Effect of Water Deficit on Germination, Growth and Biochemical Responses of Four Potentially Invasive Ornamental Grass Species
Abstract
1. Introduction
2. Results
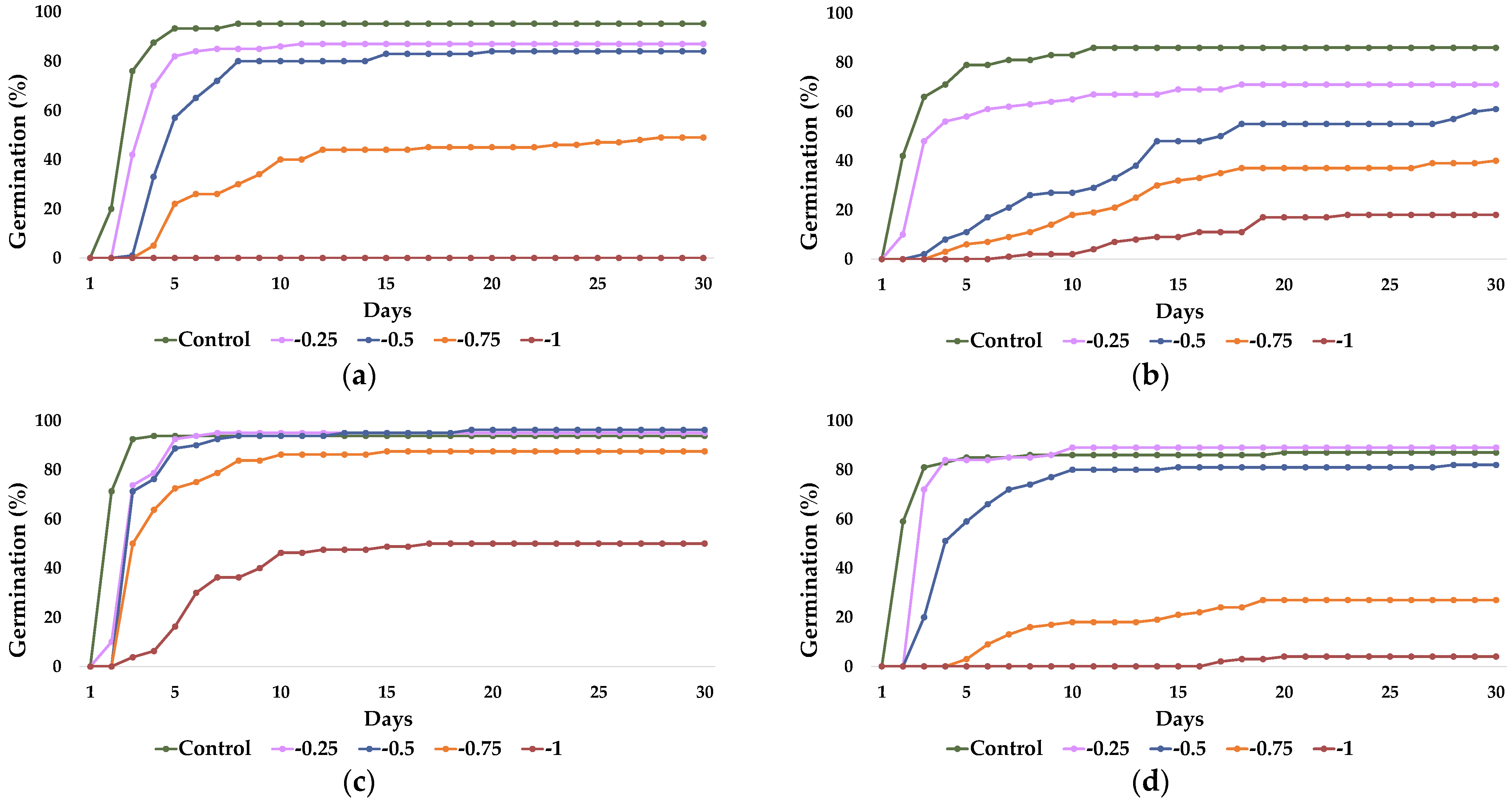
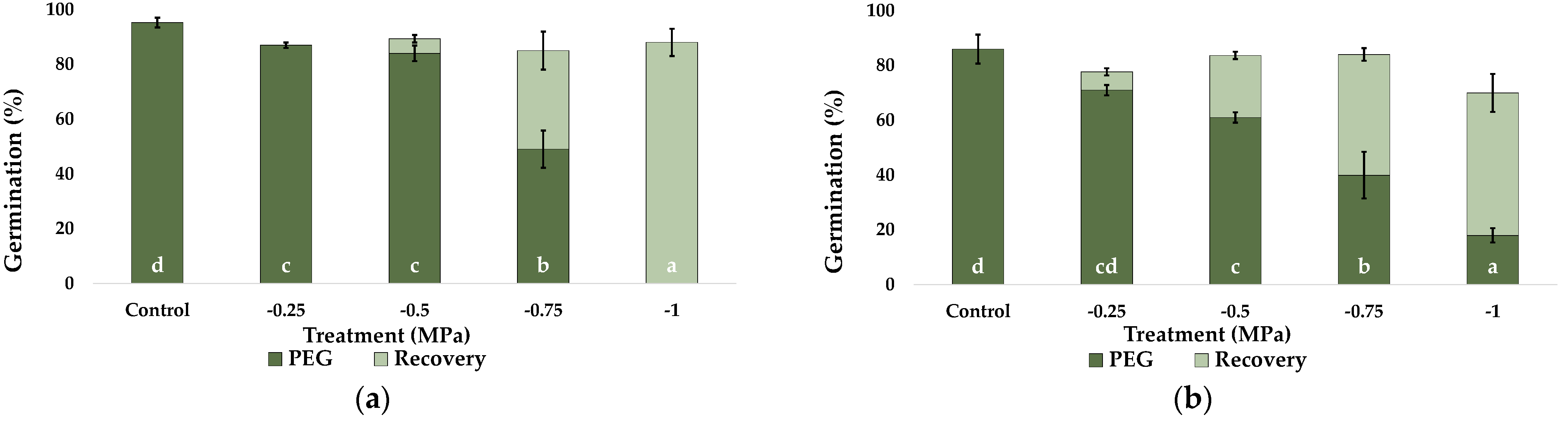
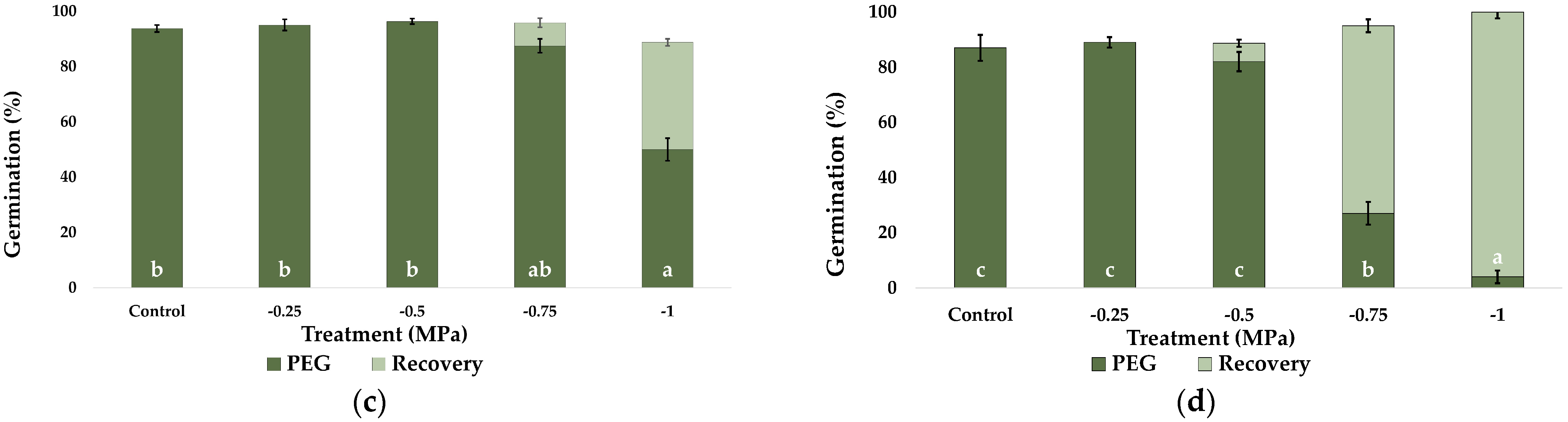
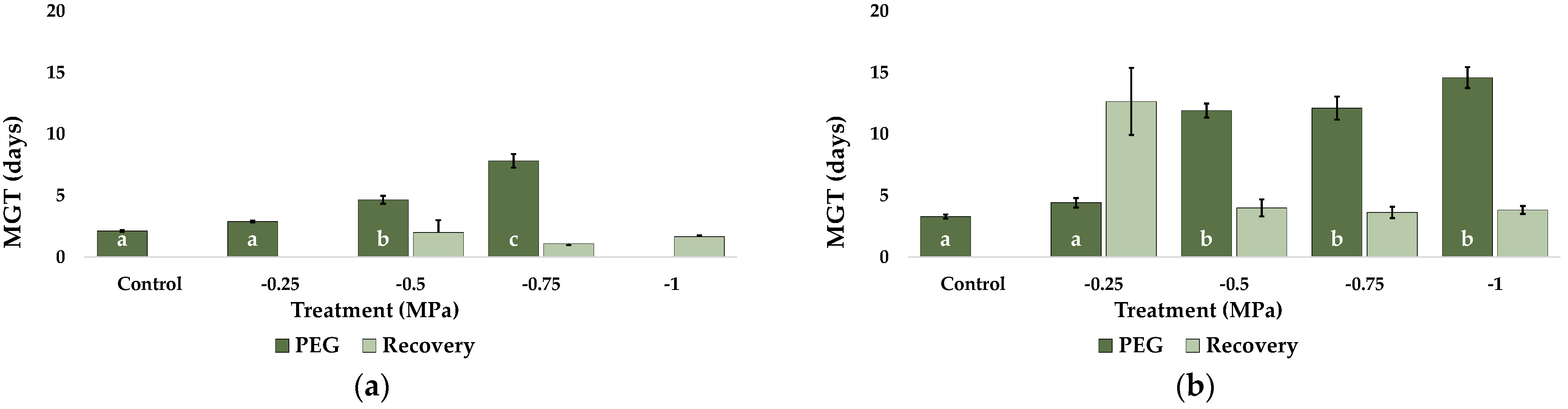
2.1. Seed Germination and Recovery of Germination
2.2. Seedling Analysis
2.3. Plant Growth
2.4. Biochemical Analyses
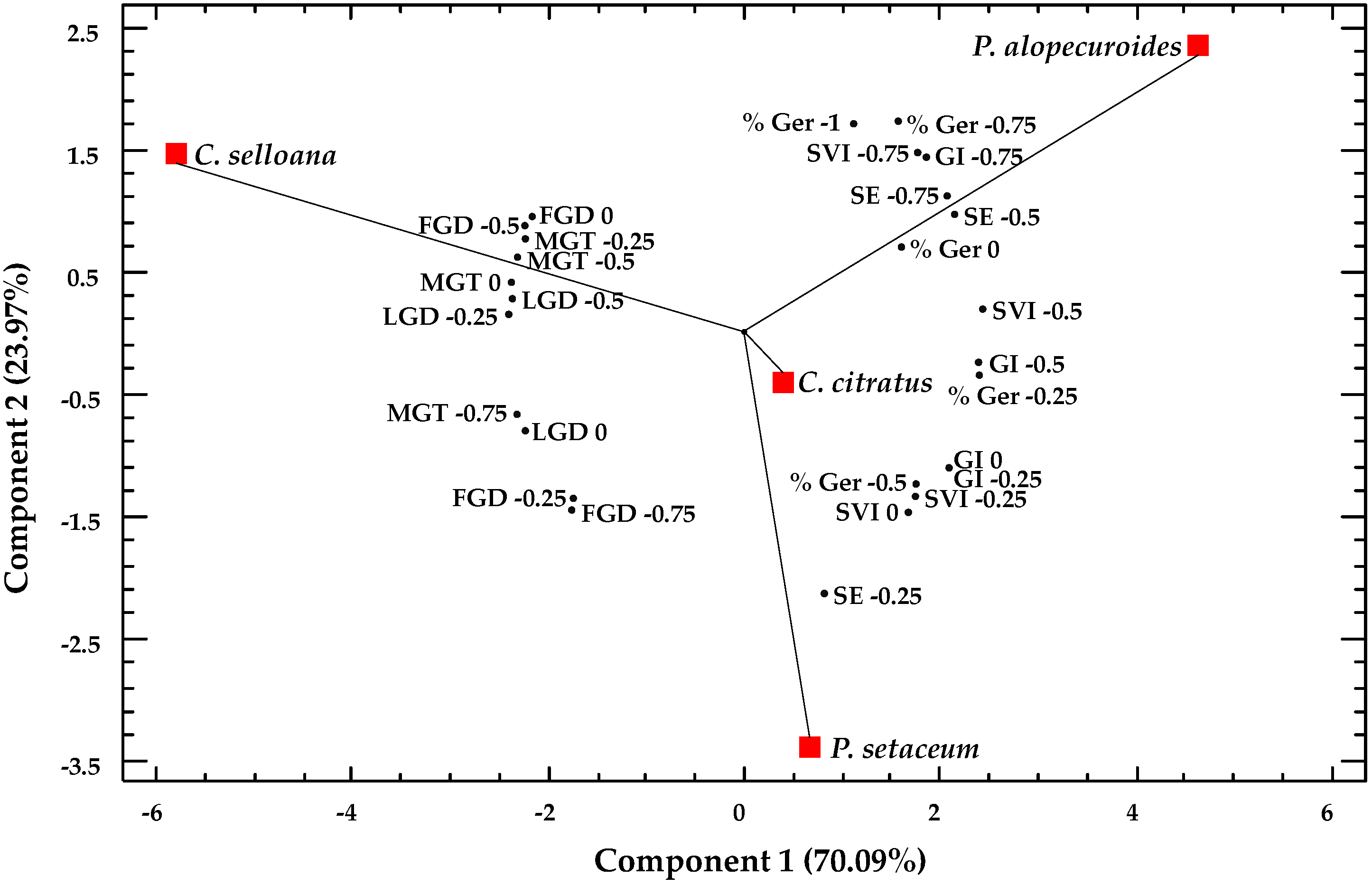
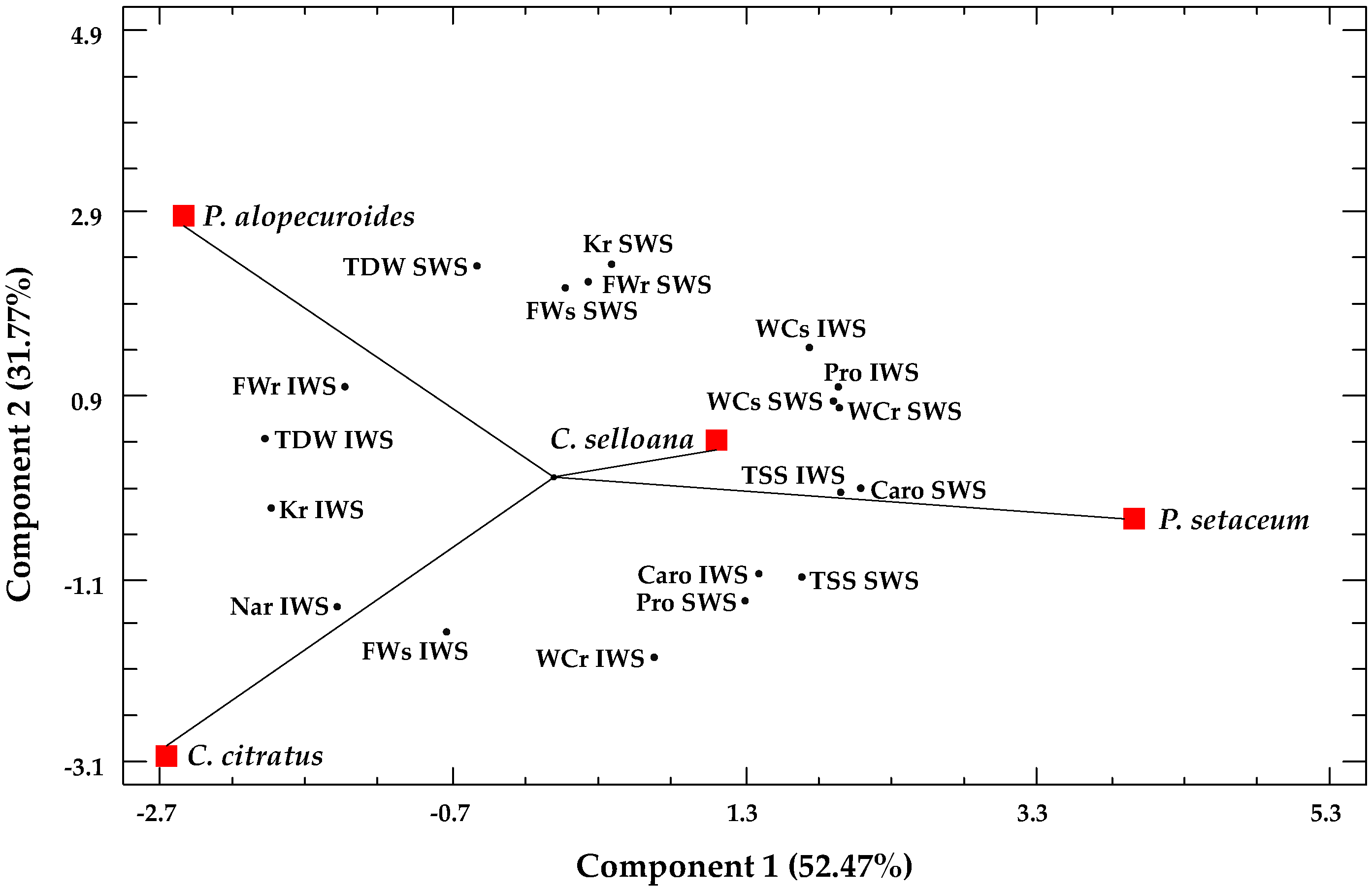
2.5. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Germination Assays
4.3. Plant Growth and Water Stress Treatments
4.4. Photosynthetic Pigments
4.5. Ion Content Measurements
4.6. Quantification of Osmolytes
4.7. Determination of Antioxidant Compounds
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulme, P.E.; Brundu, G.; Carboni, M.; Dehnen-Schmutz, K.; Dullinger, S.; Early, R.; Essl, F.; González-Moreno, P.; Groom, Q.J.; Kueffer, C.; et al. Integrating invasive species policies across ornamental horticulture supply chains to prevent plant invasions. J. Appl. Ecol. 2018, 55, 92–98. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P. Naturalization of introduced plants: Ecological drivers of biogeographical patterns. New Phytol. 2012, 196, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Gioria, M.; Osborne, B. Similarities in the impact of three large invasive plant species on soil seed bank communities. Biol. Invasions 2010, 12, 1671–1683. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. Bioscience 1998, 48, 607–615. [Google Scholar] [CrossRef]
