First Restoration Experiment for Endemic Fucus virsoides on the Western Istrian Coast—Is It Feasible?
Abstract
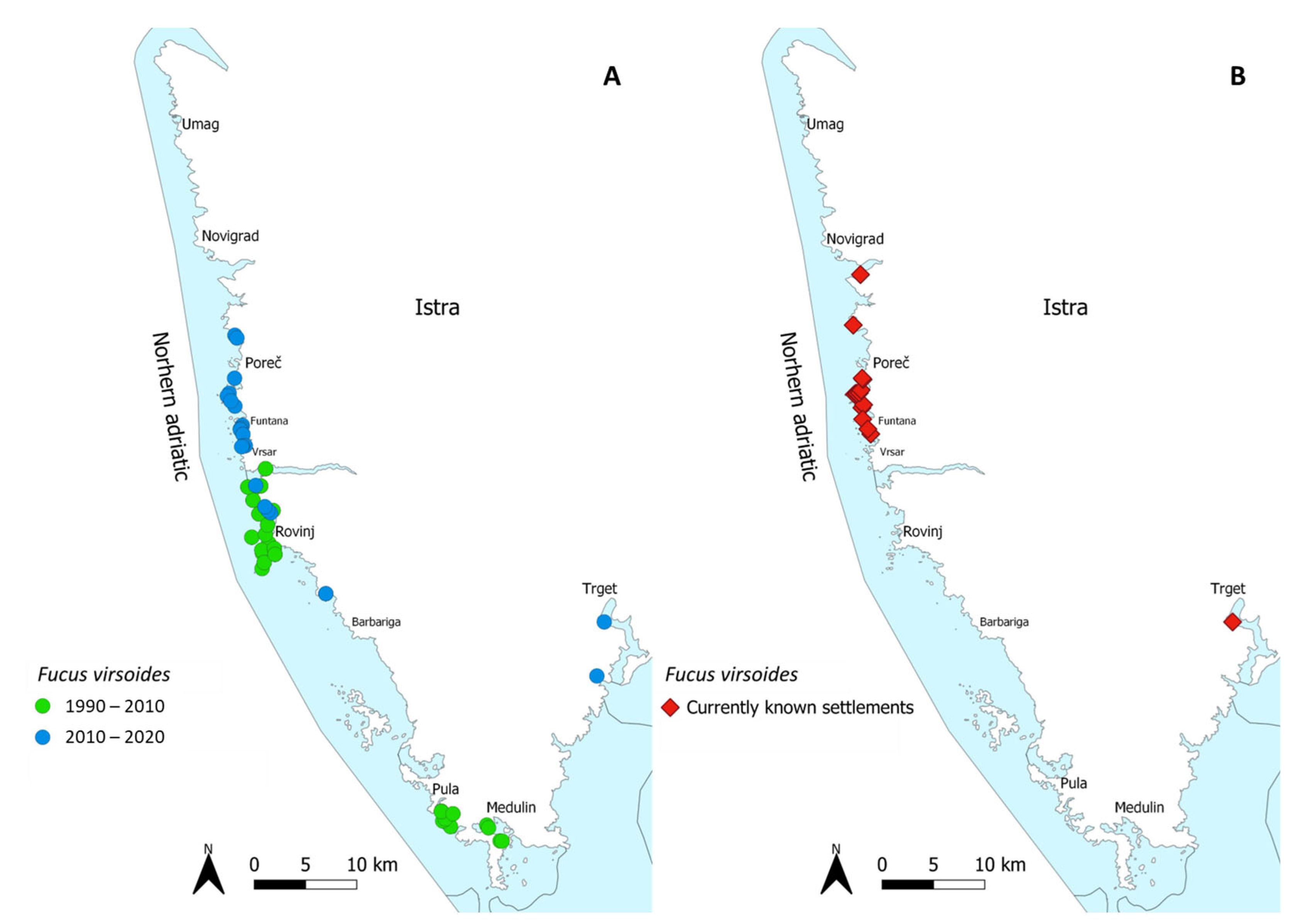
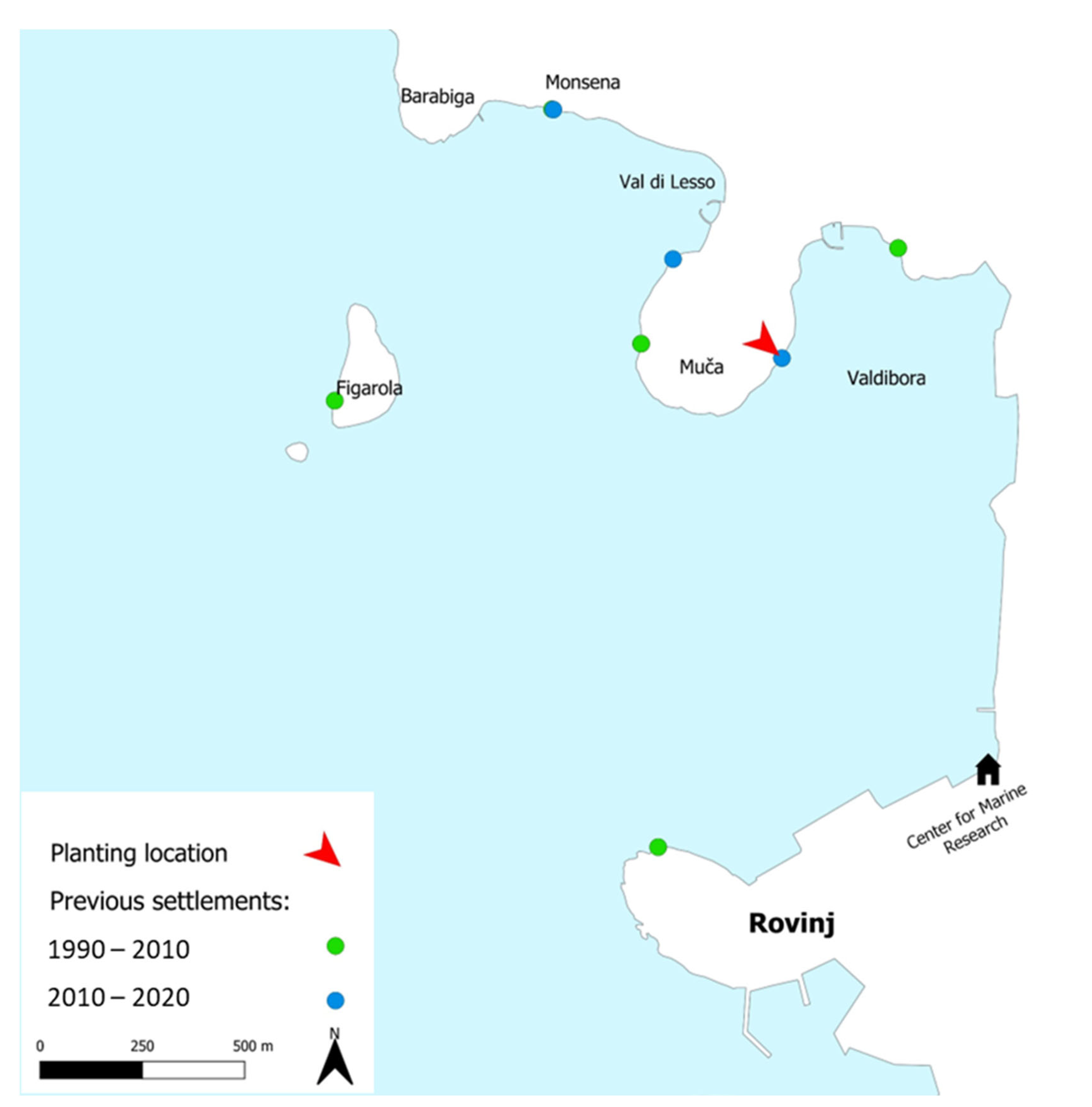
1. Introduction
2. Results
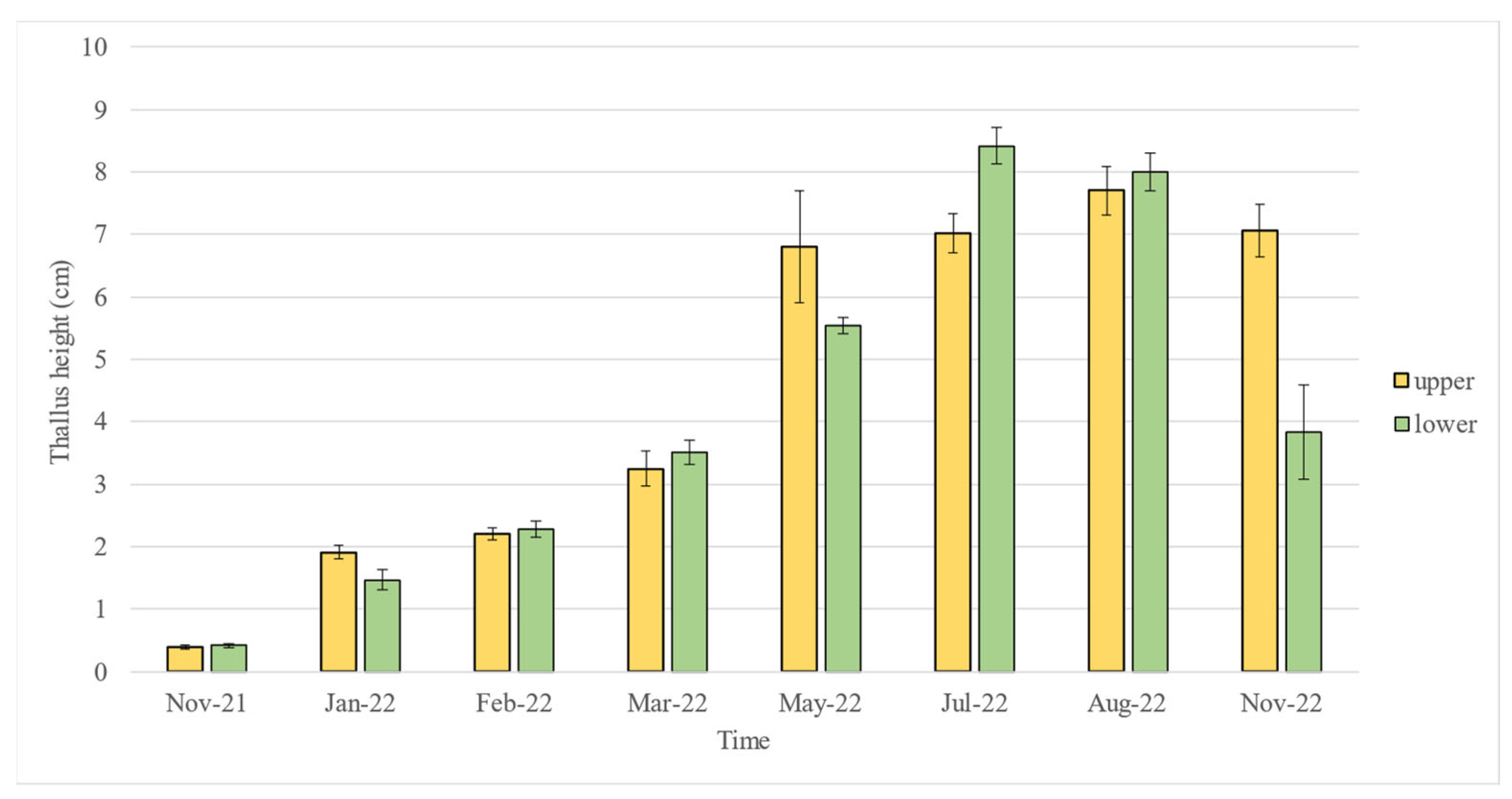
2.1. Early Growth
2.2. Post-Planting Growth
2.3. Statistical Analyses
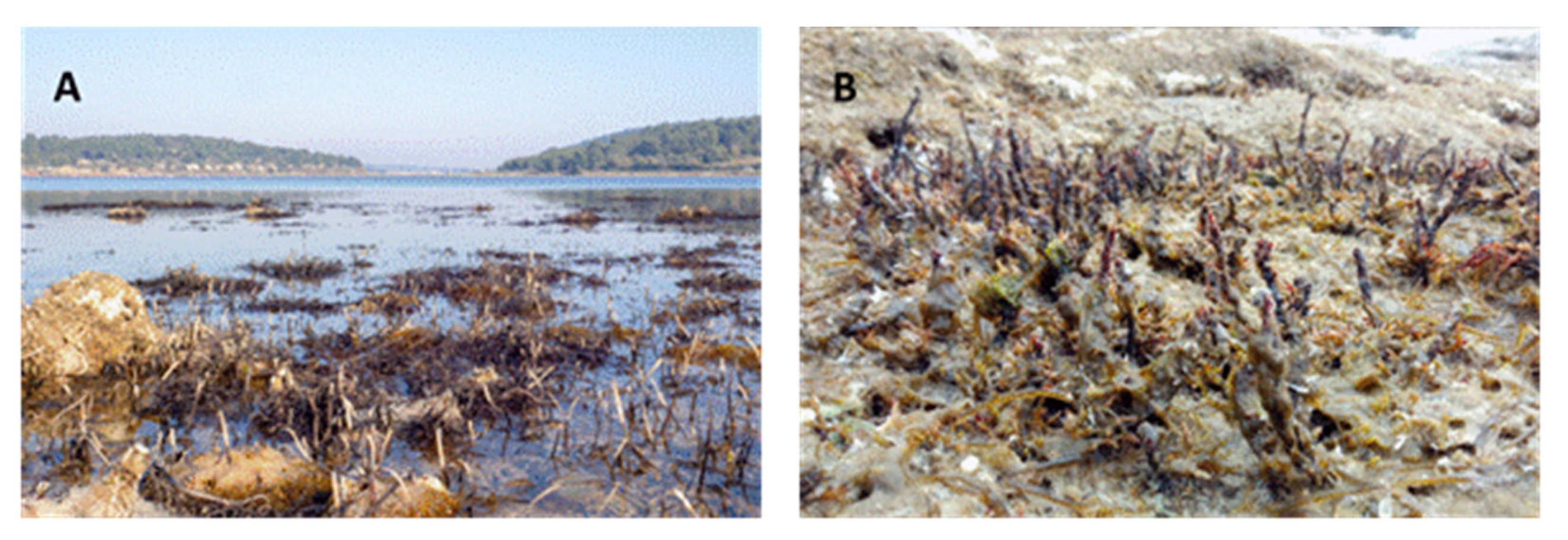
3. Discussion
4. Materials and Methods
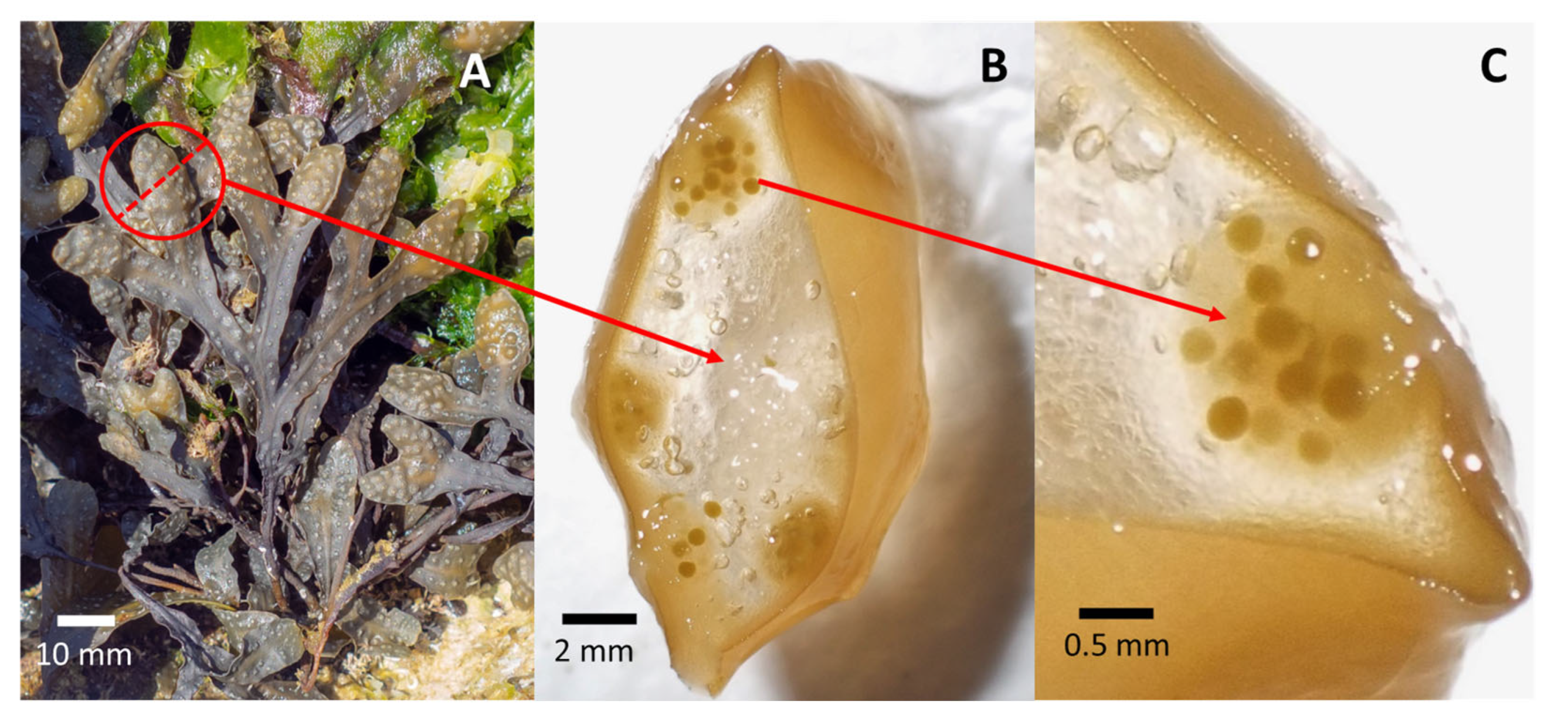
4.1. Seeding
4.2. Early Growth
4.3. Planting
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribera, M.A.; Gomez Garreta, A.; Gallardo, T.; Cormaci, M.; Furnari, G.; Giaccone, G. Check-List of Mediterranean Seaweeds I. Fucophyceae (Warming, 1884). Bot. Mar. 1992, 35, 109–130. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication. Available online: https://www.algaebase.org (accessed on 21 December 2022).
- Linardić, J. Studije o Jadranskom Fukusu (Fucus virsoides). Acta Bot. 1949, 12, 7–131. [Google Scholar]
