Integrative Analysis of Fruit Quality and Anthocyanin Accumulation of Plum cv. ‘Cuihongli’ (Prunus salicina Lindl.) and Its Bud Mutation
Abstract
1. Introduction
2. Results
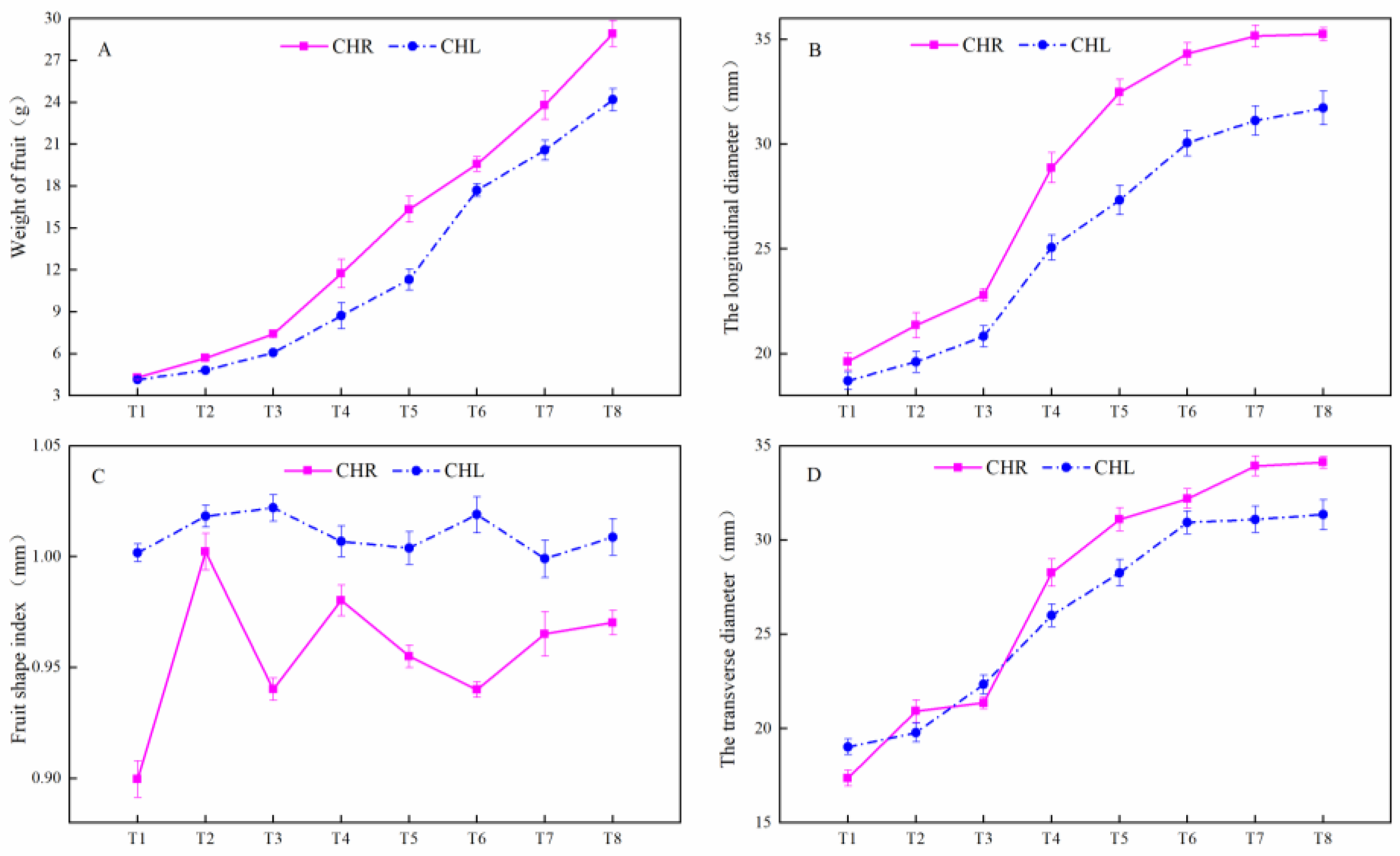
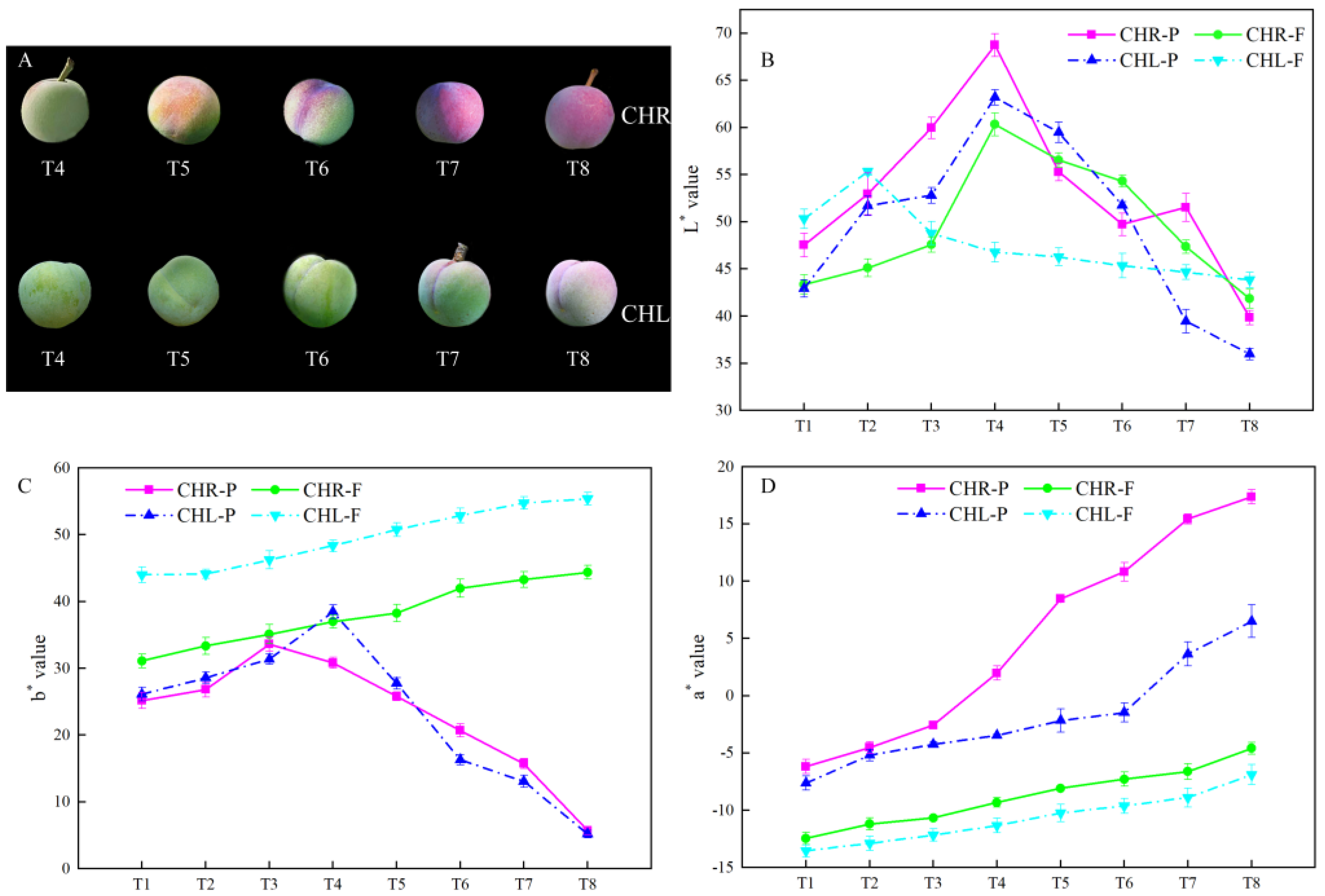
2.1. Changes of the Appearance Index in Plum Cultivars
2.2. Changes of the Soluble Sugar Contents, Total Soluble Solids, Total Acid, and Vc Contents in Plum Cultivars
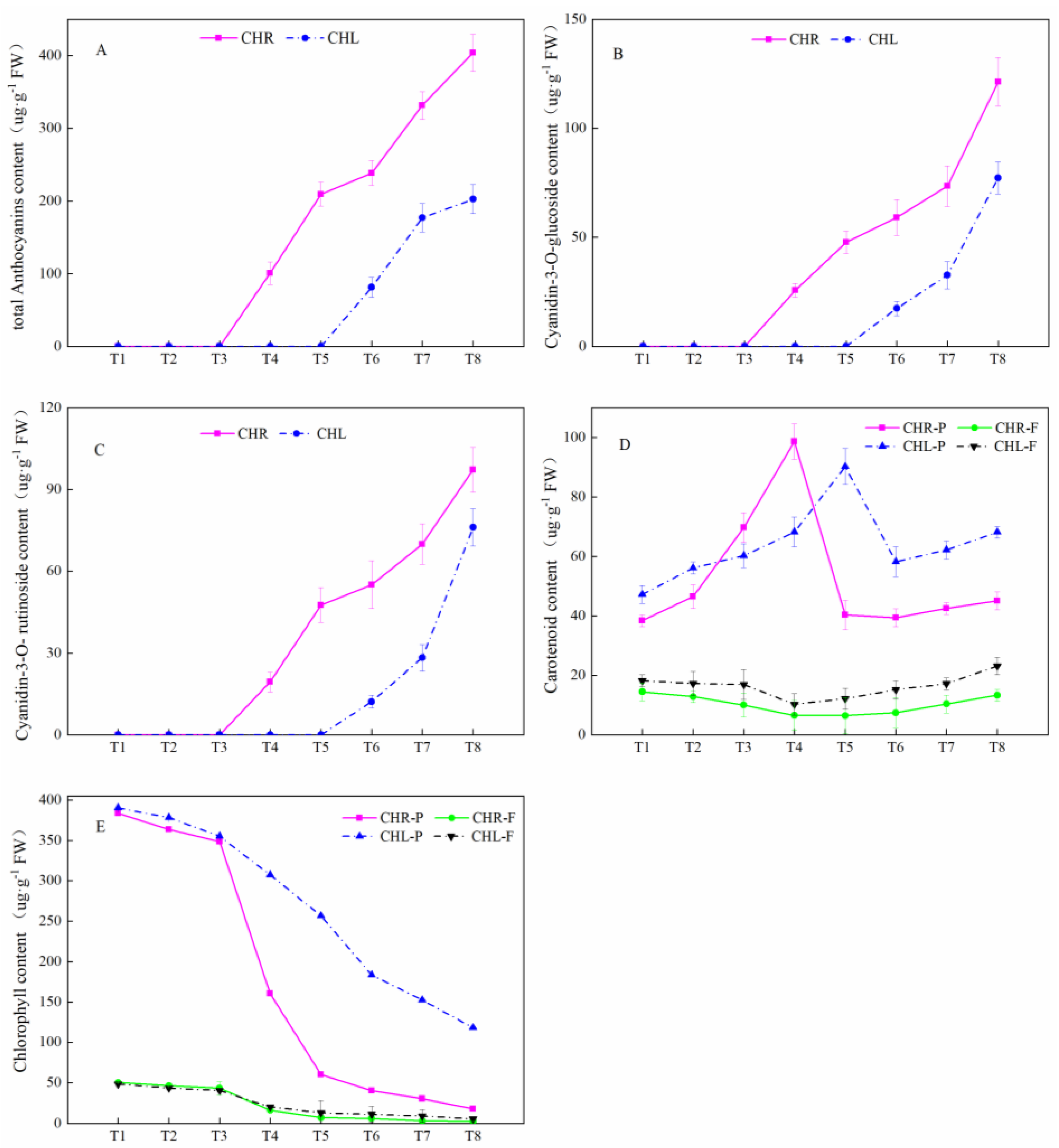
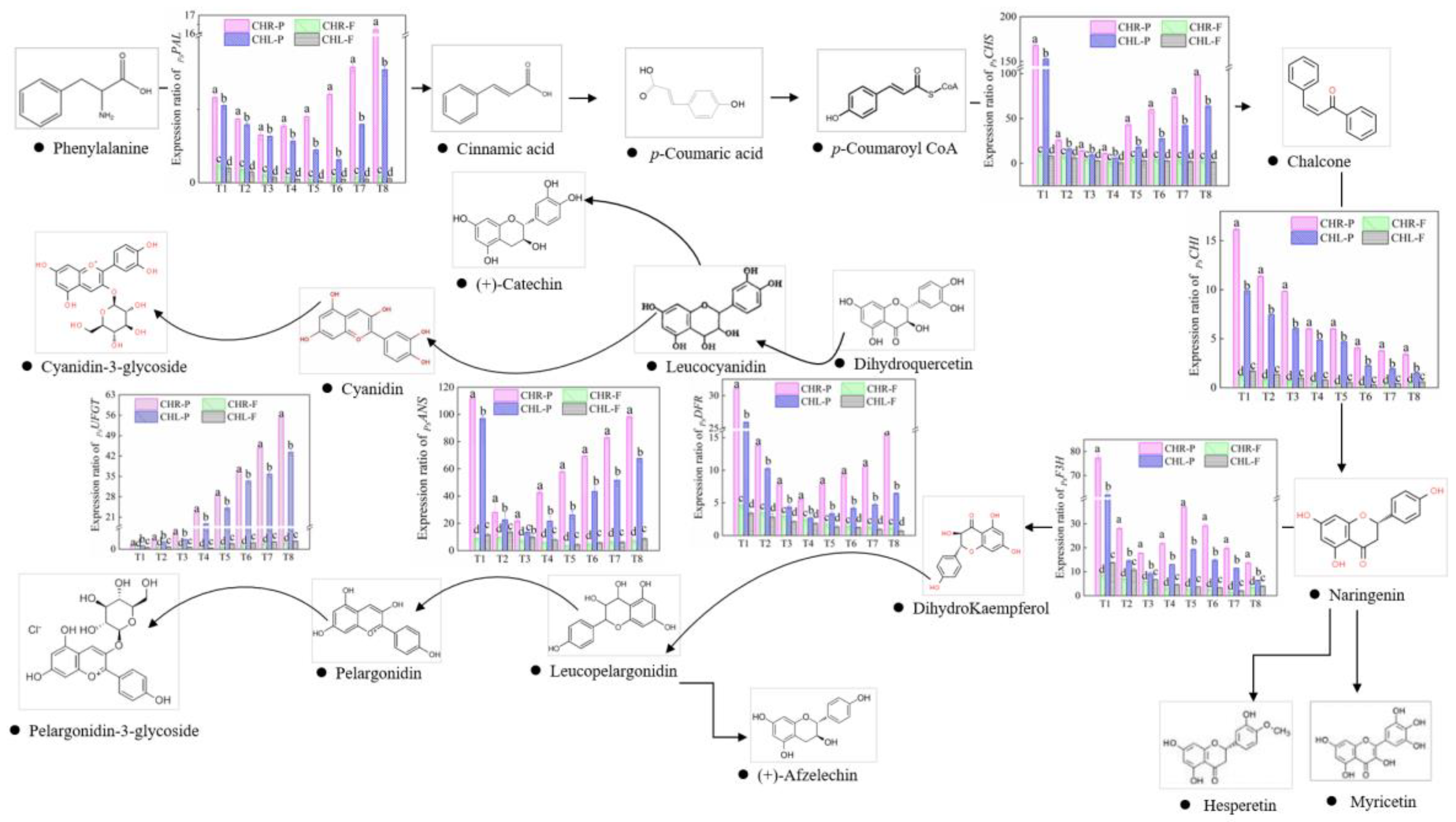
2.3. Comparison of Anthocyanin Profiles in the Skin and Flesh of Mature Plums from CHL and CHR
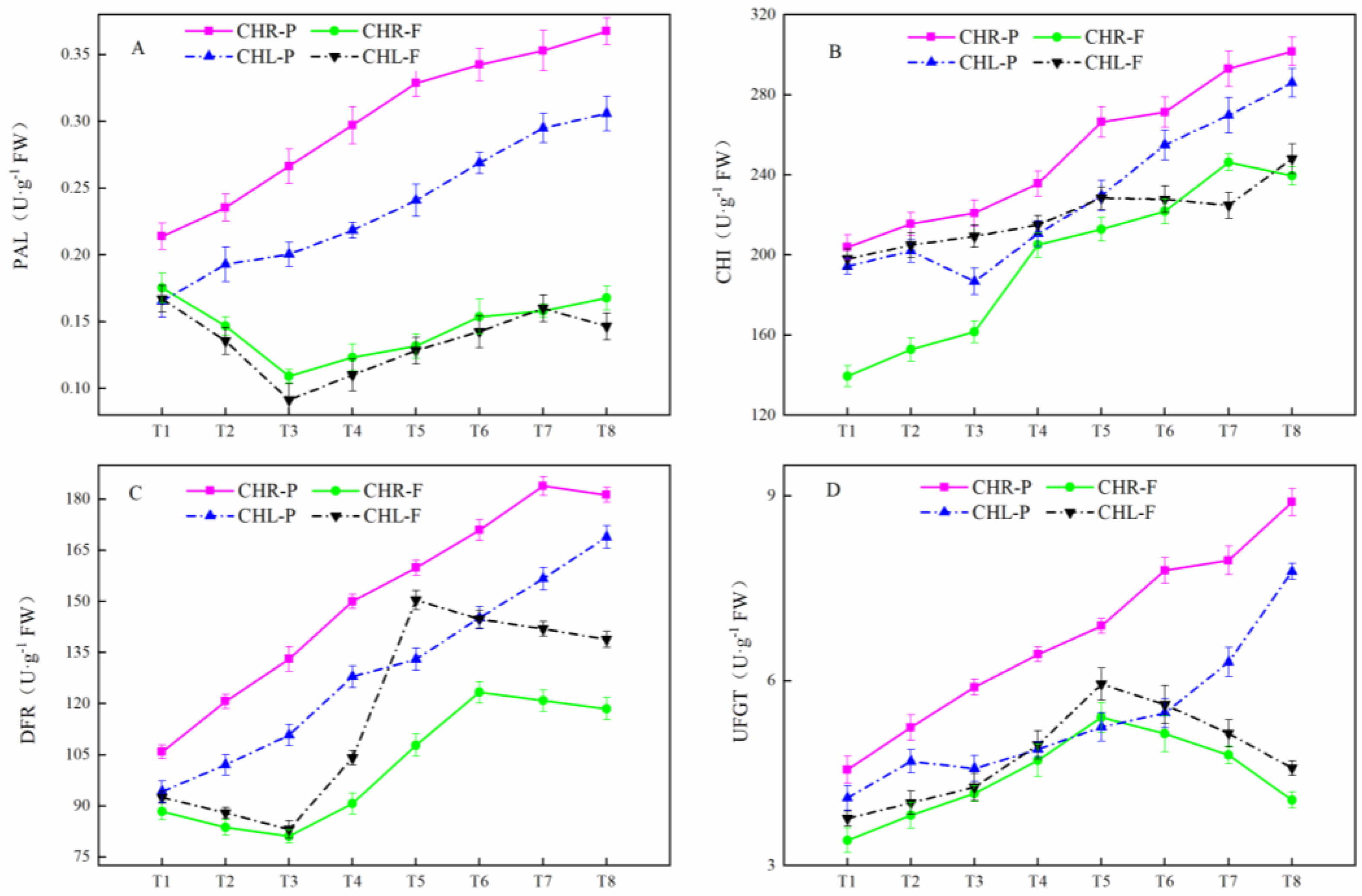
2.4. Changes of Related Enzyme Activities
2.5. Variations of Gene Expression
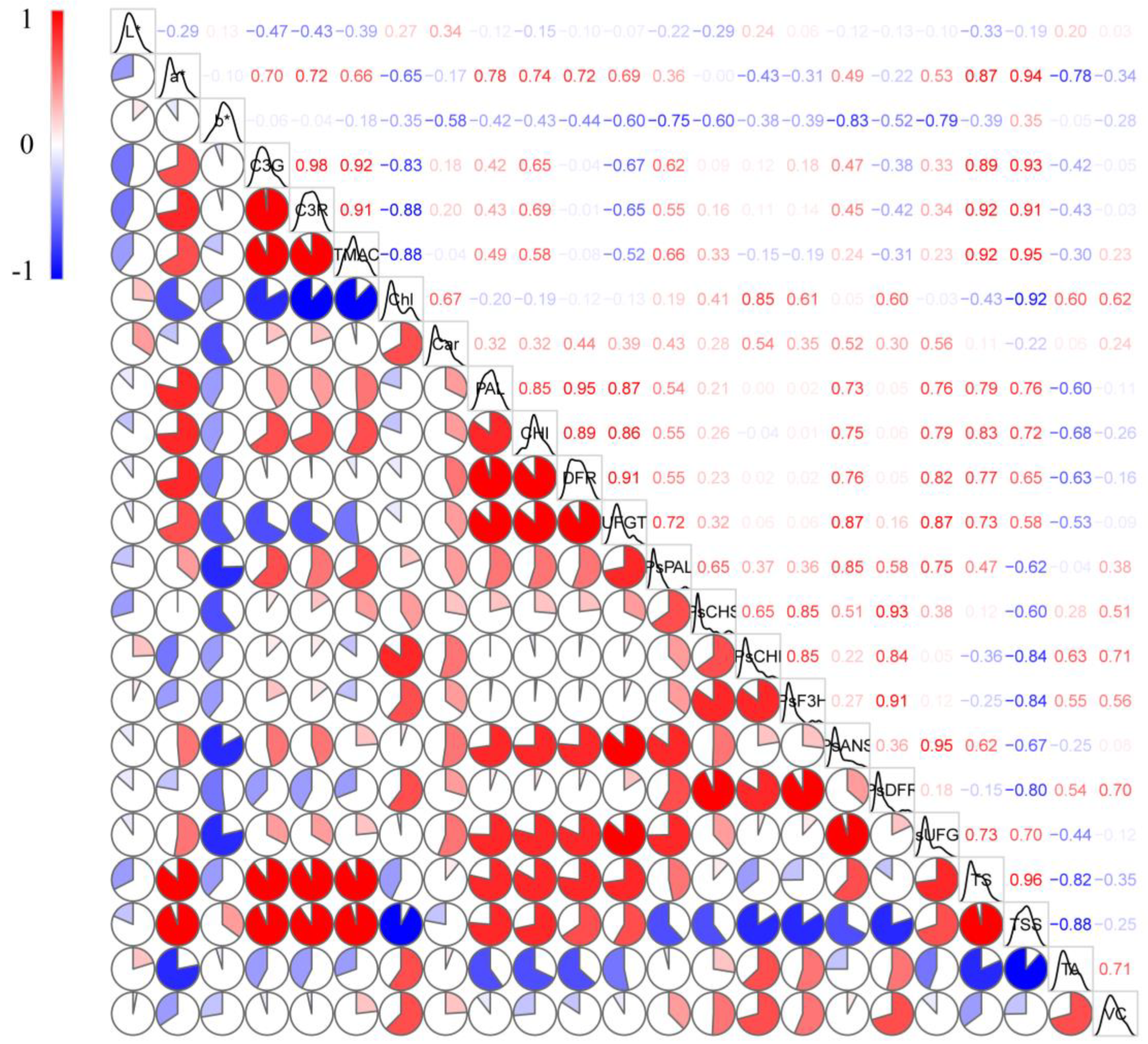
2.6. Correlation Analysis
3. Discussion
3.1. CHR Demonstrated a High Level of Fruit Quality Compared to CHL
3.2. Comparison of Colour in the Skin of CHR and CHL
3.3. Comparison of Anthocyanin Profiles in the Skin of Mature Plums from CHR and CHL
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Fruit Characteristics
4.3. Anthocyanin Analysis
4.4. Spectrophotometric Determination of Chlorophyll and Carotenoid Content
4.5. Determination of Enzyme Activity
4.6. RNA Extraction and Gene Expression Analysis
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahamishirazi, S.; Moehring, J.; Claupeina, W.; Graeff-Hoenningera, S. Quality assessment of 178 cultivars of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 2017, 214, 694–701. [Google Scholar] [CrossRef]