- Gaertner, M.; den Breeyen, A.; Hui, C.; Richardson, D.M. Impacts of alien plant invasions on species richness in Mediterranean-type ecosystems: A Meta-Analysis. Progr. Phys. Geogr. 2009, 33, 319–338. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Gioria, M.; Jarošík, V.; Pyšek, P. Impact of invasions by alien plants on soil seed bank communities: Emerging patterns. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 132–142. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Carman, J.G.; Brotherson, J.D. Comparisons of sites infested and not infested with salt cedar (Tamarix pentandra) and Russian olive (Elaeagnus angustifolia). Weed Sci. 1982, 30, 360–364. [Google Scholar] [CrossRef]
- Bock, J.H.; Bock, C.E. Vegetation responses to wildfire in native versus exotic Arizona Grassland. J. Veg. Sci. 1992, 3, 439–446. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Vitousek, P.M. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu. Rev. Ecol. Syst. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- Eviner, V.T.; Garbach, K.; Baty, J.H.; Hoskinson, S.A. Measuring the effects of invasive plants on ecosystem services: Challenges and prospects. Invasive Plant Sci. Manag. 2012, 5, 125–136. [Google Scholar] [CrossRef]
- Mack, R.N.; Erneberg, M. The United States naturalized flora: Largely the product of deliberate introductions. Ann. Mo. Bot. Gard. 2002, 89, 176–189. [Google Scholar] [CrossRef]
- Anderson, N.O.; Galatowitsch, S.M.; Gomez, N. Selection strategies to reduce invasive potential in introduced plants. Euphytica 2006, 148, 203–216. [Google Scholar] [CrossRef]
- Drew, J.; Anderson, N.; Andow, D. Conundrums of a complex vector for invasive species control: A detailed examination of the horticultural industry. Biol. Invasions 2010, 12, 2837–2851. [Google Scholar] [CrossRef]
- Barral, A. Invasive species like it hot. Nat. Plants 2019, 5, 645. [Google Scholar] [CrossRef] [PubMed]
