The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of the EO and Hydrolate
2.2. Enantioselective Analysis of the EO
3. Discussion
4. Materials and Methods
4.1. Chemicals, Materials, and Equipment
4.2. Plant Material
4.3. Obtention of the EO and Hydrolate
4.4. GC Sample Preparation
4.5. Qualitative GC-MS Analyses
4.6. Quantitative GC-FID Analyses
4.7. Enantioselective Analysis of the EO
4.8. Purification and Identification of Furanoeremophilane and Bakkenolide A
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2013; p. 743. [Google Scholar]
- Gilardoni, G.; Montalván, M.; Vélez, M.; Malagón, O. Chemical and Enantioselective Analysis of the Essential Oils from Different Morphological Structures of Ocotea quixos (Lam.) Kosterm. Plants 2021, 10, 2171. [Google Scholar] [CrossRef] [PubMed]
- Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef]
- Ramírez, J.; Andrade, M.D.; Vidari, G.; Gilardoni, G. Essential Oil and Major Non-Volatile Secondary Metabolites from the Leaves of Amazonian Piper subscutatum. Plants 2021, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A Novel Chemical Profile of a Selective In Vitro Cholinergic Essential Oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a Native Andean Species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Montalván, M.; Ortiz, M.; Vinueza, D.; Montesinos, J.V. The Flower Essential Oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, Enantioselective, and Olfactometric Analyses. Plants 2020, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef]
- Malagón, O.; Bravo, C.; Vidari, G.; Cumbicus, N.; Gilardoni, G. Essential Oil and Non-Volatile Metabolites from Kaunia longipetiolata (Sch.Bip. ex Rusby) R. M. King and H. Rob., an Andean Plant Native to Southern Ecuador. Plants 2022, 11, 2972. [Google Scholar] [CrossRef]
- Megadiverse Countries, UNEP-WCMC. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 4 January 2023).
- Malagón, O.; Ramírez, J.; Andrade, J.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef]
- Malagón, O.; Cartuche, P.; Montaño, A.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of the Endemic Andean Species Gynoxys miniphylla Cuatrec. (Asteraceae): Chemical and Enantioselective Analyses. Plants 2022, 11, 398. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of Gynoxys rugulosa Muschl. (Asteraceae) Growing in Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 849. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.J.; Tonneijk, F.H.; van der Wielen, F.W.M.; Verstraten, J.M. Can Isoprenoids in Leaves and Roots of Plants Serve as Biomarkers for Past Vegetation Changes? A Case Study from the Ecuadorian Andes. Plant Soil 2007, 291, 181. [Google Scholar] [CrossRef]
- Jakupovic, J.; Zdero, C.; King, R.M. Furanoeremophilanes from Gynoxys Species. Phytochemistry 1995, 40, 1677. [Google Scholar] [CrossRef]
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; p. 287. [Google Scholar]
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org (accessed on 4 January 2023).
- Minga, D.; Ansaloni, R.; Verdugo, A.; Ulloa Ulloa, C. Flora del Páramo del Cajas; Universidad del Azuay, Imprenta Don Bosco- Centro Gráfico Salesiano: Cuenca, Ecuador, 2016; p. 142. [Google Scholar]
- Ishii, H.; Tozyo, T.; Minato, H. Studies on Sesquiterpenoids. Part XIII. Components of the Root of Ligularia Fischeri Turcz. J. Chem. Soc. C 1966, 1545. [Google Scholar] [CrossRef]
- Sato, T.; Tada, M.; Takahashi, T.; Horibe, I.; Ishii, H.; Tori, K. Carbon-13 and Hydrogen-1 NMR Studies of Conformations of Ligularol and 6-Epiligularol, Naturally Occurring cis-Decalin Derivatives. Tetrahedron Lett. 1977, 44, 3895. [Google Scholar] [CrossRef]
- Tada, M.; Sato, T.; Takahashi, T.; Tori, K.; Horibe, I.; Kuriyama, K. Conformational Equilibria of Eremophilanes, Naturally Occurring cis-Decalin Derivatives, Studied by N.M.R. and C.D. Spectroscopy. J. Chem. Soc. Perkin I 1981, 2695–2701. [Google Scholar] [CrossRef]
- Shirahata, K.; Kato, T.; Kitahara, Y.; Abe, N. Constituents of Genus Petasites—IV. Bakkenolide A, a Sesquiterpene of Novel Carbon Skeleton. Tetrahedron 1969, 25, 3179. [Google Scholar] [CrossRef]
- Shirahata, K.; Kato, T.; Kitahara, Y.; Abe, N. Mass Spectra of Bakkenolides and Their Derivatives. Tetrahedron 1969, 25, 4671. [Google Scholar] [CrossRef]
- Constantino, M.G.; de Oliveira, K.T.; Polo, E.C.; da Silva, G.V.J.; Brocksom, T.J. Core Structure of Eremophilanes and Bakkanes through Niobium Catalyzed Diels-Alder Reaction: Synthesis of (±)-Bakkenolide A. J. Org. Chem. 2006, 71, 9880. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-193263321. [Google Scholar]
- Bendahou, M.; Muselli, A.; Grignon-Dubois, M.; Benyoucef, M.; Desjobert, J.-M.; Bernardini, A.-F.; Costa, J. Antimicrobial Activity and Chemical Composition of Origanum glandulosum Desf. Essential Oil and Extract Obtained by Microwave Extraction: Comparison with Hydrodistillation. Food Chem. 2008, 106, 132. [Google Scholar] [CrossRef]