- Simonavice, E.; Liu, P.Y.; Ilich, J.Z.; Kim, J.-S.; Arjmandi, B.; Panton, L.B. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl. Physiol. Nutr. Metab. 2013, 39, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Igwe, E.O.; Charlton, K.E.; Roodenrys, S.; Kent, K.; Fanning, K.; Netzel, M.E. Anthocyanin-rich plum juice reduces ambulatory blood pressure but not acute cognitive function in younger and older adults: A pilot crossover dose-timing study. Nutr. Res. 2017, 47, 28–43. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Kundur, A.R.; Fanning, K.; Netzel, M.; Stanley, R.; Singh, I. Consumption of anthocyanin-rich Queen Garnet plum juice reduces platelet activation related thrombogenesis in healthy volunteers. J. Funct. Foods 2015, 12, 11–22. [Google Scholar] [CrossRef]
- Muzzaffar, S.; Masoodi, F.A. Plum cultivars grown in Himalayan temperate conditions: Physicochemical, antioxidant and antiproliferative activity against three cancer cell lines. J. Food Meas. Charact. 2018, 12, 2247–2255. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Jain, S.M.; Johnson, D.V. Advances in Plant Breeding Strategies: Fruits (Volume 3)||Plum (Prunus spp.) Breeding; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Wang, K.L.; Zhou, D.R.; Lin, Y.J.; Jiang, C.C.; Pan, S.L.; Espley, R.V.; Andre, C.M.; Ye, X.F. Activation of PsMYB10.2 Transcription Causes Anthocyanin Accumulation in Flesh of the Red-Fleshed Mutant of ‘Sanyueli’ (Prunus salicina Lindl.). Front. Plant Sci. 2021, 12, 680469. [Google Scholar] [CrossRef] [PubMed]
- Tadeo, F.R.; Terol, J.; Rodrigo, M.J.; Licciardello, C.; Sadka, A. Fruit Growth and Development; Woodhead Publishing: Cambridge, UK, 2020; pp. 245–269. [Google Scholar] [CrossRef]
- Dar, J.A.; Wani, A.A.; Ahmed, M.; Nazir, R.; Zargar, S.M.; Javaid, K. Peel colour in apple (Malus × domestica Borkh.): An economic quality parameter in fruit market. Sci. Hortic. 2019, 244, 50–60. [Google Scholar] [CrossRef]
