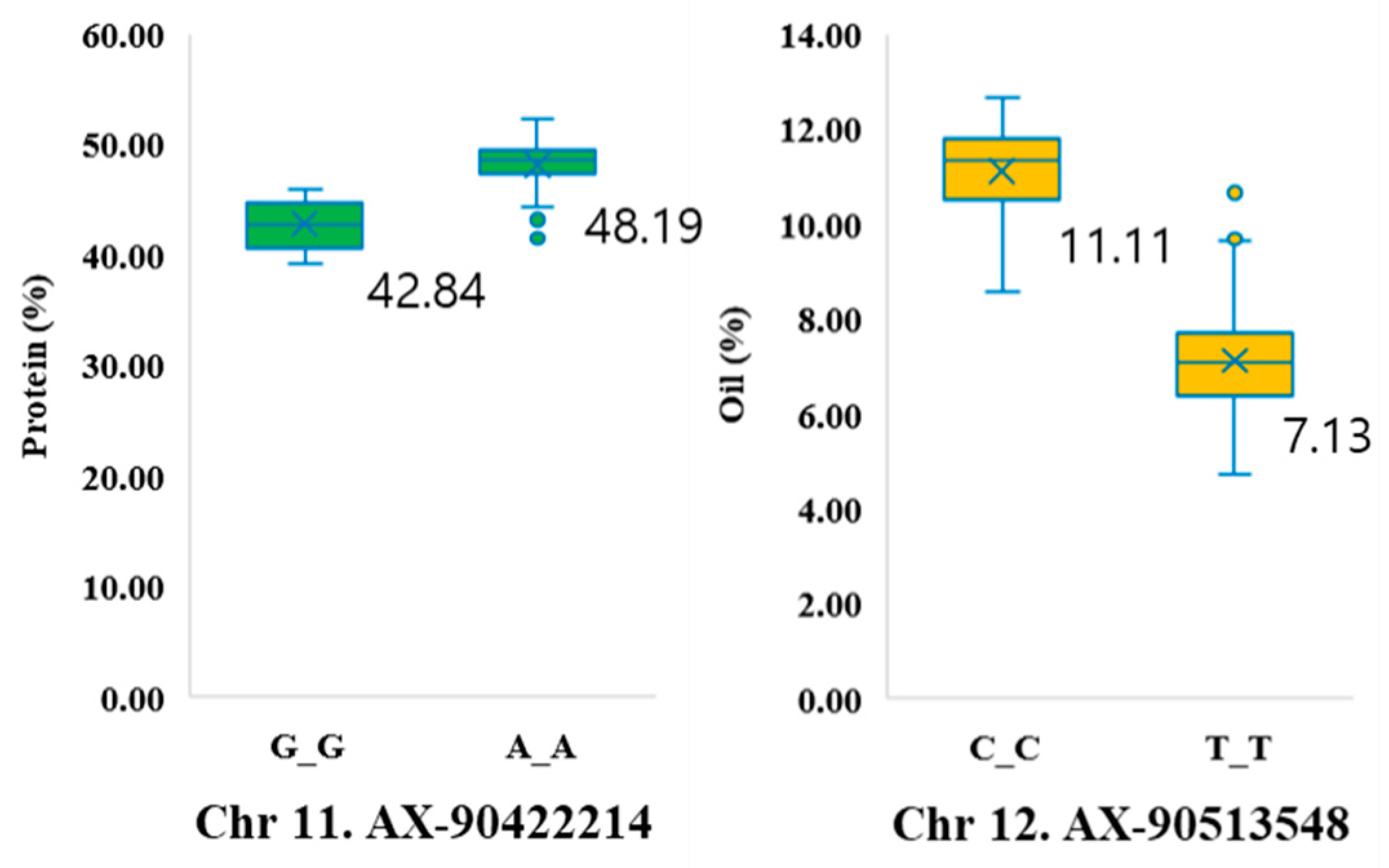
| Trait | Env. | Min. | Max. | SD | Mean | CV (%) | Skew | Kur. | h2 |
|---|---|---|---|---|---|---|---|---|---|
| Protein (%) | 2015 | 38.61 | 52.61 | 2.64 | 47.88 | 5.5 | −1.05 | 1.16 | 0.78 |
| 2016 | 38.83 | 53.21 | 2.66 | 47.92 | 5.6 | −1.01 | 1.20 | ||
| Mean | 39.17 | 52.27 | 2.43 | 47.84 | 5.1 | −1.26 | 1.33 | ||
| Oil (%) | 2015 | 4.29 | 12.86 | 1.42 | 7.11 | 19.9 | 1.16 | 2.35 | 0.83 |
| 2016 | 4.66 | 12.69 | 1.49 | 7.51 | 19.8 | 1.20 | 1.88 | ||
| Mean | 4.74 | 12.68 | 1.36 | 7.33 | 18.5 | 1.33 | 2.52 |
| Trait | Env. | Min. | Max. | SD | Mean | CV (%) | Skew | Kur. | h2 |
|---|---|---|---|---|---|---|---|---|---|
| Asp | 2015 | 4047 | 6381 | 367 | 5592 | 6.6 | −0.65 | 1.19 | 0.59 |
| (mg/g) | 2016 | 4265 | 6001 | 371 | 5242 | 7.1 | −0.50 | −0.31 | |
| Mean | 4386 | 5994 | 304 | 5417 | 5.6 | −0.79 | 0.51 | ||
| Thr | 2015 | 1406 | 2142 | 114 | 1859 | 6.1 | −0.68 | 1.54 | 0.46 |
| 2016 | 1404 | 1939 | 110 | 1716 | 6.4 | −0.53 | −0.23 | ||
| Mean | 1512 | 1954 | 87 | 1788 | 4.9 | −0.54 | 0.61 | ||
| Ser | 2015 | 1751 | 2898 | 208 | 2531 | 8.2 | −1.17 | 1.69 | 0.47 |
| 2016 | 1950 | 2736 | 170 | 2377 | 7.2 | −0.36 | −0.44 | ||
| Mean | 1915 | 2733 | 148 | 2454 | 6.0 | −0.76 | 0.69 | ||
| Glu | 2015 | 6462 | 10,548 | 641 | 9138 | 7.0 | −0.71 | 1.37 | 0.55 |
| 2016 | 6694 | 9872 | 644 | 8373 | 7.7 | −0.46 | −0.30 | ||
| Mean | 7019 | 9947 | 521 | 8754 | 5.9 | −0.82 | 0.69 | ||
| Pro | 2015 | 1372 | 3075 | 222 | 2390 | 9.3 | −0.60 | 2.71 | 0.45 |
| 2016 | 1718 | 3184 | 201 | 2237 | 9.0 | 0.31 | 2.08 | ||
| Mean | 1714 | 2730 | 164 | 2314 | 7.1 | −0.52 | 1.05 | ||
| Gly | 2015 | 1462 | 2311 | 124 | 2021 | 6.2 | −0.86 | 1.92 | 0.53 |
| 2016 | 1541 | 2171 | 119 | 1908 | 6.2 | −0.53 | −0.06 | ||
| Mean | 1644 | 2200 | 99 | 1965 | 5.0 | −0.75 | 0.45 | ||
| Ala | 2015 | 1443 | 2169 | 120 | 1946 | 6.2 | −0.91 | 1.32 | 0.44 |
| 2016 | 1437 | 2120 | 125 | 1826 | 6.8 | −0.80 | 0.74 | ||
| Mean | 1564 | 2066 | 95 | 1887 | 5.0 | −0.81 | 0.54 | ||
| Cys | 2015 | 223 | 757 | 102 | 503 | 20.2 | −0.33 | −0.35 | 0.47 |
| 2016 | 310 | 764 | 84 | 558 | 15.0 | −0.36 | −0.22 | ||
| Mean | 348 | 709 | 74 | 531 | 14.0 | −0.19 | −0.34 | ||
| Val | 2015 | 1480 | 2625 | 188 | 2163 | 8.7 | −0.74 | 2.37 | 0.46 |