- Castilho, P.; Liu, K.; Rodrigues, A.I.; Feio, S.; Tomi, F.; Casanova, J. Composición y Actividad Antimicrobiana del Aceite Esencial de Clinopodium ascendens (Jordania) Sampaio de Madeira. Diario Sabor Fraganc. 2007, 22, 139. [Google Scholar]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and Antimicrobial Activity of the Essential Oil of Six Hypericum Species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146. [Google Scholar] [CrossRef]
- Tomi, F.; Barzalona, M.; Casanova, J.; Luro, F. Chemical Variability of the Leaf Oil of 113 Hybrids from Citrus clementina (Commun) × Citrus deliciosa (Willow Leaf). Flavour Fragr. J. 2008, 23, 152. [Google Scholar] [CrossRef]
- Bianchi, F.; Cantoni, C.; Careri, M.; Chiesa, L.; Musci, M.; Pinna, A. Characterization of the Aromatic Profile for the Authentication and Differentiation of Typical Italian Dry-sausages. Talanta 2007, 72, 1552. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparison of Volatiles of Cultured and Wild Sea Bream (Sparus aurata) During Storage in Ice by Dynamic Headspace Analysis/Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 2616. [Google Scholar] [CrossRef] [PubMed]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of Lavender Essential Oils by Using Gas Chromatography-Mass Spectrometry with Correlation of Linear Retention Indices and Comparison with Comprehensive Two-Dimensional Gas Chromatography. J. Chromatogr. A 2002, 970, 225. [Google Scholar] [CrossRef]
- Muselli, A.; Rossi, P.-G.; Desjobert, J.-M.; Bernardini, A.-F.; Berti, L.; Costa, J. Chemical Composition and Antibacterial Activity of Otanthus maritimus (L.) Hoffmanns. Link Essential Oils from Corsica. Flavour Fragr. J. 2007, 22, 217. [Google Scholar]
- Maksimović, M.; Vidic, D.; Miloš, M.; Edita Šolić, M.; Abadžić, S.; Siljak-Yakovlev, S. Effect of the Environmental Conditions on Essential Oil Profile in Two Dinaric Salvia Species: S. brachyodon Vandas and S. officinalis L. Biochem. Syst. Ecol. 2007, 35, 473. [Google Scholar] [CrossRef]
- Mardarowicz, M.; Wianowska, D.; Dawidowicz, A.L.; Sawicki, R. The Influence of Sample Treatment on SPME Extracts from Conifers. I. Comparison of Terpene Composition in Engelmann Spruce (Picea engelmanii) Using Hydrodistillation, SPME and PLE. Ann. Univ. Mariae Curie-Sklodowska 2004, 59, 25. [Google Scholar]
- Pozo-Bayon, M.A.N.; Ruiz-Rodriguez, A.; Karine-Pernin, A.N.C. Influence of Eggs on the Aroma Composition of a Sponge Cake and on the Aroma Release in Model Studies on Flavored Sponge Cakes. J. Agric. Food Chem. 2007, 55, 1418. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Castola, V.; Casanova, J. Composition and Chemical Variability of the Twig Oil of Abies alba Miller from Corsica. Flavour Fragr. J. 2007, 22, 293. [Google Scholar] [CrossRef]
- Bousmaha, L.; Boti, J.B.; Bekkara, F.A.; Castola, V.; Casanova, J. Infraspecific Chemical Variability of the Essential Oil of Lavandula dentata L. from Algeria. Flavour Fragr. J. 2006, 21, 368. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Özek, T.; Akalin, E.; Özhatay, N. Micro-Distilled Volatile Compounds from Ferulago Species Growing in Western Turkey. Pharm. Biol. 2002, 40, 466. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential Oil Analysis and Antimicrobial Activity of Eight Stachys Species from Greece. Phytochemistry 2003, 64, 743. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.T. The Composition of the Volatile Fraction and the Enantiomeric Distribution of Five Volatile Components of Faustrime Oil (Monocitrus australatica × Fortunella sp. × Citrus urantifolia). J. Essent. Oil Res. 2004, 16, 328. [Google Scholar] [CrossRef]
- Marinas, M.; Sa, E.; Rojas, M.M.; Moalem, M.; Urbano, F.J.; Guillou, C.; Rallo, L. A Nuclear Magnetic Resonance (1H and 13C) and Isotope Ratio Mass Spectrometry (d13C, d2H and d18O) Study of Andalusian Olive Oils. Rapid Commun. Mass Spectrom. 2010, 24, 1457. [Google Scholar]
- Gonny, M.; Bradesi, P.; Casanova, J. Identification of the Components of the Essential Oil from Wild Corsican Daucus carota L. Using 13C-NMR Spectroscopy. Flavour Fragr. J 2004, 19, 424. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.R.; Miguel, M.G.; da Cunha, A.P. Analysis by Gas Chromatography-Mass Spectrometry of the Volatile Components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A 2004, 1033, 187. [Google Scholar] [CrossRef]
- Ledauphin, J.; Saint-Clair, J.F.; Lablanquie, O.; Guichard, H.; Founier, N.; Guichard, E.; Barillier, D. Identification of Trace Volatile Compounds in Freshly Distilled Calvados and Cognac Using Preparative Separations Coupled with Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5124. [Google Scholar] [CrossRef]
- Chisholm, M.G.; Jell, J.A.; Cass, D.M. Characterization of the Major Odorants Found in the Peel Oil of Citrus reticulata Blanco cv. Clementine Using Gas Chromatography-Olfactometry. Flavour Fragr. J 2003, 18, 275. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Essential Oil Composition of Two Variants of Prostanthera lasianthos Labill. from Australia. Biochem. Syst. Ecol. 2006, 34, 48. [Google Scholar] [CrossRef]