- Sentis, A.; Montoya, J.M.; Lurgi, M. Warming indirectly increases invasion success in food webs. Proc. R. Soc. B 2021, 288, 20202622. [Google Scholar] [CrossRef]
- Langsdorf, S.; Löschke, S.; Möller, V.; Okem, A. Climate change 2022 impacts, adaptation and vulnerability. In Working Group II. Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPC: Geneva, Switzerland, 2022; ISBN 9789291691593. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Cleland, E.E. Exotic species display greater germination plasticity and higher germination rates than native species across multiple cues. Biol. Invasions 2013, 15, 2253–2264. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Parker-Allie, F.; Musil, C.F.; Thuiller, W. Effects of climate warming on the distributions of invasive eurasian annual grasses: A South African perspective. Clim. Chang. 2009, 94, 87–103. [Google Scholar] [CrossRef]
- Voigt, T.B.; Reicher, Z.J. Selectively controlling escaped fountain grass in cool-season turf. Appl. Turfgrass Sci. 2009, 6, 1–7. [Google Scholar] [CrossRef]
- Saavedra, M.; Hidalgo, J.C.; Hidalgo, J.; Alcántara, C. Pennisetum setaceum. Planta Invasora con Gran Peligro Potencial; Junta de Andalucía; Instituto de Investigación y Formación Agraria y Pesquera: Seville, Spain, 2014; pp. 1–9. [Google Scholar]
- Navarro, M.L.R.; Trujillo, B.F. La Expansión de Cortaderia selloana (Schult. & Schult. f.) Asch. & Graebn. (Poaceae) en Tenerife, Islas Canarias. Vieraea Folia Sci. Biol. Canar. 2019, 46, 79–114. [Google Scholar] [CrossRef]
- Thomas, J.; Taylor, M. Evaluation of chemical control methods of fountain grass. Horttechnology 2021, 31, 382–384. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2022; Available online: www.cabi.org/isc (accessed on 16 November 2022).