- Zavodnik, D. Dinamika Litoralnega Fitala Na Zahodnoistrski Obali. Razpr.—Diss. 1967, 10, 7–77. [Google Scholar]
- Munda, I.M. Seasonal and Ecologicaly Conditioned Variations in the Fucus virsoides Association from the Istrian Coast (Northern Adriatic). Razpr.—Diss. 1972, 15, 1–33. [Google Scholar]
- Kashta, L. Rreth Përhapjes Dhe Ekologjisë Së Fucus virsoides J Agardh Në Brigjet e Shqipërisë. (About the Distribution and Ecology of Fucus virsoides J Agardh along the Albanian Coast). Bul. Shk. Nat. Shkodër 1996, 48, 60–65. [Google Scholar]
- Mačić, V. Distribution of Seaweed Fucus virsoides J. Agardh in Boka Kotorska Bay (South Adriatic Sea). Ann. Ser. Hist. Nat. 2006, 16, 1–4. [Google Scholar]
- Orlando-Bonaca, M.; Mannoni, P.A.; Poloniato, D.; Falace, A. Assessment of Fucus virsoides Distribution in the Gulf of Trieste (Adriatic Sea) and Its Relation to Environmental Variables. Bot. Mar. 2013, 56, 451–459. [Google Scholar] [CrossRef]