- Varming, C.; Andersen, M.L.; Poll, L. Influence of Thermal Treatment on Black Currant (Ribes nigrum L.) Juice Aroma. J. Agric. Food Chem. 2004, 52, 7628. [Google Scholar] [CrossRef]
- Perez-Cacho, P.R.; Mahattanatawee, K.; Smoot, J.M.; Rouseff, R. Identification of Sulfur Volatiles in Canned Orange Juices Lacking Orange Flavor. J. Agric. Food Chem. 2007, 55, 5761. [Google Scholar] [CrossRef] [PubMed]
- Christlbauer, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Beef and Pork Vegetable Gravies à la Chef by Application of the Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2009, 57, 19114. [Google Scholar] [CrossRef] [PubMed]
- Paolini, J.; Costa, J.; Bernardini, A.F. Analysis of the Essential Oil from the Roots of Eupatorium cannabinum subsp. corsicum (L.) by GC, GC-MS and 13C-NMR. Phytochem. Anal. 2007, 18, 235. [Google Scholar] [PubMed]
- Velasco-Negueruela, A.; Pérez-Alonso, M.J.; Pérez de Paz, P.L.; Palá-Paúl, J.; Sanz, J. Analysis by Gas Chromatography-Mass Spectrometry of the Essential Oils from the Aerial Parts of Rutheopsis herbanica (Bolle) Hans. Kunk., gathered in Fuerteventura (Canary Islands). J. Chromatogr. A 2003, 984, 159. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Gancel, A.L.; Froelicher, Y.; Luro, F.; Ollitrault, P.; Brillouet, J.M. Effects of Nucleo-Cytoplasmic Interactions on Leaf Volatile Compounds from Citrus Somatic Diploid Hybrids. J. Agric. Food Chem. 2005, 53, 4517. [Google Scholar] [CrossRef] [PubMed]
- Shellie, R.; Marriott, P.; Zappia, G.; Mondello, L.; Dugo, G. Interactive Use of Linear Retention Indices on Polar and Apolar Columns with an MS-Library for Reliable Characterization of Australian Tea Tree and Other Melaleuca sp. Oils. J. Essent. Oil Res. 2003, 15, 305. [Google Scholar] [CrossRef]
- Bertoli, A.; Pistelli, L.; Morelli, I.; Fraternale, D.; Giamperi, L.; Ricci, D. Volatile Constituents of Different Parts (Roots, Stems and Leaves) of Smyrnium olusatrum L. Flavour Fragr. J. 2004, 19, 522. [Google Scholar] [CrossRef]
- Boskovic, Z.; Radulovic, N.; Stojanovic, G. Essential oil Composition of Four Achillea Species from the Balkans and Its Chemotaxonomic Significance. Chem. Nat. Compd. 2005, 41, 674. [Google Scholar] [CrossRef]
- Gancel, A.L.; Ollitrault, P.; Froelicher, Y.; Tomi, F.; Jacquemond, C.; Luro, F.; Brillouet, J.M. Leaf Volatile Compounds of Six Citrus Somatic Allotetraploid Hybrids Originating from Various Combinations of Lime, Lemon, Citron, Sweet Orange, and Grapefruit. J. Agric. Food Chem. 2005, 53, 2224. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Rosa, P.T.V.; Menut, C.; Leydet, A.; Brat, P.; Pallet, D.; Meireles, M.A.A. Valorization of Brazilian Vetiver (Vetiveria zizanioides (L.) Nash ex Small) Oil. J. Agric. Food Chem. 2004, 52, 6578. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.L.; Ollitrault, P.; Froelicher, Y.; Tomi, F.; Jacquemond, C.; Luro, F.; Brillouet, J.M. Leaf Volatile Compounds of Seven Citrus Somatic Tetraploid Hybrids Sharing Willow Leaf Mandarin (Citrus deliciosa Ten.) as Their Common Parent. J. Agric. Food Chem. 2003, 51, 6006. [Google Scholar] [CrossRef] [PubMed]
- Jelen, H.H.; Mirocha, C.J.; Wasowicz, E.; Kaminski, E. Production of Volatile Sesquiterpenes by Fusarium sambucinum Strans with Different Abilities to Synthesize Trichothecenes. Appl. Environ. Microbiol. 1995, 61, 3815. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical Composition and Antioxidant Capacity of Free Volatile Aglycones from Basil (Ocimum basilicum L.) Compared with Its Essential Oil. Food Chem. 2007, 101, 379. [Google Scholar] [CrossRef]
- Boti, J.B.; Bighelli, A.; Cavaleiro, C.; Salgueiro, L.; Casanova, J. Chemical Variability of Juniperus oxycedrus ssp. oxycedrus Berry and Leaf Oils from Corsica, Analysed by Combination of GC, GC-MS and 13C-NMR. Flavour Fragr. J. 2006, 21, 268. [Google Scholar] [CrossRef]
- Paolini, J.; Desjobert, J.M.; Costa, J.; Bernardini, A.F.; Castellini, C.B.; Cioni, P.L.; Flamini, G.; Morelli, I. Composition of Essential Oils of Helichysum italicum (Roth) G. Don fil Subsp. italicum from Tuscan Archipelago Islands. Flavour Fragr. J. 2006, 21, 805. [Google Scholar] [CrossRef]
- Liu, J.M.; Nan, P.; Tsering, Q.; Tsering, T.; Bai, Z.K.; Wang, L.; Liu, Z.J.; Zhong, Y. Volatile Constituents of the Leaves and Flowers of Salvia przewalskii Maxim. from Tibet. Flavour Fragr. J. 2006, 21, 435. [Google Scholar] [CrossRef]
- Paolini, J.; Costa, J.; Bernardini, A.F. Analysis of the Essential Oil from Aerial Parts of Eupatorium cannabinum subsp. corsicum (L.) by Gas Chromatography with Electron Impact and Chemical Ionization Mass Spectrometry. J. Chromatogr. A 2005, 1076, 170. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L.; Bagci, Y.; Dural, H.; Ertugrul, K.; Uysal, T.; Savran, A. A Multivariate Statistical Approach to Centaurea Classification Using Essential Oil Composition Data of Some Species from Turkey. Plant Syst. Evol. 2006, 261, 217. [Google Scholar] [CrossRef]
- Andriamaharavo, N.R. Retention Data. NIST Mass Spectrometry Data Center, 2014. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Source=2014AND%2319410M&Mask=2000 (accessed on 4 January 2023).