- Clayton, W.D.; Govaerts, R.; Harman, K.T.; Williamson, H.; Vorontsova, M. World Checklist of Poaceae; Royal Botanic Gardens: Richmond, UK, 2016; Available online: http://apps.kew.org/wcsp/ (accessed on 16 November 2022).
- Ambrose, D.C.P.; Manickavasagan, A.; Naik, R. Leafy Medicinal Herbs: Botany, Chemistry, Postharvest Technology and Uses; CABI: Delémont, Switzerland, 2016; pp. xiii+282. ISBN 978-1-78064-559-9. [Google Scholar]
- Kiani, H.S.; Ali, A.; Zahra, S.; Hassan, Z.; Kubra, K.; Azam, M.; Zahid, H.F. Phytochemical composition and pharmacological potential of lemongrass (Cymbopogon) and impact on gut microbiota. Appl. Chem. 2022, 2, 229–246. [Google Scholar] [CrossRef]
- Husain, A.; Viermani, O.P.; Sharma, A.; Kumar, A.; Misra, L.N. Major Essential-Oil Bearing Plants of India; Central Institute of Medicinal and Aromatic Plants: Lucknow, India, 1988; p. 237. [Google Scholar]
- Mahmoud, N.; Abdou, M.A.H.; Salaheldin, S.; Soliman, W.S. Lemongrass Growth, Essential Oil, and Active Substances as Affected by Water Deficit. Horticulturae 2022, 8, 250. [Google Scholar] [CrossRef]
- Tesfaye, H.; Meskelu, E.; Mohammed, M. Determination of optimal soil moisture depletion level for lemongrass (Cymbopogon citratus L.). Irrig. Drain. Syst. Eng. 2017, 6, 189. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Zehra, A.; Petrik, P.; Kurjak, D. Suffer or survive: Decoding salt-sensitivity of lemongrass and its implication on essential oil productivity. Front. Plant Sci. 2022, 9, 903954. [Google Scholar] [CrossRef]
- Graveson, R. The Plants of Saint Lucia (in the Lesser Antilles of the Caribbean). 2012. Available online: http://www.saintlucianplants.com (accessed on 16 November 2022).
- Gallastegui, M.H.; Prieto, J.A.C. El Carrizo de la Pampa (Cortaderia selloana) en Bizkaia. Guía Práctica para su Control; Laboratorio de Botánica Dpto; Biología Vegetal y Ecologia: Bilbao, Spain, 2006; p. 43. [Google Scholar]
- Domènech, R.; Vilà, M. Response of the invader Cortaderia selloana and two coexisting natives to competition and water stress. Biol. Invasions 2008, 10, 903–912. [Google Scholar] [CrossRef]
- Stanton, A.E.; Di Tomaso, J.M. Growth response of Cortaderia selloana and Cortaderia jubata (Poaceae) seedlings to temperature, light and water. Madroño 2004, 51, 312–321. [Google Scholar]
- He, X.J.; Ren, G.X.; Peng, Z.L.; Jiang, K.; Zao, K.Q.; Song, F.M. Screening and evaluation of ornamental grasses with tolerance to heat and drought in South China. Acta Agric. Jiangxi 2018, 30, 64–69. [Google Scholar]
- Campos, J.A.; Herrera, M.; Biurrun, I.; Loidi, J. The role of alien plants in the natural coastal vegetation in central-northern Spain. Biodivers. Conserv. 2004, 13, 2275–2293. [Google Scholar] [CrossRef]
- Pino, J.; Vila, M.; Álvarez, N.; Segui, J.M.; Guerrero, C. Response to land-cover changes in perennial monocotyledon species of contrasting conservation concern. Appl. Veg. Sci. 2009, 12, 119–130. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, X.; Allington, G.R.H.; Wang, L.; Huang, W.; Zhang, R.; Luo, Y.; Xu, Z. Photosynthesis and growth of Pennisetum centrasiaticum (C4) is superior to Calamagrostis pseudophragmites (C3) during drought and recovery. Plants 2020, 9, 991. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Eshel, A. Ecophysiology of Pennisetum clandestinum: A valuable salt tolerant grass. Environ. Exp. Bot. 2013, 92, 55–63. [Google Scholar] [CrossRef]
- Sanz-Elorza, M.; Dana, E.D.; Vesperinas, E.S. Atlas de las Plantas Alóctonas Invasoras en España; Dirección General para la Biodiversidad; Gobierno de España: Madrid, Spain, 2004.
- “Pennisetum alopecuroides”. Missouri Botanical Gardens. Available online: http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=285289&isprofile=1&basic=Pennisetum%20alopecuroides (accessed on 5 January 2023).
- Kong, L.; Peng, W.D.; Liu, Y.F.; Zhang, Z.G. Effect of drought stress on osmotic adjustment in the leaves of three ornamental grasses. Chin. J. Grassl. 2010, 32, 82–87. [Google Scholar]
- Mane, A.; Karadge, B.; Samant, J. Salt stress induced alteration in growth characteristics of a grass Pennisetum alopecuroides. J. Env. Biol. 2011, 32, 753–758. [Google Scholar]
- Yang, J.; Yoon, Y.-H.; Ju, J.-H. Desalinization effect of Pennisetum alopecuroides and characteristics of leachate depending on calcium chloride (CaCl2) concentration. J. People Plants Environ. 2020, 23, 445–453. [Google Scholar] [CrossRef]
- Williams, D.G.; Black, R.A. Phenotypic variation in contrasting temperature environments: Growth and photosynthesis of Pennisteum setaceum from different altitudes in Hawaii. Funct. Ecol. 1993, 7, 623–633. [Google Scholar] [CrossRef]
- Badalamenti, E.; Militello, M.; la Mantia, T.; Gugliuzza, G. Seedling growth of a native (Ampelodesmos mauritanicus) and an exotic (Pennisetum setaceum) grass. Acta Oecol. 2016, 77, 37–42. [Google Scholar] [CrossRef]
- Donohue, K.; de Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Gioria, M.; Pyšek, P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol. Invasions 2017, 19, 1055–1080. [Google Scholar] [CrossRef]
- Rejmánek, M. Invasive plants: Approaches and predictions. Austral Ecol. 2000, 25, 497–506. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Wind effects on dispersal patterns of the invasive alien Cortaderia selloana in Mediterranean wetlands. Acta Oecol. 2005, 27, 129–133. [Google Scholar] [CrossRef]
- Skálová, H.; Moravcová, L.; Čuda, J.; Pyšek, P. Seed-Bank dynamics of native and invasive impatiens species during a five-year field experiment under various environmental conditions. NeoBiota 2019, 50, 75–95. [Google Scholar] [CrossRef]
- Tietze, H.S.E.; Joshi, J.; Pugnaire, F.I.; de Sá Dechoum, M. Seed germination and seedling establishment of an invasive tropical tree species under different climate change scenarios. Austral Ecol. 2019, 44, 1351–1358. [Google Scholar] [CrossRef]
- Bellache, M.; Moltó, N.; Benfekih, L.A.; Torres-Pagan, N.; Mir, R.; Verdeguer, M.; Boscaiu, M.; Vicente, O. Physiological and biochemical responses to water stress and salinity of the invasive moth plant, Araujia sericifera Brot., during seed germination and vegetative growth. Agronomy 2022, 12, 361. [Google Scholar] [CrossRef]
- Jiao, S.Y.; Li, Y.Q.; Shayila, S.; Chen, X.L. Seeds germination and seedling growth about 3 Pennisetum ornamental grasses under drought stress. Acta Bot. Boreali Occident. Sin. 2009, 29, 308–313. [Google Scholar]
- EC. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Preven-tion and Management of the Introduction and Spread of Invasive Alien Species. 2014. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:JOL_2014_317_R_0003 (accessed on 31 December 2022).
