Abstract
The intracellular accumulation of inorganic carbon (Ci) by microalgae and cyanobacteria under ambient atmospheric CO2 levels was first documented in the 80s of the 20th Century. Hence, a third variety of the CO2-concentrating mechanism (CCM), acting in aquatic photoautotrophs with the C3 photosynthetic pathway, was revealed in addition to the then-known schemes of CCM, functioning in CAM and C4 higher plants. Despite the low affinity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) of microalgae and cyanobacteria for the CO2 substrate and low CO2/O2 specificity, CCM allows them to perform efficient CO2 fixation in the reductive pentose phosphate (RPP) cycle. CCM is based on the coordinated operation of strategically located carbonic anhydrases and CO2/HCO3− uptake systems. This cooperation enables the intracellular accumulation of HCO3−, which is then employed to generate a high concentration of CO2 molecules in the vicinity of Rubisco’s active centers compensating up for the shortcomings of enzyme features. CCM functions as an add-on to the RPP cycle while also acting as an important regulatory link in the interaction of dark and light reactions of photosynthesis. This review summarizes recent advances in the study of CCM molecular and cellular organization in microalgae and cyanobacteria, as well as the fundamental principles of its functioning and regulation.
1. Introduction
Accumulation of intracellular inorganic carbon (Ci) in microalgae and cyanobacteria is one of the adaptive mechanisms available in photoautotrophs that allow them to perform efficient photosynthesis.
The central element of all types of photosynthetic carbon metabolism is the reductive pentose phosphate (RPP) cycle (Calvin-Benson-Bassham cycle, C3 cycle). Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the key enzyme of the RPP cycle, combines CO2 with ribulose-1,5-bisphosphate (RuBP). In many photoautotrophs, Rubisco is characterized by a rather low affinity for CO2 (KM (CO2)) and by a slow carboxylation turnover rate (kcatc). In addition, Rubisco is a bifunctional enzyme that performs both carboxylase and oxygenase functions. The activity of oxygenase binds oxygen to RuBP instead of CO2, reducing the efficiency of carbon fixation. Among other things, some Rubisco enzymes have low CO2/O2 specificity (Sc/o), indicating a low advantage of CO2 over O2 as a substrate. The oxygenation reaction can considerably lower photosynthesis efficiency and necessitates additional energy use for photorespiration. During photorespiration, the loss of CO2, which could be fixed in the RPP cycle, can reach 25–30% [1]. One ATP and one NADPH molecule is consumed to neutralize the products of photorespiration.
The aforementioned Rubisco properties are absolutely critical in a modern oxidative atmosphere with a low [CO2]/[O2] ratio. The low rate of diffusion of all types of Ci in water is another factor that makes the situation worse for aquatic species. For precisely these reasons, photoautotrophic cells have developed two adaptation strategies that allow them to sustain high photosynthetic productivity: (1) an increase in the affinity of Rubisco for CO2 with a simultaneous increase in the amount of this enzyme in the chloroplast; (2) an increase in the intracellular concentration of CO2 to compensate for the disadvantages of Rubisco characteristics—that is, the direct saturation of the enzyme with its substrate.
The first strategy is implemented in higher plants that fix carbon by the C3 type of photosynthesis. The second strategy is represented by three currently known ways of CO2 accumulation near Rubisco carboxylation centers. In higher land plants, it is achieved due to the schemes of C4- and CAM-photosynthesis. Aquatic photosynthetic microorganisms, namely, microalgae and cyanobacteria, perform C3-metabolism and operate another type of CO2 concentration, which is based on the direct “pumping” of Ci into a cell. All these strategies of CO2-concentration are known as “CO2-concentrating mechanisms” (CCMs). Here, we will use the term “CCM” to refer to the CO2-concentration mechanism in the cells of microalgae and cyanobacteria.
The abbreviation CCM is often deciphered as “carbon concentrating mechanism”, since its functioning solves two problems:
- Intracellular accumulation of Ci overcomes the problem of its low rate of diffusion on the way from the external environment to Rubisco. Thus, CCM solves the problem of the substrate limitation of photosynthesis;
- The concentration of CO2 molecules near the active sites of Rubisco compensates for its low substrate specificity.
CCM functions in cyanobacteria and eukaryotic algae assigned to different taxonomic groups [2,3,4]. The best studied CCMs belong to the model strains of cyanobacteria (such as Synechocystis sp. strain PCC 6803, Synechococcus elongatus PCC 7942, Synechococcus sp. strain PCC 7002) and green microalgae Chlamydomonas reinhardtii [5,6,7,8,9,10]. In addition to freshwater and marine species, CCM components are found in a number of alkaliphilic cyanobacteria [11,12]. It is noteworthy that these organisms grow in soda lakes with a very high content of bicarbonate, and, theoretically, they do not require any CO2 concentration. At the same time, alkaliphilic cyanobacteria are considered as direct relics of the ancient terrestrial microbiota that existed in the CO2-rich Archean atmosphere [13]. These circumstances raise the question about the time period and roots of the appearance of CCM on Earth.
Currently, there are several theories regarding the time of occurrence of CCM in cyanobacteria, ranging from ~350 Myr (million years) to ~3.5 Gyr (billion years) ago [2,14,15,16,17,18]. Experimental data indicate that the ancient form of CCM could function in these organisms even before the radical changes in the gas composition of the atmosphere ~2 Gyr ago [19]. Such a proto-CCM could compensate for the non-favorable [CO2]/[O2] ratio that occurs for various reasons in the pericellular layer [2,11,15,17]. It is possible that the absence of the early evolution of Rubisco [20,21] can be attributed to the presence of proto-CCM in ancient cyanobacteria.
In microalgae, the appearance of CCM is, most likely, independent. This is evidenced by the differences in the sets of CCM components of microalgae and cyanobacteria, as well as by the absence of homology between them. It was suggested that this event could have occurred about 400–500 Myr ago, after the appearance of photorespiration [22]. The development of alternate CO2-concentration strategies (C4- and CAM-photosynthesis) in terrestrial plants 20–30 Myr ago [23,24] is thought to have resulted from the inability to employ a “biophysical pump” to get Ci into the cells from the surrounding air.
The high ecological significance of CCM underpins the interest in it. Almost half of the oxygen produced on our planet is provided by oceanic phytoplankton, which includes cyanobacteria and microalgae, while the other half is provided by terrestrial plants [25,26]. The effectiveness of CO2 fixation is distributed similarly (1:1) between oceanic and terrestrial photosynthetic species in terms of their gross yearly primary output [25]. This fact becomes especially important in the present period of climate change, where mankind faces tasks of preserving the ecology of the biosphere, preventing the greenhouse effect and removing “excessive” amounts of CO2 from the atmosphere. The idea of harnessing the genetic potential of cyanobacteria and microalgae to introduce an artificial CCM into C3 plants in order to increase crop yields also dictates the relevance of versatile CCM research [10,27,28,29,30].
2. Rubisco, Types of Photosynthetic Carbon Metabolism and CO2-Concentrating Mechanisms
The interaction of organic and inorganic carbon cycles in autotrophic organisms occurs through photosynthetic Ci fixation in the RPP cycle with Rubisco as the key enzyme. The structure of Rubisco, as well as the phylogeny of these proteins, are fairly well investigated [21,31,32,33]. All Rubisco are divided into four basic forms (I–IV), each of which catalyzes the identical reaction, but has very different structural and kinetic characteristics [31]. An exception is Rubisco IV, which lacks catalytic activity. To distinguish the latter from active enzymes, proteins that belong to Rubisco IV are referred to as Rubisco-like-proteins. It is assumed that form IV is the progenitor of the CO2-fixing Rubisco [34].
Cells of higher plants, cyanobacteria and eukaryotic algae (along with proteobacteria) carry Rubisco form I [31]. Proteins of this group consist of large (L) (~55 kDa) and small (S) (~15 kDa) subunits, which form the L8S8 superstructure. The large subunit contains the enzyme’s active center, while the small subunit appears to provide structural stability and catalytic efficiency [32]. Rubisco forms I are subdivided into four groups (IA-ID) comprising enzymes with a different primary sequence of the large subunit. Of these, two forms, IA and IB, are present in proteobacteria, cyanobacteria, green algae and higher plants, while IC and ID are present in non-green algae and proteobacteria [35].
According to the mode of CO2 fixation, the following types of photosynthetic carbon metabolism are distinguished: C3, C4 and CAM. Only C3 higher plants do not require RPP cycle additions to maintain high photosynthetic productivity. In these organisms, Rubisco has a rather low turnover rate (kcatc), performing, on average, about three carboxylation reactions per second [21]. At the same time, these enzymes are characterized by a high affinity for CO2 as a substrate (avg. KM (CO2)~14 μM) and by high CO2/O2 specificity (avg. Sc/o~98). Furthermore, the amount of Rubisco in the chloroplast stroma in C3 higher plants is up to three orders of magnitude higher than that of CO2 and approximately equal to that of RuBP, which provides a high concentration of the enzyme’s active sites in vivo, reaching 10 mM [36,37]. These particular characteristics allow C3 higher plants to saturate the carboxylation reaction with CO2.
In contrast to C3 higher plants, cyanobacteria and microalgae cannot saturate Rubisco active centers at the current atmospheric CO2 concentration. Cyanobacterial Rubisco has a high carboxylation turnover rate (kcatc up to 14 s−1), but a very low affinity for CO2 as a substrate (KM (CO2) over 300 μM) and a low CO2/O2 specificity (avr. Sc/o~48), indicating that CO2 has little advantage over O2 as a substrate [21,38]. The Rubisco of green microalgae are characterized by mean Sc/o~62, with a higher affinity for CO2 than in cyanobacteria (avr. KM (CO2)~32 μM), but a lower carboxylation turnover rate (avr. kcatc~3 s−1) [21]. In microalgae cells, Rubisco accounts on average for about 5% of the total cellular protein. The content of cyanobacterial Rubisco rarely exceeds 10%, which is five times less than in the photosynthetic tissues of C3 higher plants [39,40,41].
The Rubisco of CAM and C4 plants generally have kinetic parameters similar to those of C3 plants [21]. However, due to the peculiarities of their habitat and unique life strategies, these species exhibit significant diurnal changes in CO2 levels (CAM), or excessive photorespiration (C4) [23].
Thus, all photoautotrophs, other than C3 higher plants, require the adaptive mechanisms to maintain the efficiency of the RuBP carboxylation reaction. Those organisms use a common, most obvious strategy—the concentration of CO2 molecules near the active centers of Rubisco is used to saturate the enzyme. This strategy can be implemented in three different ways, which are discussed further below.
In higher terrestrial plants, CO2 concentration is achieved through the operation of C4- and CAM-photosynthesis, which differs from C3-photosynthesis in that they have the metabolic add-ons to the RPP cycle that complicate the biochemical pathways of CO2 fixation and reduction. This is why this type of CO2 fixation is called “biochemical” [3,18]. Certain species of freshwater aquatic plants also belong to CAM plants [23]. The processes of CO2 assimilation in C4- and CAM-photosynthesis are similar in many respects. In both C4- and CAM-plants, the first CO2 acceptor is phosphoenolpyruvate (PEP), which binds to the bicarbonate produced by the hydration of the CO2 molecule entering the cell (Figure 1). The primary products of photosynthesis here are C4-dicarboxylic acids, and the formation of CO2 for its incorporation into the RPP cycle (with a simultaneous concentration near Rubisco) results from subsequent decarboxylation reactions. Due to C4-photosynthesis, the concentration of CO2 at Rubisco localization sites is increased approximately 10–20-fold compared to the atmospheric level [42,43] resulting in the suppression of photorespiration. However, the contribution of C4 plants to the gross Earth photosynthesis is small, as they account for only 3% of all known species [44]. In terms of biochemistry and the general scheme, CAM photosynthesis is very similar to C4 photosynthesis, but it is slightly inferior in terms of the net CO2 fixation [45].
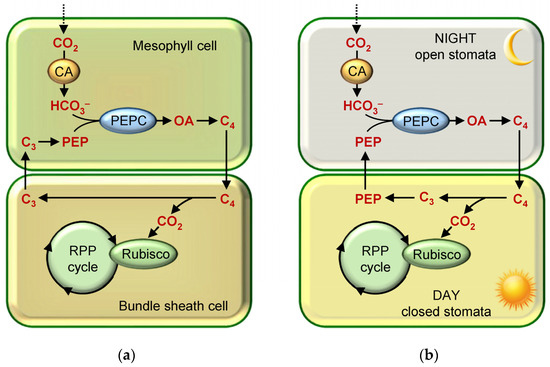
Figure 1.
Schematic representation of dark phase photosynthesis reactions in (a) C4 plant cells; (b) CAM plants. Abbreviations: C3/C4—C3/C4-dicarboxylic acids; OA—oxaloacetate; PEP—phosphoenolpyruvate; PEPC—phosphoenolpyruvate carboxylase.
In cells of microalgae and cyanobacteria with a C3-type of photosynthesis, the CO2 concentration is achieved by the CCM, which ensures the active “pumping” of exogenous Ci into cells, with the subsequent accumulation of CO2 molecules at carboxylation sites. CCM was discovered in the 1980s [46,47,48,49,50,51,52]. Its scheme is referred to as a “biophysical” type of concentration [3,18,29,53]. In contrast to the constitutive mechanisms of CO2 concentration that function in C4- and CAM-plants, CCM is an inducible process that is activated only when the Ci concentration in the environment decreases. CCM is also an add-on to the RPP cycle with the exception that the Ci pool inside the cell does not enter into biochemical transformations before the RPP cycle, and the first CO2 acceptor is RuBP.
Characteristics of cyanobacterial and microalgal cells adapted to the atmospheric CO2 concentration are similar to that of C4 plants in terms of some photosynthetic parameters (reduced inhibition of photosynthesis by O2, reduced photorespiration and reduced CO2 compensation point). Cells grown at high CO2, however, show photosynthetic characteristics similar to those of C3 plants. CCM allows more efficient CO2 fixation in the RPP cycle [30] as compared to C4- and CAM-plants. Therefore, microalgae and cyanobacteria are the essential contributors to the gross photosynthesis, organic matter and oxygen formation in our planet [25,26].
It should be emphasized that the literature currently refers to C3 higher plants as having the so-called “passive CO2-concentrating mechanism” (pCCM) [54]. This term refers to the ATP-independent biological transport processes that ensure the capture of CO2 released during respiration and photorespiration, its subsequent delivery to the Rubisco and re-fixation. Moreover, it has been proposed that cells of C3 higher plants may employ a “basal CCM” depending on the functioning of mitochondrial CAs [55]. This scheme also implies the prevention of CO2 leakage from a cell and allows the efficient recycling of CO2 released due to the reactions of the tricarboxylic acid (TCA) cycle and photorespiration. However, the two above-mentioned schemes cannot be considered as a true CO2-concentration, which implies the energy-dependent transformation of the low concentration of exogenous CO2 into its high intracellular concentration near the active centers of Rubisco. Instead, the so-called “basal” and “passive” CCM are linked to resource conservation and its reasonable use in cells.
3. General Principles of CCM Operation
The CCM is based on the co-operation of the Ci (HCO3−/CO2) uptake systems and carbonic anhydrases (CAs). A simplified scheme of the CCM operation is shown in Figure 2. The first step is the creation of a HCO3− pool, the intracellular content of which may be several orders of magnitude higher than the concentration of Ci in the environment. In the second step, the stored HCO3− is used to generate a high concentration of CO2 molecules near the active centers of Rubisco.

Figure 2.
Schematic representation of the CCM in microalgal and cyanobacterial cells. The font size reflects the relative concentrations of CO2 and HCO3− in the external environment and inside the cell. Thick arrows show the main direction of Ci flow. * Energy requirements for the promoted CO2 uptake are currently approved only for cyanobacteria.
In general, the CCM of cyanobacteria and microalgae is characterized by the following structural and physiological features:
- The CCM is organized within a single cell;
- The CCM is an inducible process: it is activated when the concentration of exogenous Ci is insufficient to ensure the efficient photosynthesis;
- The CCM induction requires light;
- In contrast to the C4- and CAM-photosynthesis, and the C3-photosynthesis of higher plants, where Ci enters the cell only by CO2 diffusion, microalgae and cyanobacteria additionally possess the systems of the active (energy-consuming) uptake of both CO2 molecules and HCO3− ions;
- During the operation of the CCM, the rapid interconversion of Ci species (CO2/HCO3−) both outside and inside the cell is maintained by CAs;
- The efficient incorporation of CO2 into the RPP cycle is achieved through the joint localization of Rubisco and CA in special microcompartments, such as carboxysomes in cyanobacteria or pyrenoid in microalgae. The cooperation between Rubisco and CA is the fundamental basis for the functioning of the CCM;
- The CCM includes the prevention of CO2 leakage from the cell;
- The structural features of the CCM allow one to protect Rubisco from O2 and to minimize the oxygenase reaction.
The CCM in cyanobacteria and microalgae is known as the “biophysical” CO2 concentration because it is primarily based on the activation of systems for an active physical uptake of exogenous Ci. At the same time, cells under conditions of Ci starvation undergo rather complex rearrangements in structural organization and biochemical processes [6,22,56,57,58,59,60,61].
It should be emphasized that CCM is a strictly energy-dependent and light-regulated process, since the absorption and accumulation of Ci by the cell does not occur in the dark [50]. In addition to intracellular Ci accumulation, light regulates many other processes related to CCM functioning—such as the transcription of CCM-associated genes, relocalization of the CCM components with the cell, formation of carboxysomes and pyrenoids, etc. [6,7,22,58,61,62,63]. It is currently unknown how the light regulation of the CCM induction and operation is performed. It has been suggested that the activity of Ci uptake systems can be regulated allosterically depending on changes in the [ATP]/[ATP + ADP + AMP] pools’ ratio under transitions from the light to darkness [63].
The energy expenditure of the cell for the CCM requires special consideration. Ci-limiting conditions cause a significant decrease in the growth rate, which is most likely due to the CCM’s high energy requirements, which are primarily related to the operation of the induced Ci transporters [6]. At the same time, the CCM is generally an energetically advantageous mode of photosynthetic assimilation. Indeed, by inhibiting photorespiration, CCM helps to save energy: the energy cost for the CCM functioning is lower than for the maintenance of photorespiratory metabolism [64]. The CCM also reduces (compared to C3 plants) the amount of Rubisco per unit biomass required to ensure a given rate of CO2 fixation. As a result, the amount of cell resources used for Rubisco synthesis is similarly decreased [64].
In addition, the functioning of the CCM helps to maintain an equilibrium between light and dark photosynthetic reactions. With the onset of Ci starvation, the activity of RPP cycle decreases, which leads to a decrease in the available NADP+ and ADP molecules. A lack of NADP+, which is required to keep the electron transport chain running, leads to the suppression of light processes by the inhibition of the excessive activity of the photosynthetic apparatus (photoinhibition). The suppression of the electron transport can also result from excessive proton accumulation in the thylakoid lumen due to a shortage of ADP molecules and a decrease in ATP synthase activity. The activation of CCM leads to the restoration of RPP cycle activity. Simultaneously, the conversion of bicarbonate to CO2 for Rubisco using lumen protons minimizes their excessive accumulation [60,65,66,67]. Thus, CCM can improve the efficiency of light utilization and, as a result, the overall efficiency of the photosynthetic machinery [64].
3.1. Exogenous Sources of Ci, Ci Uptake Systems and the Formation of the Intracellular HCO3− Pool
Microalgae and cyanobacteria live in an aquatic environment where Ci exists in three different forms: CO2, HCO3− or CO32−, whose ratio depends on the pH of a solution [68] (Figure 3). Seawater with alkaline pH values contains a high pool of bicarbonate ions (1.8 mM, 90%) along with 10% (0.35 mM) of CO32– and a small amount (0.01–0.02 mM) of dissolved CO2 in the equilibrium with air [49,69]. The Ci total concentration in seawater can reach 2 mM. Freshwater contains only 10 μM Ci, and its available forms are represented by CO2 and HCO3−. As an external source of Ci, microalgae and cyanobacteria use CO2 and/or HCO3−. Many microalgae and cyanobacteria exhibit species-specific preferences for some forms of Ci [70]. The preferential form of exogenous Ci, as well as the set of Ci uptake systems specific to a particular cell, determine the preference for CO2 or HCO3− uptake.