- Hochmannova, J.; Novotny, L.; Herout, V. On Terpenes. CXXXVIII. Sesquiterpenic Hydrocarbons from Coltsfoot Rhizomes (Petasites officinalis Moench.). Collect. Czechoslov. Chem. Commun. 1962, 27, 1870. [Google Scholar] [CrossRef]
- Novotny, L.; Herout, V.; Sorm, F. Substances from Petasites officinalis Moench. and Petasites albus (L.) Gaertn. Tetrahedron Lett. 1961, 20, 697. [Google Scholar] [CrossRef]
- Hochmannova, J.; Novotny, L.; Herout, V. On Terpenes. CXXLIV. Hydrocarbons from Petasites albus (L.) Gaertn. Rhizomes. Collect. Czechoslov. Chem. Commun. 1962, 27, 2711. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Mahanta, P.K. Neue Eremophilan-Derivate aus Lopholaena-Arten. Phytochemitry 1977, 16, 1796. [Google Scholar] [CrossRef]
- Bohlmann, F.; Le Van, N. Neue Sesqui- und Diterpene aus Bedfordia salicina. Phytochemistry 1978, 17, 1173. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. New Furanoeremophilanes from South African Senecio Species. Phytochemitry 1978, 17, 1161. [Google Scholar] [CrossRef]
- Saritas, Y.; von Reuβ, S.H.; Koenig, W.A. Sesquiterpene Constituents in Petasites hybridus. Phytochemitry 2002, 59, 795. [Google Scholar] [CrossRef]
- Abe, N.; Onoda, R.; Shirahata, K.; Kato, T.; Woods, M.C.; Kitahara, Y. The Structure of Bakkenolide A. Tetrahedron Lett. 1968, 3, 369. [Google Scholar] [CrossRef]
- Novotny, L.; Kotva, K.; Toman, J.; Herout, V. Sesquiterpenes from Petasites. Phytochemistry 1972, 11, 2795. [Google Scholar] [CrossRef]
- Jamieson, G.R.; Reid, E.H.; Turner, B.P.; Jamieson, A.T. Bakkenolide A. Its Distribution in Petasites Species and Cytotoxic Properties. Phytochemistry 1976, 15, 1713. [Google Scholar] [CrossRef]
- Kulinowski, Ł.; Luca, S.V.; Minceva, M.; Skalicka-Wozniak, K. A Review on the Ethnobotany, Phytochemistry, Pharmacology and Toxicology of Butterbur Species (Petasites L.). J. Ethnopharmacol. 2022, 293, 115263. [Google Scholar] [CrossRef]
- Chen, H.M.; Cai, M.S.; Jia, Z.J. Sesquiterpenes from Ligularia intermedia. Phytochemistry 1997, 7, 1441. [Google Scholar] [CrossRef]
- Hanai, R.; Watanabe, S.; Matsushima, M.; Nagano, H.; Kuroda, C.; Gong, X. Diversity in Eremophilane Components of Ligularia dictyoneura in Yunnan and Sichuan Provinces of China. Nat. Prod. Commun. 2019, 14, 1. [Google Scholar] [CrossRef]
- Jakupovic, J.; Grenz, M.; Bohlmann, F. A Further Bakenolide A Derivative from Homogyne alpina. Planta Med. 1989, 55, 571. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F., Jr. Total Synthesis of Bakkanes. Synthesis 2001, 5, 671. [Google Scholar] [CrossRef]
- Jerkovic, I.; Males, Z.; Friscic, M. Actualities in the Phytochemical Research on Selected Terpenes. Acta Pharm. 2019, 69, 533. [Google Scholar] [CrossRef]