- Fanning, K.J.; Topp, B.; Russell, D.; Stanley, R.; Netzel, M. Japanese plums (Prunus salicina Lindl.) and phytochemicals- breeding, horticultural practice, postharvest storage, processing and bioactivity. J. Sci. Food Agric. 2014, 94, 2137–2147. [Google Scholar] [CrossRef]
- Wang, L.; Sang, W.; Xu, R.; Cao, J. Alteration of flesh color and enhancement of bioactive substances via the stimulation of anthocyanin biosynthesis in ‘Friar’plum fruit by low temperature and the removal. Food Chem. 2020, 310, 125862. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, L.; Yuan, C.; Guan, J. Molecular characterization of ethylene-regulated anthocyanin biosynthesis in plums during fruit ripening. Plant Mol. Biol. Rep. 2016, 34, 777–785. [Google Scholar] [CrossRef]
- González-Villagra, J.; Kurepin, L.V.; Reyes-Díaz, M.M. Evaluating the involvement and interaction of abscisic acid and miRNA156 in the induction of anthocyanin biosynthesis in drought-stressed plants. Planta 2017, 246, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Einhorn, T.C.; Cheng, L. Comparison of phenolic metabolism and primary metabolism between green ‘Anjou’pear and its bud mutation, red ‘Anjou’. Physiol. Plant. 2014, 150, 339–354. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, M.; Zou, C.; Liu, X.; Shen, X.; Hayward, A.; Wang, Y. Comparative analysis of anthocyanin biosynthesis during fruit development in two Lycium species. Physiol. Plant. 2014, 150, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Busatto, N.; Trainotti, L. Regulation of anthocyanin biosynthesis in peach fruits. Planta 2014, 240, 913–929. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- González, M.; Salazar, E.; Cabrera, S.; Olea, P.; Carrasco, B. Analysis of anthocyanin biosynthesis genes expression profiles in contrasting cultivars of Japanese plum (Prunus salicina L.) during fruit development. Gene. Expr. Patterns 2016, 21, 54–62. [Google Scholar] [CrossRef]
- Petit, R.J.; Hampe, A. Some Evolutionary Consequences of Being a Tree. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 187–214. [Google Scholar] [CrossRef]
- Li, W.F.; Mao, J.; Yang, S.J.; Guo, Z.G.; Ma, Z.H.; Dawuda, M.M.; Chen, B.H. Anthocyanin accumulation correlates with hormones in the fruit skin of ‘Red Delicious’ and its four generation bud sport mutants. BMC Plant Biol. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Guan, L.; Zheng, T.; Jiu, S.; Zhu, X.; Jia, S.T.; Fang, J. Changes of anthocyanin component biosynthesis in ‘Summer Black’grape berries after the red flesh mutation occurred. J. Agric. Food Chem. 2018, 66, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.H.; Tong, L.; Liu, M.Z.; Zhou, X.K.; Tahir, M.M.; Xing, L.B.; Ma, J.J.; An, N.; Zhao, C.P.; et al. Multi-omics analyses reveal MdMYB10 hypermethylation being responsible for a bud sport of apple fruit color. Hortic. Res. 2022, 9, uhac179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, J.; Liu, Y.; Zhao, X.; Deng, X.; Guo, L.; Gu, J. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Xi, F.F.; Guo, L.L.; Yu, Y.H.; Wang, Y.; Li, Q.; Zhao, H.L.; Zhang, G.H.; Guo, D.L. Comparison of reactive oxygen species metabolism during grape berry development between ‘Kyoho’ and its early ripening bud mutant ‘Fengzao’. Plant Physiol. Biochem. 