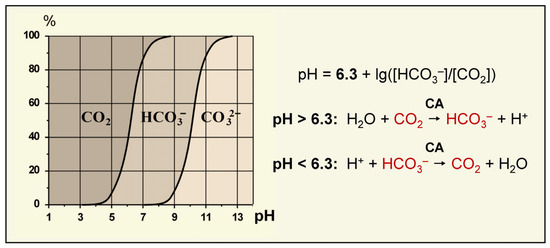
Figure 3.
The concentration ratio and pH dependence of Ci forms in water solutions [65]; the Henderson–Hasselbach equation for [CO2]/[HCO3−] ratio; and the preferential direction of the reaction ensured by CA as a function of pH.
The outer membrane is the first barrier for exogenous Ci, which can pass it through aquaporin channels [2,5]. Ci must then cross the plasma membrane and, in eukaryotic algae, the chloroplast membranes. Due to its high solubility in lipids, the CO2 molecule can enter the cell by direct diffusion. In contrast to CO2, negatively charged HCO3− can only cross the cell membranes via active transport. At the same time, even in the presence of a concentration gradient, HCO3− is well retained in a cell. The presence of bicarbonate uptake systems in microalgae and cyanobacteria is critical, because these organisms live in aquatic environments where CO2 diffusion rates are much lower than in air, and HCO3− often becomes the predominant form of exogenous Ci [4].
In general, three fluxes can cause the intracellular accumulation of Ci (Figure 2):
- Active transport of HCO3− into a cell;
- Entry of CO2 into a cell by the diffusion of dissolved gas;
- Increased CO2 diffusion into a cell as a result of the quick conversion of CO2 (which has already entered the cell) into HCO3−.
Operation of the Ci uptake system results in the intracellular accumulation of Ci as a pool of HCO3−. The conversion of CO2 to HCO3− in intracellular compartments is dictated by their alkaline pH values and by the catalytic action of Ci conversion systems based on the activity of CAs (Section 3.2). In general, the intracellular Ci concentration accumulated due to CCM can be 10–1000 times higher than that in the environment [49,71].
The active cellular uptake of Ci against the concentration gradient is an energy-dependent process [5,64]. This uptake is supported by ATP molecules, which are generated during photosynthetic electron transport, both cyclic and linear. When cells adapt to low CO2 concentrations, the cyclic electron transport activity increases, allowing an increase in the ratio of ATP/NADPH produced by the electron transport chain and providing an additional ATP influx for CCM [35,72]. An increase in the ratio of photosystem activities (PSI/PSII) in microalgae has been demonstrated under Ci-limiting conditions [73,74]. In cyanobacteria, the activity of NADPH dehydrogenase complexes, located in the thylakoid membrane and participating in the cyclic electron transport around PSI, also increased [35].
3.2. Carbonic Anhydrases and CO2/HCO3− Conversion Systems as an Element of the CCM
The carbonic anhydrase (CA, EC 4.2.1.1) system, consisting of external and intracellular forms of the enzyme, is the second important element of the CCM of microalgae and cyanobacteria. The reaction catalyzed by this enzyme determines the CA participation in biological processes: CO2 + H2O ⇆ HCO3− + H+. Such an interconversion is possible without a CA, but it is extremely slow. For example, the value of the rate constant for the spontaneous reaction of CO2 hydration is 0.037 s−1 [75], whereas for the reverse reaction of the HCO3− dehydration it is 20 s−1 [76]. Such a circumstance could significantly slow down biochemical processes. This is why CA is such an important enzyme of carbon metabolism with a fundamental significance of carbon-based life [77]. CAs are found in all presently known systematic groups of living organisms; they are present in all organs, tissues and compartments, where the acceleration of CO2/HCO3− interconversions or a rapid change in concentration of any of the four reaction components is required. The detailed information regarding the mechanism of catalysis, diversity of structural organization, phylogeny and biological functions of CAs in living organisms is properly reviewed in several books and articles [78,79,80,81].
Depending on the primary protein sequence, its three-dimensional structure, active site organization and catalytic properties, all known CAs are divided into eight classes—α, β, γ, δ, ζ, η, θ and ι [79]. Cyanobacteria and microalgae possess three classes of CAs—α, β and γ. In addition to these, microalgae cells contain CAs of δ, ζ and θ-classes [82]. CAs of the same class may be represented by several proteins with different biological relevance. The most prominent physiological roles of CAs in cyanobacteria and microalgae are related to the maintenance of CCM and photosynthesis in their cells. The enzymes take part or play the key roles in the following processes associated with these global mechanisms:
- Ci uptake by supplying CO2/HCO3− molecules for their transport across cell membranes;
- Photosynthetic Ci fixation by generating the CO2-substrate for Rubisco;
- Prevention of CO2 leakage from the cell by the operation of intracellular Ci conversion systems possessing CA activity;
- Regulation of photosynthesis by the modulation of the PSII activity and electron flow rate via the protection of the microalgal water oxidizing complex (WOC) from excess protons.
The fundamental basis for the efficient operation of CCM is the rapid compartment-specific transformation of the Ci species with the participation of CAs. Equilibrium concentrations of CO2 and HCO3− in the enzymatic reaction correlates with the distribution of Ci forms in the solution depending on its pH, as described by the Henderson–Hasselbach equation: pH = 6.3 + lg([HCO3−]/[CO2]) [68]. Thus, at pH < 6.3, the equilibrium shifts toward CO2 formation, whereas at pH > 6.3, the equilibrium shifts toward the predominant generation of HCO3− (Figure 3). Similarly, the predominance of a specific Ci form in a particular cellular compartment depends on its pH. The cytoplasmic pH in different species of microalgae and cyanobacteria ranges from 7.1 to 8.2 [5,83,84,85]; the pH of chloroplast stroma is about 8 [85]. In these compartments, Ci is mainly represented by bicarbonate (about 80–90%). Whereas, in lumen, when a proton gradient is created in the illuminated thylakoid membranes, the pH reaches 4–5 units [85], and CO2 becomes the main form of Ci. Mathematical modeling shows that the cyanobacterial carboxysome matrix also possesses an acidic pH [86], which can be formed as a result of proton release during the RuBP carboxylation reaction [87,88,89]. Lower pH values of the internal contents of carboxysomes (in comparison to the cytoplasm) have recently been demonstrated [90].
Thus, the direction of Ci flow from the environment to Rubisco is insured by the difference in the physico-chemical properties of CO2 and HCO3−, as well as by the difference in pH in the cellular compartments and rapid transformation of Ci with the participation of CAs (Figure 2). The involvement of CAs in the CO2/HCO3− delivery for their uptake systems is mediated by the local pH values of the periplasmic or pericellular space and by the advantage of a hydration or dehydration direction in the enzymatic reaction. The weakly alkaline pH of the cytoplasm and chloroplast stroma promotes the preferential accumulation of Ci as a lipophilic-neutral pool of HCO3−. The latter significantly reduces the risk of the spontaneous leakage of Ci from a cell. Conversely, under the acidic pH of the carboxysomes matrix or the lumen of the intrapyrenoid thylakoids, CAs “release” CO2 molecules from the HCO3− pool for their use by Rubisco [85,86,91,92]. The benefit of the bicarbonate dehydration reaction under acidic lumen pH values enables the enzyme’s protective activity in H+-removing from WOC in stromal thylakoids [92,93,94].
The operation of intracellular Ci conversion systems, presumably based on their CA activity, is critical for preventing CO2 molecule leakage into the external environment (Section 4.2 and Section 5.3.4). These systems, located in the cytoplasm in cyanobacteria and in the stroma of chloroplasts in microalgae, convert CO2 molecules into HCO3−, promoting the “locking” of Ci inside the cell. Concurrently, the Ci conversion systems capture CO2 molecules that have not been incorporated into the RPP cycle.
3.3. Rubisco-Containing Microcompartments: Carboxysomes and Pyrenoids. The Principles of Cooperation between CA and Rubisco
All of the stages of CCM described above (exogenous Ci uptake with the participation of external CAs, and its accumulation as HCO3− in the cytoplasm in cyanobacteria or in the cytoplasm and stroma of chloroplast in microalgae) are prerequisites for the final stage: the conversion of the accumulated pool of HCO3− into CO2 molecules that serve as Rubisco substrate. To effectively engage CO2 in the RPP cycle and minimize the leakage of these molecules from a cell, CO2 concentration increases only locally, near Rubisco active centers (Figure 2). The intracellular CAs are responsible for the conversion of HCO3− to CO2. A microcompartment, where the majority of Rubisco is concentrated and where this enzyme is co-localized with CA, is a structural feature of CCM [35,74,95,96]. In cyanobacteria, these microcompartments are known as carboxysomes, while in microalgae, they are known as pyrenoids. The Rubisco content in the inner phase of pyrenoids and carboxysomes reaches 70–98% of its total amount in the cell [57,97]. Thus, Rubisco and CA form the central element of the CCM, “for whose benefit” this mechanism functions. The interplay of the organic and inorganic carbon cycles in microalgae and cyanobacteria is mostly mediated by this tandem. A CCM model, in which CA and Rubisco were co-localized in carboxysomes, was first proposed in 1989 [98].
Depending on the Ci availability, both carboxysomes and pyrenoids undergo significant changes in size, composition and structure. The pyrenoid structure becomes more apparent under low carbon conditions, and it is typically surrounded by a thick starch sheath [58,85,99]. Under these conditions, the majority of the entire amount of cellular Rubisco is present in the pyrenoid [61,99]. At a high CO2, however, less than 50% of the enzyme occurred in the pyrenoid, and the remaining percentage is found in the chloroplast stroma together with the accumulated starch grains. In cyanobacteria, Ci-limiting conditions lead to an increase in the number of carboxysomes; they become more contrasting due to the synthesis of a protein shell [57,100]. Rubisco is mostly concentrated in the carboxysomes during Ci deficiency, much like a pyrenoid.
New pyrenoids are formed by both a fission and de novo assembly [101]. In contrast to carboxysomes, pyrenoids are a transient subcellular feature. The CCM is present in all pyrenoid-containing microalgae, but not all microalgae that accumulate Ci under low carbon conditions have pyrenoids [2]. As a result, the subtleties of pyrenoid involvement in CO2 concentration have yet to be evaluated. The identification of the factors that regulate the pyrenoid assembly and functioning is currently an active area of study [102,103,104,105,106].
4. CCM of Cyanobacteria
Photosynthetic gram-negative bacteria, namely, cyanobacteria, are among the oldest organisms on our planet, and the only prokaryotes capable of oxygenic photosynthesis. Due to the CCM, cyanobacteria have extremely high photosynthetic productivity. According to various estimates, these organisms contribute 10–30% of the global net primary carbon fixation [107,108,109].
The current knowledge of cyanobacterial CCM is based on studies of model laboratory strains such as the freshwater Synechocystis sp. strain PCC 6803 and Synechococcus elongatus PCC 7942, or the marine Synechococcus spp. and Prochlorococcus spp. Principles of the CCM function have been outlined in a number of previous reviews [5,6,7,17,110]. Here, we will discuss only the recent progress in the field and review the general scheme of the induction and functioning of the cyanobacterial CCM, taking into account the principles of operation of its individual components.
4.1. CCM Induction in Cyanobacteria
As previously stated, the cyanobacterial CCM is an inducible mechanism, which is solely activated when the exogenous Ci is insufficient to saturate the dark phase of photosynthesis. In cyanobacteria, allophycocyanin may play the role of the Ci sensor [111]. Alternatively, this role is assigned to the soluble adenylate cyclase, whose activity is directly proportional to the concentration of Ci in the environment [112,113,114]. The cAMP synthesized by adenylate cyclase can work as a Ci-sensing signal.
Two states, “low-affinity” (basal) and “high-affinity” (induced), are characteristic of the cyanobacterial CCM [5,6]. The basal level of CCM is kept in cells that grow at the so-called “high” Ci level, and is capable of maintaining the efficiency of the RuBP carboxylation reaction in the RPP cycle. In the laboratory, these conditions correspond to the growth of cultures at 1.5–2% CO2 in a gas–air mixture. Here, the CCM operation is ensured by its constitutive components, which include low-affinity high-rate Ci uptake systems (NDH-14 and BicA), Rubisco and carboxysomal CA. The accumulation of intracellular Ci and concentration of CO2 molecules near Rubisco do not occur under these conditions.
The induced state of the CCM corresponds to the Ci-limiting conditions. These usually refer to the growth in the most natural aquatic environments, including seawater. High-affinity Ci uptake systems (NDH-13, SbtA and BCT1) are primarily responsible for the operation of the induced CCM [5], ensuring the accumulation of the intracellular HCO3− pool. In addition, the increased level of the thylakoid β-CA, EcaB, is recorded under these conditions [115]. This occurrence is explained by the involvement of EcaB in the operation of the NDH-13 complex.
The CCM induction entails for optimizing the set of its components and/or changing their activity. This is ensured by three levels of regulatory processes: (1) transcriptional, which regulates the mRNA level of the CCM components; (2) post-transcriptional, which regulates the translation efficiency from mRNA; and (3) post-translational, which tunes the activity of already existing protein components through their modifications or by allosteric regulation.
The most important biochemical changes in response to low-Ci stress have been analyzed in recent reviews [6,7,116]. Carbon deficiency inhibits the RPP cycle and causes an imbalance in photosynthesis’s light and dark reactions. Photodynamic disturbances lead to changes in the redox state of a cell. Protein synthesis becomes suppressed. Simultaneously, photorespiration is activated as a result of a shift of Rubisco activity towards oxygenation. Glycolysis, the TCA cycle, and the oxidative pentose phosphate cycle are activated, allowing the additional synthesis of NADPH and ATP, as well as the release of carbon from organic compounds for its utilization in de novo protein synthesis. Intermediates in these metabolic pathways can act as effectors, correcting the ability of the CCM-associated transcriptional factors (TFs) to bind to DNA and exert control over the gene expression [6,7]. Among these metabolites with established regulatory functions are RuBP, 2-phosphoglycolate (2-PG) and α-ketoglutarate (α-KG; also known as 2-oxoglutarate). Additionally, the regulation of gene expression can be achieved by altering the DNA topology [117,118].
The cyanobacterial cell acclimation to low Ci is controlled by three known TFs [6,7,116]: CmpR, NdhR (CcmR), and CyAbrB2. CmpR (cmp operon regulator) acts as a transcriptional activator of the cmp operon, encoding the high-affinity bicarbonate transporter, BCT1, of freshwater cyanobacteria [119]. CmpR binding to the promoter is enhanced in the presence of RuBP and 2-PG, whose level is expected to increase under low CO2 [120].
Another TF, NdhR (CcmR) (NDH-1 genes regulator/carbon concentrating mechanism regulator), acts as a repressor of genes, encoding: (a) Ci uptake systems SbtA, BicA and NDH-13; (b) SbtB regulator protein; and (c) mnh operon for the complex involved in the generation of an electrochemical Na+ gradient required for the Na+-dependent HCO3− uptake [121,122,123,124]. A molecule of α-KG acts as a co-repressor and enhances the promoter affinity of NdhR, whereas 2-PG is a CCM inducer [125]. It was originally reported that NADP+ could also act as a co-repressor of NdhR [126], but further studies have not confirmed this fact [125]. The concentration of α-KG is related to the carbon/nitrogen balance of the cell. These molecules are accumulated under the Ci excess due to the active TCA cycle and under a nitrogen limitation [116,127,128]. At the same time, the 2-PG molecules generated by the Rubisco oxygenation reaction serve as a signal of Ci deficiency. When 2-PG appears, it antagonizes the effect of α-KG, leading to the dissociation of NdhR from its target repressing site [125].
A third TF, cyAbrB2 (cyanobacterial AbrB-like protein 2), acts complementarily to CmpR and NdhR, through controlling several CCM-related genes encoding BCT1, NDH-13 and SbtA/B [129,130]. The effectors that regulate this TF are currently unknown.
At the post-transcriptional level, the variation in the composition of CCM components is regulated translationally by small regulatory RNAs [131], which help to enhance or weaken the synthesis of certain proteins. Small RNAs may control the increase in the number of carboxysomes under Ci deficiency, since the expression of the ccm gene cluster was not up-regulated under these conditions [6].
At the post-translational level, the regulation may operate via changes in the thiol groups status due to changes in the redox state of a cell [7], phosphorylation [132] or other structural modifications of proteins. An important regulatory element is the SbtB protein, a member of the PII superfamily of multifunctional signal processing proteins [133,134]. Previously, SbtB was considered only as a regulator, which allosterically controls the transport activity of the HCO3− transporter, SbtA [63,116,135,136]. The binding of SbtB to various adenyl-nucleotides, including cAMP—a potential signaling molecule involved in Ci acclimation [113,114], suggested its involvement in the modulation of the CCM activity [133]. The participation of SbtB in the regulation of the expression of a number of CCM-associated genes (regardless of the involvement of secondary messengers) has recently been reported [137]. The mechanism of this regulation remains to be evaluated.
4.2. Ci Uptake in Cyanobacteria and Ci Conversion System in the Cytoplasm
There are five systems known in cyanobacteria model strains that allow Ci entry into the cell [7,138]. These include three transporters of HCO3− and two CO2 uptake systems. The latter ensure the diffusion of CO2 inside the cell due to its rapid conversion to HCO3− and creation of a negative gradient (Figure 4). The auxiliary elements (Figure 4) supporting the Ci transport systems in cyanobacteria are: (1) the Na+/H+ antiporter NhaS3 and (2) the specialized NDH-1 complex, Mnh; both participate in the creation of a Na+ gradient during the Na+-dependent transport of bicarbonate; as well as (3) the proton pump PcxA, which works to release H+ from the cytoplasm and contributes to the maintenance of cellular pH at weakly alkaline values, thus ensuring the accumulation of the intracellular pool of Ci in the form of HCO3−.
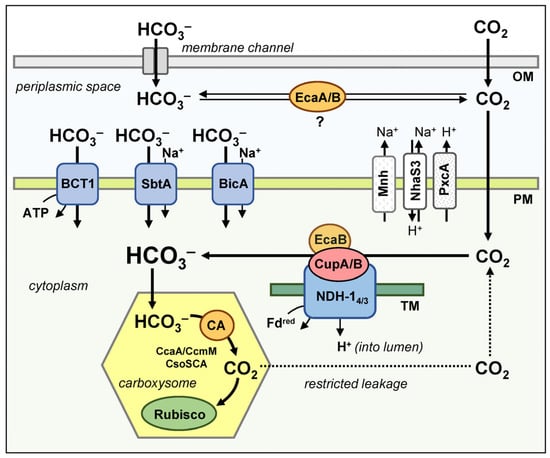
Figure 4.
CCM in model strains of cyanobacteria. The periplasmic CAs, EcaA and EcaB are also shown, but their involvement in the CCM has not yet been confirmed. Abbreviations: OM—outer membrane; PM—plasma membrane; TM—thylakoid membrane.
Bicarbonate transporters are represented by the proteins SbtA, BicA and the BCT1 complex located in the plasma membrane [138]. Notably, cyanobacteria lack the DAB complex responsible for Ci uptake in many prokaryotes, including chemolithoautotrophs [139,140]. Energy equivalents for the active HCO3− uptake are ATP molecules (required for BCT1 operation) or the electrochemical gradient of Na+ that enables the SbtA and BicA operation [7].
The high-affinity (KM (HCO3−)~15 μM) transporter BCT1 (bicarbonate transporter 1) with a medium flux rate is encoded by the cmpABCD operon [141]. BCT1 is expressed only under conditions of strict Ci limitation [100,142,143]. In marine and alkaliphilic species inhabiting environments with high HCO3− concentrations, this high-affinity transporter is detected quite rarely [5,12,144].