- Payne, J.E. The Total Synthesis of +/- Bakkenolide-A. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 1999. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Zhang, L.; Hong, Z.; Zhang, R.R.; Sun, X.Z.; Yuan, Y.F.; Hu, J.; Wang, X. Bakkenolide A Inhibits Leukemia by Regulation of HDAC3 and PI3K/Akt-related Signaling Pathways. Biomed. Pharmacother. 2016, 83, 958. [Google Scholar] [CrossRef]
- Nawrot, J.; Bloszyk, E.; Harmatha, J.; Novotny, L. The Effect of Bisaboloangelone, Helenalin and Bakkenolide A on Development and Behaviour of Some Stored Product Beetles. Z. Ang. Ent. 1984, 98, 394. [Google Scholar] [CrossRef]
- Isman, M.B.; Brard, N.L.; Nawrot, J.; Harmatha, J. Antifeedant and Growth Inhibitory Effects of Bakkenolide-A and Other Sesquiterpene Lactones on the Variegated Cutworm, Peridroma saucia Hubner (Lep., Noctuidae). J. Appl. Enthomol. 1989, 107, 524. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohnb, S.; Rimbach, G. Thermal Stability, Antioxidant, and Anti-inflammatory Activity of Curcumin and Its Degradation Product 4-Vinylguaiacol. Food Funct. 2015, 6, 887. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Serra, S. Enantioselective Perception of Chiral Odorants. Tetrahedron Asymmetry 2003, 14, 1. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290. [Google Scholar] [CrossRef]

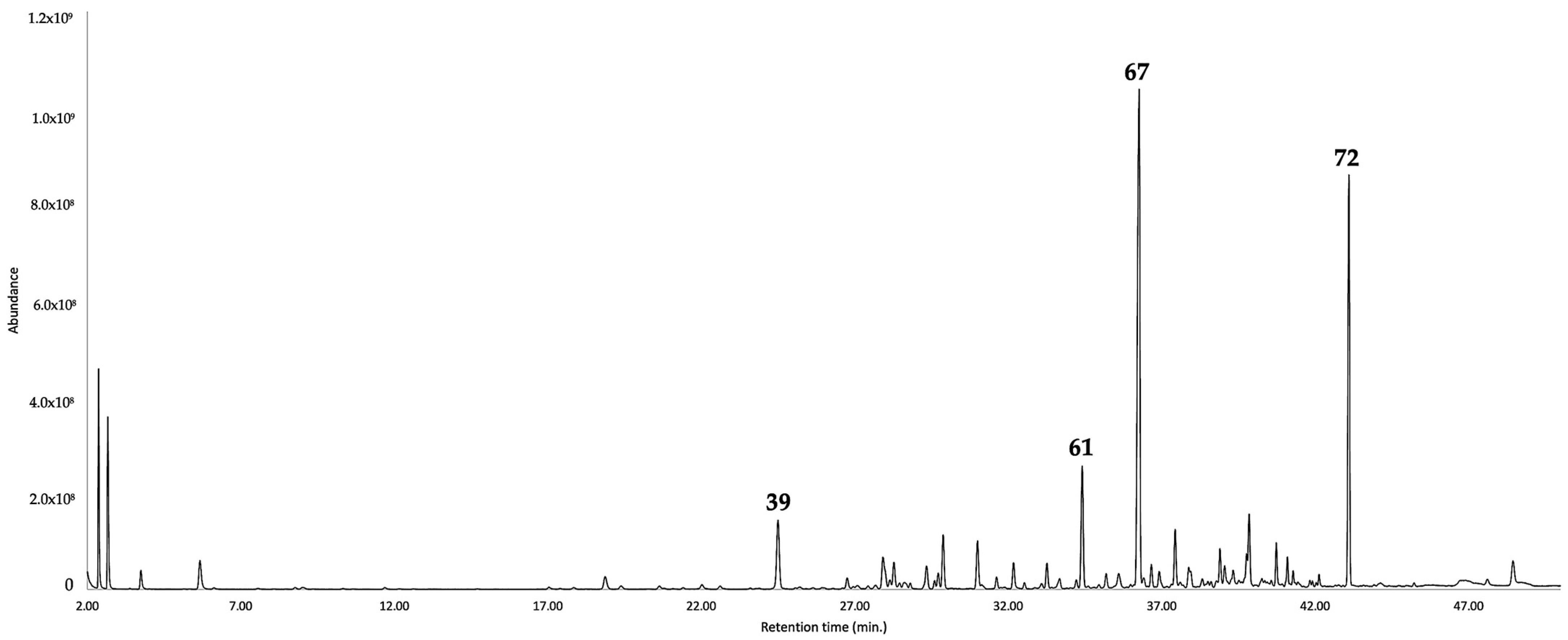
| N. | Compound | 5%-Phenyl-Methylpolysiloxane | Polyethylene Glycol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRI 1 | LRI 2 | Reference | Essential Oil | Hydrolate | LRI 1 | LRI 2 | Reference | Essential Oil | Hydrolate | ||||||
| % | σ | mg/100 mL | σ | % | σ | mg/100 mL | σ | ||||||||
| 1 | α-pinene | 933 | 932 | [25] | 1.1 | 0.01 | - | - | 1015 | 1022 | [26] | 1.3 | 0.10 | - | - |
| 2 | sabinene | 974 | 969 | [25] | 0.1 | 0.01 | - | - | 1115 | 1120 | [27] | trace | - | - | - |
| 3 | β-pinene | 979 | 974 | [25] | 2.7 | 0.05 | - | - | 1102 | 1105 | [28] | 2.6 | 0.17 | - | - |
| 4 | myrcene | 992 | 988 | [25] | trace | - | - | - | 1161 | 1161 | [29] | trace | - | - | - |
| 5 | n-decane | 1000 | 1000 | [25] | 0.1 | 0.01 | - | - | 1000 | - | * | trace | - | - | - |
| 6 | α-phellandrene | 1009 | 1002 | [25] | trace | - | - | - | 1156 | 1164 | [30] | trace | - | - | - |
| 7 | (2E,4E)-heptadienal | 1024 | 1017 | [25] | - | - | 0.4 | 0.03 | 1484 | 1492 | [31] | - | - | 0.5 | 0.06 |
| 8 | ο-cymene | 1029 | 1022 | [25] | 0.1 | 0.01 | - | - | 1262 | 1266 | [32] | trace | - | - | - |
| 9 | limonene | 1032 | 1024 | [25] | 0.1 | 0.01 | - | - | 1190 | 1197 | [30] | trace | - | - | - |
| 10 | β-phellandrene | 1034 | 1025 | [25] | 0.1 | 0.01 | - | - | 1197 | 1195 | [33] | trace | - | - | - |
| 11 | 1,8-cineole | 1036 | 1026 | [25] | 0.1 | 0.01 | 0.6 | 0.13 | 1195 | 1190 | [34] | trace | 0.01 | 1.1 | 0.15 |