2017, 118, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, C.C.; Gribaudo, I.; Schneider, A.; Wehrens, R.; Grando, M.S.; Costantini, L. Transcriptome analysis during berry development provides insights into co-regulated and altered gene expression between a seeded wine grape variety and its seedless somatic variant. BMC Genom. 2014, 15, 1030. [Google Scholar] [CrossRef]
- Foster, T.M.; Aranzana, M.J. Attention sports fans! The far-reaching contributions of bud sport mutants to horticulture and plant biology. Hortic. Res. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.C.; Fang, Z.Z.; Zhou, D.R.; Pan, S.L.; Ye, X.F. Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv.‘Furongli’ (Prunus salicina Lindl). J. Sci. Food Agric. 2019, 99, 1010–1019. [Google Scholar] [CrossRef]
- Durmaz, G.; Cam, M.; Kutlu, T.; HIşIL, Y. Some physical and chemical changes during fruit development of five common apricot (Prunus armeniaca L.) cultivars. Food Sci. Technol. Res. 2010, 16, 71–78. [Google Scholar] [CrossRef]
- Dai, Z.W.; Meddar, M.; Renaud, C.; Merlin, I.; Hilbert, G.; Delrot, S.; Gomès, E. Long-term in vitro culture of grape berries and its application to assess the effects of sugar supply on anthocyanin accumulation. J. Exp. Bot. 2013, 65, 4665–4677. [Google Scholar] [CrossRef]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Han, J.; Song, C.N.; Cheng, Z.M.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plant. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- Steyn, W.J. Prevalence and function of anthocyanins in fruits. In Anthocyanins: Biosynthesis, Functions and Applications; Gould, K., Davis, K., Chris, W., Eds.; Springer: New York, NY, USA, 2009; pp. 85–100. [Google Scholar] [CrossRef]