SbtA (sodium-bicarbonate transport A) is an inducible, high-affinity (KM (HCO3−)~5 μM) Na+-dependent transporter with a relatively low flux rate [145]. Cyanobacterial genomes may contain several copies of the sbtA gene with varying degrees of homology. The SbtA transport activity is allosterically regulated by the PII-like signaling protein SbtB, which can bind a number of adenyl nucleotides [63,133]. Two corresponding genes, sbtA and sbtB, are often adjacent in a single operon and are expressed together under Ci-limiting conditions [133]. The binding of SbtB to SbtA suppresses the bicarbonate uptake function of SbtA [135]. The formation and dissociation of the SbtA/SbtB complex depends on the nature of the SbtB-bound adenyl nucleotide. There are two different views on the nature of the regulation of SbtA activity by SbtB. One of them [116,136] suggests that SbtB acts as a Ci sensor distinguishing between [ADP + AMP] and [cAMP] concentration differences under low and high Ci, respectively. The second model [63] implies that SbtB performs a light-dependent regulation of SbtA via changes in the adenylate charge ratio ([ATP]/[ATP + ADP + AMP]) in a cell under its transition from light to darkness.
A third HCO3− transporter, BicA (bicarbonate transporter A), was characterized by low-affinity (KM (HCO3−) 90–170 μM) and supported a high flux rate [146]. In different species of cyanobacteria, BicA can be expressed constitutively or be highly inducible under a Ci limitation [100,123,146,147]. Often, similarly to SbtA, cells contain several BicA proteins with varying homology. Remarkably, BicA is the only Ci transport system usually detected in marine cyanobacteria [5].
Two systems, NDH-13 (NDH1-MS) and NDH-14 (NDH1-MS′), are the special modification of NADPH dehydrogenase respiratory complexes (NDH-1) that occur in eubacteria and eukaryotic mitochondria [148]. NDH-13/4 is responsible for CO2 uptake in cyanobacteria [149,150]. In contrast to the mitochondrial complexes, which generate a H+ gradient for ATP synthesis simultaneously with NADPH oxidation, NDH-13/4 use the reduced ferredoxin as the energy equivalent. In this regard, the designation for cyanobacterial “NDH-1” has recently been changed to “photosynthetic complex I” [151].
In microalgae, both complexes NDH-13 and NDH-14 are localized in thylakoid membranes [152,153]. Low-affinity (KM (CO2)~10 μM) NDH-14 is expressed constitutively, whereas high-affinity (KM (CO2)~1–2 μM) NDH-13 is induced by the Ci limitation [5]. It is thought that NDH-13/4 converts CO2 entering the cells into HCO3− due to the CA activity of their unique (in comparison with other NDH-1 complexes in cyanobacteria) constituent proteins, CupA/B, which are also known as ChpY/X [149,150,154]. Although the CA specific activity of CupA/B has not been confirmed experimentally, this hypothesis was well supported by the computer simulations as well as by a number of indirect data [154,155,156,157]. Two alternative hypotheses have been proposed regarding the functioning of NDH-13/4 complexes. Their fundamental difference lies in the assumption of whether the CO2 substrate is supplied to the CupA/B active centers: (a) from the cytoplasm [115] or (b) from the thylakoid lumen [158]. It has also been suggested that the CA activity of NDH-13/4 may be maintained or regulated by β-CA and EcaB, whose specific activity and interaction with CupA/B proteins have been demonstrated [115].
In addition to the CO2 uptake, NDH-13/4 complexes act as Ci conversion systems and prevent the leakage of CO2 molecules that escape from carboxysomes (Figure 4). Under high-light stress, NDH-13/4 can participate in the dissipation of excess HCO3− and light energy by relieving the plastoquinone pool over-reduction and preventing photoinhibition [115]. This phenomenon of Ci backflow into the environment to dissipate the excess light energy by the rapid recycling of ATP molecules or their equivalent (proton gradient) was first described about 25 years ago [159,160] and designated as “Ci cycling”. Moreover, NDH-13/4 complexes participate in cycling the electron flow around PSI [156,161].
4.3. Cyanobacterial Carbonic Anhydrases
Cyanobacterial cells contain external (located in the outer layers relative to the plasma membrane), thylakoid and carboxysomal forms of CAs. However, not all of them are involved in the CCM.
The EcaA (external carbonic anhydrase, alpha class) protein, which possesses a leader sequence for transport through the plasma membrane, is the only CA of α-class in cyanobacteria. The ecaA gene is found in the genomes of many cyanobacteria, but the presence of EcaA in cells has been confirmed only in two species, Anabaena sp. PCC 7120 [162] and Cyanothece sp. ATCC 51142 [147]. Earlier studies also reported the presence of EcaA in Synechococcus elongatus PCC 7942 [162]. This conclusion was based on the Western blot analysis of the Synechococcus total proteins with antibodies against EcaA from Anabaena. However, the specific antibodies could not locate EcaA in Synechococcus cells, regardless of the Ci supply [163].
The EcaB protein (external carbonic anhydrase, beta class) was also previously referred to as the external CA, because of the presence of a hypothetical lipoprotein lipid attachment site [164] and a leader sequence for transport through the plasma membrane [165]. Although the presence of EcaB in the periplasmic space of Synechocystis sp. PCC 6803 was previously directly confirmed by proteomics’ methods [165], a recent study demonstrated that the bulk of this protein is associated with thylakoid membranes, and only a small part is associated with the plasma membrane [115].
The specific activities of EcaA and EcaB have only recently been confirmed [115,147,163], more than 20 years after the discovery of these proteins. The physiological role of EcaA, as well as that of periplasmic EcaB, has not yet been defined. The biological role of the thylakoid form of EcaB is probably related to the function of NDH-13/4 systems responsible for CO2 uptake and Ci conversion in the cytoplasm [115].
Another external β-CA mentioned in the literature is the protein CahB1 (carbonic anhydrase, beta class, protein 1) from the haloalkaliphilic cyanobacterium Sodalinema gerasimenkoae IPPAS B-353 (formerly, Microcoleus chthonoplastes), which appeared in the cell envelopes and exopolysaccharide layer [166,167]. While CahB1 has an extremely high degree of homology with the carboxysomal β-CA, CcaA, the presence of CahB1 in S. gerasimenkoae carboxysomes was not confirmed. The paradox was reinforced by the fact that CahB1 does not contain any classical leader peptide for transport through the plasma membrane. Moreover, it appears that CahB1 may be the only active CA in the IPPAS B-353 strain [144]. If so, the question about the CA that delivers CO2 to Rubisco in S. gerasimenkoae carboxysomes remains open. Notably, when expressed heterologously in Synechocystis sp. PCC 6803, CahB1 acts as a functional carboxysomal CA [168]. Theoretically, the overall data [144,166,168] could be explained by the potential variations in the architecture of the CAs’ system in cyanobacteria from different habitats. The “non-model” strains, including S. gerasimenkoae, may exhibit different schemes of CA involvement in the photosynthetic carbon metabolism compared to “model” strains. At the same time, S. gerasimenkoae belongs to a group of the so-called “relict” cyanobacteria [11,13]. Therefore, there is a possibility that those cells preserved a variant of the ancient proto-secretion system [168].
The carboxysomal CAs of cyanobacteria are represented by the following enzymes: β-CA CcaA (carboxysomal carbonic anhydrase protein A), formerly IcfA (inorganic carbon fixation protein A); β-CA CsoSCA (carboxysome shell carbonic anhydrase), formerly CsoS3 (carboxysome shell protein 3); and γ-class CA, CcmM (carbon concentrating mechanism, protein M) [169,170,171,172]. The role of these CAs is to convert HCO3− to CO2 for its further fixation by Rubisco and involvement in the RPP cycle.
4.4. Carboxysomes
The Rubisco-containing microcompartments in cyanobacteria are called carboxysomes. Carboxysomes are polyhedral protein microbodies, usually of 100–400 nm in diameter, located in the cytoplasm and covered by a single-layer protein shell [173,174,175,176,177,178]. On the transmission electron microscopy images, carboxysomes appear as polyhedral electron-dense inclusions in the cytoplasm (Figure 5a). The presence of carboxysomes is also a characteristic feature in many autotrophic bacteria [177].

Figure 5.
Cyanobacterial carboxysomes. (a) Carboxysomes (arrows) in the cytoplasm of the cyanobacterium Synechocystis sp. strain PCC 6803. Image was kindly provided by Dr. M.A. Sinetova (K.A. Timiryazev Institute of Plant Physiology, RAS, Moscow). Scale bar is 0.5 µm. (b) Structural components of α- and β-carboxysomes in Prochlorococcus marinus MED4 (α-cyanobacteria) and Synechococcus elongatus PCC 7942 (β-cyanobacteria). Modified from [174]; supplemented from [89,175]. The proteins of the inner layer of the β-carboxysome shell form a bicarbonate-dehydrating complex that converts HCO3− to CO2 for the RPP cycle [184]. The organizing link of the complex is a full-length form of the CcmM protein, which simultaneously binds Rubisco, CcmN and CcaA (β-CA). The complex is associated with the carboxysome shell through specific interactions of CcmM with proteins in outer shell layer, CcmK and CcmL. Bicarbonate dehydration is performed by the CcaA or CcmM CAs.
Carboxysomes may contain two Rubisco subclasses, IA and IB, according to which they are called α- or β-carboxysomes, and the organisms containing them are called α- and β-cyanobacteria, accordingly [174,175]. These two types of carboxysomes are structural and functional analogues, and are composed of homologous proteins and enzymes. In addition, carboxysomes of α- and β-type differ in their organization at the genome level. The genes encoding polypeptides included in α-carboxysomes are usually organized in a single cso operon, whereas the genes for the components of β-type carboxysomes are clustered in several genomic locations [174,175]. The clustering of β-carboxysome genes implies their independent regulation and plasticity of expression in response to environmental changes [177].
Recent studies suggest the independent origin of α- and β-carboxysomes as a result of convergent evolution [179]. Little is known about the potential ecological benefits of each type of these microbodies. Until recently, it was thought that cyanobacteria with the Rubisco form IA mostly inhabit sea waters, whereas most cyanobacteria with the Rubisco form IB prefer fresh water environments. However, recent studies challenge this paradigm [180].
Both types of carboxysomes consist of a matrix and a protein shell (Figure 5b). The matrix includes Rubisco and structuring proteins that support the three-dimensional organization of the enzyme (CsoS2 or CcmM in α- and β-carboxysomes, respectively). Structural units of the carboxysome’s shell are several types of homo-oligomeric proteins that form shell vertices or shell facets with pores, through which metabolite molecules can pass. It is suggested that the pores facilitate the selective entry of HCO3− and RuBP inside this microcompartment and the simultaneous release of 3-phosphoglyceric acid (3-PGA) into the cytoplasm [181]. Molecular simulations indicate that the carboxysome shell functions as a barrier for CO2 and O2 [182,183]. It blocks CO2 leakage from the matrix and, at the same time, prevents O2 entry, thus keeping carboxylase activity of Rubisco at the high level.
The carboxysomal β-CAs, CsoSCA and CcaA are functional analogues. CsoSCA is bound to the inner side of the α-carboxysomal shell, and probably carries out the conversion of HCO3− to CO2 independently from other protein components [171]. Unlike CsoSCA, CcaA functions as part of the so-called “bicarbonate dehydration complex” located on the inner layer of the β-carboxysomal shell [184]. Another important protein of β-carboxysomes is CcmM. CcmM is expressed in two forms [185]. The full-length form consists of an N-terminal γ-CA domain followed by several RbcS-like domains attached by linkers. The short form of CcmM contains only RbcS-like domains. The full-length CcmM is part of the bicarbonate dehydration complex, and it can also function as CA in the absence of the ccaA gene [186,187]. The short form of CcmM serves as the structural element responsible for the paracrystalline organization of Rubisco IB [174,188]. Recently, along with the short form, the presence of full-length CcmM was shown in the carboxysome matrix, which can probably also participate in the three-dimensional organization of Rubisco [189].
Another component of cyanobacterial carboxysomes is Rubisco activase. In α-carboxysomes, this component is represented by the CbbX protein [190]. Cells of β-cyanobacteria can contain ALC (activase-like cyanobacterial protein), which does not function as a canonical Rubisco activase, but its presence is important for the proper assembly of carboxysomes [191].
The impairments in carboxysome distribution in cells cause their elongation, asymmetric division, increased Rubisco levels and growth retardation [192]. These observations suggest that carboxysomes may act not only as CO2 fixation centers, but also participate in other physiological processes. The spatial distribution of carboxysomes in β-cyanobacteria is achieved through the operation of a two-component system, McdAB, that presumably employs a Brownian-ratchet mechanism to position these microbodies [193,194]. McdAB-like systems are also found in α-cyanobacteria [195].
The structural organization of carboxysomes undergoes significant changes depending on the level of Ci supply to a cell. Under Ci excess, pro-carboxysomes are found in the cytoplasm of β-cyanobacteria. They represent Rubisco and CA aggregates assembled together with CcmM [196,197,198]. Upon a decrease in Ci in the environment, these conglomerates become coated with a protein shell and are converted into carboxysomes. The acquisition of the shell with its selective properties allows these microparticles to become the full-fledged participants of the CCM.
4.5. The Model of Cyanobacterial CCM
The general scheme of the CCM operation in cyanobacteria is shown in Figure 4. As the CCM enters the high-affinity state, active Ci accumulation by the cell begins with the participation of three HCO3− transporters (BCT1, SbtA and BicA) and two CO2 uptake systems (NDH-13 and NDH-14) (Section 4.2). The structure of the Ci-uptake complex is significantly influenced by the habitats of each particular cyanobacterial species. Its composition, as well as the affinity of Ci-uptake systems to their substrates, and their intrinsic flux rate, strictly corresponds to the needs of the species to ensure an inflow of Ci sufficient to saturate photosynthesis.
Regardless of the type of exogenous substrate, Ci enter a cell is accumulated as a pool of HCO3− in the cytoplasm with a slightly alkaline pH around 7.4, which significantly reduces the risk of the spontaneous leakage of CO2. The concentration of HCO3− in the cytoplasm of cells growing under a carbon limitation reaches 20–40 mM [71,74,199,200].
The final stage of CO2 concentration in cyanobacteria is carried out in carboxysomes. HCO3− and RuBP enter this microcompartment through pores formed by the shell proteins [181]. Conversion of accumulated HCO3− into CO2 molecules occurs with the participation of the carboxysomal CAs (CsoSCA, CcaA or CcmM) (Section 4.3), under presumably acidic pH values (<6.3) of the carboxysomal matrix [86]. The produced CO2 is used by Rubisco to carboxylate RuBP. The synthesized 3-PGA backflows from the carboxysome to the cytoplasm, where the rest of the RPP cycle reactions take place. The co-localization of Rubisco and CA in carboxysomes significantly reduces the leakage of CO2 molecules. The additional minimization of CO2 leakage with the simultaneous reduction of photorespiration is achieved due to the selective properties of the protein shell of carboxysomes. Limited amounts of CO2 leaked from carboxysomes are converted into HCO3− by NDH-13/4 complexes that act as intracellular Ci conversion systems along with a participation in CO2 uptake (Section 4.2). Through the work of NDH-13/4, CO2 molecules are returned to the total cytoplasmic pool of Ci.
5. CCM of Chlamydomonas reinhardtii
The CCM of microalgae is more complicated than that of cyanobacteria due to a larger number of subcellular compartments in the eukaryotic cell. Its structure and principles of functioning have been best studied in the model laboratory strain of the unicellular green microalga, Chlamydomonas reinhardtii. The effect of intracellular Ci accumulation under limiting concentrations of exogenous CO2 was also first discovered in C. reinhardtii [46]. The CCM organization of other eukaryotic microalgae is much less studied. The available information concerns mainly the individual components presumably involved in Ci concentration or some particular aspects of this process. A notable exception is the marine diatom algae, for which the CCM architecture and functioning was reconstructed using the whole genome sequence; and it appeared to be quite similar to the CCM of C. reinhardtii [53].
The CCM of C. reinhardtii requires at least 17 components to function properly [9,28,201,202]. These include nine systems for Ci transport across cell membranes (HLA3, LCI1, LCIA, CCP1, CCP2, CIA8 and BST1–3); three CAs (CAH1, CAH3 and CAH6); two peripheral pyrenoid proteins (LCIB and LCIC), presumably also possessing the CA activity; a methyltransferase homolog protein, CIA6; and two nuclear transcriptional regulators (CIA5 and LCR1). The exact function and importance of these individual participants is still not fully understood. The unambiguous necessity for providing directional Ci flow from the external environment to Rubisco is demonstrated for the following five proteins: HLA3, LCI1, LCIA, CAH3 and LCIB.
5.1. CCM: Induction and Two Photosynthetic Acclimation States of C. reinhardtii at Low Ci
In C. reinhardtii, three physiological states of cells are distinguished during their acclimation to various CO2 concentrations in the environment [9,10]:
- L-cells (low-CO2 cells) are acclimated to low CO2 (0.04–0.5%). A total of 0.04% corresponds to the natural content of CO2 in the modern atmosphere.
- VL-cells (very-low-CO2 cells) are acclimated to very low (<0.02%) CO2 concentrations.
- H-cells (high-CO2 cells) are acclimated to elevated CO2 concentrations (2–5%).
Different sets of CCM components are responsible for the L- and VL-cells’ adaptation, resulting in their different photosynthetic and physiological properties [9]. Mainly, it concerns VL-cells with a higher affinity to CO2 and simultaneous decrease in the photosynthetic activity. In comparison to L-cells, VL-cells are distinguished by their small size, slow growth rate and low chlorophyll content. Despite such differences in physiological characteristics, L- and VL-cells displayed no differences in the level of the transcription of CCM-associated genes [203], pointing to some non-transcriptional switch that regulates the acclimation.
The mechanisms underpinning the microalgal CCM activation and transition to a high-affinity state have been significantly less studied than those in cyanobacteria. To date, it is evident that the CCM activity in C. reinhardtii does not depends only on the CO2 content in the external environment [22]. As with cyanobacteria (Section 4.1), it is assumed that a decrease in CO2 is perceived by microalgal cells via indirect signals, the appearance of which is associated with an imbalance between the light (photosynthetic electron transport) and dark (RPP cycle) phases of photosynthesis. Such signals include photorespiratory metabolites or changes in the redox status of cells. These are involved in retrograde signaling via specific mediators, such as CAS or GUN4, and serve as effectors for CCM induction [22].
The majority of the genes encoding the confirmed CCM components are practically not transcribed under elevated CO2, showing an increase in the expression level only under Ci-limiting conditions. The exceptions are CAH3 and LCIB, which show low and moderate expression, respectively, under high CO2 with little or high increase in mRNA levels under carbon limitation ([203], supplementary data; [204], supplementary data). This may indicate that the corresponding proteins, the thylakoid CA CAH3 and the LCIB protein, which is a part of the Ci conversion system in the chloroplast stroma, are required for the successful growth of C. reinhardtii in a wide range of exogenous Ci concentrations.
The most complete picture of the C. reinhardtii cellular response to Ci limitation has been obtained only with the development of transcriptomics methods. A comprehensive analysis [203,204] revealed that at the beginning of the adaptation to the new environment, there is a global decrease in photosynthesis, a decrease in protein synthesis and in cell energy level, with a concurrent increase in photorespiration. An induction in the expression of the genes encoding the CCM components was observed after a short (~30 min) period.
CIA5 (also known as CCM1) and LCR1 are two CCM-associated regulatory proteins that have been identified in C. reinhardtii. The activity of other potential regulatory elements that could participate in the transcriptional regulation of the CCM activity is currently questionable [22]. CIA5 (Ci accumulation protein 5) is the major regulator of the CCM expression [205,206,207,208]. CIA5 controls all confirmed components of the CCM (Table 1 and Table 2), as well as a second regulatory protein, LCR1 (low CO2 stress response) [209]. Thus, the LCR1-regulated genes are ultimately controlled by CIA5. CIA5 is constitutively expressed regardless of the exogenic CO2 concentration, and it requires a post-translational activation under Ci-limiting conditions [206,207,208]. CIA5 may function as a TF, or it can exert a regulatory function by modifying or interacting with other regulatory proteins [9,22]. LCR1 functions as a traditional TF [209] activating transcription with the participation of CIA5.

Table 1.
Confirmed and potential Ci transport systems in C. reinhardtii.

Table 2.
Carbonic anhydrases and Ci conversion system with putative CA activity in C. reinhardtii.