| 12 | ο-tolualdehyde | 1058 | 1068 | [35] | - | - | 0.6 | 0.06 | 1638 | 1636 | [36] | - | - | 0.2 | 0.23 |
| 13 | γ-terpinene | 1061 | 1054 | [25] | trace | - | - | - | 1244 | 1244 | [37] | trace | - | - | - |
| 14 | linalool oxide (furanoid) | 1075 | 1067 | [25] | - | - | 1.9 | 0.56 | 1436 | 1439 | [38] | - | - | 0.7 | 0.32 |
| 15 | p-mentha-2,4(8)-diene | 1088 | 1085 | [25] | trace | - | - | - | 1287 | 1286 | [39] | trace | - | - | - |
| 16 | linalool | 1106 | 1095 | [25] | 0.1 | 0.01 | 1.0 | 0.08 | 1553 | 1554 | [40] | trace | - | 1.0 | 0.29 |
| 17 | n-nonanal | 1112 | 1100 | [25] | 0.4 | 0.01 | - | - | 1388 | 1387 | [28] | trace | - | - | - |
| 18 | trans-p-menth-2-en-1-ol | 1150 | 1136 | [25] | 0.1 | 0.05 | - | - | 1604 | 1609 | [41] | 0.1 | 0.05 | - | - |
| 19 | ethyl benzoate | 1167 | 1169 | [25] | 0.2 | 0.01 | - | - | - | - | - | - | - | - | - |
| 20 | p-mentha-1,5-dien-8-ol | 1184 | 1185 | [42] | - | - | 0.8 | 0.10 | 1724 | 1725 | [43] | - | - | 0.7 | 0.06 |
| 21 | terpinen-4-ol | 1188 | 1174 | [25] | 0.1 | 0.01 | 1.3 | 0.09 | 1594 | 1595 | [44] | trace | - | 1.6 | 0.52 |
| 22 | n-dodecane | 1200 | 1200 | [25] | 0.1 | 0.05 | - | - | - | - | - | - | - | - | - |
| 23 | 2-allylphenol | 1199 | 1189 | [25] | - | - | 0.8 | 0.08 | - | - | - | - | - | - | - |
| 24 | γ-terpineol | 1205 | 1199 | [25] | trace | - | 1.4 | 0.06 | 1692 | 1696 | [45] | trace | - | 0.9 | 0.17 |
| 25 | n-decanal | 1215 | 1201 | [25] | 0.3 | 0.01 | - | - | 1493 | 1502 | [46] | trace | - | - | - |
| 26 | geraniol | 1262 | 1249 | [25] | trace | - | - | - | 1850 | 1851 | [47] | 0.4 | 0.03 | - | - |
| 27 | carvacrol | 1312 | 1298 | [25] | 0.9 | 0.05 | - | - | 2196 | 2189 | [48] | 1.3 | 0.10 | - | - |
| 28 | silphiperfol-5-ene | 1325 | 1326 | [25] | trace | 0.01 | - | - | 1407 | 1407 | [33] | - | - | - | - |
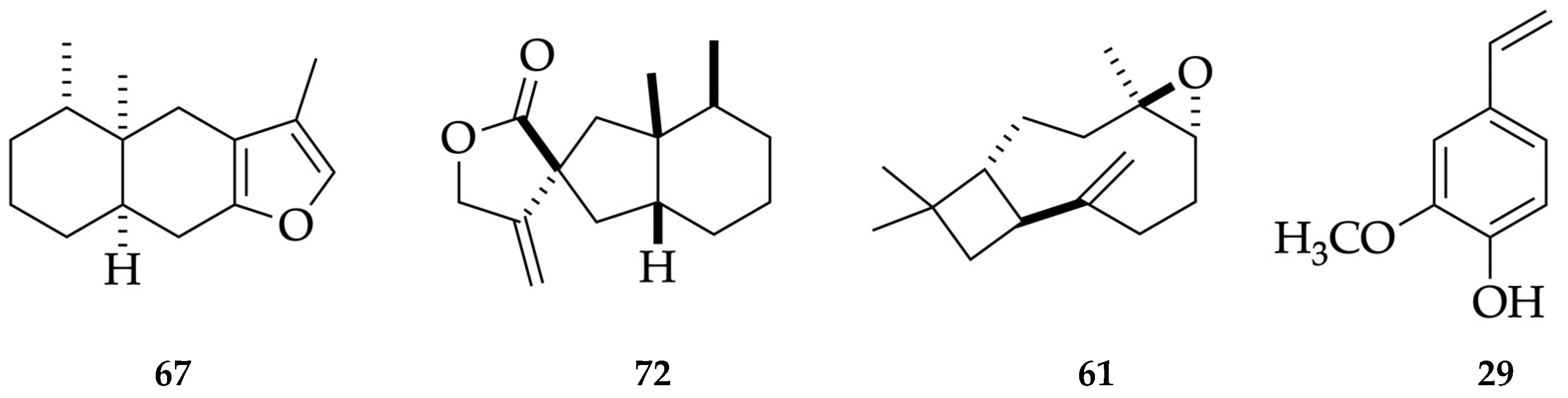
| 29 | p-vinylguaiacol | 1326 | 1309 | [49] | - | - | 25.4 | 1.33 | 2196 | 2196 | [50] | - | - | 29.9 | 1.15 |
| 30 | presilphiperfol-7-ene | 1336 | 1334 | [25] | 0.2 | 0.01 | - | - | - | - | - | - | - | - | - |
| 31 | undetermined (mw = 204) | 1347 | - | - | 0.9 | 0.05 | - | - | 1432 | - | - | 1.3 | 0.10 | - | - |
| 32 | 7-epi-silphiperfol-5-ene | 1350 | 1345 | [25] | 0.1 | 0.05 | - | - | 1444 | 1454 | [51] | trace | - | - | - |
| 33 | silphiperfol-5,7(14)-diene | 1361 | 1358 | [25] | 0.3 | 0.05 | - | - | 1509 | 1523 | [51] | trace | - | - | - |
| 34 | α-copaene | 1378 | 1374 | [25] | trace | - | - | - | 1525 | 1525 | [52] | trace | - | - | - |
| 35 | geranyl acetate | 1385 | 1379 | [25] | 0.7 | 0.01 | - | - | 1756 | 1752 | [51] | 1.2 | 0.26 | - | - |
| 36 | β-cubebene | 1391 | 1387 | [25] | 0.2 | 0.01 | - | - | 1526 | 1522 | [53] | trace | - | - | - |