- Battelli, C. Disappearance of Fucus virsoides J. Agardh from the Slovenian Coast (Gulf of Trieste, Northern Adriatic). Ann. Ser. Hist. Nat. 2016, 26, 1–12. [Google Scholar] [CrossRef]
- Rindi, F.; Gavio, B.; Díaz-Tapia, P.; Di Camillo, C.G.; Romagnoli, T. Long-Term Changes in the Benthic Macroalgal Flora of a Coastal Area Affected by Urban Impacts (Conero Riviera, Mediterranean Sea). Biodivers. Conserv. 2020, 29, 2275–2295. [Google Scholar] [CrossRef]
- Munda, I.M. The Production of Biomass in the Settlements of Benthic Marine Algae in the Northern Adriatic. Bot. Mar. 1973, 15, 218–244. [Google Scholar] [CrossRef]
- Čelig, A. Mapping of Brown Alga Fucus virsoides J. Agardh along the Coast of Southern Istria. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 2010. [Google Scholar]
- Kučinar, I. Kartiranje Naselja Smeđe Alge Fucus virsoides J. Agardh u Priobalju Rovinja (Mapping of Brown Algae Fucus virsoides J. Agardh in the Rovinj Area). Bachellor’s Thesis, Juraj Dobrila University, Pula, Croatia, 2014. [Google Scholar]