At the post-transcriptional level, the modulation of CCM activity in C. reinhardtii is accomplished by varying the translation efficiency via small regulatory RNAs [22] or post-translational modifications [9]. The role of phosphorylation in the activation of algal CCM components is well established. Phosphorylation affects both the CCM components and their immediate protein environment. The phosphorylation of the linker protein EPYC1 (LCI5), which is critical for pyrenoid assembly, regulates its interaction with Rubisco by altering the availability of protein-binding domains [102,210]. The phosphorylation of the lumenal CAH3 leads to a significant increase in its CA activity and migration from the stromal thylakoids to the pyrenoid tubules [67]. Diffusion of the LCIB/LCIC complex (a stromal Ci conversion system) toward the pyrenoid at extremely low CO2 [211,212] is apparently also associated with the phosphorylation of its protein components [213]. The global analysis of the C. reinhardtii phosphoproteome revealed that numerous other CCM-related proteins, such as HLA3, LCIC and several CAs (CAH6-9 and CAG2), were phosphorylated [214]. Another way of the post-translational modification is glutathionylation, which has been demonstrated for the LCIB and proteins involved in the RPP cycle [215]. This may indicate the role of oxidative stress and changes in the redox state of the cell under conditions of Ci starvation in the CCM regulation.
5.2. Transport of Ci in C. reinhardtii
Microalgae, like cyanobacteria, use both CO2 molecules and bicarbonate ions from the aqueous environment. On its way to Rubisco, Ci must overcome the plasma membrane, the chloroplast envelope and the thylakoid membrane. C. reinhardtii cells have a variety of experimentally proven and potential Ci transport systems across membranes (Table 1). Until recently, the MITC11 (mitochondrial carrier protein), a homolog to mitochondrial carrier proteins, was also considered as a potential Ci transporter in the chloroplast of C. reinhardtii [8]. However, to date, this role of MITC11 has neither been confirmed nor disproved. The chloroplast envelope protein CemA encoded by the ycf10 gene [216] was also excluded from the list of potential Ci transporters. CemA is now assumed to work as a Na+-dependent proton pump, which removes H+ from the stroma [9].
Notably, the transcription of many C. reinhardtii genes involved in the function of the Ci uptake systems (HLA3, LCI1, LCIA, CCP1/2, and BST1–3) is enhanced upon a decrease in CO2. These genes are directly, or via LCR1, controlled by CIA5, the master regulator of the CCM genes’ expression [202,203,217] (Table 1). The involvement in the CCM-associated Ci uptake is now fairly well confirmed for HLA3, LCI1 and LCIA proteins. Impairments in the expression of the CIA8 and BST1–3 genes reduce the growth of cells and cause a decrease in their affinity to Ci under the CO2 limitation [201,202]. However, the activities of CIA8 and BST1–3 as the HCO3− transporters still require the experimental confirmation. The transport of Ci across mitochondrial membranes with the help of potential transporters, CCP1 and CCP2, is thought to be important in coordinating CCM with photorespiration [10,22].
5.2.1. Transport of Ci through the Plasma Membrane
C. reinhardtii has several proteins that have been proven or are suspected of being involved in Ci transport through the first barrier to the cell, the plasma membrane.
HLA3 (high light-activated protein) belongs to the family of ABC transporters with an ATP-binding cassette [208,218,219]. The HLA3 was originally described as the gene induced by high-light intensity. The location of HLA3 in the plasma membrane is well documented [28]. Several works are devoted to elucidating its function [220,221,222]. The ability of HLA3 to transport HCO3− has been unequivocally demonstrated in vitro in the Xenopus oocytes model system [28]. HLA3 is important for the adaptation of VL-cells and is inhibited by low CO2 in L-cells [223]. The enhanced transcription of the HLA3 gene at low CO2 occurs through the CAS (calcium sensing) protein involved in the Ca2+-dependent retrograde signaling [224,225]. Recently, the SAGA1 protein, whose function was previously attributed only to the starch sheath formation and maintenance of the pyrenoid structure [104], was demonstrated to play the important role in this process [106].
LCI1 (low-CO2-inducible protein) was first identified as a plasma membrane protein that was expressed only at reduced CO2 concentrations [226,227]. LCI1 ensures an active CO2 uptake in L-cells, whereas its role in VL-cells is minimal [228]. Structural studies of LCI1 suggest that it may function as a gated plasma membrane CO2 channel [229].
In the plasma membrane of C. reinhardtii, HLA3 and LCI1 form a joint complex interacting with several other proteins [102,222]. In particular, both HLA3 and LCI1 interact with ACA4, which is a putative H+-exporting ATPase. It has been suggested that ACA4 may contribute to HCO3− uptake, either by maintaining a proton gradient or by generating local cytosolic alkaline regions [102].
Two Rhesus-like proteins, RHP1 and RHP2, are found in the plasma membrane of C. reinhardtii and are thought to be potential CO2 channels [230,231,232]. The available information is mostly attributed to RHP1. The enhancement of the RHP1 gene expression and the appearance of the corresponding protein in cells occurs only at high CO2 [203,233]. Earlier studies [217] supposed that the RHP1 expression is not controlled by CIA5. Other studies, however, suggest the involvement of CIA5 in the regulation of RHP1 [203]. Although RHP1 and RHP2 are not directly appointed to CCM, they may be important for the Chlamydomonas vital activity under elevated CO2 concentrations [233].
5.2.2. Transport of Ci through the Chloroplast Envelope
The chloroplast envelope of C. reinhardtii forms a second barrier for the Ci transport toward Rubisco. Even recently, three possible candidates that may be associated with the HCO3− transport into the chloroplast have been considered: the LCIA protein and the CCP1/CCP2 proteins [9]. Finally, CCP1/CCP2 were allocated to mitochondria [28], although they still retain their former acronyms (CCP, chloroplast carrier proteins).
The LCIA (low-CO2-inducible protein A) protein was originally annotated as a putative nitrite transporter based on its structural similarity to the formate/nitrite transporters (FNTs) [208,234]. This is why LCIA often appears under another name, NAR1.2 (nitrite assimilation-related). The ability of LCIA to assimilate both HCO3− and NO2− has been confirmed [28,234]. LCIA, like other bacterial FNTs, it is thought to act as a channel facilitating HCO3− entry into the Chlamydomonas chloroplast by collaborating with the plasma membrane protein HLA3 [28,102,220,222].
Studies show that LCIA is physiologically relevant in VL-cells (0.01% CO2), and it is inhibited by ambient CO2 (0.04–0.5%) levels [204,223]. The activation of LCIA gene transcription, as well as that of HLA3, occurs at conditions of Ci deficiency and is mediated by the CAS protein [224].
The presence of a specialized CO2 transporter in the chloroplast envelope has not yet been reported. Thus, CO2 molecules transported by LCI1 into the cytosol may further enter the chloroplast stroma through an unidentified transporter or by passive diffusion [228].
5.2.3. Bicarbonate Transporters in Thylakoid Membranes
For a long time, one of the main missing links in the scheme of photosynthetic Ci assimilation in C. reinhardtii was the HCO3− transporter(s) from the chloroplast stroma to the thylakoid lumen, which supply bicarbonate ions to CAH3. The existence of such a transporter was a key argument for approving the CAH3 involvement in the CO2 supply to Rubisco, as well as in the WOC stabilization [65,87,93]. Therefore, the recent discovery of these CCM components became an eagerly anticipated event.
The CIA8 (Ci accumulation protein 8) gene encodes a transmembrane protein belonging to the sodium/bile acid symporter family (SBF), which may also participate in the bicarbonate transport [201]. The CIA8 protein possesses a leader peptide that may targets this protein to the thylakoid membrane or to the chloroplast envelope. CIA8, expressed in-frame as a GFP fusion in C. reinhardtii cells, was not associated with the chloroplast envelope, but rather dispersed throughout the organelle [201], suggesting the thylakoid location of CIA8.
The accumulation of CIA8 transcripts at very low (0.01%) CO2 concentrations suggested its involvement in CCM [201]. Under CO2-limiting conditions, the CIA8 mutant showed a reduced growth, decreased affinity for Ci and decreased photosynthetic oxygen evolution rate. However, CIA8 expression is not regulated by the CCM master regulator, CIA5. The precise location of CIA8 in Chlamydomonas cells, as well as the physiological role played by this protein, remain unknown.
Three bestrophin-like proteins, BST1, BST2, and BST3 (LCI11), represent additional potential bicarbonate transporters in C. reinhardtii thylakoid membranes [202]. These proteins interact with each other [102]. BST1–3 is found in both stromal and intrapyrenoid thylakoid membranes, with a marked concentration at the periphery of the pyrenoid [202]. It has been proposed that BST1–3, like a human bestrophin [235], functions as an anion channel. However, the ability of BST1–3 to transport HCO3− needs to be confirmed.
The involvement of BST1–3 in the CCM of C. reinhardtii was suggested because BST1–3 genes expression, being under the control of CIA5, strongly increased under low and very low CO2 [202,203,204]. Mutants with reduced BST1–3 expression were unable to grow at low CO2 exhibiting a reduced affinity to Ci and a reduced Ci uptake [202]. Interaction of BST1 and BST3 with the Ci conversion system in the stroma of chloroplasts and with the LCIB/LCIC complex [102] also suggested their involvement in CCM.
5.2.4. Mitochondrial Ci Transporters
The mitochondrial membranes of C. reinhardtii contain CCP1 and CCP2 proteins [28], which belong to the MCP (mitochondrial carrier proteins) superfamily. Previously, CCP1 and CCP2 (chloroplast carrier proteins) were referred to as chloroplast membrane proteins, because they were originally found in the fraction of isolated chloroplasts [236,237,238].
CCP1 and CCP2 expression, which is under control of CIA5, significantly increased in VL-cells at extremely low CO2 [203,204]. Knock-outs deficient in CCP1 and CCP2 displayed growth retardation, whereas Ci accumulation and photosynthetic affinity for Ci were not affected [236].
It was suggested that CCP1 and CCP2 are involved in the transport of Ci from mitochondria to chloroplasts [22] during the acclimation to Ci deficiency. It is assumed that mitochondrial CAs (Section 5.3.3) participates in the conversion of CO2 released during respiration and photorespiration. The resulting HCO3− can return to the chloroplast for recycling in the RPP cycle. This process should be especially important in VL-cells, allowing them to conserve the resources of intracellular Ci.
5.3. Carbonic Anhydrases and Ci Conversion System in the Stroma of C. reinhardtii Chloroplast
The CA enzyme, which interconverts two forms of Ci, CO2 and HCO3−, is the second important element of microalgal CCM. Fourteen genes were found in C. reinhardtii genome that encode the proven and potential CAs (Table 2). Three of these proteins belong to the α-class (CAH1, CAH2, and CAH3), six—to the β-class (CAH4-9), and three—to the γ-class of the enzyme (CAG1-3). Another two proteins are LCIB and LCIC, which probably belong to θ-class of CAs [82,213,239]. The CAs listed above have different locations in the cell and play different physiological roles [82,240] are discussed further below.
The master CCM regulator, CIA5, regulates the transcription of a number of C. reinhardtii CAs genes, which is enhanced under carbon-limiting conditions (Table 2). To date, only the following proteins have been experimentally attributed to the CCM: CAH1, CAH3, and the LCIB/LCIC complex [9,102,240]. It was suggested that other proteins (CAH2, CAH4, CAH5, CAH6, CAG1, CAG2, and CAG3) may indirectly participate in CCM.
5.3.1. CAs of Periplasmic Space
The genome of C. reinhardtii includes two genes, CAH1 and CAH2 (carbonic anhydrase 1/2), that encode highly homologous α-CAs located in the periplasmic space [241,242,243]. Numerous studies reviewed in [9,240,244], show that CAH1 is involved in the maintenance of CCM functioning under low or very-low CO2. The function of CAH1 has been attributed to the delivery of HCO3−/CO2 to HLA3 and LCI1 transporters in the plasma membrane. Compared to CAH1, the CAH2 content in C. reinhardtii cells is rather low [244]. It should be noted that the CAH2 gene is transcribed at a very low level, and this process is not regulated by CIA5 or reduced CO2 concentrations (Table 2). CAH2 was not thought to play a role in the CCM of C. reinhardtii and it is rather required for the assimilation of high CO2 concentrations [204,241].
In addition to α-class CAH1 and CAH2, two homologous β-class CAs, CAH7 and CAH8, were found in C. reinhardtii cells, and their specific activities were confirmed [245]. CAH8 is located in the periplasmic space, in close proximity to the plasma membrane [245]. If CAH8 is really associated with the membrane, it is still unknown which side of the membrane its active center is on; it was previously believed that it faces toward the periplasmic space [244]. Although the precise position of CAH7 has not been established, CAH8′s analogous extracellular location has been assumed [245]. There is ongoing debate over the CAH7/8 physiological functions, especially their role in CCM.
5.3.2. Putative Cytosolic CA
The CAH9 gene of C. reinhardtii encodes β-class CAs, the activity of which, however, has not been confirmed [85,244]. It should be noted that the name “CAH9” originally referred to the mitochondrial γ-class CA, now called CAG3 (GenBank IDs AAS48197 and AY463240). Nowadays, the name “CAH9” refers to the protein with GenBank ID ADW08083. This protein lacks a leader sequence, and it was localized in the microalgal cytoplasm [102]. CAH9 is very weakly transcribed in C. reinhardtii cells at both low and high CO2 concentrations; the content of the corresponding protein in cells is also low [244]. Currently, this CA is not thought to play an important role in Ci accumulation and CO2 fixation [203,244].
5.3.3. Mitochondrial CAs
Mitochondria of C. reinhardtii contain CAH4 and CAH5 proteins that belong to β-class CAs [102,246]. The CAH4/5 transcription is strictly regulated by the CO2 concentrations [203,204,247]. Both proteins are found only in L-cells and are absent in H-cells [244].
The direct involvement of CAH4/5 in CCM is questionable [9]. These proteins, together with the mitochondrial bicarbonate transporters CCP1/2 (Section 5.2.4), may prevent CO2 (generated in mitochondria during respiration and photorespiration) leakage from cells, directing it for further fixation in the RPP cycle [248]. Involvement in such an economical intracellular Ci consumption may explain the accumulation of CAH4/5 under low CO2 conditions.
Additionally, three potential γ-class CAs, and CAG1, CAG2 and CAG3 (Glp1), have been found in C. reinhardtii mitochondria [102,249,250]. The CAG1-3 genes are expressed constitutively, without any response to decreasing CO2 concentrations [204]. The CA activity was only assessed for the recombinant CAG3, but it was not confirmed [250]. If CAG1-3s do have enzymatic activity, their presumed function, like that of CAH4/5, would be to prevent the leakage of CO2 generated during respiration and photorespiration [240].
5.3.4. Chloroplast CAs and Ci Conversion System
CAH3—Carbonic Anhydrase of the Thylakoid Lumen
α-class CA, CAH3, is located in the thylakoid lumen of C. reinhardtii [251]. The amount of CAH3 mRNA increases slightly with a decrease in CO2 concentration [203,204]. CAH3 expression level does not change visibly in the CIA5 disrupted mutant of C. reinhardtii, suggesting that this CCM regulator is not involved in CAH3 regulation ([203], supplementary data).
CAH3 is a constitutive protein that is required at both low and high CO2 concentrations [251]. CAH3 plays a key role in CCM: it supplies CO2 molecules to Rubisco, generating them from the HCO3− in the lumen of interpyrenoid thylakoids [60,91,252,253]. In stromal thylakoids, where primary photosynthetic light reactions are carried out, CAH3 is bound to the polypeptides of the PSII reaction center [65]. CAH3 aids in the stabilization of the manganese cluster of PSII by hastening the removal of H+ released during the water-oxidizing reaction [92,93,94,254,255,256]. Thereby, CAH3 helps to facilitate the electron donation from the water to PSII and participates in the regulation of the light reaction of the photosynthesis.
Constitutive Complex LCIB/LCIC—Putative θ-Class CAs and Ci Conversion System in Chloroplast Stroma
Five proteins of the LCI family are encoded in the C. reinhardtii genome: LCIA, LCIB, LCIC, LCID and LCIE [9]. LCIA is a chloroplast envelope protein that transports HCO3− from the cytosol to the plastid stroma (Section 5.2.2). In contrast to LCIA, LCIB–E proteins are soluble proteins and lack transmembrane domains. Among those, LCIB and LCIC are the most studied. Little information is available about LCID and LCIE: their subcellular localization and function, including the involvement in photosynthetic Ci assimilation and CCM, are currently unknown.
Crystallographic data revealed that LCIB and LCIC are homologues and structurally analogous to the active θ-class CA from the diatom alga P. tricornutum [213,239]. Therefore, LCIB and LCIC are regarded as potential θ-class CAs in C. reinhardtii; however, their specific enzymatic activity has yet to be confirmed.
Both proteins, LCIB and LCIC, have been found in the stroma of the C. reinhardtii chloroplast, where they form a heteromultimeric complex [102,211,212]. LCIB and LCIC are among the low CO2-induced, CIA5-regulated genes [203]. Notably, the LCIB/LCIC complex is also detected in H-cells, but its content is significantly increased in L- and VL-cells under CO2-limiting conditions. The complex is diffusely distributed in the stroma of L-cells, whereas it is concentrated near the pyrenoid in VL-cells [211,212,257]. The migration of the complex likely occurs with the assistance of LCIC and a starch sheath of the pyrenoid [258,259].
The involvement of LCIB/LCIC in the CCM of C. reinhardtii has been well documented. If LCIB and LCIC really possess the CA activity, the LCIB/LCIC complex should convert the CO2 entering the chloroplast into HCO3−, thereby maintaining a CO2 gradient for its facilitated uptake in both L- and VL-cells [9]. Such a function is similar to that of the cyanobacterial NDH-13/4 systems (Section 4.2). In VL-cells, LCIB/LCIC can also act as a CO2 recapture system, preventing the CO2 leakage from the pyrenoid by its conversion into HCO3− [212,257]. It is suggested that the CA reaction is carried out by LCIB, while LCIC serves as the integrating structural component [9].
5.3.5. Flagellar CA
CAH6, an active β-class CA, was previously described as a chloroplast stromal enzyme in C. reinhardtii [260]. The recent in vivo analysis of the subcellular location of CAH6 using immunofluorescence microscopy allocated this protein to the flagella [102]. This finding confirmed the earlier data on flagella proteomes [261], as well as the whole proteome of Chlamydomonas chloroplast [262]. The CAH6 protein is found in both L- and H-cells [260]. CAH6 is slightly up-regulated in low CO2 environments, and its transcription is not regulated by the CIA5 [203]. CAH6 may be involved in the Chlamydomonas chemotaxis toward bicarbonate while determining the amount of Ci in the environment [102].
5.4. Pyrenoids
The Rubisco-containing microcompartment in microalgae is the pyrenoid, which serves as a functional analogue of cyanobacterial carboxysomes. Pyrenoids are protein complexes located in the chloroplasts of many eukaryotic microalgae [103,263,264]. These microbodies appear as electron-dense inclusions in the chloroplast, often surrounded by a starch sheath that becomes more pronounced under low CO2 (Figure 6a). The three-dimensional structure of pyrenoid, its protein composition and function, as well as the mutual arrangement of the CCM components associated with this microcompartment in C. reinhardtii, are well studied [101,102,103,105,265,266].

Figure 6.
Pyrenoid in C. reinhardtii cells. (a) The TEM image showing the pyrenoid inside the chloroplast of Chlamydomonas was kindly provided by Dr. Maria A. Sinetova (K.A. Timiryazev Institute of Plant Physiology, RAS, Moscow). Scale bar is 1 µm. (b) Schematic cross-sectional view of a Chlamydomonas cell showing the branching of cylindrical pyrenoid tubules from the thylakoid stacks and their entrance into the pyrenoid matrix through fenestrations in the starch sheath.