- Ranganath, K.G. Pigments that colour our fruits: An overview. Erwerbs-Obstbau 2022, 64, 535–547. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Grant, J.E.; Lister, C.E.; Taylor, M.C. Skin color in apples-influence of copigmentation and plastid pigments on shade and darkness of red color in five genotypes. J. Am. Soc. Hortic. Sci. 1994, 119, 63–69. [Google Scholar] [CrossRef]
- Yang, T.; Ma, H.; Zhang, J.; Wu, T.; Song, T.; Tian, J.; Yao, Y. Systematic identification of long noncoding RNA s expressed during light-induced anthocyanin accumulation in apple fruit. Plant J. 2019, 100, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Infante, R.; Rubio, P.; Contador, L.; Noferini, M.; Costa, G. Determination of harvest maturity of D’Agen plums using the chlorophyll absorbance index. Cienc. E Investig. Agrar. Rev. Latinoam. Cienc. Agric. 2011, 38, 199–203. [Google Scholar] [CrossRef]
- Alina, V.R.; Vlad, M.; Elena, M.A.; Carmen, M.C.; Adriana, P.; Viorel, M.; Simona, C.M.; Muste, S. The changes of polyphenols, flavonoids, anthocyanins and chlorophyll content in plum peels during growth phases: From fructification to ripening. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 46, 148. [Google Scholar] [CrossRef]
- Chaves-Silva, S.; Dos Santos, A.L.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants-tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef]
- Usenik, V.; Tampar, F.; Veberi, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Roussos, P.A.; Efstathios, N.; Intidhar, B.; Denaxa, N.K.; Tsafouros, A. Plum (Prunus domestica L. and P. salicina Lindl.). In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, UK, 2016; pp. 639–666. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Venter, A.; Joubert, E.; Beer, D.D. Nutraceutical Value of Yellow-and Red-Fleshed South African Plums (Prunus salicina Lindl.): Evaluation of Total Antioxidant Capacity and Phenolic Composition. Molecules 2014, 19, 3084–3109. [Google Scholar] [CrossRef]