- Da Re, D.; Tordoni, E.; De Pascalis, F.; Negrín-Pérez, Z.; Fernández-Palacios, J.M.; Arévalo, J.R.; Rocchini, D.; Medina, F.M.; Otto, R.; Arlé, E.; et al. Invasive fountain grass (Pennisetum setaceum (Forssk.) Chiov.) increases its potential area of distribution in Tenerife Island under future climatic scenarios. Plant Ecol. 2020, 221, 867–882. [Google Scholar] [CrossRef]
- Poulin, J.; Sakai, A.K.; Weller, S.G.; Nguyen, T. Phenotypic plasticity, precipitation, and invasiveness in the fire-promoting grass Pennisetum setaceum (Poaceae). Am. J. Bot. 2007, 94, 533–541. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Montoya, N.; Soriano, P.; Estrelles, E. An electrochemical analysis suggests role of gynodioecy in adaptation to stress in Cortaderia selloana. Curr. Plant Biol. 2018, 16, 9–14. [Google Scholar] [CrossRef]
- Bacchetta, G.; Dettori, C.A.; Mascia, F.; Meloni, F.; Podda, L. Assessing the potential invasiveness of Cortaderia selloana in sardinian wetlands through seed germination study. Plant Biosyst. 2010, 144, 518–527. [Google Scholar] [CrossRef]
- Domènech, R.; Vilà, M. Cortaderia selloana seed germination under different ecological conditions. Acta Oecol. 2008, 33, 93–96. [Google Scholar] [CrossRef]
- Springer, T.L.; Goldman, J.J. Germination of Saccharum ravennae (L.) L. (Poaceae) caryopses and intact spikelets. Crop Sci. 2016, 56, 682–688. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource-use efficiency and plant invasion in low-resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef]
- Vourlitis, G.; Kroon, J. Growth and resource use of the invasive grass, pampas grass (Cortaderia selloana), in response to nitrogen and water availability. Weed Sci. 2013, 61, 117–125. [Google Scholar] [CrossRef]
- Mundada, P.S.; Sonawane, M.M.; Shaikh, S.S.; Barvkar, V.T.; Kumar, S.A.; Umdale, S.D.; Suprasanna, P.; Barmukh, R.B.; Nikam, T.D.; Ahire, M.L. Silicon alleviates PEG-induced osmotic stress in finger millet by regulating membrane damage, osmolytes, and antioxidant defense. Not. Sci. Biol. 2022, 14, 11097. [Google Scholar] [CrossRef]
- Hadinezhad, P.; Payamenur, V.; Mohamadi, J.; Ghaderifar, F. The effect of priming on seed germination and seedling growth in Quercus castaneifolia. Seed Sci. Technol. 2013, 41, 121–124. [Google Scholar] [CrossRef]
- Noori, H.; Moosavi, S.G.; Seghatoleslami, M.; Rostampour, M.F. Responses of cumin (Cuminum cyminum L.) to different seed priming methods under osmotic stress. Not. Bot. Horti Agrobot. 2022, 50, 12600. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with polyethylene glycol (PEG) for abiotic stress tolerance in germinating crop seeds: A review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- Bittencourt, H.V.H.; Bonome, L.T.S.; Trezzi, M.M.; Vidal, R.A.; Lana, M.A. Seed germination ecology of Eragrostis plana, an invasive weed of South American pasture lands. S. Afr. J. Bot. 2017, 109, 246–252. [Google Scholar] [CrossRef]
- Otieno, D.O.; Kurz-Besson, C.; Liu, J.; Schmidt, M.W.T.; Vale-Lobo do, R.; David, T.S.; Siegwolf, R.; Pereira, J.S.; Tenhunen, J.D. Seasonal variations in soil and plant water status in a Quercus suber L. stand: Roots as determinants of tree productivity and survival in the Mediterranean-type ecosystem. Plant Soil 2006, 283, 119–135. [Google Scholar] [CrossRef]