| 37 | β-elemene | 1393 | 1389 | [25] | 0.4 | 0.01 | - | - | 1561 | 1563 | [33] | 0.1 | 0.15 | - | - |
| 38 | n-tetradecane | 1400 | 1400 | [25] | 0.1 | 0.01 | - | - | 1400 | - | * | trace | - | - | - |
| 39 | (E)-β-caryophyllene | 1425 | 1417 | [25] | 4.4 | 0.06 | - | - | 1578 | 1572 | [54] | 4.4 | 0.40 | - | - |
| 40 | γ-elemene | 1432 | 1434 | [25] | 0.1 | 0.01 | - | - | 1645 | 1644 | [55] | trace | - | - | - |
| 41 | α-funebrene | 1436 | 1438 | [56] | 0.1 | 0.05 | - | - | 1529 | - | * | trace | - | - | - |
| 42 | cis-cadina-1(6),4-diene | 1456 | 1461 | [25] | 0.1 | 0.01 | - | - | 1558 | - | * | 0.1 | 0.15 | - | - |
| 43 | α-humulene | 1463 | 1452 | [25] | 0.8 | 0.06 | - | - | 1651 | 1650 | [57] | 1.2 | 0.19 | - | - |
| 44 | cis-muurola-4(14),5-diene | 1471 | 1465 | [25] | 0.1 | 0.01 | - | - | 1657 | - | * | 0.1 | 0.15 | - | - |
| 45 | α-neocallitropsene | 1482 | 1474 | [25] | 0.2 | 0.10 | - | - | - | - | * | trace | - | - | - |
| 46 | ar-curcumene | 1487 | 1479 | [25] | 1.5 | 0.05 | - | - | 1766 | 1763 | [51] | 1.4 | 0.22 | - | - |
| 47 | trans-muurola-4(14),5-diene | 1489 | 1493 | [25] | 2.8 | 0.08 | - | - | 1691 | - | * | 2.5 | 0.24 | - | - |
| 48 | cis-β-guaiene | 1490 | 1492 | [25] | 2.1 | 0.10 | - | - | 1704 | 1702 | [58] | 2.4 | 0.38 | - | - |
| 49 | valencene | 1495 | 1496 | [25] | - | - | 1691 | 1689 | [54] | 2.5 | 0.24 | - | - | ||
| 50 | β-selinene | 1497 | 1489 | [25] | - | - | 1698 | 1698 | [59] | 0.1 | 0.25 | - | - | ||
| 51 | β-himachalene | 1513 | 1510 | [60] | 0.4 | 0.01 | - | - | 1622 | 1632 | [51] | 0.1 | 0.10 | - | - |
| 52 | (Z)-γ-bisabolene | 1516 | 1514 | [25] | 0.3 | 0.06 | - | - | 1878 | - | * | trace | - | - | - |
| 53 | n-tridecanal | 1519 | 1509 | [25] | 0.3 | 0.05 | - | - | - | - | - | - | - | - | - |
| 54 | γ-cadinene | 1521 | 1513 | [25] | 0.3 | 0.01 | - | - | 1716 | 1716 | [61] | 0.1 | 0.15 | - | - |
| 55 | δ-cadinene | 1525 | 1522 | [25] | 0.3 | 0.01 | - | - | 1745 | 1745 | [62] | 0.1 | 0.25 | - | - |
| 56 | zonarene | 1531 | 1528 | [25] | 1.3 | 0.01 | - | - | 1760 | - | * | 1.2 | 0.22 | - | - |
| 57 | undetermined (mw = 202) | 1541 | - | - | 0.7 | 0.01 | - | - | 1863 | - | 0.4 | 0.03 | - | - | |
| 58 | italicene ether | 1546 | 1536 | [25] | 0.3 | 0.01 | - | - | 1845 | 1830 | [63] | 0.4 | 0.03 | - | - |
| 59 | β-vetivenene | 1551 | 1554 | [25] | 0.2 | 0.01 | - | - | 2074 | - | * | 0.1 | 0.15 | - | - |
| 60 | spathulenol | 1591 | 1577 | [25] | 2.7 | 0.30 | - | - | 2115 | 2121 | [28] | 2.7 | 0.24 | - | - |
| 61 | caryophyllene oxide | 1599 | 1595 | [64] | 6.0 | 0.10 | 0.5 | 0.03 | 1963 | 1960 | [51] | 5.8 | 0.54 | 0.3 | 0.12 |
| 62 | muurola-4,10(14)-dien-1-β-ol | 1622 | 1630 | [25] | 0.3 | 0.05 | - | - | 2259 | - | * | 0.1 | 0.15 | - | - |
