Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease
Abstract
1. Introduction
2. Results
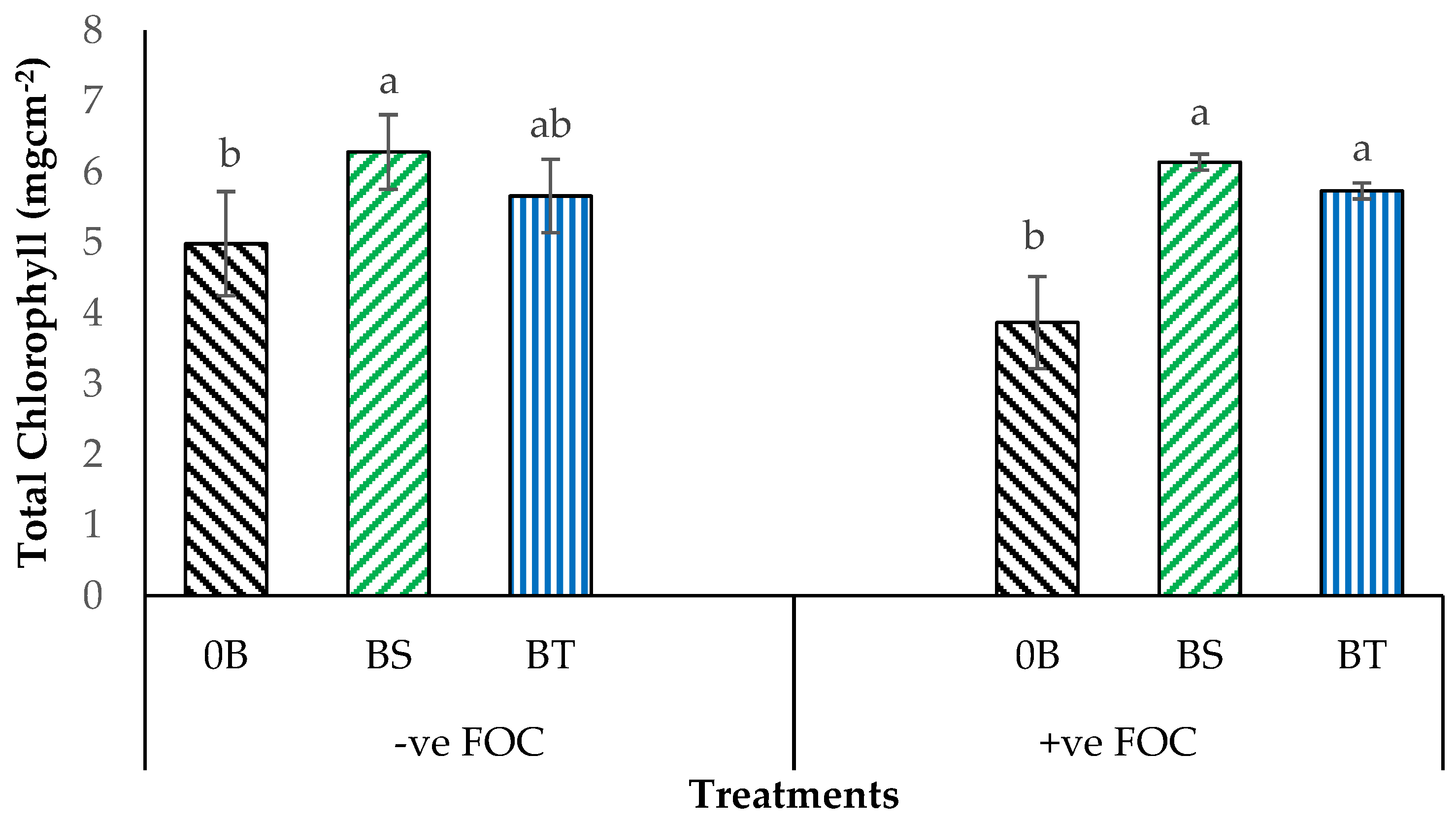
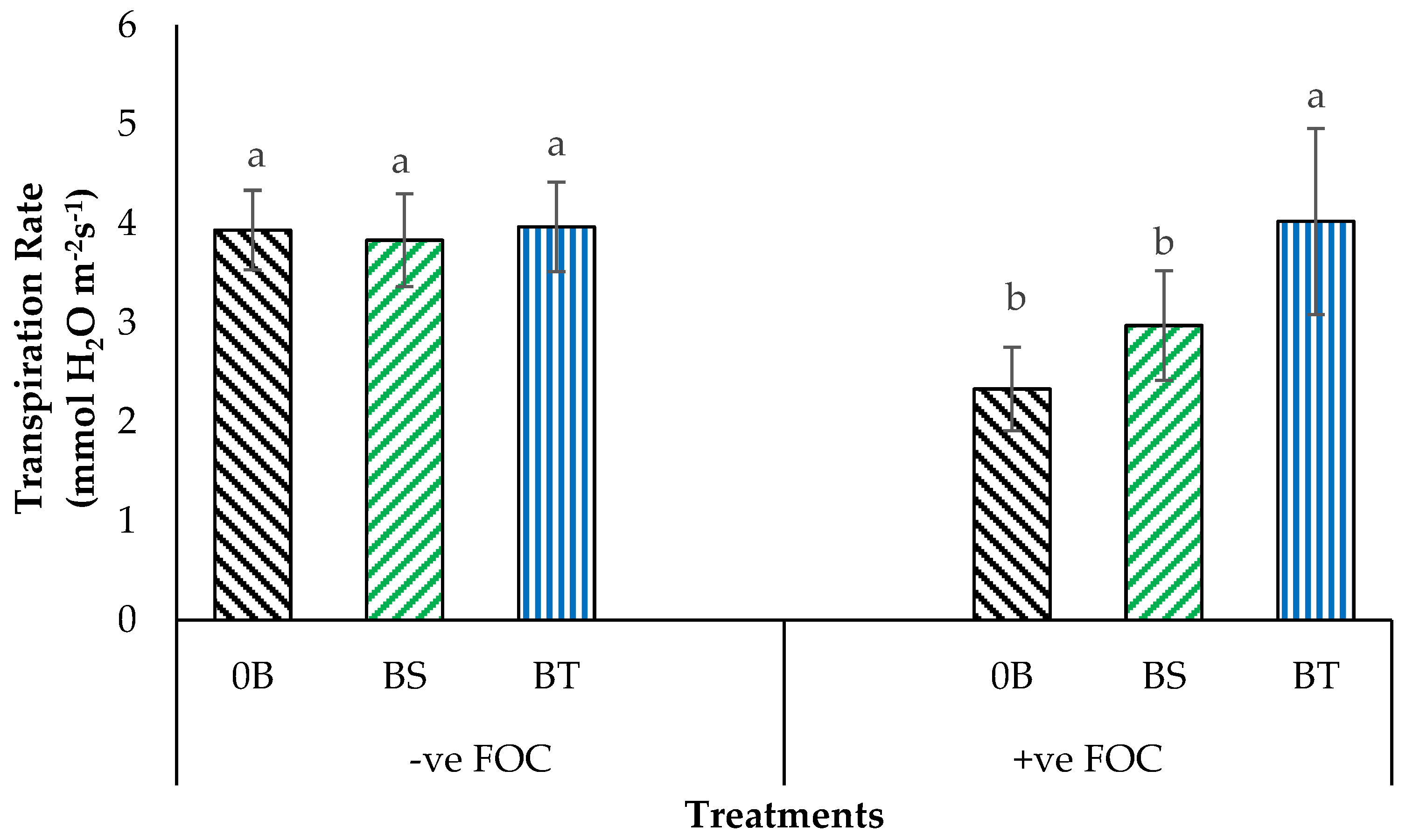