- López, A.S.; López, D.R.; Arana, M.v.; Batlla, D.; Marchelli, P. Germination response to water availability in populations of Festuca pallescens along a Patagonian rainfall gradient based on hydrotime model parameters. Sci. Rep. 2021, 11, 10653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Luo, K.; Chen, D.; Baskin, J.; Baskin, C.; Wang, Y.; Hu, X. Comparison of thermal and hydrotime requirements for seed germination of seven Stipa Species from cool and warm habitats. Front. Plant Sci. 2020, 11, 560714. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Plants in Changing Environments: Linking Physiological, Population, and Community Ecology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Kassahun, B.M.; Mekonen, S.A.; Abdena, Z.T.; Kidanemariam, H.G.; Yalemtesfa, B.; Atnafu, G.; Melka, B.; Mengesha, W.K.; Silva, J.A.T. Performance of lemongrass (Cymbopogon citratus L. (DC) Stapf) agronomic and chemical traits in different agro-ecologies of Ethiopia. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 133–138. [Google Scholar]
- González-Rodríguez, A.M.; Baruch, Z.; Palomo, D.; Cruz-Trujillo, G.; Jiménez, M.S.; Morales, D. Ecophysiology of the invader Pennisetum setaceum and three native grasses in the Canary Islands. Acta Oecol. 2010, 36, 248–254. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Domènech, R.; Vilà, M.; Pino, J.; Gesti, J. Historical land-use legacy and Cortaderia selloana invasion in the Mediterranean region. Glob. Chang. Biol. 2005, 11, 1054–1064. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lloret, F.; Vilà, M. Simulating the effects of different disturbance regimes on Cortaderia selloana invasion. Biol. Conserv. 2006, 128, 128–135. [Google Scholar] [CrossRef]
- Domènech, R.; Vilà, M. Cortaderia selloana Invasion across a Mediterranean coastal strip. Acta Oecol. 2007, 32, 255–261. [Google Scholar] [CrossRef]
- Domènech, R.; Vilà, M.; Gesti, J.; Serrasolses, I. Neighbourhood Association of Cortaderia selloana invasion, soil properties and plant community structure in Mediterranean coastal grasslands. Acta Oecol. 2006, 29, 171–177. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Boscaiu Neagu, M.; Lull, C.; Bautista, I.; Lidón, A.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The physiology of channel-mediated K+ acquisition in roots of higher plants. Physiol. Plant 2014, 151, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Chakraborty, K.; Bose, J.; Shabala, L.; Shabala, S. Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. J. Exp. Bot. 2016, 67, 4611–4625. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Borochov-Neori, H.; Yermiyahu, U.; Shani, U. Is osmotic potential a more appropriate property than electrical conductivity for evaluating whole-plant response to salinity? Environ. Exp. Bot. 2009, 65, 232–237. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Madidi, S.; Barudi, B.; Ameur, F.B. Effects of salinity on germination and early growth of barley (Hordeum vulgare L.) cultivars. Int. J. Agric. Biol. 2004, 6, 767–770. [Google Scholar]
- Kader, M. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. NSW 2005, 138, 65–75. [Google Scholar]
- Islam, A.K.M.A.; Anuar, N.; Yaakob, Z. Effect of genotypes and pre-sowing treatments on seed germination behavior of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Zaman, S.; Padmesh, S.; Tawfiq, H. Seed germination and viability of Salsola imbricata Forssk. Int. J. Biodiv. Conserv. 2010, 2, 388–394. [Google Scholar]
- Gummerson, R.J. The effect of constant temperatures and osmotic potentials on the germination of sugar beet. J. Exp. Bot. 1986, 37, 729–741. [Google Scholar] [CrossRef]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef]
- Kebreab, E.; Murdoch, A.J. Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J. Exp. Bot. 1999, 50, 655–664. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; Mello, J. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]

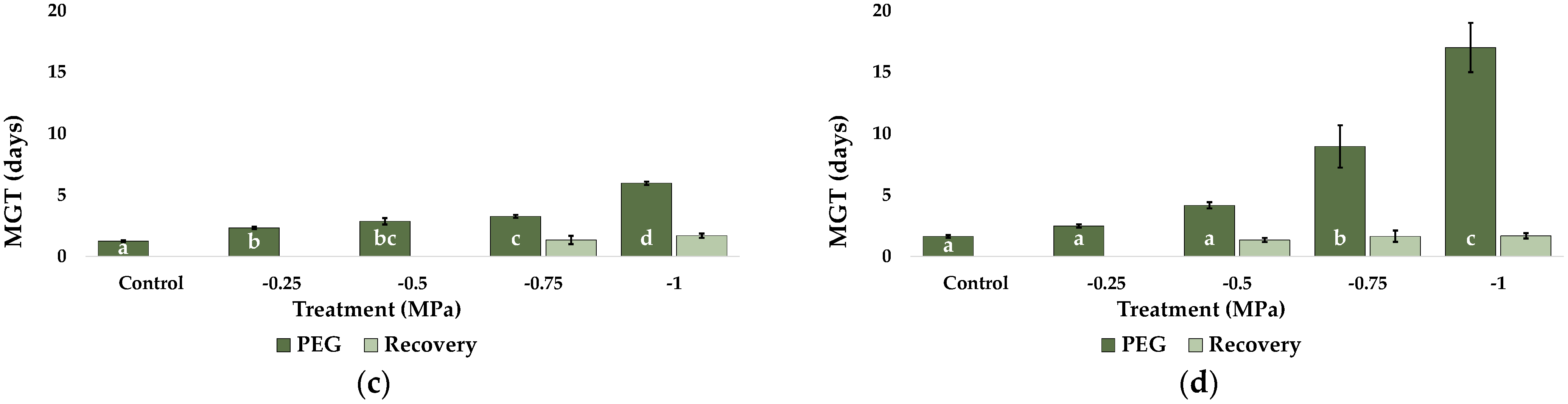
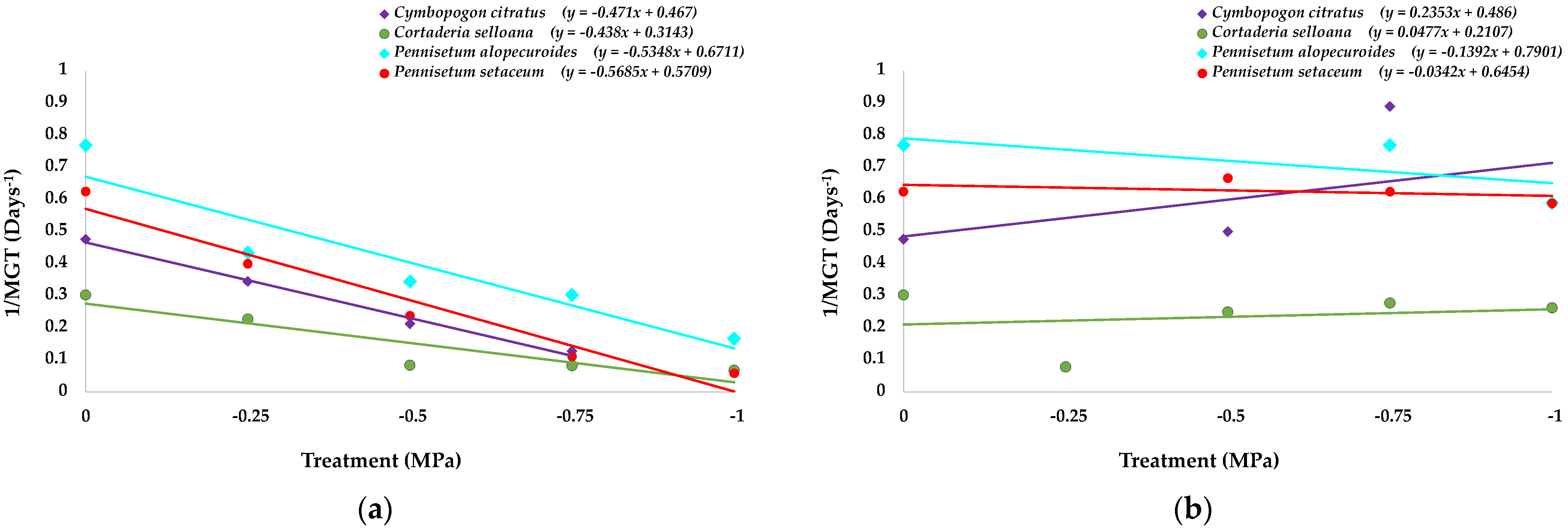
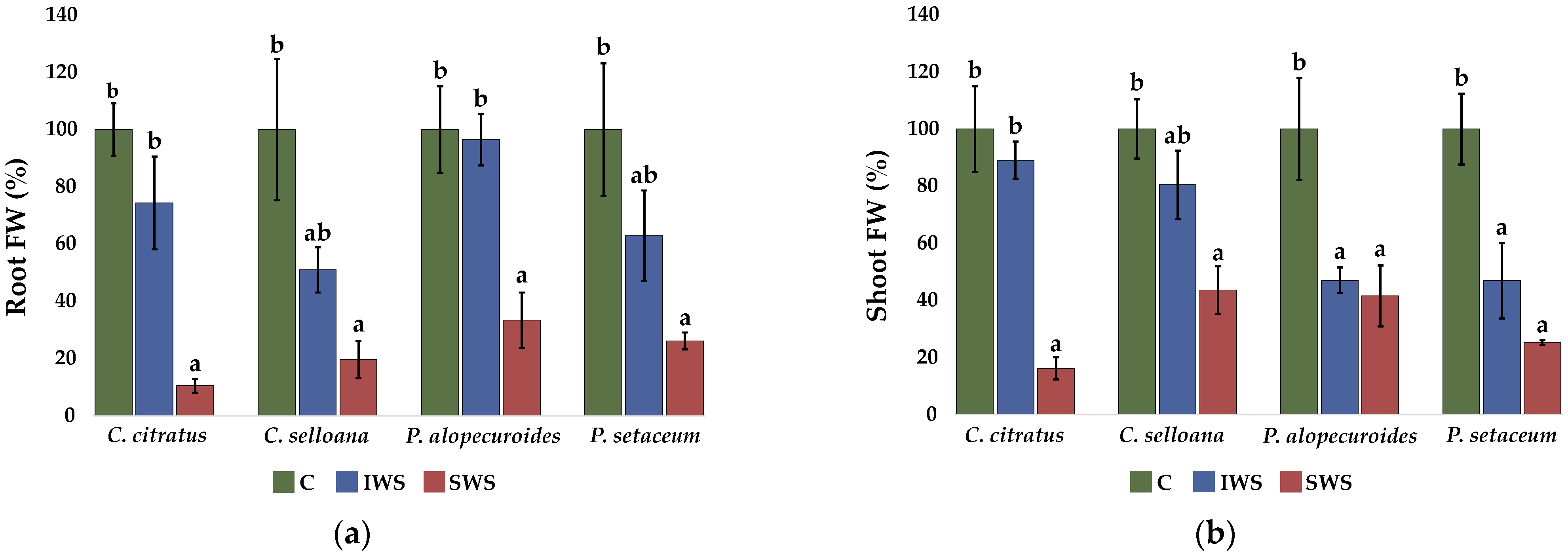
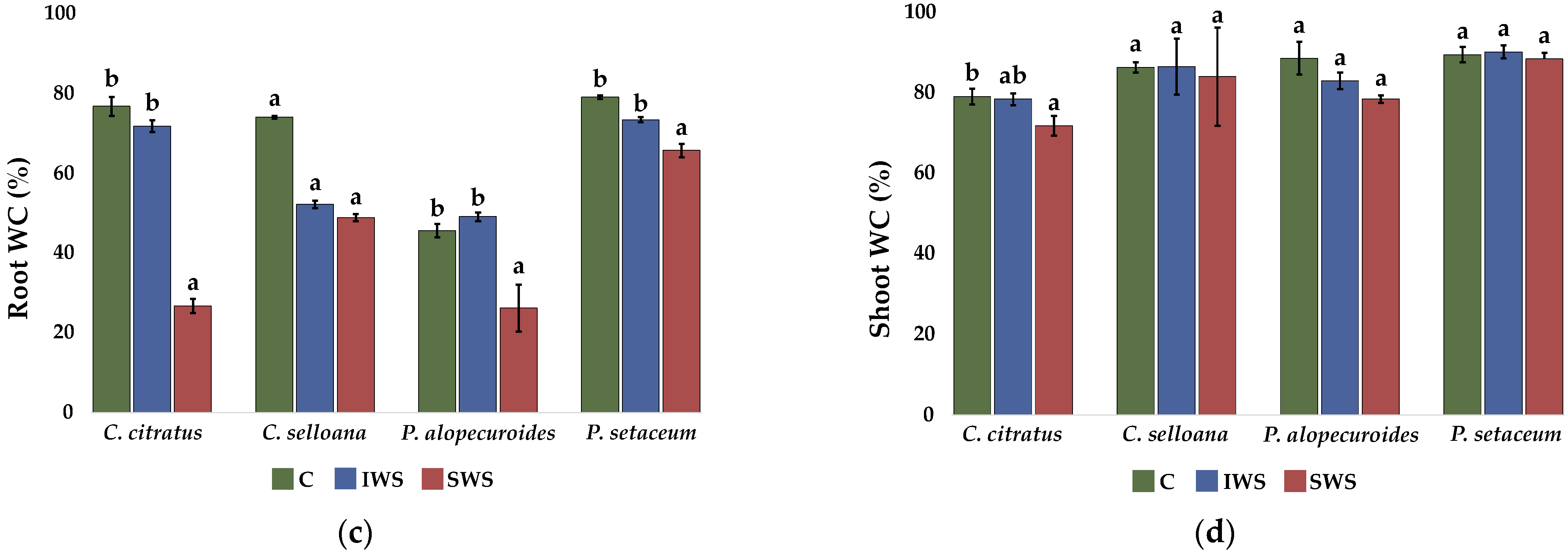