The proteomic analysis of the C. reinhardtii pyrenoid demonstrated that, in addition to Rubisco, it contains about 200 individual proteins [266]. Among them are pyrenoid matrix proteins, which are necessary for the packaging and efficient operation of Rubisco. These include Rubisco activase (chaperone protein RCA1), which ensures the carboxylation reaction at the highest possible rate [95], and the structural protein EPYC1 (essential pyrenoid component), which serves as a linker to promote the Rubisco aggregation in the pyrenoid [267] (previously known as LCI5—low-CO2-inducible protein 5). The presence of a variety of enzymes unrelated to the CO2 and starch metabolism in the C. reinhardtii pyrenoids suggests that these microcompartments may conduct previously unknown functions. Notably, the putative methyltransferase CIA6, which was attributed to the pyrenoid assembly [268], was not reported in the pyrenoid proteome ([266], supplementary data). Simultaneously, the intracellular location of CIA6 in Chlamydomonas cells is uncertain [102].
Thylakoid stacks in the stroma and the pyrenoid are connected by cylindrical pyrenoid tubules, which pass between starch grains and enter the pyrenoid matrix [265] (Figure 6b). Each pyrenoid tubule of C. reinhardtii contains 2–8 parallel minitubules inside, which are formed when two or more thylakoids merge into a common large tubule. Thus, the internal content of the minitubules is filled with chloroplast stroma; this solves the problem of the apparent physical isolation of Rubisco from other enzymes of the RPP cycle. The lumen of pyrenoid tubules is connected with the lumen of stromal thylakoids, allowing coordination of the chloroplasts’ light and dark reactions (Figure 7).

Figure 7.
Pyrenoid tubules with minitubules: schematic representation of the cross-sectional plane of C. reinhardtii cells at the entrance of pyrenoid tubules into the pyrenoid matrix.
It was suggested that HCO3− enters inside the thylakoids from the stroma side via CIA8 and BST1–3 transporters [201,202] and further diffuses inside the pyrenoid through the lumen of large pyrenoid tubules [265]. Alternatively, bicarbonate can diffuse to the interior of the pyrenoid directly via minitubules or enter into the lumen of pyrenoid tubules via its transporters. The minitubules also carry the unidirectional diffusion of ATP molecules required for Rubisco activase activity and exchange of RuBP and 3-PGA, which are substrates and products of the Rubisco carboxylase activity [95,264,269].
CAH3, which supplies CO2 to Rubisco, is located in the lumen of large pyrenoid tubules [60,92,250,251] (Figure 7). CAH3 is crucial for cell growth at low CO2 levels. Under these conditions, CAH3 is phosphorylated and transferred from stromal thylakoids to pyrenoid tubules [67]. Simultaneously, phosphorylation leads to a significant increase in the CA activity of CAH3. In the lumen of the pyrenoid thylakoid tubules, HCO3− is converted to CO2 by CAH3 (Figure 7). This reaction is facilitated by the high concentration of H+ in the lumen of illuminated cells, which shifts the CO2/HCO3− interconversion reaction towards the CO2 generation. The resulting lipophilic CO2 molecules can diffuse from the thylakoids into the pyrenoid matrix, where they are fixed by Rubisco in the RPP cycle. It should be highlighted that contrary to long-held beliefs, the pyrenoid matrix acts more like a liquid than a crystal-like or amorphous solid structure [101,270]. This knowledge may aid in imagining how the CO2 substrate penetrates into Rubisco’s active centers, despite its apparent density.
The pyrenoid is thought to spatially separate Rubisco from the oxygen-evolving complex because the thylakoid membranes of the pyrenoids are enriched in active PSI complexes and devoid of PSII [95,266]. This arrangement results in a low O2/CO2 ratio that favors the carboxylase function to Rubisco and reduces its oxygenase activity. Although fluorescently labeled components of PSI and PSII, cytochrome b6/f-complex and ATP synthase have been detected within C. reinhardtii pyrenoids [102], these results contradict with the proteomic data obtained on the isolated pyrenoids [266].
Pyrenoids are often surrounded by a starch sheath whose thickness increases during the acclimation to low CO2 [58]. The pyrenoid matrix and starch sheath bind together through the protein SAGA1 (starch granules abnormal 1), which also regulates the starch sheath morphology [104]. It is believed that the starch sheath can serve as a barrier, preventing CO2 leakage from the pyrenoid. However, surprisingly, the absence of the sheath does not interfere with the CCM activity [66,74,271]. To date, the barrier role of the starch sheath has not either been confirmed or disproved. However, its shape (morphology) has been demonstrated to be closely tied to the CCM’s functioning [104] and to the correct localization of the LCIB protein [259]. In addition, the pyrenoid-localized protein SAGA1 is necessary for the CAS-dependent expression of HLA3 and LCIA genes, encoding Ci transporters [106].
5.5. The Models of C. reinhardtii CCM under Varying Availability of Ci
The currently accepted scheme of CCM in microalgal cells, in general, does not substantially differ from the initial models [87,91,252,272,273,274]. However, it is now much better resolved due to the information acquired over more than 40 years of research regarding its molecular components, subcellular location and functions. Figure 8 shows a scheme of photosynthetic Ci assimilation under a different CO2 supply in C. reinhardtii.

Figure 8.
Schematic of photosynthetic Ci assimilation in C. reinhardtii under different CO2 supply. (a) H-cells growing under conditions of elevated CO2 concentrations (2–5%), with inactive CCM; Generalized model of CCM functioning in C. reinhardtii cells grown at: (b) low (0.03–0.5%; L cells) and (c) very low (<0.02%; VL cells) CO2 concentrations. The scheme shows bicarbonate entry into the lumen of intrapyrenoid thylakoids (pyrenoid tubules) from minitubules in L- and VL-cells, and does not take into account the participation of transporters located on membranes on the stroma side. Abbreviations: ChE—chloroplast envelope; PM—plasma membrane; SS—starch sheath; TM—thylakoid membrane; CAH3*, activated enzyme. The preferential pathways of Ci fluxes in the corresponding acclimation state of the cell are shown in bold.
Figure 8a shows the photosynthetic CO2 fixation pathway in H-cells grown at elevated CO2 concentrations (2–5%) in the absence of the active Ci uptake. Under these conditions, the photosynthesis is ensured by the following CCM components: CAs CAH2 and CAH3, CO2-channels RHP1/2, and the LCIB/LCIC complex. CO2 can enter the cell by a direct diffusion through cell membranes. The CO2 transport channels (RHP1/2) located in the plasma membrane may exert their functions with the participation of CAH2 [241]. At elevated CO2 concentrations, the pyrenoid structures are weak, the starch sheath is absent and Rubisco is located mainly in the stroma. Thus, the CO2 entering the cell is fixed in the RPP cycle directly in the chloroplast stroma. At the same time, the LCIB/LCIC complex can convert the CO2 entering the cell into HCO3−; this process is facilitated by slightly alkaline stromal pH values. Under the Ci excess, the main physiological role of the lumenal CA of thylakoids, CAH3, is obviously not in generation of CO2 for Rubisco, but in the protection of WOC of PSII from the excess of protons generated due to the activity of the electron transport chain [94]. HCO3− molecules enter into the lumen of stromal thylakoids via BST1–3 and CIA8 transporters. CAH3 catalyzes the interaction of HCO3− with protons to form CO2, which diffuses according to a concentration gradient into the stroma, where it can be consumed for RuBP carboxylation. The constitutive expression of LCIB and CAH3 proteins under high CO2 allows cells to respond immediately to the CO2 shortage, avoiding the delay associated with the de novo synthesis of the complete set of CCM proteins [9].
Figure 8b,c shows the organization of the CCM in the L- and VL-cells of C. reinhardtii adapted to low (0.03–0.5%) and very low (<0.02%) CO2, respectively. It is assumed that the high CO2 concentration near the Rubisco active centers under these conditions is achieved, as follows.
- (1)
- CAH6, located in the flagella of C. reinhardtii [102], may estimate an ambient Ci concentration, providing the orientation of cells relative to its gradient and enabling the directional chemotaxis (not shown in Figure 8).
- (2)
- Ci enters into the cell and further into the chloroplast by active transport in two forms, HCO3− and CO2. The periplasmic CA, CAH1, evidently contributes to the interconversion of Ci forms and participates in the supply of substrates for the plasma membrane transporters. The transport of HCO3− through the plasma membrane and chloroplast envelope is facilitated by the cooperative operation of HLA3 and LCIA [28,102,222]. It appears that the active HCO3− uptake occurs only in VL-cells, whereas in L-cells, the HLA3 and LCIA transporters are inhibited by CO2 [223]. The CO2 uptake in both L- and VL-cells may be due to its facilitated diffusion, driven by light-dependent stromal alkalinization and the operation of the LCIB/LCIC complex [9,257]. In L-cells, the flow of CO2 inside the cell is additionally provided by the LCI1 channel [228]. CO2 can enter the chloroplast not only by direct diffusion, but also through some, as of yet, unidentified transporter. The absorbed Ci accumulates in the chloroplast stroma in the form of HCO3−, which is facilitated by its slightly alkaline pH values.
- (3)
- Stromal HCO3− enters inside the thylakoids with the participation of CIA8 and BST1–3 transporters located in membranes on the stroma side, followed by a diffusion deep into the pyrenoid along the lumen of large pyrenoid tubules [201,202]. Simultaneously, HCO3− can diffuse inside the pyrenoid along the stroma inside the minitubules and then enter the lumen of intrapyrenoid thylakoids via transporters. The conversion of HCO3− into CO2 in the lumen of intrapyrenoid thylakoids is performed by CAH3, which exploits the inherently acidic pH value of this compartment [9,60,85,92].
- (4)
- CO2 formed in the intrapyrenoid thylakoid lumen diffuses into the pyrenoid matrix, where it is fixed by Rubisco in the RuBP carboxylation reaction. The co-localization of Rubisco and CA in the pyrenoid is the cornerstone of the CCM scheme, necessary for the efficient incorporation of CO2 into the initial chain of organic carbon transformations in the cell.
- (5)
- The prevention of CO2 leakage from the chloroplast is also very important. The current CCM model suggests that this process is primarily important in VL-cells [9,10]. This function is performed by the LCIB/LCIC protein complex (Ci conversion system) located in the chloroplast stroma. In VL-cells, the complex migrates to the periphery of the pyrenoid and acts as a vector module for the conversion of CO2 that escaped from the pyrenoid into HCO3− [211] (Figure 8c). This assumption is based on the high probability that LCIB/LCIC possess the CA activity. Under low CO2, the main function of the LCIB/LCIC complex is probably to hydrate the incoming cellular CO2 with the formation of HCO3− in the stroma [212].
- (6)
- It is assumed that mitochondrial CAs and Ci transporters may participate, although indirectly, in the CCM of C. reinhardtii [22,244,248] (not shown in Figure 8). The CO2 generated during photorespiration and respiration in these organelles can be converted by CAH4 and CAH5 into HCO3−, with the latter released into the cytosol via CCP1/2 transporters. Then, HCO3− can be returned to the chloroplast and reintroduced into the dark phase of photosynthesis.
6. Conclusions
The CCM of microalgae and cyanobacteria has been actively studied over the past 40 years after its discovery in the 1980s. The most significant progress in the study of its cellular and molecular organization (especially in microalgae), and understanding the subtleties of this mechanism functioning and regulation, have been made in the last decade due to the development of omics methodology and structural analysis. Many aspects of the CCM organization and regulation are being revised and evolved on a constant basis. Yet, some critical parts and characteristics of CCM operation remain unexplored. The following are the primary “hot spots” that warrant more investigation:
- More nuanced understanding of the mechanisms of CCM regulation. In particular, it remains largely unclear how its light regulation is performed;
- In-depth study of the functioning of the CA system and Ci transport;
- Determination of the mechanisms by which CO2 enters the algal chloroplast;
- Confirmation of the CA activity of the LCIB/LCIC complex in algae and CupA/B proteins in cyanobacteria;
- Evaluation of external, mitochondrial, and flagella CAs for their participation in photosynthetic assimilation of Ci and CCM;
- More direct experimental measurements of pH values in the periplasmic space of microalgae and cyanobacterial cells, as well as the pH of the carboxysomal matrix in cyanobacteria, would aid in understanding the role of CAs in Ci flow control;
- Clarification of the involvement of CIA6 protein in pyrenoid biogenesis, and the exact role of the starch sheath in the CCM;
- Currently, the CCM has been studied in detail only in model laboratory species of cyanobacteria and microalgae. However, the sets of CCM components and the significance of this mechanism may differ in species inhabiting different ecological niches;
- In addition, special attention should be paid to the study of the evolutionary origin of CCM in interrelation with the geological history of Earth and the history of the biosphere.
Author Contributions
Conceptualization, E.V.K. and N.A.P.; writing—original draft preparation, E.V.K., N.A.P. and D.A.L.; writing—review and editing, E.V.K. and D.A.L.; visualization, E.V.K.; funding acquisition, D.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from Russian Science Foundation (grant RSF # 21-74-30003) and partially supported by the Ministry of Science and Higher Education of the Russian Federation (theme no. 122042700043-9).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their sincere gratitude to Maria A. Sinetova (K.A. Timiryazev Institute of Plant Physiology, Moscow, Russian Federation) for the images of transmission electron microscopy included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raines, C.A. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: Current and future strategies. Plant Physiol. 2011, 155, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanism in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Meyer, M.; Griffiths, H. Origins and diversity of eukaryotic CO2-concentrating mechanisms: Lessons for the future. J. Exp. Bot. 2013, 64, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Maberly, S.C.; Gontero, B. Ecological imperatives for aquatic CO2-concentrating mechanisms. J. Exp. Bot. 2017, 68, 3797–3814. [Google Scholar] [CrossRef]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef]
- Burnap, R.L.; Hagemann, M.; Kaplan, A. Regulation of CO2 concentrating mechanism in cyanobacteria. Life 2015, 5, 348–371. [Google Scholar] [CrossRef]
- Hagemann, M.; Song, S.; Brouwer, E.M. Inorganic carbon assimilation in cyanobacteria: Mechanisms, regulation, and engineering. In Cyanobacteria Biotechnology, 1st ed.; Hudson, P., Lee, S.Y., Nielsen, J., Eds.; Wiley-VCH: Weinheim, Germany, 2021; pp. 1–31. [Google Scholar]
- Jungnick, N.; Ma, Y.; Mukherjee, B.; Cronan, J.C.; Speed, D.J.; Laborde, S.M.; Longstreth, D.J.; Moroney, J.V. The carbon concentrating mechanism in Chlamydomonas reinhardtii: Finding the missing pieces. Photosynth. Res. 2014, 121, 159–173. [Google Scholar] [CrossRef]
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Mackinder, L.C. The Chlamydomonas CO2-concentrating mechanism and its potential for engineering photosynthesis in plants. New Phytol. 2018, 217, 54–61. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Samylina, O.S. CO2-concentrating mechanism and its traits in haloalkaliphilic cyanobacteria. Microbiology 2015, 84, 112–124. [Google Scholar] [CrossRef]
- Klanchui, A.; Cheevadhanarak, S.; Prommeenate, P.; Meechai, A. Exploring Components of the CO2-concentrating mechanism in alkaliphilic cyanobacteria through genome-based analysis. Comput. Struct. Biotechnol. J. 2017, 15, 340–350. [Google Scholar] [CrossRef]
- Zavarzin, G.A. Epicontinental soda lakes as probable relict biotopes of terrestrial biota formation. Microbiology 1993, 62, 473–479. [Google Scholar]
- Badger, M.R.; Price, G.D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J. Exp. Bot. 2003, 54, 609–622. [Google Scholar] [CrossRef]
- Raven, J.A.; Cockell, C.S.; De La Rocha, C.L. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2641–2650. [Google Scholar] [CrossRef]
- Hagemann, M.; Kern, R.; Maurino, V.G.; Hanson, D.T.; Weber, A.P.; Sage, R.F.; Bauwe, H. Evolution of photorespiration from cyanobacteria to land plants, considering protein phylogenies and acquisition of carbon concentrating mechanisms. J. Exp. Bot. 2016, 67, 2963–2976. [Google Scholar] [CrossRef]
- Pronina, N.A.; Kupriyanova, E.V.; Igamberdiev, A.U. Photosynthetic carbon metabolism and CO2 concentration mechanism of cyanobacteria. In Modern Topics in the Phototrophic Prokaryotes—Metabolism, Bioenergetics, and Omics; Hallenbeck, P.C., Ed.; Springer: Cham, Switzerland, 2017; pp. 271–303. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J.; Sánchez-Baracaldo, P. The possible evolution and future of CO2-concentrating mechanisms. J. Exp. Bot. 2017, 68, 3701–3716. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.J.; Wing, B.A.; Jasper, C.E.; Hill, N.C.; Cameron, J.C. Carbon isotope evidence for the global physiology of Proterozoic cyanobacteria. Sci. Adv. 2021, 7, eabc8998. [Google Scholar] [CrossRef] [PubMed]
- Kroth, P.G. The biodiversity of carbon assimilation. J. Plant Physiol. 2015, 172, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, C.; Capó-Bauçà, S.; Niinemets, Ü.; Stoll, H.; Aguiló-Nicolau, P.; Galmés, J. Evolutionary trends in RuBisCO kinetics and their co-evolution with CO2 concentrating mechanisms. Plant J. 2020, 101, 897–918. [Google Scholar] [CrossRef]
- Santhanagopalan, I.; Wong, R.; Mathur, T.; Griffiths, H. Orchestral manoeuvres in the light: Crosstalk needed for regulation of the Chlamydomonas carbon concentration mechanism. J. Exp. Bot. 2021, 72, 4604–4624. [Google Scholar] [CrossRef]
- Keeley, J.E.; Rundel, P.W. Evolution of CAM and C4 carbon-concentrating mechanisms. Int. J. Plant Sci. 2003, 164, S55–S77. [Google Scholar] [CrossRef]