| 2016 | 1599 | 2338 | 143 | 1980 | 7.2 | −0.24 | 0.02 | ||
| Mean | 1700 | 2354 | 132 | 2071 | 6.4 | −0.50 | 0.24 | ||
| Met | 2015 | 339 | 735 | 62 | 499 | 12.5 | 0.31 | 0.35 | 0.35 |
| 2016 | 341 | 602 | 56 | 478 | 11.8 | −0.02 | −0.51 | ||
| Mean | 369 | 584 | 44 | 489 | 9.0 | −0.05 | −0.38 | ||
| Iso | 2015 | 1505 | 2536 | 148 | 2107 | 7.0 | −0.14 | 1.07 | 0.53 |
| 2016 | 1480 | 2269 | 143 | 1904 | 7.5 | −0.51 | 0.17 | ||
| Mean | 1638 | 2231 | 117 | 2006 | 5.8 | −0.56 | 0.13 | ||
| Leu | 2015 | 2587 | 4035 | 220 | 3546 | 6.2 | −0.69 | 1.43 | 0.50 |
| 2016 | 2632 | 3802 | 228 | 3269 | 7.0 | −0.43 | −0.25 | ||
| Mean | 2817 | 3784 | 177 | 3407 | 5.2 | −0.78 | 0.55 | ||
| Tyr | 2015 | 947 | 1719 | 137 | 1460 | 9.4 | −1.01 | 1.85 | 0.48 |
| 2016 | 1101 | 1660 | 109 | 1429 | 7.6 | −0.69 | 0.16 | ||
| Mean | 1106 | 1627 | 98 | 1445 | 6.8 | −0.91 | 1.11 | ||
| Phe | 2015 | 1657 | 2722 | 163 | 2338 | 7.0 | −0.65 | 1.36 | 0.60 |
| 2016 | 1501 | 2459 | 181 | 2140 | 8.5 | −0.85 | 0.72 | ||
| Mean | 1677 | 2522 | 142 | 2238 | 6.3 | −0.97 | 1.12 | ||
| His | 2015 | 1048 | 1752 | 108 | 1496 | 7.2 | −0.24 | 0.96 | 0.54 |
| 2016 | 922 | 1545 | 114 | 1308 | 8.7 | −0.81 | 0.82 | ||
| Mean | 1090 | 1603 | 90 | 1403 | 6.4 | −0.68 | 0.66 | ||
| Lys | 2015 | 2235 | 3420 | 186 | 3048 | 6.1 | −0.64 | 1.15 | 0.50 |
| 2016 | 1943 | 3285 | 254 | 2777 | 9.2 | −1.12 | 1.20 | ||
| Mean | 2249 | 3279 | 177 | 2913 | 6.1 | −1.04 | 1.07 | ||
| Arg | 2015 | 2387 | 5128 | 451 | 3873 | 11.6 | −0.28 | 0.52 | 0.71 |
| 2016 | 1993 | 4648 | 486 | 3622 | 13.4 | −0.64 | 0.54 | ||
| Mean | 2273 | 4613 | 411 | 3747 | 11.0 | −0.63 | 0.60 |
| Trait | SNP | Chr | Position | −log10(P) | Reference + | Minor | Major | MAF |
|---|---|---|---|---|---|---|---|---|
| Protein | AX-90486230 | 3 | 39,216,582 | 7.70 | C | C | T | 0.05 |
| AX-90422214 | 11 | 1,067,467 | 8.87 | G | G | A | 0.06 | |
| AX-90436656 | 12 | 4,681,230 | 8.42 | T | T | A | 0.05 | |
| AX-90336510 | 13 | 24,160,907 | 6.66 | C | C | T | 0.05 | |
| AX-90450715 | 14 | 44,061,946 | 9.43 | T | T | G | 0.06 | |
| AX-90368184 | 15 | 4,332,019 | 7.70 | T | T | A | 0.07 | |
| Oil | AX-90408186 | 12 | 6,713,247 | 7.24 | A | A | C | 0.06 |
| AX-90513548 | 12 | 34,460,746 | 8.82 | C | C | T | 0.06 | |
| AX-90440743 | 13 | 21,445,098 | 9.08 | C | C | T | 0.08 | |