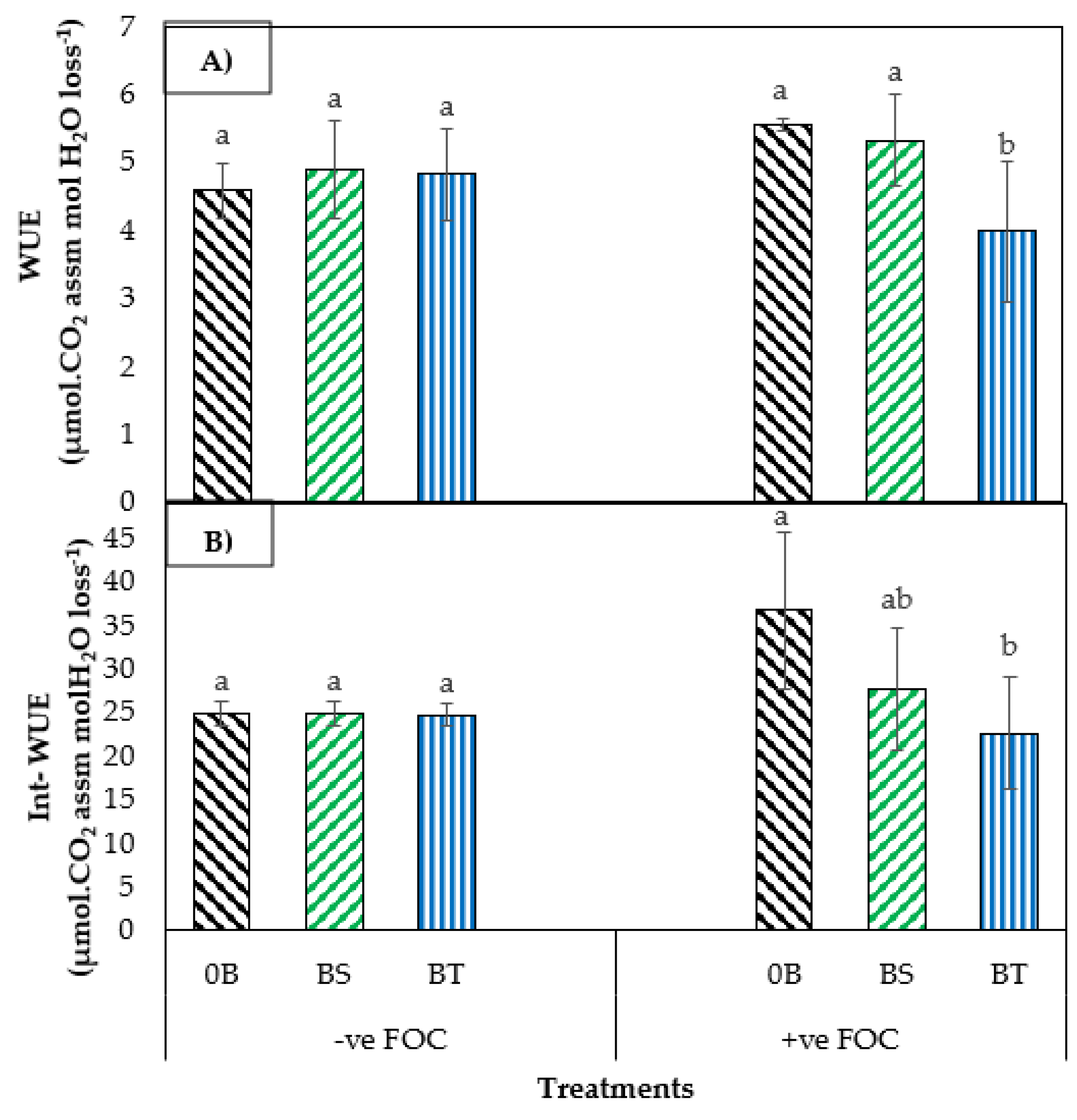
2.1. Total Chlorophyll