- Heyduk, K. Evolution of Crassulacean acid metabolism in response to the environment: Past, present, and future. Plant Physiol. 2022, 190, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Randerson, J.T.; McClain, C.R.; Feldman, G.C.; Los, S.O.; Tucker, C.J.; Falkowski, P.G.; Field, C.B.; Frouin, R.; Esaias, W.E.; et al. Biospheric primary production during an ENSO transition. Science 2001, 291, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.M.; Long, S.P. Can the cyanobacterial carbon-concentrating mechanism increase photosynthesis in crop species? A theoretical analysis. Plant Physiol. 2014, 164, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.; Feike, D.; Mackinder, L.C.; Meyer, M.T.; Griffiths, H.; Jonikas, M.C.; Smith, A.M.; McCormick, A.J. Introducing an algal carbon concentrating mechanism into higher plants: Location and incorporation of key components. Plant Biotechnol. J. 2016, 14, 1302–1315. [Google Scholar] [CrossRef]
- Rae, B.D.; Long, B.M.; Förster, B.; Nguyen, N.D.; Velanis, C.N.; Atkinson, N.; Hee, W.Y.; Mukherjee, B.; Price, G.D.; McCormick, A.J. Progress and challenges of engineering a biophysical CO2-concentrating mechanism into higher plants. J. Exp. Bot. 2017, 68, 3717–3737. [Google Scholar] [CrossRef]
- Hennacy, J.H.; Jonikas, M.C. Prospects for engineering biophysical CO2 concentrating mechanisms into land plants to enhance yields. Annu. Rev. Plant Biol. 2020, 71, 461–485. [Google Scholar] [CrossRef]
- Tabita, F.R.; Satagopan, S.; Hanson, T.E.; Kreel, N.E.; Scott, S.S. Distinct form I, II, III, and IV RuBisCO proteins from the three kingdoms of life provide clues about RuBisCO evolution and structure/function relationships. J. Exp. Bot. 2008, 59, 1515–1524. [Google Scholar] [CrossRef]
- Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008, 59, 1555–1568. [Google Scholar] [CrossRef]
- Andersson, I.; Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 2008, 46, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Ashida, H.; Saito, Y.; Kojima, C.; Kobayashi, K.; Ogasawara, N.; Yokota, A. A functional link between RuBisCO-like protein of Bacillus and photosynthetic RuBisCO. Science 2003, 302, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Spalding, M.H. CO2 acquisition, concentration and fixation in cyanobacteria and algae. In Photosynthesis: Physiology and Metabolism; Leegood, R.C., Sharkey, T.D., von Caemmerer, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 369–397. [Google Scholar]
- Igamberdiev, A.U.; Kleczkowski, L.A. Optimization of CO2 fixation in photosynthetic cells via thermodynamic buffering. Biosystems 2011, 103, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, R.W. An upper limit to the active site concentration of ribulose bisphosphate carboxylase in chloroplasts. Biochem. J. 1986, 236, 311. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.G.B.; Farquhar, G.D.; Andrews, T.J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Raven, J.A. Rubisco: Still the most abundant protein of Earth? New Phytol. 2013, 198, 1–3. [Google Scholar] [CrossRef]
- Flynn, K.J.; Raven, J.A. What is the limit for photoautotrophic plankton growth rates? J. Plankton Res. 2017, 39, 13–22. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Furbank, R.T.; Hatch, M.D. Mechanism of C4 photosynthesis: A model describing the inorganic carbon pool in bundle sheath cells. Plant Physiol. 1989, 91, 1372–1381. [Google Scholar] [CrossRef]
- Ludwig, M. Carbonic anhydrase and the molecular evolution of C4 photosynthesis. Plant Cell Environ. 2012, 35, 22–37. [Google Scholar] [CrossRef]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef] [PubMed]
- Nobel, P.S. Achievable productivities of certain CAM plants: Basis for high values compared with C3 and C4 plants. New Phytol. 1991, 119, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Kaplan, A.; Berry, J.A. Internal inorganic carbon pool of Chlamydomonas reinhardtii. Evidence for a carbon dioxide concentrating mechanism. Plant Physiol. 1980, 66, 407–413. [Google Scholar] [CrossRef]
- Beardall, J.; Raven, J.A. Transport of inorganic carbon and the ‘CO2 concentrating mechanism’ in Chlorella emersonii (Chlorophyceae). J. Phycol. 1981, 17, 134–141. [Google Scholar] [CrossRef]
- Pronina, N.A.; Ramazanov, Z.M.; Semenenko, V.E. Carbonic anhydrase activity of Chlorella cells as a function of CO2 concentration. Sov. Plant Physiol. 1981, 28, 345–351. [Google Scholar]
- Aizawa, K.; Miyachi, S. Carbonic anhydrase and CO2-concentrating mechanism in microalgae and cyanobacteria. FEMS Microbiol. Rev. 1986, 39, 215–233. [Google Scholar] [CrossRef]
- Spalding, M.H.; Ogren, W.L. Photosynthesis is required for induction of the CO2-concentrating system in Chlamydomonas reinhardtii. FEBS Lett. 1982, 145, 41–44. [Google Scholar] [CrossRef]
- Moroney, J.V.; Tolbert, N.E.; Sears, B.B. Complementation analysis of the inorganic carbon concentrating mechanism of Chlamydomonas reinhardtii. Mol. Gen. Genet. 1986, 204, 199–203. [Google Scholar] [CrossRef]
- Palmqvist, K.; Sjöberg, S.; Samuelsson, G. Induction of inorganic carbon accumulation in the unicellular green algae Scenedesmus obliquus and Chlamydomonas reinhardtii. Plant Physiol. 1988, 87, 437–442. [Google Scholar] [CrossRef]
- Tsuji, Y.; Nakajima, K.; Matsuda, Y. Molecular aspects of the biophysical CO2-concentrating mechanism and its regulation in marine diatoms. J. Exp. Bot. 2017, 68, 3763–3772. [Google Scholar] [CrossRef]
- Sage, R.F.; Khoshravesh, R. Passive CO2 concentration in higher plants. Curr. Opin. Plant. Biol. 2016, 31, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zabaleta, E.; Martin, M.V.; Braun, H.P. A basal carbon concentrating mechanism in plants? Plant Sci. 2012, 187, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, M.; Ohnuma, E.; Sato, N.; Takaku, T.; Kawaguchi, A. Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol. 1990, 93, 851–856. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.M.L.; Gibbs, S.P.; Espie, G.S. Effect of dissolved inorganic carbon on the expression of carboxysomes, localization of Rubisco and the mode of inorganic carbon transport in cells of the cyanobacterium Synechococcus UTEX 625. Arch. Microbiol. 1993, 159, 21–29. [Google Scholar] [CrossRef]
- Ramazanov, Z.; Rawat, M.; Henk, M.C.; Mason, C.B.; Matthews, S.W.; Moroney, J.V. The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta 1994, 195, 210–216. [Google Scholar] [CrossRef]
- Geraghty, A.M.; Spalding, M.H. Molecular and structural changes in Chlamydomonas under limiting CO2: A possible mitochondrial role in adaptation. Plant Physiol. 1996, 111, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Kupriyanova, E.V.; Markelova, A.G.; Allakhverdiev, S.I.; Pronina, N.A. Identification and functional role of the carbonic anhydrase Cah3 in thylakoid membranes of pyrenoid of Chlamydomonas reinhardtii. Biochim. Biophys. Acta 2012, 1817, 1248–1255. [Google Scholar] [CrossRef]
- Mitchell, M.C.; Meyer, M.T.; Griffiths, H. Dynamics of carbon-concentrating mechanism induction and protein relocalization during the dark-to-light transition in synchronized Chlamydomonas reinhardtii. Plant Physiol. 2014, 166, 1073–1082. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, F.; Dykes, G.F.; Liu, L.N. Diurnal regulation of in vivo localization and CO2-fixing activity of carboxysomes in Synechococcus elongatus PCC 7942. Life 2020, 10, 169. [Google Scholar] [CrossRef]
- Kaczmarski, J.A.; Hong, N.S.; Mukherjee, B.; Wey, L.T.; Rourke, L.; Förster, B.; Peat, T.S.; Price, G.D.; Jackson, C.J. Structural basis for the allosteric regulation of the SbtA bicarbonate transporter by the PII-like protein, SbtB, from Cyanobium sp. PCC7001. Biochemistry 2019, 58, 5030–5039. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J.; Giordano, M. Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth. Res. 2014, 121, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Karlsson, J.; Rojdestvenski, I.; Pronina, N.; Klimov, V.; Oquist, G.; Samuelsson, G. Role of a novel photosystem II-associated carbonic anhydrase in photosynthetic carbon assimilation in Chlamydomonas reinhardtii. FEBS Lett. 1999, 444, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, A.; Martinez, F.; Plumed, M.d.P.; Ramazanov, Z. The induction of the CO2 concentrating mechanism in a starch-less mutant of Chlamydomonas reinhardtii. Physiol. Plant. 1996, 98, 798–802. [Google Scholar] [CrossRef]
- Blanco-Rivero, A.; Shutova, T.; Roman, M.J.; Villarejo, A.; Martinez, F. Phosphorylation controls the localization and activation of the lumenal carbonic anhydrase in Chlamydomonas reinhardtii. PLoS ONE 2012, 7, e49063. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitch, E.I. Photosynthesis and Related Processes; Interscience Publishers: New York, NY, USA, 1945; Volume 1, p. 177. [Google Scholar]
- Dickson, A.G. The carbon dioxide system in seawater: Equilibrium chemistry and measurements. In Guide to Best Practices for Ocean Acidification Research and Data Reporting; Riebesell, U., Fabry, V.J., Hansson, L., Gattuso, J.-P., Eds.; Publications Office of the European Union: Luxembourg, Luxembourg, 2010; pp. 17–40. [Google Scholar]
- Fukuzawa, H.; Ogawa, T.; Kaplan, A. The uptake of CO2 by cyanobacteria and microalgae. In Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation, Advances in Photosynthesis and Respiration; Eaton-Rye, J., Tripathy, B., Sharkey, T., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 34, pp. 625–650. [Google Scholar] [CrossRef]
- Price, G.D.; Sültemeyer, D.; Klughammer, B.; Ludwig, M.; Badger, M.R. The functioning of the CO2 concentrating mechanism in several cyanobacterial strains: A review of general physiological characteristics, genes, proteins and recent advances. Can. J. Bot. 1998, 76, 973–1002. [Google Scholar] [CrossRef]
- Lucker, B.; Kramer, D.M. Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth. Res. 2013, 117, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, K.; Sundblad, L.G.; Wingsle, G.; Samuelsson, G. Acclimation of photosynthetic light reactions during induction of inorganic carbon accumulation in the green alga Chlamydomonas reinhardtii. Plant Physiol. 1990, 94, 357–366. [Google Scholar] [CrossRef]
- Kaplan, A.; Reinhold, L. CO2 concentrating mechanism in photosynthetic microorganisms. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 539–570. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef]
- Kern, D.M. The hydration of carbon dioxide. J. Chem. Educ. 1960, 37, 14–23. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Pronina, N.A.; Los, D.A. Carbonic anhydrase—A universal enzyme of the carbon-based life. Photosynthetica 2017, 55, 3–19. [Google Scholar] [CrossRef]
- Chegwidden, W.R.; Carter, N.D.; Edwards, Y.H. (Eds.) The Carbonic Anhydrases: New Horizons; Birkhäuser Verlag: Basel, Switzerland, 2013; 619p. [Google Scholar]
- Supuran, C.T. A story on carbon dioxide and its hydration. In New Trends in Macromolecular and Supramolecular Chemistry for Biological Applications; Abadie, M.J.M., Pinteala, M., Rotaru, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 115–131. [Google Scholar] [CrossRef]
- Frost, S.C.; McKenna, R. (Eds.) Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Springer Science & Business Media: Dordrecht, The Netherlands, 2014; Subcellular Biochemistry; Volume 75, 430p. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Ignatova, L.K.; Zhurikova, E.M.; Yanyushin, M.F.; Ivanov, B.N. The multiplicity of functions of carbonic anhydrases in higher plants. In Carbonic Anhydrases: Biochemistry, Mechanism of Action and Therapeutic Applications; Penttinen, J., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 111–137. [Google Scholar]
- DiMario, R.J.; Machingura, M.C.; Waldrop, G.L.; Moroney, J.V. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 2018, 268, 11–17. [Google Scholar] [CrossRef]
- Braun, F.J.; Hegemann, P. Direct measurement of cytosolic calcium and pH in living Chlamydomonas reinhardtii cells. Eur. J. Cell Biol. 1999, 78, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Messerli, M.A.; Amaral-Zettler, L.A.; Zettler, E.; Jung, S.K.; Smith, P.J.; Sogin, M.L. Life at acidic pH imposes an increased energetic cost for a eukaryotic acidophile. J. Exp. Biol. 2005, 208, 2569–2579. [Google Scholar] [CrossRef] [PubMed]
- Moroney, J.V.; Ynalvez, R.A. Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot. Cell 2007, 6, 1251–1259. [Google Scholar] [CrossRef]
- Long, B.M.; Förster, B.; Pulsford, S.B.; Price, G.D.; Badger, M.R. Rubisco proton production can drive the elevation of CO2 within condensates and carboxysomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2014406118. [Google Scholar] [CrossRef]
- Pronina, N.A.; Semenenko, V.E. Localization of membrane-associated and soluble forms of carbonic anhydrase in Chlorella cells. Sov. Plant Physiol. 1984, 31, 187–196. [Google Scholar]
- Shively, J.M.; Vankeulen, G.; Meijer, W.G. Something from almost nothing: Carbon dioxide fixation in chemoautotrophs. Annu. Rev. Microbiol. 1998, 52, 191–230. [Google Scholar] [CrossRef]
- Espie, G.S.; Kimber, M.S. Carboxysomes: Cyanobacterial RubisCO comes in small packages. Photosynth. Res. 2011, 109, 7–20. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Q.; Yang, M.; Dykes, G.F.; Weetman, S.L.; Xin, W.; He, H.L.; Liu, L.N. Probing the internal pH and permeability of a carboxysome shell. Biomacromolecules 2022, 23, 4339–4348. [Google Scholar] [CrossRef]
- Raven, J.A. CO2-concentrating mechanisms: A direct role for thylakoid lumen acidification? Plant Cell Environ. 1997, 20, 147–154. [Google Scholar] [CrossRef]
- Markelova, A.G.; Sinetova, M.P.; Kupriyanova, E.V.; Pronina, N.A. Distribution and functional role of carbonic anhydrase Cah3 associated with thylakoid membranes in the chloroplast and pyrenoid of Chlamydomonas reinhardtii. Russ. J. Plant Physiol. 2009, 56, 761–768. [Google Scholar] [CrossRef]
- Villarejo, A.; Shutova, T.; Moskvin, O.; Forssen, M.; Klimov, V.V.; Samuelsson, G. A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J. 2002, 21, 1930–1938. [Google Scholar] [CrossRef]
- Shutova, T.; Kenneweg, H.; Buchta, J.; Nikitina, J.; Terentyev, V.; Chernyshov, S.; Andersson, B.; Allakhverdiev, S.I.; Klimov, V.V.; Dau, H.; et al. The photosystem II-associated Cah3 in Chlamydomonas enhance the O2 evolution rate by proton removal. EMBO J. 2008, 27, 782–791. [Google Scholar] [CrossRef]
- McKay, R.M.L.; Gibbs, S.P. Composition and function of pyrenoids: Cytochemical and immunocytochemical approaches. Can. J. Bot. 1991, 69, 1040–1052. [Google Scholar] [CrossRef]
- Pronina, N.A.; Semenenko, V.E. Role of the pyrenoid in concentration, generation and fixation of CO2 in the chloroplast of microalgae. Sov. Plant Physiol. 1992, 39, 470–476. [Google Scholar]
- Kuchitsu, K.; Tsuzuki, M.; Miyachi, S. Polypeptide composition and enzyme activities of the pyrenoid and its regulation by CO2 concentration in unicellular green algae. Can. J. Bot. 1991, 69, 1062–1069. [Google Scholar] [CrossRef]
- Reinhold, L.; Zviman, M.; Kaplan, A. A quantitative model for inorganic carbon fluxes and photosynthesis in cyanobacteria. Plant Physiol. 1989, 27, 945–954. [Google Scholar]
- Borkhsenious, O.N.; Mason, C.B.; Moroney, J.V. The intracellular localization of ribulose-1,5-bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. Plant Physiol. 1998, 116, 1585–1591. [Google Scholar] [CrossRef]
- Eisenhut, M.; Aguirre von Wobeser, E.; Jonas, L.; Schubert, H.; Ibelings, B.W.; Bauwe, H.; Matthijs, H.C.; Hagemann, M. Long-term response toward inorganic carbon limitation in wild type and glycolate turnover mutants of the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 2007, 144, 1946–1959. [Google Scholar] [CrossRef]
- Freeman Rosenzweig, E.S.; Xu, B.; Kuhn Cuellar, L.; Martinez-Sanchez, A.; Schaffer, M.; Strauss, M.; Cartwright, H.N.; Ronceray, P.; Plitzko, J.M.; Förster, F.; et al. The eukaryotic CO2-concentrating organelle is liquid-like and exhibits dynamic reorganization. Cell 2017, 171, 148–162.e19. [Google Scholar] [CrossRef] [PubMed]
- Mackinder, L.C.M.; Chen, C.; Leib, R.D.; Patena, W.; Blum, S.R.; Rodman, M.; Ramundo, S.; Adams, C.M.; Jonikas, M.C. A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 2017, 171, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Whittaker, C.; Griffiths, H. The algal pyrenoid: Key unanswered questions. J. Exp. Bot. 2017, 68, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Itakura, A.K.; Chan, K.X.; Atkinson, N.; Pallesen, L.; Wang, L.; Reeves, G.; Patena, W.; Caspari, O.; Roth, R.; Goodenough, U.; et al. A Rubisco-binding protein is required for normal pyrenoid number and starch sheath morphology in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2019, 116, 18445–18454. [Google Scholar] [CrossRef]
- Barrett, J.; Girr, P.; Mackinder, L.C.M. Pyrenoids: CO2-fixing phase separated liquid organelles. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118949. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, D.; Yamano, T.; Niikawa, Y.; Hu, D.; Fukuzawa, H. A pyrenoid-localized protein SAGA1 is necessary for Ca2+-binding protein CAS-dependent expression of nuclear genes encoding inorganic carbon transporters in Chlamydomonas reinhardtii. Photosynth. Res. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Liu, H.; Landry, M.R.; Vaulot, D.; Campbell, L. Prochlorococcus growth rates in the central equatorial Pacific: An application of the fmax approach. J. Geophys. Res. 1999, 104, 3391–3399. [Google Scholar] [CrossRef]
- Rae, B.D.; Long, B.M.; Whitehead, L.F.; Förster, B.; Badger, M.R.; Price, G.D. Cyanobacterial carboxysomes: Microcompartments that facilitate CO2 fixation. J. Mol. Microbiol. Biotechnol. 2013, 23, 300–307. [Google Scholar] [CrossRef]
- Mangan, N.M.; Flamholz, A.; Hood, R.D.; Milo, R.; Savage, D.F. pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, E5354–E5362. [Google Scholar] [CrossRef]
- Long, B.M.; Rae, B.D.; Rolland, V.; Förster, B.; Price, G.D. Cyanobacterial CO2-concentrating mechanism components: Function and prospects for plant metabolic engineering. Curr. Opin. Plant Biol. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- Guillén-García, A.; Gibson, S.E.R.; Jordan, C.J.C.; Ramaswamy, V.K.; Linthwaite, V.L.; Bromley, E.H.C.; Brown, A.P.; Hodgson, D.R.W.; Blower, T.R.; Verlet, J.R.R.; et al. Allophycocyanin A is a carbon dioxide receptor in the cyanobacterial phycobilisome. Nat Commun. 2022, 13, 5289. [Google Scholar] [CrossRef]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Cann, M.J.; Hammer, A.; Zhou, J.; Kanacher, T.A. A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J. Biol. Chem. 2003, 278, 35033–35038. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Hodgson, D.R.; Cann, M.J. Regulation of prokaryotic adenylyl cyclases by CO2. Biochem. J. 2006, 396, 215–218. [Google Scholar] [CrossRef]