| AX-90525501 | 14 | 8,179,883 | 7.32 | T | T | A | 0.08 | |
| AX-90387626 | 20 | 4,185,011 | 9.76 | C | C | G | 0.06 | |
| AX-90339137 | 20 | 10,234,522 | 7.06 | A | A | C | 0.05 | |
| AX-90513791 | 20 | 32,766,317 | 7.06 | T | T | C | 0.05 |
| Trait | SNP | Chr | Position | −log10(P) | Reference + | Minor | Major | MAF |
|---|---|---|---|---|---|---|---|---|
| Ala | AX-90332294 | 1 | 6,633,674 | 5.19 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 6.73 | G | G | T | 0.08 | |
| AX-90390655 | 12 | 5,348,412 | 6.39 | G | G | T | 0.16 | |
| AX-90434969 | 15 | 1,252,401 | 5.87 | A | A | G | 0.15 | |
| Arg | AX-90392478 | 7 | 36,935,345 | 5.15 | T | C | T | 0.10 |
| AX-90422214 | 11 | 1,067,467 | 6.20 | G | G | A | 0.06 | |
| AX-90311210 | 20 | 2,947,656 | 5.42 | G | G | T | 0.05 | |
| Asp | AX-90332294 | 1 | 6,633,674 | 6.03 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 6.77 | G | G | T | 0.08 | |
| Cys | AX-90358159 | 7 | 18,191,224 | 5.12 | T | T | C | 0.10 |
| Glu | AX-90332294 | 1 | 6,633,674 | 6.16 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 6.67 | G | G | T | 0.08 | |
| Gly | AX-90332294 | 1 | 6,633,674 | 5.50 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 6.01 | G | G | T | 0.08 | |
| His | AX-90422214 | 11 | 1,067,467 | 5.10 | G | G | A | 0.06 |
| Leu | AX-90332294 | 1 | 6,633,674 | 6.41 | G | T | G | 0.07 |
| AX-90397199 | 3 | 43,985,440 | 6.67 | C | C | T | 0.08 | |
| Lys | AX-90332294 | 1 | 6,633,674 | 6.02 | G | T | G | 0.07 |
| AX-90485849 | 1 | 53,039,571 | 5.63 | T | T | C | 0.08 | |
| AX-90397199 | 3 | 43,985,440 | 6.00 | C | C | T | 0.08 | |
| AX-90440743 | 13 | 21,445,098 | 6.08 | C | C | T | 0.08 | |
| AX-90525380 | 20 | 2,448,280 | 6.48 | A | A | T | 0.10 | |
| Phe | AX-90525380 | 20 | 2,448,280 | 5.44 | A | A | T | 0.10 |
| Pro | AX-90332294 | 1 | 6,633,674 | 6.53 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 7.58 | G | G | T | 0.08 | |
| AX-90374011 | 19 | 43,104,938 | 5.39 | A | A | G | 0.27 | |
| Ser | AX-90332294 | 1 | 6,633,674 | 5.43 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 5.57 | G | G | T | 0.08 | |
| Thr | AX-90332294 | 1 | 6,633,674 | 5.50 | G | T | G | 0.07 |
| AX-90522787 | 3 | 43,983,721 | 6.26 | G | G | T | 0.08 | |