| Parameter | C. citratus | C. selloana | P. alopecuroides | P. setaceum |
|---|---|---|---|---|
| Ψb (MPa) | −1.0 | −0.7 | −1.3 | −1.0 |
| Ψb50 (MPa) | −1.4 | −1.1 | −2.6 | −1.2 |
| θ (MPa·days) | 2.1 | 2.3 | 1.9 | 1.8 |
| Parameter | Treatment (MPa) | C. citratus | C. selloana | P. alopecuroides | P. setaceum |
|---|---|---|---|---|---|
| Radicle length (mm) | Control | 19.3 ± 0.4 d | 4.4 ± 0.3 ab | 23.9 ± 0.5 d | 19.7 ± 2.7 c |
| −0.25 | 15.6 ± 0.2 b | 5.7 ± 0.3 b | 20.6 ± 0.4 c | 17.2 ± 0.7 c | |
| −0.5 | 18.3 ± 0.2 c | 4.3 ± 0.2 a | 17.9 ± 0.1 b | 9.9 ± 0.2 b | |
| −0.75 | 12.6 ± 0.2 a | 3.8 ± 0.3 a | 17.4 ± 0.0 b | 3.9 ± 0.1 a | |
| –1.0 | n.g. | 3.5 ± 0.3 a | 3.7 ± 0.0 a | 0.9 ± 0.5 a | |
| Radicle length reduction (%) | −0.25 | 18.8 ± 1.2 a | –28.1 ± 7.7 a | 14.2 ± 1.8 b | 13.0 ± 3.6 a |
| −0.5 | 5.2 ± 1.1 b | 3.4 ± 3.6 b | 25.1 ± 0.2 b | 49.7 ± 1.1 b | |
| −0.75 | 34.8 ± 0.9 c | 12.9 ± 6.5 b | 27.4 ± 0.1 c | 80.3 ± 0.7 bc | |
| −1.0 | n.g. | 19.8 ± 7.3 c | 84.4 ± 0.2 d | 95.6 ± 2.5 c | |
| Hypocotyl length (mm) | Control | 18.2 ± 0.4 c | 10.4 ± 0.4 c | 24.5 ± 0.3 e | 36.1 ± 1.4 c |
| −0.25 | 15.3 ± 0.2 b | 10.0 ± 0.3 c | 20.9 ± 0.1d | 29.2 ± 0.5 c | |
| −0.5 | 14.2 ± 0.1 b | 4.9 ± 0.4 b | 19.8 ± 0.3 c | 13.9 ± 0.3 b | |
| −0.75 | 10.8 ± 0.3 a | 3.9 ± 0.7 ab | 11.0 ± 0.1 b | 2.4 ± 0.2 a | |
| −1.0 | n.g. | 1.3 ± 0.2 a | 2.3 ± 0.03 a | 0.7 ± 0.5 a | |
| Hypocotyl length reduction (%) | −0.25 | 15.7 ± 0.9 b | 3.8 ± 3.3 a | 14.6 ± 0.6 b | 19.2 ± 1.3 b |
| −0.5 | 22.0 ± 0.6 c | 52.2 ± 3.9 b | 19.1 ± 1.2 b | 61.3 ± 0.9 c | |
| −0.75 | 40.3 ± 1.5 d | 62.3 ± 6.4 b | 55.1 ± 0.4 c | 93.3 ± 0.6 d | |
| −1.0 | n.g. | 86.6 ± 1.7 c | 90.3 ± 0.1 d | 97.9 ± 1.3 d | |
| Seedling vigour index | Control | 17.3 ± 0.4 c | 8.9 ± 0.7 d | 23 ± 0.5 e | 31.5 ± 2.7 c |
| −0.25 | 13.3 ± 0.1 b | 7.1 ± 0.3 c | 19.9 ± 0.5 d | 25.9 ± 0.4 c | |
| −0.5 | 11.9 ± 0.4 b | 3 ± 0.3 b | 19.1 ± 0.4 c | 11.4 ± 0.6 b | |
| −0.75 | 5.3 ± 0.7 a | 1.4 ± 0.3 ab | 9.6 ± 0.3 b | 0.6 ± 0.1 a | |
| −1.0 | n.g. | 0.2 ± 0.0 a | 1.1 ±0.1 a | 0.1 ± 0.0 a |
| Parameter | Treat. | C. citratus | C. selloana | P. alopecuroides | P. setaceum |
|---|---|---|---|---|---|
| Dry weight roots (g) | CON | 1.1 ± 0.1 b | 0.3 ± 0.0 a | 0.6 ± 0.1 b | 0.8 ± 0.2 b |
| IWS | 1.0 ± 0.2 b | 0.2 ± 0.0 ab | 0.5 ± 0.1 b | 0.7 ± 0.1 ab | |
| SWS | 0.4 ± 0.0 a | 0.1 ± 0.0 a | 0.3 ± 0.1 a | 0.3 ± 0.0 a | |
| Dry weight shoots (g) | CON | 2.3 ± 0.3 b | 0.8 ± 0.1 a | 0.5 ± 0.1 a | 2.1 ± 0.3 b |
| IWS | 2.0 ± 0.3 b | 0.6 ± 0.1 ab | 0.4 ± 0.0 a | 1.0 ± 0.3 a | |
| SWS | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.6 ± 0.1 a | |
| Total dry weight (g) | CON | 3.4 ± 0.5 b | 1.0 ± 0.1 b | 1.1 ± 0.1 a | 2.9 ± 0.5 b |
| IWS | 3.0 ± 0.5 b | 0.8 ± 0.1 b | 0.9 ± 0.1 a | 1.7 ± 0.4 b | |
| SWS | 0.8 ± 0.1 a | 0.5 ± 0.1 a | 0.7 ± 0.2 a | 0.9 ± 0.1 a |
| Parameter | Treat. | C. citratus | C. selloana | P. alopecuroides | P. setaceum |
|---|---|---|---|---|---|
| Chl a (mg g−1 DW) | CON | 7.3 ± 1.5 a | 8.4 ± 0.8 a | 12.9 ± 1.6 b | 6.0 ± 0.7 a |
| IWS | 7.1 ± 1.8 a | 10.6 ± 1.2 a | 6.8 ± 0.5 a | 6.8 ± 0.8 a | |
| SWS | 4.3 ± 0.8 a | 7.1 ± 0.9 a | 8.5 ± 1.2 ab | 7.5 ± 0.8 a | |
| Chl b (mg g−1 DW) | CON | 2.1 ± 0.5 a | 2.7 ± 0.2 a | 3.6 ± 0.4 b | 1.5 ± 0.2 a |
| IWS | 2.0 ± 0.5 a | 3.6 ± 0.3 a | 1.9 ± 0.1 a | 1.6 ± 0.2 a | |
| SWS | 1.1 ± 0.2 a | 2.5 ± 0.4 a | 2.5 ± 0.4 ab | 2.0 ± 0.2 a | |
| Caro (mg g−1 DW) | CON | 1.6 ± 0.3 a | 1.5 ± 0.1 ab | 2.4 ± 0.2 b | 1.0 ± 0.13 a |
| IWS | 1.5 ± 0.2 a | 2.0 ± 0.2 b | 1.3 ± 0.1 a | 1.2 ± 0.2 a | |
| WS | 0.9 ± 0.1 a | 1.2 ± 0.2 a | 1.5 ± 0.2 a | 1.3 ± 0.1 a | |
| Pro (µmol g−1 DW) | CON | 3.1. ± 0.8 a | 4.3 ± 1.1 a | 1.1 ± 0.3 a | 1.6 ± 0.3 a |
| IWS | 2.2 ± 0.4 a | 6.8 ± 0.9 a | 1.5 ± 0.2 a | 3.8 ± 0.8 ab | |