- Gljušćić, E. Distribution and Ecology of Endemic Macroalga Fucus virsoides J. Agardh in the Area of Poreč, Funtana and Vrsar. Bachellor’s Thesis, Juraj Dobrila University, Pula, Croatia, 2016. [Google Scholar]
- Schiffner, V. Studien Tiber Die Algen Des Adriatischen Meeres. Helgoländer Wiss. Meeresunters. 1916, 11, 129–198. [Google Scholar]
- Vatova, A. Compendio Della Flora e Fauna Del Mare Adriatico Presso Rovigno Memoria. Mem. R Com. Talassogr. Ital. 1928, 143, 1–613. [Google Scholar]
- Munda, I.M. Long-Term Marine Floristic Changes around Rovinj (Istrian Coast, North Adriatic) Estimated on the Basis of Historical Data Estimated on the Basis of Historical Data from Paul Kuckuck’s Field Diaries from the End of the 19th Century. Nov. Hedwigia 2000, 71, 1–36. [Google Scholar] [CrossRef]
- Battelli, C.; Alberti, G. Antonio Zaratin (1846–1923): Raccoglitore e Preparatore d’alghe Dell’Istria e Del Quarnero. Atti 2003, 33, 643–684. [Google Scholar]
- Zavodnik, N.; Iveša, L.; Travizi, A. Note on Recolonisation by Fucoid Algae Cystoseira spp. and Fucus virsoides in the North Adriatic Sea. Acta Adriat. 2002, 43, 25–32. [Google Scholar]
- Munda, I.M. Changes in the Benthic Algal Associations of the Vicinity of Rovinj (Istrian Coast, North Adriatic) Caused by Organic Wastes. Acta Adriat. 1980, 21, 299–323. [Google Scholar]
- Munda, I.M. Survey of the Algal Biomass Inthe Polluted Area around Rovinj (Istrian Coast, Nothern Adriatic). Acta Adriat. 1980, 21, 333–354. [Google Scholar]
- Munda, I.M. Changes and Degradation of Seaweed Stands in the Northern Adriatic. Hydrobiologia 1993, 260, 239–253. [Google Scholar] [CrossRef]
- Hanel, R. Recovery of Fucacean Associations and Associated Fish Assemblages in the Vicinity of Rovinj, Istrian Coast, Northern Adriatic Sea. Period. Biol. 2002, 104, 159–163. [Google Scholar]
- Iveša, L.; Djakovac, T. Recovery of Cystoseira Forests Along the West Istrian Coast (Northern Adriatic Sea, Croatia). Eur. J. Phycol. 2015, 50, 88–89. [Google Scholar]
- Rindi, F.; Falace, A.; Moro, I.; Iveša, L.; Caragnano, A.; Sfriso, A. Diversity Patterns and Long-Term Changes in the Benthic Macroalgal Vegetation of the Northern Adriatic. Eur. J. Phycol. 2019, 54, 98. [Google Scholar]
- Gljušćić, E.; Bilajac, A.; Smith, S.; Iveša, L.; Bošković, I.R.; Paliaga, G. Jadranski Fukus (Fucus virsoides J. In Agardh) Na Obali Istre: Prošlost, Sadašnjost i (Možda) Budućnost. In Proceedings of the Climate change and preservation of marine ecosystems of the Adriatic Sea, Krk, Croatia, 30 September–2 October 2022. [Google Scholar]
- Miočić-Stošić, J.; Pleslić, G.; ECOSS: Ecological observing System in the Adriatic Sea: Oceanographic Observations for Biodiversity Priority. D4.2.1 Review of the knowledge of the target species at the selected Natura 2000 sites. 29 June 2020. Available online: https://www.italy-croatia.eu/web/ecoss (accessed on 15 December 2022).