| Val | AX-90333532 | 6 | 4,923,700 | 5.10 | G | A | G | 0.15 |
| Trait | SNP | Chr | Position | Candidate Gene + | Location (bp) | Gene Description |
|---|---|---|---|---|---|---|
| Protein | AX-90422214 | 11 | 1,067,467 | Glyma.11g015500 | 1,066,054..1,068,916 | RNA-binding (RRM/RBD/RNP motifs) family protein |
| AX-90436656 | 12 | 4,681,230 | Glyma.12g063800 | 4,680,402..4,682,246 | OBF-binding protein 3 | |
| AX-90336510 | 13 | 24,160,907 | Glyma.13g128700 | 24,151,824..24,170,639 | Myosin 2 | |
| AX-90450715 | 14 | 44,061,946 | Glyma.14g179200 | 44,060,802..44,063,722 | 10-formyltetrahydrofolate | |
| AX-90368184 | 15 | 4,332,019 | Glyma.15g055200 | 4,330,953..4,332,402 | F-box and associated interaction domain-containing protein | |
| Oil | AX-90513548 | 12 | 34,460,746 | Glyma.12g183500 | 34,455,733..34,463,207 | Homolog of histone chaperone HIRA |
| AX-90440743 | 13 | 21,445,098 | Glyma.13g099400 | 21,442,358..21,445,246 | Metaxin-related | |
| AX-90525501 | 14 | 8,179,883 | Glyma.14g089900 | 8,175,666..8,180,225 | SWIM zinc finger family protein | |
| AX-90387626 | 20 | 4,185,011 | Glyma.20g032400 | 4,183,317..4,191,520 | EXS (ERD1/XPR/SYG1) family protein | |
| AX-90339137 | 20 | 10,234,522 | Glyma.20g050300 | 10,226,320..10,241,515 | Zinc-binding alcohol dehydrogenase family protein | |
| AX-90513791 | 20 | 32,766,317 | Glyma.20g087700 | 32,765,127..32,768,858 | Protein of unknown function (DUF668) |
| Trait | SNP | Chr | Position | Candidate Gene + | Location (bp) | Gene Description |
|---|---|---|---|---|---|---|
| Ala, Asp, Glu, Gly, Leu, Lys, Pro, Ser, Thr | AX-90332294 | 1 | 6,633,674 | Glyma.01g053100 | 6,620,086..6,625,502 | Translation initiation factor 2C (eIF-2C) and related proteins |
| Glyma.01g053200 | 6,632,524..6,638,087 | Prefoldin chaperone subunit family protein | ||||
| Glyma.01g053900 | 6,835,875..6,836,372 | Aconitase | ||||
| Ala, Asp, Glu, Gly, Leu, Lys, Pro, Ser, Thr | AX-90522787 | 3 | 43,983,721 | Glyma.03g239700 | 43,835,476..43,838,866 | Aspartyl protease/7S seed globulin precursor |
| Glyma.03g240100 | 43,884,764..43,895,501 | D-amino acid aminotransferase-like PLP-dependent enzyme superfamily protein | ||||
| Glyma.03g242400 | 44,043,853..44,051,476 | Shikimate dehydrogenase | ||||