- Hyun, T.K.; Lee, S.; Rim, Y.; Kumar, R.; Han, X.; Lee, S.Y.; Kim, J.Y. De-novo RNA sequencing and metabolite profiling to identify genes involved in anthocyanin biosynthesis in Korean black raspberry (Rubus coreanus Miquel). PLoS ONE 2014, 9, e88292. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E.; Culbertson, J.D.; Corn Well, C.J.; Mattick, L.R. Detection of adulteration in blackberry juice concentrates and wines. J. Assoc. Off. Anal. Chem. 1982, 65, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Alan, R.W. The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Cheng, L. Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol. Plant. 2013, 148, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Lister, C.E.; Lancaster, J.E.; Walker, J. Developmental Changes in Enzymes of Flavonoid Biosynthesis in the Skins of Red and Green Apple Cultivars. J. Sci. Food Agric. 2015, 71, 313–320. [Google Scholar] [CrossRef]
- Duan, B.; Song, C.; Zhao, Y.; Jiang, Y.; Shi, P.; Meng, J.; Zhang, Z. Dynamic changes in anthocyanin biosynthesis regulation of Cabernet Sauvignon (Vitis vinifera L.) grown during the rainy season under rain-shelter cultivation. Food Chem. 2019, 283, 404–413. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Primer Sequence (5′–3′) |
|---|---|
| Actin | Forward: GCAGACAGGATGAGCAAGGAGATTAC |
| Reverse: TCTGTTGGAAGGTACTGAGGGATG | |
| PsPAL | Forward: CCTCCCACAGAAGAACAAAGCAAG |
| Reverse: GCCTGACTCTTTCGTGCTCCC | |
| PsCHS | Forward: GCGGACTACCAGCTCACCAAG |
| Reverse: CACACAACAAGAACACGAGCAC | |