- Eger, A.M.; Layton, C.; McHugh, T.A.; Gleason, M.; Eddy, N. Kelp Restoration Guidebook: Lessons Learned from Kelp Projects around the World; Caselle, J., DeAngelis, B., Eds.; The Nature Conservancy: Arlington, TX, USA, 2022; ISBN 9780578381237. [Google Scholar]
- Cebrian, E.; Tamburello, L.; Verdura, J.; Guarnieri, G.; Medrano, A.; Linares, C.; Hereu, B.; Garrabou, J.; Cerrano, C.; Galobart, C.; et al. A Roadmap for the Restoration of Mediterranean Macroalgal Forests. Front. Mar. Sci. 2021, 8, 709219. [Google Scholar] [CrossRef]
- Siméon, A.; Hervé, C. Isolation of Fucus serratus Gametes and Cultivation of the Zygotes. Bio-Protoc. 2017, 7, e2408. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Dulčić, J. Relationships among Predatory Fish, Sea Urchins and Barrens in Mediterranean Rocky Reefs across a Latitudinal Gradient. Mar. Environ. Res. 2007, 63, 168–184. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Mangialajo, L. Reduction of Herbivorous Fish Pressure Can Facilitate Focal Algal Species Forestation on Artificial Structures. Mar. Environ. Res. 2018, 138, 102–109. [Google Scholar] [CrossRef]
- Martone, P.; Alyono, M.; Stites, S. Bleaching of an Intertidal Coralline Alga: Untangling the Effects of Light, Temperature, and Desiccation. Mar. Ecol. Prog. Ser. 2010, 416, 57–67. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of Climate Change On Global Seaweed Communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef]
- Saada, G.; Nicastro, K.R.; Jacinto, R.; McQuaid, C.D.; Serrão, E.A.; Pearson, G.A.; Zardi, G.I. Taking the Heat: Distinct Vulnerability to Thermal Stress of Central and Threatened Peripheral Lineages of a Marine Macroalga. Divers. Distrib. 2016, 22, 1060–1068. [Google Scholar] [CrossRef]
- Hanžek, N. Fiziološke Prilagodbe Jadranskog Bračića (Fucus virsoides J. Agardh) Na Isušivanje i Ultraljubičasto Zračenje. Master’s Thesis, University of Zagreb, Zagreb, Croatia, 2014. [Google Scholar]
- Vouk, V. Bilješke o Jadranskom Fukusu (Fucus virsoides (Don.) J. Ag.). Godišnjak Oceanogr. Inst. 1938, 1, 153–159. [Google Scholar]
- Cánovas, F.G.; Mota, C.F.; Serrão, E.A.; Pearson, G.A. Driving South: A Multi-Gene Phylogeny of the Brown Algal Family Fucaceae Reveals Relationships and Recent Drivers of a Marine Radiation. BMC Evol. Biol. 2011, 11, 371. [Google Scholar] [CrossRef]
- Lipizer, M.; Bressan, G.; Catalano, G.; Ghirardelli, L.A. Adaptability of Fucus virsoides J.AG. (Fucales, Chromophycophyta) to Habitat Variations in the Gulf of Trieste, North Adriatic Sea. Oebalia 1995, 21, 51–59. [Google Scholar]
- Vukovič, A. Bentoška Vegetacija Koprskega Zaliva. Acta Adriat. 1982, 23, 227–235. [Google Scholar]
- Rindi, F.; Battelli, C. Spatio-Temporal Variability of Intertidal Algal Assemblages of the Slovenian Coast (Gulf of Trieste, Northern Adriatic Sea). Bot. Mar. 2005, 48, 96–105. [Google Scholar] [CrossRef]
- Battelli, C. Structure and Dynamic of Midlittoral Macrobenthic Algal Communities of the Slovenian Sea; University of Ljubljana: Ljubljana, Slovenia, 2013. [Google Scholar]