| SWS | 7.2 ± 1.6 b | 5.9 ± 0.7 a | 1.2 ± 0.2 a | 5.8 ± 0.7 b | |
| TSS (mg eq. glucose g−1 DW) | CON | 28.9 ± 4.1 a | 28.6 ± 6.8 a | 9.8 ± 2.0 a | 5.6 ± 0.8 a |
| IWS | 23.6 ± 5.2 a | 45.4 ± 3.3 a | 6.9 ± 0.9 a | 9.3 ± 1.5 ab | |
| SWS | 43.5 ± 3.2 b | 35.4 ± 7.7 a | 6.9 ± 0.4 a | 15.9 ± 4.2 b | |
| TPC (mg eq. GA g−1 DW) | CON | 11.6 ± 1.1 a | 7.9 ± 0.3 a | 8.9 ± 1.0 a | 4.8 ± 0.5 a |
| IWS | 9.9 ± 1.8 a | 9.4 ± 0.5 a | 6.9 ± 0.8 a | 5.7 ± 0.9 a | |
| SWS | 13.7 ± 1.3 a | 6.9 ± 1.8 a | 5.5 ± 1.2 a | 6.4 ± 1.8 a | |
| TF (mg eq. C g−1 DW) | CON | 6.6 ± 0.7 a | 5.5 ± 0.6 a | 3.7 ± 0.4 a | 3.1 ± 0.3 a |
| IWS | 4.9 ± 0.9 a | 6.9 ± 0.7 a | 2.6 ± 0.4 a | 4.2 ± 0.5 a | |
| SWS | 6.7 ± 0.9 a | 4.3 ± 0.8 a | 2.5 ± 0.6 a | 3.8 ± 1.3 a |
| Parameter | Treat. | C. citratus | C. selloana | P. alopecuroides | P. setaceum |
|---|---|---|---|---|---|
| K+ shoot (µmol g−1 DW) | CON | 569.1 ± 125.6 a | 785.7 ± 67.0 a | 975.9 ± 46.8 a | 1104.9 ± 79.7 a |
| IWS | 458.4 ± 67.1 a | 882.8 ± 60.1 a | 864.1 ± 91.2 a | 1290.7 ± 87 a | |
| SWS | 432.4 ± 45.9 a | 934.4 ± 34.7 a | 792.7 ± 89.4 a | 1164.1 ± 30 a | |
| K+ root (µmol g−1 DW) | CON | 252.5 ± 54.3 a | 500 ± 25.3 a | 316.2 ± 14.6 a | 744.5 ± 46.5 ab |
| IWS | 336.6 ± 65.5 a | 448 ± 16.3 a | 400.4 ± 19.3 b | 670.0 ± 36.9 a | |
| SWS | 210.3 ± 19.6 a | 476.9 ± 37.6 a | 397.9 ± 15.2 b | 826.2 ± 21.1 b | |
| Na+ shoot (µmol g−1 DW) | CON | 273.4 ± 52.7 a | 399.4 ± 25.1 a | 417.9 ± 31.1 a | 561.7 ± 51.9 a |
| IWS | 216.6 ± 38 a | 422.7 ± 27.2 a | 526.6 ± 49.8 a | 619 ± 48.1 a | |
| WS | 214.0 ± 42.2 a | 433.7 ± 12.9 a | 524.8 ± 32.6 a | 521.9 ± 14.3 a | |
| Na+ root (µmol g−1 DW) | CON | 160.1 ± 30.2 ab | 199.3 ± 12.7 a | 189.1 ± 10.4 a | 315.6 ± 19.3 a |
| IWS | 211.6 ± 36.4 b | 172.5 ± 13.2 a | 200.5 ± 24.6 a | 303.2 ± 14.7 a | |
| SWS | 81.1 ± 6.5 a | 165.2 ± 19.4 a | 203.1 ± 16.6 a | 418.2 ± 28.0 b |
| Plant Species | Cymbopogoncitratus | Cortaderiaselloana | Pennisetumalopecuroides | Pennisetumsetaceum |
|---|---|---|---|---|
| Source of the seeds | CANTUESO Natural Seeds | The Botanical Garden, Valencia, Spain | CANTUESO Natural Seeds | Collected from the wild, Valencia, Spain |
| Native distribution | Sri Lanka | Asia and Africa | Eastern Africa | South America |
| Environmental requirements | Full sun, moist soil | Full sun, sandy soil | Full sun, moist soil | Medium shade |
| Invasive potential | High | High | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mircea, D.M.; Estrelles, E.; Al Hassan, M.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Effect of Water Deficit on Germination, Growth and Biochemical Responses of Four Potentially Invasive Ornamental Grass Species. Plants 2023, 12, 1260. https://doi.org/10.3390/plants12061260
Mircea DM, Estrelles E, Al Hassan M, Soriano P, Sestras RE, Boscaiu M, Sestras AF, Vicente O. Effect of Water Deficit on Germination, Growth and Biochemical Responses of Four Potentially Invasive Ornamental Grass Species. Plants. 2023; 12(6):1260. https://doi.org/10.3390/plants12061260
Chicago/Turabian StyleMircea, Diana M., Elena Estrelles, Mohamad Al Hassan, Pilar Soriano, Radu E. Sestras, Monica Boscaiu, Adriana F. Sestras, and Oscar Vicente. 2023. "Effect of Water Deficit on Germination, Growth and Biochemical Responses of Four Potentially Invasive Ornamental Grass Species" Plants 12, no. 6: 1260. https://doi.org/10.3390/plants12061260
APA StyleMircea, D. M., Estrelles, E., Al Hassan, M., Soriano, P., Sestras, R. E., Boscaiu, M., Sestras, A. F., & Vicente, O. (2023). Effect of Water Deficit on Germination, Growth and Biochemical Responses of Four Potentially Invasive Ornamental Grass Species. Plants, 12(6), 1260. https://doi.org/10.3390/plants12061260