| Val | AX-90333532 | 6 | 4,923,700 | Glyma.06g061700 | 4,644,103..4,646,096 | Chorismate mutase |
| Glyma.06g064700 | 4,921,259..4,925,737 | Xanthine/uracil permease family protein | ||||
| Arg, His | AX-90422214 | 11 | 1,067,467 | Glyma.11g014900 | 1,029,017..1,031,439 | Acyl-activating enzyme |
| Glyma.11g015500 | 1,066,054..1,068,916 | RNA-binding (RRM/RBD/RNP motifs) family | ||||
| Ala | AX-90390655 | 12 | 5,348,412 | Glyma.12g072500 | 5,344,610..5,350,276 | Arogenate dehydratase 2 |
| Lys | AX-90440743 | 13 | 21,445,098 | Glyma.13g099400 | 21,442,358..21,445,246 | Metaxin-related |
| Ala | AX-90434969 | 15 | 1,252,401 | Glyma.15g012300 | 980,507..987,672 | Aminotransferase |
| Glyma.15g015800 | 1,250,483..1,253,447 | |||||
| Pro | AX-90374011 | 19 | 43,104,938 | Glyma.19g164800 | 42,559,312..42,561,641 | Glycinin subunit G7 |
| Glyma.19g164900 | 42,567,390..42,570,310 | Glycinin A1bB2-784 | ||||
| Arg, Lys, Phe | AX-90311210 | 20 | 2,947,656 | Glyma.20g025400 | 2,768,770..2,781,380 | Asparagine synthase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.J.; Kang, B.H.; Kang, S.; Shin, S.; Chowdhury, S.; Jeong, S.-C.; Choi, M.-S.; Park, S.-K.; Moon, J.-K.; Ryu, J.; et al. A Genome-Wide Association Study of Protein, Oil, and Amino Acid Content in Wild Soybean (Glycine soja). Plants 2023, 12, 1665. https://doi.org/10.3390/plants12081665
Kim WJ, Kang BH, Kang S, Shin S, Chowdhury S, Jeong S-C, Choi M-S, Park S-K, Moon J-K, Ryu J, et al. A Genome-Wide Association Study of Protein, Oil, and Amino Acid Content in Wild Soybean (Glycine soja). Plants. 2023; 12(8):1665. https://doi.org/10.3390/plants12081665
Chicago/Turabian StyleKim, Woon Ji, Byeong Hee Kang, Sehee Kang, Seoyoung Shin, Sreeparna Chowdhury, Soon-Chun Jeong, Man-Soo Choi, Soo-Kwon Park, Jung-Kyung Moon, Jaihyunk Ryu, and et al. 2023. "A Genome-Wide Association Study of Protein, Oil, and Amino Acid Content in Wild Soybean (Glycine soja)" Plants 12, no. 8: 1665. https://doi.org/10.3390/plants12081665
APA StyleKim, W. J., Kang, B. H., Kang, S., Shin, S., Chowdhury, S., Jeong, S.-C., Choi, M.-S., Park, S.-K., Moon, J.-K., Ryu, J., & Ha, B.-K. (2023). A Genome-Wide Association Study of Protein, Oil, and Amino Acid Content in Wild Soybean (Glycine soja). Plants, 12(8), 1665. https://doi.org/10.3390/plants12081665