| 63 | dillapiole | 1636 | 1620 | [25] | 0.8 | 0.01 | - | - | 2318 | 2327 | [65] | 1.2 | 0.22 | - | - |
| 64 | cis-cadin-4-en-7-ol | 1642 | 1635 | [25] | 0.2 | 0.01 | - | - | 2045 | - | * | 0.6 | 0.18 | - | - |
| 65 | α-muurolol (=torreyol) | 1658 | 1644 | [25] | 0.4 | 0.01 | - | - | 2170 | 2173 | [51] | 0.1 | 0.20 | - | - |
| 66 | α-cadinol | 1661 | 1652 | [25] | 0.6 | 0.10 | - | - | 2180 | 2188 | [66] | 1.2 | 0.31 | - | - |
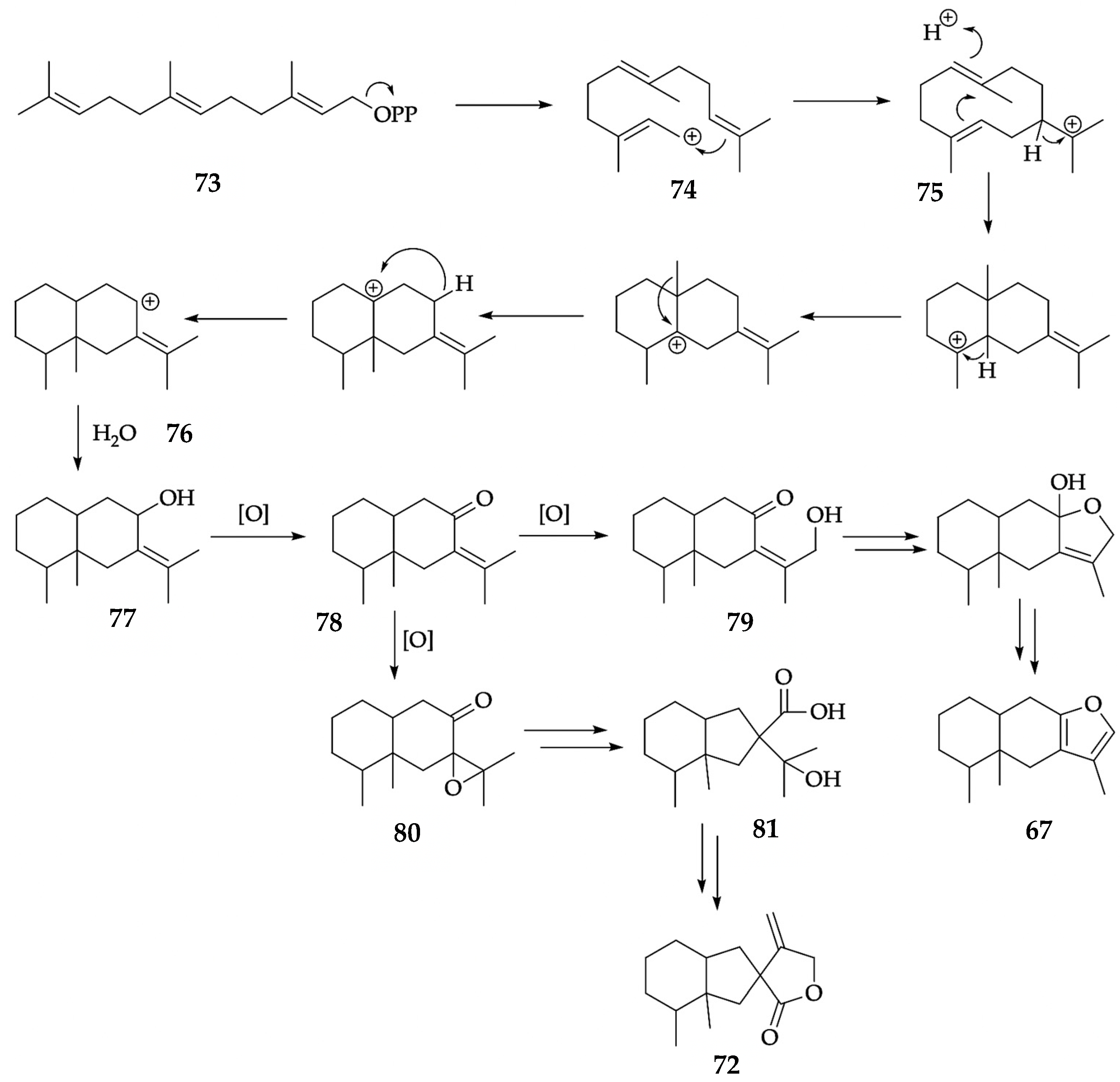
| 67 | furanoeremophilane | 1676 | - | § | 31.3 | 0.41 | - | - | 2054 | - | § | 28.3 | 4.00 | - | - |
| 68 | cyperotundone | 1721 | 1718 | [67] | 0.9 | 0.10 | - | - | 2375 | - | * | 1.2 | 0.31 | - | - |
| 69 | cyclocolorenone | 1761 | 1759 | [25] | 1.3 | 0.01 | 0.5 | 0.06 | 2298 | - | * | 1.3 | 0.08 | 0.6 | 0.26 |
| 70 | xanthorrhizol | 1766 | 1751 | [25] | - | - | 2547 | - | * | 0.1 | 0.05 | - | - | ||
| 71 | eremophilone | 1726 | 1734 | [25] | 0.5 | 0.06 | 2299 | - | * | - | - | 0.4 | 0.10 | ||
| 72 | bakkenolide A | 1845 | - | § | 17.6 | 0.34 | 5.0 | 0.36 | 2430 | - | § | 16.3 | 0.55 | 5.5 | 0.56 |
| monoterpene hydrocarbons | 4.3 | - | 3.9 | - | |||||||||||
| oxygenated monoterpenes | 1.6 | 7.0 | 1.8 | 6.0 | |||||||||||
| sesquiterpene hydrocarbons | 17.5 | - | 18.1 | - | |||||||||||
| oxygenated sesquiterpenes | 61.9 | 6.5 | 58.1 | 6.8 | |||||||||||
| other compounds | 2.6 | 27.2 | 2.4 | 30.6 | |||||||||||
| total amount | 87.9 | 40.7 | 84.3 | 43.4 | |||||||||||
| Enantiomers | LRI | Enantiomeric Distribution (%) | e.e. (%) |
|---|---|---|---|
| (1S,5S)-(−)-α-pinene | 925 | 100.0 | 100.0 |
| (1S,5S)-(−)-β-pinene | 977 | 100.0 | 100.0 |
| (R)-(+)-sabinene | 1007 | 15.4 | 69.2 |
| (S)-(−)-sabinene | 1012 | 84.6 | |
| (S)-(+)-α-phellandrene | 1025 | 100.0 | 100.0 |
| (S)-(−)-limonene | 1051 | Inseparable | - |
| (R)-(+)-limonene | |||
| (S)-(+)-β-phellandrene | 1071 | 100.0 | 100.0 |
| (S)-(−)-terpinen-4-ol | 1379 | 100.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cumbicus, C.; Malagón, O.; Cumbicus, N.; Gilardoni, G. The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants 2023, 12, 1323. https://doi.org/10.3390/plants12061323
Cumbicus C, Malagón O, Cumbicus N, Gilardoni G. The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants. 2023; 12(6):1323. https://doi.org/10.3390/plants12061323
Chicago/Turabian StyleCumbicus, Carolina, Omar Malagón, Nixon Cumbicus, and Gianluca Gilardoni. 2023. "The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A" Plants 12, no. 6: 1323. https://doi.org/10.3390/plants12061323
APA StyleCumbicus, C., Malagón, O., Cumbicus, N., & Gilardoni, G. (2023). The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants, 12(6), 1323. https://doi.org/10.3390/plants12061323