| PsCHI | Forward: AGGTGACAACGATACTGCCA |
| Reverse: CGCCAGGTGGGAAGTTTT | |
| PsF3H | Forward: CAATGGGAGGTTCAAGAATG |
| Reverse: TCTGGAATGTGGCTATGG | |
| PsDFR | Forward: GGCTGACCTGGCGGACGAG |
| Reverse: CACTTCGTTCTCGGGGTCTTTGG | |
| PsANS | Forward: GAGTACATCAGACCCAAGGAAGAGC |
| Reverse: GCCTTCTTCAACTCCTCCCTGC | |
| PsUFGT | Forward: GTACCCTTCTTATGGTCTCTCA |
| Reverse: CCATCCCATTGCTTGCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Li, Y.; Lan, X.; Yang, Y.; Wei, W.; Ai, J.; Feng, X.; Chen, H.; Tang, Y.; Xi, L.; et al. Integrative Analysis of Fruit Quality and Anthocyanin Accumulation of Plum cv. ‘Cuihongli’ (Prunus salicina Lindl.) and Its Bud Mutation. Plants 2023, 12, 1357. https://doi.org/10.3390/plants12061357
Liao L, Li Y, Lan X, Yang Y, Wei W, Ai J, Feng X, Chen H, Tang Y, Xi L, et al. Integrative Analysis of Fruit Quality and Anthocyanin Accumulation of Plum cv. ‘Cuihongli’ (Prunus salicina Lindl.) and Its Bud Mutation. Plants. 2023; 12(6):1357. https://doi.org/10.3390/plants12061357
Chicago/Turabian StyleLiao, Ling, Yaman Li, Xuejiao Lan, Yiyue Yang, Wen Wei, Jinglan Ai, Xiangning Feng, Hongyu Chen, Yuhang Tang, Lijuan Xi, and et al. 2023. "Integrative Analysis of Fruit Quality and Anthocyanin Accumulation of Plum cv. ‘Cuihongli’ (Prunus salicina Lindl.) and Its Bud Mutation" Plants 12, no. 6: 1357. https://doi.org/10.3390/plants12061357
APA StyleLiao, L., Li, Y., Lan, X., Yang, Y., Wei, W., Ai, J., Feng, X., Chen, H., Tang, Y., Xi, L., & Wang, Z. (2023). Integrative Analysis of Fruit Quality and Anthocyanin Accumulation of Plum cv. ‘Cuihongli’ (Prunus salicina Lindl.) and Its Bud Mutation. Plants, 12(6), 1357. https://doi.org/10.3390/plants12061357