- Sun, N.; Han, X.; Xu, M.; Kaplan, A.; Espie, G.S.; Mi, H. A thylakoid-located carbonic anhydrase regulates CO2 uptake in the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2019, 222, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, K.; Selim, K.A. Carbon/nitrogen homeostasis control in cyanobacteria. FEMS Microbiol. Rev. 2020, 44, 33–53. [Google Scholar] [CrossRef]
- Gabay, C.; Lieman-Hurwitz, J.; Hassidim, M.; Ronen-Tarazi, M.; Kaplan, A. Modification of topA in Synechococcus sp. PCC 7942 resulted in mutants capable of growing under low but not high concentration of CO2. FEMS Microbiol. Lett. 1998, 159, 343–347. [Google Scholar] [CrossRef]
- Prakash, J.S.S.; Sinetova, M.; Kupriyanova, E.; Zorina, A.; Suzuki, I.; Murata, N.; Los, D.A. DNA supercoiling regulates the stress-inducible expression of genes in the cyanobacterium. Mol. Biosyst. 2009, 5, 1904–1912. [Google Scholar] [CrossRef]
- Omata, T.; Gohta, S.; Takahashi, Y.; Harano, Y.; Maeda, S. Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J. Bacteriol. 2001, 183, 1891–1898. [Google Scholar] [CrossRef]
- Nishimura, T.; Takahashi, Y.; Yamaguchi, O.; Suzuki, H.; Maeda, S.I.; Omata, T. Mechanism of low CO2-induced activation of the cmp bicarbonate transporter operon by a LysR family protein in the cyanobacterium Synechococcus elongatus strain PCC 7942. Mol. Microbiol. 2008, 68, 98–109. [Google Scholar] [CrossRef]
- Figge, R.M.; Cassier-Chauvat, C.; Chauvat, F.; Cerff, R. Characterization and analysis of an NAD(P)H dehydrogenase transcriptional regulator critical for the survival of cyanobacteria facing inorganic carbon starvation and osmotic stress. Mol. Microbiol. 2001, 39, 455–469. [Google Scholar] [CrossRef]
- Wang, H.L.; Postier, B.L.; Burnap, R.L. Alterations in global patterns of gene expression in Synechocystis sp. PCC 6803 in response to inorganic carbon limitation and the inactivation of ndhR, a LysR family regulator. J. Biol. Chem. 2004, 279, 5739–5751. [Google Scholar] [CrossRef]
- Woodger, F.J.; Bryant, D.A.; Price, G.D. Transcriptional regulation of the CO2-concentrating mechanism in a euryhaline, coastal marine cyanobacterium, Synechococcus sp. strain PCC 7002: Role of NdhR/CcmR. J. Bacteriol. 2007, 189, 3335–3347. [Google Scholar] [CrossRef] [PubMed]
- Klähn, S.; Orf, I.; Schwarz, D.; Matthiessen, J.K.; Kopka, J.; Hess, W.R.; Hagemann, M. Integrated transcriptomic and metabolomic characterization of the low-carbon response using an ndhR mutant of Synechocystis sp. PCC 6803. Plant Physiol. 2015, 169, 1540–1556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Wang, X.P.; Sun, H.; Han, S.J.; Li, W.F.; Cui, N.; Lin, G.M.; Zhang, J.Y.; Cheng, W.; Cao, D.D.; et al. Coordinating carbon and nitrogen metabolic signaling through the cyanobacterial global repressor NdhR. Proc. Natl. Acad. Sci. USA 2018, 115, 403–408. [Google Scholar] [CrossRef]
- Daley, S.M.; Kappell, A.D.; Carrick, M.J.; Burnap, R.L. Regulation of the cyanobacteria CO2-concentrating mechanism involves internal sensing of NADP+ and alpha-ketogutarate levels by transcription factor CcmR. PLoS ONE 2012, 7, e41286. [Google Scholar] [CrossRef] [PubMed]
- Muro-Pastor, M.I.; Reyes, J.C.; Florencio, F.J. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J. Biol. Chem. 2001, 276, 38320–38328. [Google Scholar] [CrossRef]
- Zhang, C.C.; Zhou, C.Z.; Burnap, R.L.; Peng, L. Carbon/nitrogen metabolic balance: Lessons from cyanobacteria. Trends Plant. Sci. 2018, 23, 1116–1130. [Google Scholar] [CrossRef]
- Lieman-Hurwitz, J.; Haimovich, M.; Shalev-Malul, G.; Ishii, A.; Hihara, Y.; Gaathon, A.; Lebendiker, M.; Kaplan, A. A cyanobacterial AbrB-like protein affects the apparent photosynthetic affinity for CO2 by modulating low-CO2-induced gene expression. Environ. Microbiol. 2009, 11, 927–936. [Google Scholar] [CrossRef]
- Orf, I.; Schwarz, D.; Kaplan, A.; Kopka, J.; Hess, W.R.; Hagemann, M.; Klähn, S. CyAbrB2 Contributes to the transcriptional regulation of low CO2 acclimation in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2016, 57, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Georg, J.; Hess, W.R. cis-Antisense RNA, another level of gene regulation in bacteria. Microbiol. Mol. Biol. Rev. 2011, 75, 286–300. [Google Scholar] [CrossRef]
- Spät, P.; Barske, T.; Maček, B.; Hagemann, M. Alterations in the CO2 availability induce alterations in the phosphoproteome of the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2021, 231, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; Haase, F.; Hartmann, M.D.; Hagemann, M.; Forchhammer, K. PII-like signaling protein SbtB links cAMP sensing with cyanobacterial inorganic carbon response. Proc. Natl. Acad. Sci. USA 2018, 115, E4861–E4869. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, K.; Selim, K.A.; Huergo, L.F. New views on PII signaling: From nitrogen sensing to global metabolic control. Trends Microbiol. 2022, 30, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Förster, B.; Rourke, L.; Howitt, S.M.; Price, G.D. Characterisation of cyanobacterial bicarbonate transporters in E. coli shows that SbtA homologs are functional in this heterologous expression system. PLoS ONE 2014, 9, e115905. [Google Scholar] [CrossRef]
- Fang, S.; Huang, X.; Zhang, X.; Zhang, M.; Hao, Y.; Guo, H.; Liu, L.N.; Yu, F.; Zhang, P. Molecular mechanism underlying transport and allosteric inhibition of bicarbonate transporter SbtA. Proc. Natl. Acad. Sci. USA 2021, 118, e2101632118. [Google Scholar] [CrossRef]
- Mantovani, O.; Reimann, V.; Haffner, M.; Herrmann, F.P.; Selim, K.A.; Forchhammer, K.; Hess, W.R.; Hagemann, M. The impact of the cyanobacterial carbon-regulator protein SbtB and of the second messengers cAMP and c-di-AMP on CO2-dependent gene expression. New Phytol. 2022, 234, 1801–1816. [Google Scholar] [CrossRef]
- Price, G.D. Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism. Photosynth. Res. 2011, 109, 47–57. [Google Scholar] [CrossRef]
- Desmarais, J.J.; Flamholz, A.I.; Blikstad, C.; Dugan, E.J.; Laughlin, T.G.; Oltrogge, L.M.; Chen, A.W.; Wetmore, K.; Diamond, S.; Wang, J.Y.; et al. DABs are inorganic carbon pumps found throughout prokaryotic phyla. Nat. Microbiol. 2019, 4, 2204–2215. [Google Scholar] [CrossRef]
- Price, G.D.; Long, B.M.; Förster, B. DABs accumulate bicarbonate. Nat. Microbiol. 2019, 4, 2029–2030. [Google Scholar] [CrossRef]
- Omata, T.; Price, G.D.; Badger, M.R.; Okamura, M.; Gohta, S.; Ogawa, T. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc. Natl. Acad. Sci. USA 1999, 96, 13571–13576. [Google Scholar] [CrossRef]
- McGinn, P.J.; Price, G.D.; Maleszka, R.; Badger, M.R. Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol. 2003, 132, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Woodger, F.J.; Badger, M.R.; Price, G.D. Inorganic carbon limitation induces transcripts encoding components of the CO2-concentrating mechanism in Synechococcus sp. PCC7942 through a redox-independent pathway. Plant Physiol. 2003, 133, 2069–2080. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Cho, S.M.; Park, Y.I.; Pronina, N.A.; Los, D.A. The complete genome of a cyanobacterium from a soda lake reveals the presence of the components of CO2-concentrating mechanism. Photosynth. Res. 2016, 130, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Katoh, H.; Sonoda, M.; Ohkawa, H.; Shimoyama, M.; Fukuzawa, H.; Kaplan, A.; Ogawa, T. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: Function and phylogenetic analysis. J. Biol. Chem. 2002, 277, 18658–18664. [Google Scholar] [CrossRef]
- Price, G.D.; Woodger, F.J.; Badger, M.R.; Howitt, S.M.; Tucker, L. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 18228–18233. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Mironov, K.S.; Novikova, G.V.; Dykman, L.A.; Rodionova, M.V.; Gabrielyan, D.A.; Los, D.A. Highly active extracellular α-class carbonic anhydrase of Cyanothece sp. ATCC 51142. Biochimie 2019, 160, 200–209. [Google Scholar] [CrossRef]
- Battchikova, N.; Eisenhut, M.; Aro, E.M. Cyanobacterial NDH-1 complexes: Novel insights and remaining puzzles. Biochim. Biophys. Acta 2011, 1807, 935–944. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkawa, H.; Kaneko, T.; Fukuzawa, H.; Tabata, S.; Kaplan, A.; Ogawa, T. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: Genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. USA 2001, 98, 11789–11794. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Badger, M.R.; Price, G.D. Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC 7942. Mol. Microbiol. 2002, 43, 425–435. [Google Scholar] [CrossRef]
- Schuller, J.M.; Birrell, J.A.; Tanaka, H.; Konuma, T.; Wulfhorst, H.; Cox, N.; Schuller, S.K.; Thiemann, J.; Lubitz, W.; Sétif, P.; et al. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 2019, 363, 257–260. [Google Scholar] [CrossRef]
- Folea, I.M.; Zhang, P.; Nowaczyk, M.M.; Ogawa, T.; Aro, E.M.; Boekema, E.J. Single particle analysis of thylakoid proteins from Thermosynechococcus elongatus and Synechocystis 6803: Localization of the CupA subunit of NDH-1. FEBS Lett. 2008, 582, 249–254. [Google Scholar] [CrossRef]
- Xu, M.; Ogawa, T.; Pakrasi, H.B.; Mi, H. Identification and localization of the CupB protein involved in constitutive CO2 uptake in the cyanobacterium, Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 2008, 49, 994–997. [Google Scholar] [CrossRef]
- Price, G.D.; Maeda, S.I.; Omata, T.; Badger, M.R. Modes of inorganic carbon uptake in the cyanobacterium Synechococcus sp. PCC 7942. Funct. Plant Biol. 2002, 29, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, N.; Xu, M.; Hualing, M. Co-ordination of NDH and Cup proteins in CO2 uptake in cyanobacterium Synechocystis sp. PCC 6803. J. Exp. Bot. 2017, 68, 3869–3877. [Google Scholar] [CrossRef]
- Schuller, J.M.; Saura, P.; Thiemann, J.; Schuller, S.K.; Gamiz-Hernandez, A.P.; Kurisu, G.; Nowaczyk, M.M.; Kaila, V.R.I. Redox-coupled proton pumping drives carbon concentration in the photosynthetic complex I. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Artier, J.; Walker, R.M.; Miller, N.T.; Zhang, M.; Price, G.D.; Burnap, R.L. Modeling and mutagenesis of amino acid residues critical for CO2 hydration by specialized NDH-1 complexes in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148503. [Google Scholar] [CrossRef]
- Hagemann, M.; Kaplan, A. Is the structure of CO2-hydrating complex I compatible with the cyanobacterial CO2-concentrating mechanism? Plant Physiol. 2020, 183, 460–463. [Google Scholar] [CrossRef]
- Tchernov, D.; Hassidim, M.; Luz, B.; Sukenik, A.; Reinhold, L.; Kaplan, A. Sustained net CO2 evolution during photosynthesis by marine microorganisms. Curr. Biol. 1997, 7, 723–728. [Google Scholar] [CrossRef]
- Tchernov, D.; Silverman, J.; Luz, B.; Reinhold, L.; Kaplan, A. Massive light-dependent cycling of inorganic carbon between oxygenic photosynthetic microorganisms and their surroundings. Photosynth. Res. 2003, 77, 95–103. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, M.; Wu, Y.; Lv, J.; Fu, P.; Mi, H. NdhM subunit is required for the stability and the function of NAD(P)H dehydrogenase complexes involved in CO2 uptake in Synechocystis sp. strain PCC 6803. J. Biol. Chem. 2016, 291, 5902–5912. [Google Scholar] [CrossRef]
- Soltes-Rak, E.; Mulligan, M.E.; Coleman, J.R. Identification and characterization of gene encoding a vertebrate-type carbonic anhydrase in cyanobacteria. J. Bacteriol. 1997, 179, 769–774. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Bedbenov, V.S.; Pronina, N.A.; Los, D.A. Putative extracellular α-class carbonic anhydrase, EcaA, of Synechococcus elongatus PCC 7942 is an active enzyme: A sequel to an old story. Microbiology 2018, 164, 576–586. [Google Scholar] [CrossRef] [PubMed]
- So, A.K.; Van Spall, H.G.C.; Coleman, J.R.; Espie, O.S. Catalytic exchange of 18O from 13C18O-labelled CO2 by wild type cells and ecaA, ecaB, and ccaA mutants of the cyanobacteria Synechococcus PCC 7942 and Synechocystis PCC 6803. Can. J. Bot. 1998, 76, 1153–1160. [Google Scholar] [CrossRef]
- Fulda, S.; Huang, F.; Nilsson, F.; Hagemann, M.; Norling, B. Proteomics of Synechocystis sp. strain PCC 6803. Identification of periplasmic proteins in cells grown at low and high salt concentrations. Eur. J. Biochem. 2000, 267, 5900–5907. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Sinetova, M.A.; Markelova, A.G.; Allakhverdiev, S.I.; Los, D.A.; Pronina, N.A. Extracellular β-class carbonic anhydrase of the alkaliphilic cyanobacterium Microcoleus chthonoplastes. J. Photochem. Photobiol. B. 2011, 103, 78–86. [Google Scholar] [CrossRef]
- Samylina, O.S.; Sinetova, M.A.; Kupriyanova, E.V.; Starikov, A.Y.; Sukhacheva, M.V.; Dziuba, M.V.; Tourova, T.P. Ecology and biogeography of the ‘marine Geitlerinema’ cluster and a description of Sodalinema orleanskyi sp. nov., Sodalinema gerasimenkoae sp. nov., Sodalinema stali sp. nov. and Baaleninema simplex gen. et sp. nov. (Oscillatoriales, Cyanobacteria). FEMS Microbiol. Ecol. 2021, 97, fiab104. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, J.; Dann, M. Extracellular CahB1 from Sodalinema gerasimenkoae IPPAS B-353 acts as a functional carboxysomal β-carbonic anhydrase in Synechocystis sp. PCC6803. Plants 2023, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, H.; Suzuki, E.; Komukai, Y.; Miyachi, S. A gene homologous to chloroplast carbonic anhydrase (icfA) is essential to photosynthetic carbon dioxide fixation by Synechococcus PCC 7942. Proc. Natl. Acad. Sci. USA 1992, 89, 4437–4441. [Google Scholar] [CrossRef]
- So, A.K.; Espie, G.S. Cloning, characterization and expression carbonic anhydrase from the cyanobacterium Synechocystis PCC 6803. Plant Mol. Biol. 1998, 37, 205–215. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Cannon, G.C.; Heinhorst, S.; Tanaka, S.; Williams, E.B.; Yeates, T.O.; Kerfeld, C.A. The structure of beta-carbonic anhydrase from the carboxysomal shell reveals a distinct subclass with one active site for the price of two. J. Biol. Chem. 2006, 17, 7546–7555. [Google Scholar] [CrossRef]
- Ludwig, M.; Sültemeyer, D.; Price, G.D. Isolation of ccmKLMN genes from the marine cyanobacterium, Synechococcus sp. PCC 7002 (Cyanophyceae), and evidence that CcmM is essential for carboxysome assembly. J. Phycol. 2000, 36, 1109–1118. [Google Scholar] [CrossRef]
- Yeates, T.O.; Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C.; Shively, J.M. Protein-based organelles in bacteria: Carboxysomes and related microcompartments. Nat. Rev. Microbiol. 2008, 6, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.D.; Long, B.M.; Badger, M.R.; Price, G.D. Functions, compositions, and evolution of the two types of carboxysomes: Polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 357–379. [Google Scholar] [CrossRef]
- Turmo, A.; Gonzalez-Esquer, C.R.; Kerfeld, C.A. Carboxysomes: Metabolic modules for CO2 fixation. FEMS Microbiol. Lett. 2017, 364, fnx176. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Aussignargues, C.; Zarzycki, J.; Cai, F.; Sutter, M. Bacterial microcompartments. Nat. Rev. Microbiol. 2018, 16, 277–290. [Google Scholar] [CrossRef]
- Liu, L.N. Advances in the bacterial organelles for CO2 fixation. Trends Microbiol. 2022, 30, 567–580. [Google Scholar] [CrossRef]
- Huffine, C.A.; Zhao, R.; Tang, Y.J.; Cameron, J.C. Role of carboxysomes in cyanobacterial CO2 assimilation: CO2 concentrating mechanisms and metabolon implications. Environ. Microbiol. 2023, 25, 219–228. [Google Scholar] [CrossRef]
- Melnicki, M.R.; Sutter, M.; Kerfeld, C.A. Evolutionary relationships among shell proteins of carboxysomes and metabolosomes. Curr. Opin. Microbiol. 2021, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Yeves, P.J.; Scanlan, D.J.; Callieri, C.; Picazo, A.; Schallenberg, L.; Huber, P.; Roda-Garcia, J.J.; Bartosiewicz, M.; Belykh, O.I.; Tikhonova, I.V.; et al. α-cyanobacteria possessing form IA RuBisCO globally dominate aquatic habitats. ISME J. 2022, 16, 2421–2432. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.G.; Zwart, P.; Bagby, S.C.; Cai, F.; Chisholm, S.W.; Heinhorst, S.; Cannon, G.C.; Kerfeld, C.A. Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J. Mol. Biol. 2009, 392, 319–333. [Google Scholar] [CrossRef]
- Mahinthichaichan, P.; Morris, D.M.; Wang, Y.; Jensen, G.J.; Tajkhorshid, E. Selective permeability of carboxysome shell pores to anionic molecules. J. Phys. Chem. B 2018, 122, 9110–9118. [Google Scholar] [CrossRef]
- Faulkner, M.; Szabó, I.; Weetman, S.L.; Sicard, F.; Huber, R.G.; Bond, P.J.; Rosta, E.; Liu, L.N. Molecular simulations unravel the molecular principles that mediate selective permeability of carboxysome shell protein. Sci. Rep. 2020, 10, 17501. [Google Scholar] [CrossRef] [PubMed]
- Cot, S.S.; So, A.K.; Espie, G.S. A multiprotein bicarbonate dehydration complex essential to carboxysome function in cyanobacteria. J. Bacteriol. 2008, 190, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Long, B.M.; Tucker, L.; Badger, M.R.; Price, G.D. Functional cyanobacterial β-carboxysomes have an absolute requirement for both long and short forms of the CcmM protein. Plant Physiol. 2010, 153, 285–293. [Google Scholar] [CrossRef]
- Peña, K.L.; Castel, S.E.; de Araujo, C.; Espie, G.S.; Kimber, M.S. Structural basis of the oxidative activation of the carboxysomal gamma-carbonic anhydrase, CcmM. Proc. Natl. Acad. Sci. USA 2010, 107, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, C.; Arefeen, D.; Tadesse, Y.; Long, B.M.; Price, G.D.; Rowlett, R.S.; Kimber, M.S.; Espie, G.S. Identification and characterization of a carboxysomal γ-carbonic anhydrase from the cyanobacterium Nostoc sp. PCC 7120. Photosynth. Res. 2014, 1201, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, X.; Aigner, H.; Bracher, A.; Nguyen, N.D.; Hee, W.Y.; Long, B.M.; Price, G.D.; Hartl, F.U.; Hayer-Hartl, M. Rubisco condensate formation by CcmM in β-carboxysome biogenesis. Nature 2019, 566, 131–135. [Google Scholar] [CrossRef]
- Niederhuber, M.J.; Lambert, T.J.; Yapp, C.; Silver, P.A.; Polka, J.K. Superresolution microscopy of the β-carboxysome reveals a homogeneous matrix. Mol. Biol. Cell 2017, 28, 2734–2745. [Google Scholar] [CrossRef]
- Roberts, E.W.; Cai, F.; Kerfeld, C.A.; Cannon, G.C.; Heinhorst, S. Isolation and characterization of the Prochlorococcus carboxysome reveal the presence of the novel shell protein CsoS1D. J. Bacteriol. 2012, 194, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Lechno-Yossef, S.; Rohnke, B.A.; Belza, A.C.O.; Melnicki, M.R.; Montgomery, B.L.; Kerfeld, C.A. Cyanobacterial carboxysomes contain an unique rubisco-activase-like protein. New Phytol. 2020, 225, 793–806. [Google Scholar] [CrossRef]
- Rillema, R.; Hoang, Y.; MacCready, J.S.; Vecchiarelli, A.G. Carboxysome mispositioning alters growth, morphology, and Rubisco level of the cyanobacterium Synechococcus elongatus PCC 7942. mBio 2021, 12, e0269620. [Google Scholar] [CrossRef]