- Vergés, A.; Alcoverro, T.; Ballesteros, E. Role of Fish Herbivory in Structuring the Vertical Distribution of Canopy Algae Cystoseira spp. in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2009, 375, 1–11. [Google Scholar] [CrossRef]
- Antolić, B.; Skaramuca, B.; Špan, A.; Mušin, D.; Sanko-Njire, J. Food and Feeding Habits of a Herbivore Fish Sarpa salpa (L.) (Teleostei, Sparidae) in the Southern Adriatic (Croatia). Acta Adriat. 1994, 35, 45–52. [Google Scholar]
- Sala, E.; Boudouresque, C.F.; Harmelin-Vivien, M. Fishing, Trophic Cascades, and the Structure of Algal Assemblages: Evaluation of an Old but Untested Paradigm. Oikos 1998, 82, 425–439. [Google Scholar] [CrossRef]
- Iveša, L.; Djakovac, T.; Devescovi, M. Long-Term Fluctuations in Cystoseira Populations along the West Istrian Coast (Croatia) Related to Eutrophication Patterns in the Northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Cogoni, D.; Fenu, G.; Dessi, C.; Deidda, A.; Giotta, C.; Piccitto, M.; Bacchetta, G. Importance of Plants with Extremely Small Populations (PSESPs) in Endemic-Rich Areas, Elements Often Forgotten in Conservation Strategies. Plants 2021, 10, 1504. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. U.S. National Institutes of Health, Bethesda, Maryland, USA. Available online: https://imagej.nih.gov/ij/ (accessed on 1 March 2023).




| Source | df | MS | F | p |
|---|---|---|---|---|
| Position | 1 | 2.4337 | 0.38 | 0.5556 |
| Time | 7 | 160.2287 | 104.77 | 0.0000 |
| Position × Time | 7 | 6.3555 | 4.16 | 0.0003 |
| Residual | 144 | 1.5293 | ||
| Cochran’s test: 0.33, p < 0.05 | ||||
| SNK test for the interaction Position x Time: | ||||
| November 2021: Upper = Lower January 2022: Upper = Lower February 2022: Upper = Lower March 2022: Upper = Lower | May 2022: Upper > Lower July 2022: Upper < Lower August 2022: Upper = Lower November 2022: Upper > Lower | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gljušćić, E.; Bilajac, A.; Smith, S.M.; Najdek, M.; Iveša, L. First Restoration Experiment for Endemic Fucus virsoides on the Western Istrian Coast—Is It Feasible? Plants 2023, 12, 1445. https://doi.org/10.3390/plants12071445
Gljušćić E, Bilajac A, Smith SM, Najdek M, Iveša L. First Restoration Experiment for Endemic Fucus virsoides on the Western Istrian Coast—Is It Feasible? Plants. 2023; 12(7):1445. https://doi.org/10.3390/plants12071445
Chicago/Turabian StyleGljušćić, Edi, Andrea Bilajac, Shannen Maree Smith, Mirjana Najdek, and Ljiljana Iveša. 2023. "First Restoration Experiment for Endemic Fucus virsoides on the Western Istrian Coast—Is It Feasible?" Plants 12, no. 7: 1445. https://doi.org/10.3390/plants12071445
APA StyleGljušćić, E., Bilajac, A., Smith, S. M., Najdek, M., & Iveša, L. (2023). First Restoration Experiment for Endemic Fucus virsoides on the Western Istrian Coast—Is It Feasible? Plants, 12(7), 1445. https://doi.org/10.3390/plants12071445