Content and Leaf Gas Exchange
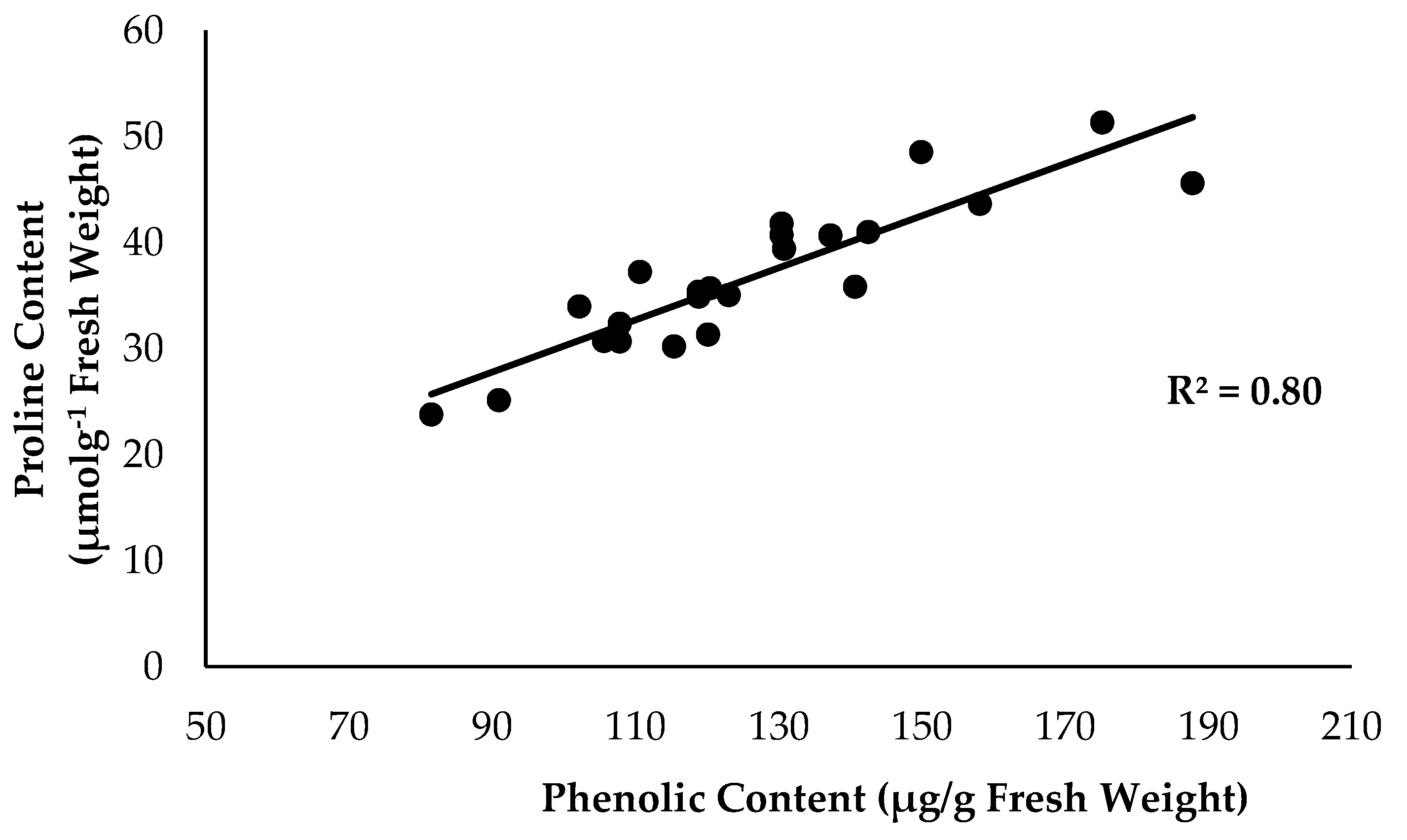