- Savage, D.F.; Afonso, B.; Chen, A.H.; Silver, P.A. Spatially ordered dynamics of the bacterial carbon fixation machinery. Science 2010, 327, 1258–1261. [Google Scholar] [CrossRef]
- MacCready, J.S.; Hakim, P.; Young, E.J.; Hu, L.; Liu, J.; Osteryoung, K.W.; Vecchiarelli, A.G.; Ducat, D.C. Protein gradients on the nucleoid position the carbon-fixing organelles of cyanobacteria. eLife 2018, 7, e39723. [Google Scholar] [CrossRef] [PubMed]
- MacCready, J.S.; Tran, L.; Basalla, J.L.; Hakim, P.; Vecchiarelli, A.G. The McdAB system positions α-carboxysomes in proteobacteria. Mol. Microbiol. 2021, 116, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.C.; Wilson, S.C.; Bernstein, S.L.; Kerfeld, C.A. Biogenesis of a bacterial organelle: The carboxysome assembly pathway. Cell 2013, 155, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Robinson-Mosher, A.; Savage, D.F.; Silver, P.A.; Polka, J.K. The bacterial carbon-fixing organelle is formed by shell envelopment of preassembled cargo. PloS ONE 2013, 8, e76127. [Google Scholar] [CrossRef]
- Zang, K.; Wang, H.; Hartl, F.U.; Hayer-Hartl, M. Scaffolding protein CcmM directs multiprotein phase separation in β-carboxysome biogenesis. Nat. Struct. Mol. Biol. 2021, 28, 909–922. [Google Scholar] [CrossRef]
- Sültemeyer, D.; Price, G.D.; Yu, J.W.; Badger, M.R. Characterization of carbon dioxide and bicarbonate transport during steady-state photosynthesis in the marine cyanobacterium Synechococcus strain PCC7002. Planta 1995, 197, 597–607. [Google Scholar] [CrossRef]
- Woodger, F.J.; Badger, M.R.; Price, G.D. Sensing of inorganic carbon limitation in Synechococcus PCC 7942 is correlated with the size of the internal inorganic carbon pool and involves oxygen. Plant Physiol. 2005, 139, 1959–1969. [Google Scholar] [CrossRef]
- Machingura, M.C.; Bajsa-Hirschel, J.; Laborde, S.M.; Schwartzenburg, J.B.; Mukherjee, B.; Mukherjee, A.; Pollock, S.V.; Förster, B.; Price, G.D.; Moroney, J.V. Identification and characterization of a solute carrier, CIA8, involved in inorganic carbon acclimation in Chlamydomonas reinhardtii. J. Exp. Bot. 2017, 68, 3879–3890. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lau, C.S.; Walker, C.E.; Rai, A.K.; Prejean, C.I.; Yates, G.; Emrich-Mills, T.; Lemoine, S.G.; Vinyard, D.J.; Mackinder, L.C.M.; et al. Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2019, 116, 16915–16920. [Google Scholar] [CrossRef]
- Fang, W.; Si, Y.; Douglass, S.; Casero, D.; Merchant, S.S.; Pellegrini, M.; Ladunga, I.; Liu, P.; Spalding, M.H. Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. Plant Cell 2012, 24, 1876–1893. [Google Scholar] [CrossRef]
- Brueggeman, A.J.; Gangadharaiah, D.S.; Cserhati, M.F.; Casero, D.; Weeks, D.P.; Ladunga, I. Activation of the carbon concentrating mechanism by CO2 deprivation coincides with massive transcriptional restructuring in Chlamydomonas reinhardtii. Plant Cell 2012, 24, 1860–1875. [Google Scholar] [CrossRef]
- Moroney, J.V.; Husic, H.D.; Tolbert, N.E.; Kitayama, M.; Manuel, L.J.; Togasaki, R.K. Isolation and characterization of a mutant of Chlamydomonas reinhardtii deficient in the CO2 concentrating mechanism. Plant Physiol. 1989, 89, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, H.; Miura, K.; Ishizaki, K.; Kucho, K.I.; Saito, T.; Kohinata, T.; Ohyama, K. Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc. Natl. Acad. Sci. USA 2001, 98, 5347–5352. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.B.; Zhang, J.; Weeks, D.P. The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2001, 98, 5341–5346. [Google Scholar] [CrossRef]
- Miura, K.; Yamano, T.; Yoshioka, S.; Kohinata, T.; Inoue, Y.; Taniguchi, F.; Asamizu, E.; Nakamura, Y.; Tabata, S.; Yamato, K.T.; et al. Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol. 2004, 135, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Taniguchi, F.; Miura, K.; Inoue, T.; Yamano, T.; Fukuzawa, H. The novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1, encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Cell 2004, 16, 1466–1477. [Google Scholar] [CrossRef]
- Turkina, M.V.; Blanco-Rivero, A.; Vainonen, J.P.; Vener, A.V.; Villarejo, A. CO2 limitation induces specific redox-dependent protein phosphorylation in Chlamydomonas reinhardtii. Proteomics 2006, 6, 2693–2704. [Google Scholar] [CrossRef]
- Yamano, T.; Tsujikawa, T.; Hatano, K.; Ozawa, S.I.; Takahashi, Y.; Fukuzawa, H. Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 2010, 51, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spalding, M.H. LCIB in the Chlamydomonas CO2 concentrating mechanism. Photosynth. Res. 2014, 121, 185–192. [Google Scholar] [CrossRef]
- Jin, S.; Sun, J.; Wunder, T.; Tang, D.; Cousins, A.B.; Sze, S.K.; Mueller-Cajar, O.; Gao, Y.G. Structural insights into the LCIB protein family reveals a new group of β-carbonic anhydrases. Proc. Natl. Acad. Sci. USA 2016, 113, 14716–14721. [Google Scholar] [CrossRef]
- Wang, H.; Gau, B.; Slade, W.O.; Juergens, M.; Li, P.; Hicks, L.M. The global phosphoproteome of Chlamydomonas reinhardtii reveals complex organellar phosphorylation in the flagella and thylakoid membrane. Mol. Cell Proteomics. 2014, 13, 2337–2353. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, M.; Bedhomme, M.; Groni, H.; Marchand, C.H.; Puppo, C.; Gontero, B.; Cassier-Chauvat, C.; Decottignies, P.; Lemaire, S.D. Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: A proteomic survey. Mol. Cell Proteomics 2012, 11, M111-014142. [Google Scholar] [CrossRef]
- Rolland, N.; Dorne, A.J.; Amoroso, G.; Sültemeyer, D.F.; Joyard, J.; Rochaix, J.D. Disruption of the plastid ycf10 open reading frame affects uptake of inorganic carbon in the chloroplast of Chlamydomonas. EMBO J. 1997, 16, 6713–6726. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Horken, K.M.; Im, C.S.; Xiang, Y.; Grossman, A.R.; Weeks, D.P. Analyses of CIA5, the master regulator of the carbon concentrating mechanism in Chlamydomonas reinhardtii, and its control of gene expression. Can. J. Bot. 2005, 83, 765–779. [Google Scholar] [CrossRef]
- Im, C.S.; Grossman, A.R. Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii. Plant J. 2002, 30, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Im, C.S.; Zhang, Z.; Shrager, J.; Chang, C.W.; Grossman, A.R. Analysis of light and CO2 regulation in Chlamydomonas reinhardtii using genome-wide approaches. Photosynth. Res. 2003, 75, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, D.; Miller, A.R.; Horken, K.M.; Weeks, D.P.; Spalding, M.H. Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3− transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2009, 106, 5990–5995. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Fei, X.; Wright, D.A.; Spalding, M.H. Expression activation and functional analysis of HLA3, a putative inorganic carbon transporter in Chlamydomonas reinhardtii. Plant J. 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Yamano, T.; Sato, E.; Iguchi, H.; Fukuda, Y.; Fukuzawa, H. Characterization of cooperative bicarbonate uptake into chloroplast stroma in the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2015, 112, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spalding, M.H. Acclimation to very-low CO2: Contribution of LCIB and LCIA to inorganic carbon uptake in Chlamydomonas reinhardtii. Plant Physiol. 2014, 166, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yamano, T.; Takane, S.; Niikawa, Y.; Toyokawa, C.; Ozawa, S.I.; Tokutsu, R.; Takahashi, Y.; Minagawa, J.; Kanesaki, Y.; et al. Chloroplast-mediated regulation of CO2-concentrating mechanism by Ca2+-binding protein CAS in the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 12586–12591. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Toyokawa, C.; Fukuzawa, H. High-resolution suborganellar localization of Ca2+-binding protein CAS, a novel regulator of CO2-concentrating mechanism. Protoplasma 2018, 255, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.D.; Chen, Z.Y.; Mouton, T.M.; Moroney, J.V. Isolation of cDNA clones of genes induced upon transfer of Chlamydomonas reinhardtii cells to low CO2. Plant Mol. Biol. 1996, 31, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Mukherjee, B.; Tsujikawa, T.; Yanase, M.; Nakano, H.; Moroney, J.V.; Fukuzawa, H. Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. Plant Cell 2010, 22, 3105–3117. [Google Scholar] [CrossRef]
- Kono, A.; Spalding, M.H. LCI1, a Chlamydomonas reinhardtii plasma membrane protein, functions in active CO2 uptake under low CO2. Plant J. 2020, 102, 1127–1141. [Google Scholar] [CrossRef]
- Kono, A.; Chou, T.H.; Radhakrishnan, A.; Bolla, J.R.; Sankar, K.; Shome, S.; Su, C.C.; Jernigan, R.L.; Robinson, C.V.; Yu, E.W.; et al. Structure and function of LCI1: A plasma membrane CO2 channel in the Chlamydomonas CO2 concentrating mechanism. Plant J. 2020, 102, 1107–1126. [Google Scholar] [CrossRef]
- Soupene, E.; King, N.; Field, E.; Liu, P.; Niyogi, K.K.; Huang, C.H.; Kustu, S. Rhesus expression in a green alga is regulated by CO2. Proc. Natl Acad. Sci. USA 2002, 99, 7769–7773. [Google Scholar] [CrossRef]
- Yoshihara, C.; Inoue, K.; Schichnes, D.; Ruzin, S.; Inwood, W.; Kustu, S. An Rh1-GFP fusion protein is in the cytoplasmic membrane of a white mutant strain of Chlamydomonas reinhardtii. Mol. Plant 2008, 1, 1007–1020. [Google Scholar] [CrossRef]
- Kustu, S.; Inwood, W. Biological gas channels for NH3 and CO2: Evidence that Rh (Rhesus) proteins are CO2 channels. Transfus. Clin. Biol. 2006, 13, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Soupene, E.; Inwood, W.; Kustu, S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc. Natl. Acad. Sci. USA 2004, 101, 7787–7792. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, V.; Moulin, P.; Orsel, M.; Miller, A.J.; Fernandez, E.; Galvan, A. Differential regulation of the Chlamydomonas Nar1 gene family by carbon and nitrogen. Protist 2006, 157, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Hartzell, H.C. Bestrophin Cl− channels are highly permeable to HCO3−. Am. J. Physiol. Cell Physiol. 2008, 294, 1371–1377. [Google Scholar] [CrossRef]
- Pollock, S.V.; Prout, D.L.; Godfrey, A.C.; Lemaire, S.D.; Moroney, J.V. The Chlamydomonas reinhardtii proteins Ccp1 and Ccp2 are required for long-term growth, but are not necessary for efficient photosynthesis, in a low-CO2 environment. Plant Mol. Biol. 2004, 56, 125–132. [Google Scholar] [CrossRef]
- Ramazanov, Z.; Mason, C.B.; Geraghty, A.M.; Spalding, M.H.; Moroney, J.V. The low CO2-inducible 36-kilodalton protein is localized to the chloroplast envelope of Chlamydomonas reinhardtii. Plant Physiol. 1993, 101, 1195–1199. [Google Scholar] [CrossRef]
- Mason, C.B.; Manuel, L.J.; Moroney, J.V. A new chloroplast protein is induced by growth on low CO2 in Chlamydomonas reinhardtii. Plant Physiol. 1990, 93, 833–836. [Google Scholar] [CrossRef]
- Kikutani, S.; Nakajima, K.; Nagasato, C.; Tsuji, Y.; Miyatake, A.; Matsuda, Y. Thylakoid luminal θ-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. USA 2016, 113, 9828–9833. [Google Scholar] [CrossRef]
- Aspatwar, A.; Haapanen, S.; Parkkila, S. An update on the metabolic roles of carbonic anhydrases in the model alga Chlamydomonas reinhardtii. Metabolites 2018, 8, 22. [Google Scholar] [CrossRef]
- Fujiwara, S.; Fukuzawa, H.; Tachiki, A.; Miyachi, S. Structure and differential expression of two genes encoding carbonic anhydrase in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1990, 87, 9779–9783. [Google Scholar] [CrossRef]
- Fukuzawa, H.; Fujiwara, S.; Yamamoyo, Y.; Dionisio-Sese, M.L.; Miyachi, S. cDNA cloning, sequence, and expression of carbonic anhydrase in Chlamydomonas reinhardtii: Regulation by environmental CO2 concentration. Proc. Natl. Acad. Sci. USA 1990, 87, 4383–4387. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Moroney, J.V. Partial, characterization of a new isoenzyme of carbonic anhydrase isolated from Chlamydomonas reinhardtii. J. Biol. Chem. 1991, 266, 9719–9723. [Google Scholar] [CrossRef]
- Moroney, J.V.; Ma, Y.; Frey, W.D.; Fusilier, K.A.; Pham, T.T.; Simms, T.A.; DiMario, R.J.; Yang, J.; Mukherjee, B. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosynth. Res. 2011, 109, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Ynalvez, R.A.; Xiao, Y.; Ward, A.S.; Cunnusamy, K.; Moroney, J.V. Identification and characterization of two closely related β-carbonic anhydrases from Chlamydomonas reinhardtii. Physiol. Plant. 2008, 133, 15–26. [Google Scholar] [CrossRef]
- Eriksson, M.; Karlsson, J.; Ramazanov, Z.; Gardeström, P.; Samuelsson, G. Discovery of an algal mitochondrial carbonic anhydrase: Molecular cloning and characterization of a low-CO2-induced polypeptide in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1996, 93, 12031–12034. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Villand, P.; Gardeström, P.; Samuelsson, G. Induction and regulation of expression of a low-CO2-induced mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol. 1998, 116, 637–641. [Google Scholar] [CrossRef]
- Rai, A.K.; Chen, T.; Moroney, J.V. Mitochondrial carbonic anhydrases are needed for optimal photosynthesis at low CO2 levels in Chlamydomonas. Plant Physiol. 2021, 187, 1387–1398. [Google Scholar] [CrossRef]
- Cardol, P.; Gonzalez-Halphen, D.; Reyes-Prieto, A.; Baurain, D.; Matagne, R.F.; Remacle, C. The mitochondrial oxidative phosphorylation proteome of Chlamydomonas reinhardtii deduced from the Genome Sequencing Project. Plant Physiol. 2005, 137, 447–459. [Google Scholar] [CrossRef]
- Mitra, M.; Mason, C.; Lato, S.M.; Ynalvez, R.A.; Xiao, Y.; Moroney, J.V. The carbonic anhydrase gene families of Chlamydomonas reinhardtii. Can. J. Bot. 2005, 83, 780–795. [Google Scholar] [CrossRef]
- Karlsson, J.; Clarke, A.K.; Chen, Z.Y.; Hugghins, S.Y.; Park, Y.I.; Husic, H.D.; Moroney, J.V.; Samuelsson, G. A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 1998, 10, 1208–1216. [Google Scholar] [CrossRef]
- Moroney, J.V.; Somanchi, A. How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol. 1999, 119, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.T.; Franklin, L.A.; Samuelsson, G.; Badger, M.R. The Chlamydomonas reinhardtii cia3 mutant lacking a thylakoid lumen-localized carbonic anhydrase is limited by CO2 supply to Rubisco and not photosystem II function in vivo. Plant Physiol. 2003, 132, 2267–2275. [Google Scholar] [CrossRef]
- van Hunnik, E.; Sültemeyer, D. A possible role for carbonic anhydrase in the lumen of chloroplast thylakoids in green algae. Funct. Plant Biol. 2002, 29, 243–249. [Google Scholar] [CrossRef]
- Shitov, A.V.; Terentyev, V.V.; Zharmukhamedov, S.K.; Rodionova, M.V.; Karacan, M.; Karacan, N.; Klimov, V.V.; Allakhverdiev, S.I. Is carbonic anhydrase activity of photosystem II required for its maximum electron transport rate? Biochim. Biophys. Acta Bioenerg. 2018, 1859, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, V.V.; Shukshina, A.K.; Shitov, A.V. Carbonic anhydrase CAH3 supports the activity of photosystem II under increased pH. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Duanmu, D.; Wang, Y.; Spalding, M.H. Thylakoid lumen carbonic anhydrase (CAH3) mutation suppresses air-dier phenotype of LCIB mutant in Chlamydomonas reinhardtii. Plant Physiol. 2009, 149, 929–937. [Google Scholar] [CrossRef]
- Yamano, T.; Toyokawa, C.; Shimamura, D.; Matsuoka, T.; Fukuzawa, H. CO2-dependent migration and relocation of LCIB, a pyrenoid-peripheral protein in Chlamydomonas reinhardtii. Plant Physiol. 2022, 188, 1081–1094. [Google Scholar] [CrossRef]
- Toyokawa, C.; Yamano, T.; Fukuzawa, H. Pyrenoid starch sheath is required for LCIB localization and the CO2-concentrating mechanism in green algae. Plant Physiol. 2020, 182, 1883–1893. [Google Scholar] [CrossRef]
- Mitra, M.; Lato, S.M.; Ynalvez, R.A.; Xiao, Y.; Moroney, J.V. Identification of a new chloroplast carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol. 2004, 135, 173–182. [Google Scholar] [CrossRef]
- Pazour, G.J.; Agrin, N.; Leszyk, J.; Witman, G.B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 2005, 170, 103–113. [Google Scholar] [CrossRef]
- Terashima, M.; Specht, M.; Naumann, B.; Hippler, M. Characterizing the anaerobic response of Chlamydomonas reinhardtii by quantitative proteomics. Mol. Cell Proteomics 2010, 9, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, I.A.; Boldina, O.N. Comparative analysis of the pyrenoid ultrastructure in green monad and coccoid algae. Russ. J. Plant Physiol. 2000, 47, 655–659. [Google Scholar]
- Wunder, T.; Oh, Z.G.; Mueller-Cajar, O. CO2 -fixing liquid droplets: Towards a dissection of the microalgal pyrenoid. Traffic 2019, 20, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Engel, B.D.; Schaffer, M.; Cuellar, L.K.; Villa, E.; Plitzko, J.M.; Baumeister, W. Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife 2015, 4, e04889. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Marchand, C.H.; Maes, A.; Mauries, A.; Sun, Y.; Dhaliwal, J.S.; Uniacke, J.; Arragain, S.; Jiang, H.; Gold, N.D.; et al. Pyrenoid functions revealed by proteomics in Chlamydomonas reinhardtii. PLoS ONE 2018, 13, e0185039. [Google Scholar] [CrossRef]
- Mackinder, L.C.M.; Meyer, M.T.; Mettler-Altmann, T.; Chen, V.K.; Mitchell, M.C.; Caspari, O.; Freeman Rosenzweig, E.S.; Pallesen, L.; Reeves, G.; Itakura, A.; et al. A repeat protein links Rubisco to form the eukaryotic carbon-concentrating organelle. Proc. Natl. Acad. Sci. USA 2016, 113, 5958–5963. [Google Scholar] [CrossRef]
- Ma, Y.; Pollock, S.V.; Xiao, Y.; Cunnusamy, K.; Moroney, J.V. Identification of a novel gene, CIA6, required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol. 2011, 156, 884–896. [Google Scholar] [CrossRef]
- Streusand, V.J.; Portis, A.R. Rubisco activase mediates ATP-dependent activation of ribulose bisphosphate carboxylase. Plant Physiol. 1987, 85, 152–154. [Google Scholar] [CrossRef]
- Wunder, T.; Cheng, S.L.H.; Lai, S.K.; Li, H.Y.; Mueller-Cajar, O. The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Plumed, M.P.; Villarejo, A.; de los Rios, A.; Garcia-Reina, G.; Ramazanov, Z. The CO2-concentrating mechanism in a starchless mutant of the green unicellular alga Chlorella pyrenoidosa. Planta 1996, 200, 28–31. [Google Scholar] [CrossRef]
- Pronina, N.A.; Borodin, V.B. CO2 stress and CO2 concentration mechanism: Investigation by means of photosystem-deficient and carbonic anhydrase-deficient mutants of Chlamydomonas reinhardtii. Photosynthetica 1993, 28, 515–522. [Google Scholar]
- Thielmann, J.; Tolbert, N.E.; Goyal, A.; Senger, H. Two systems for concentrating CO2 and bicarbonate during photosynthesis by Scenedesmus. Plant Physiol. 1990, 92, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.H.; Portis, A.R. A model of carbon dioxide assimilation in Chlamydomonas reinhardii. Planta 1985, 164, 308–320. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).