2.2. Physiological Attribute and Biochemical Changes
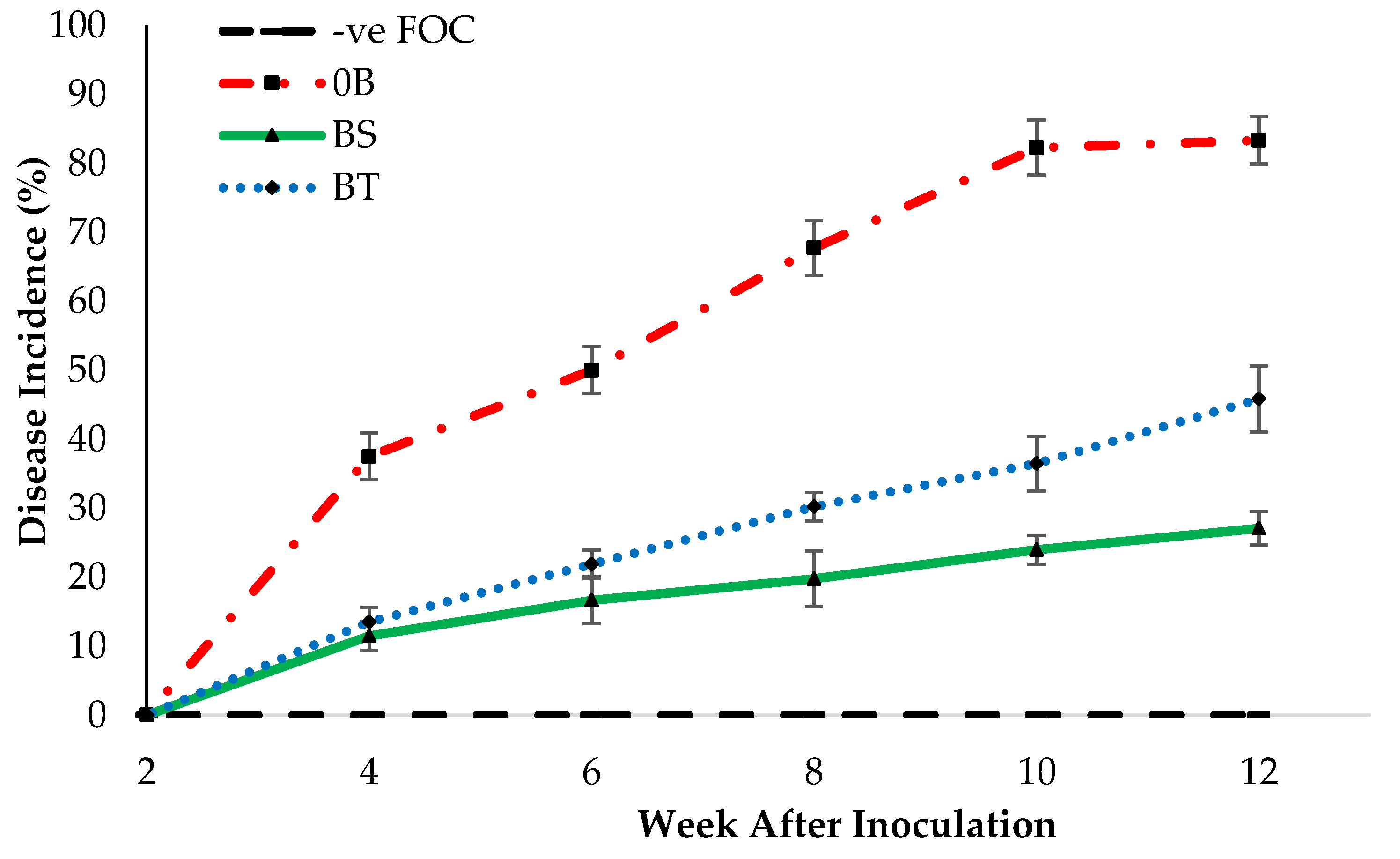
2.3. Disease Assessment
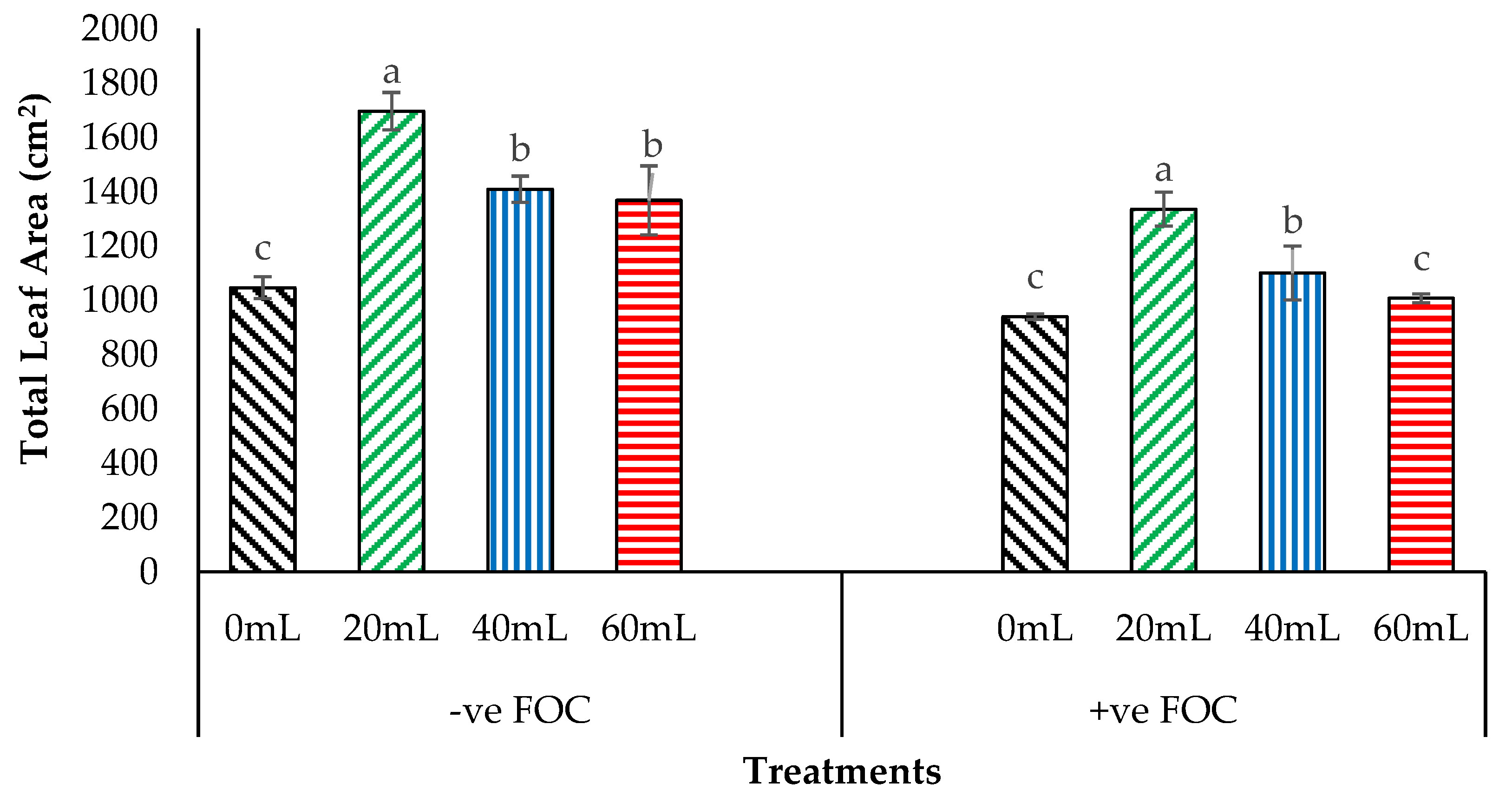
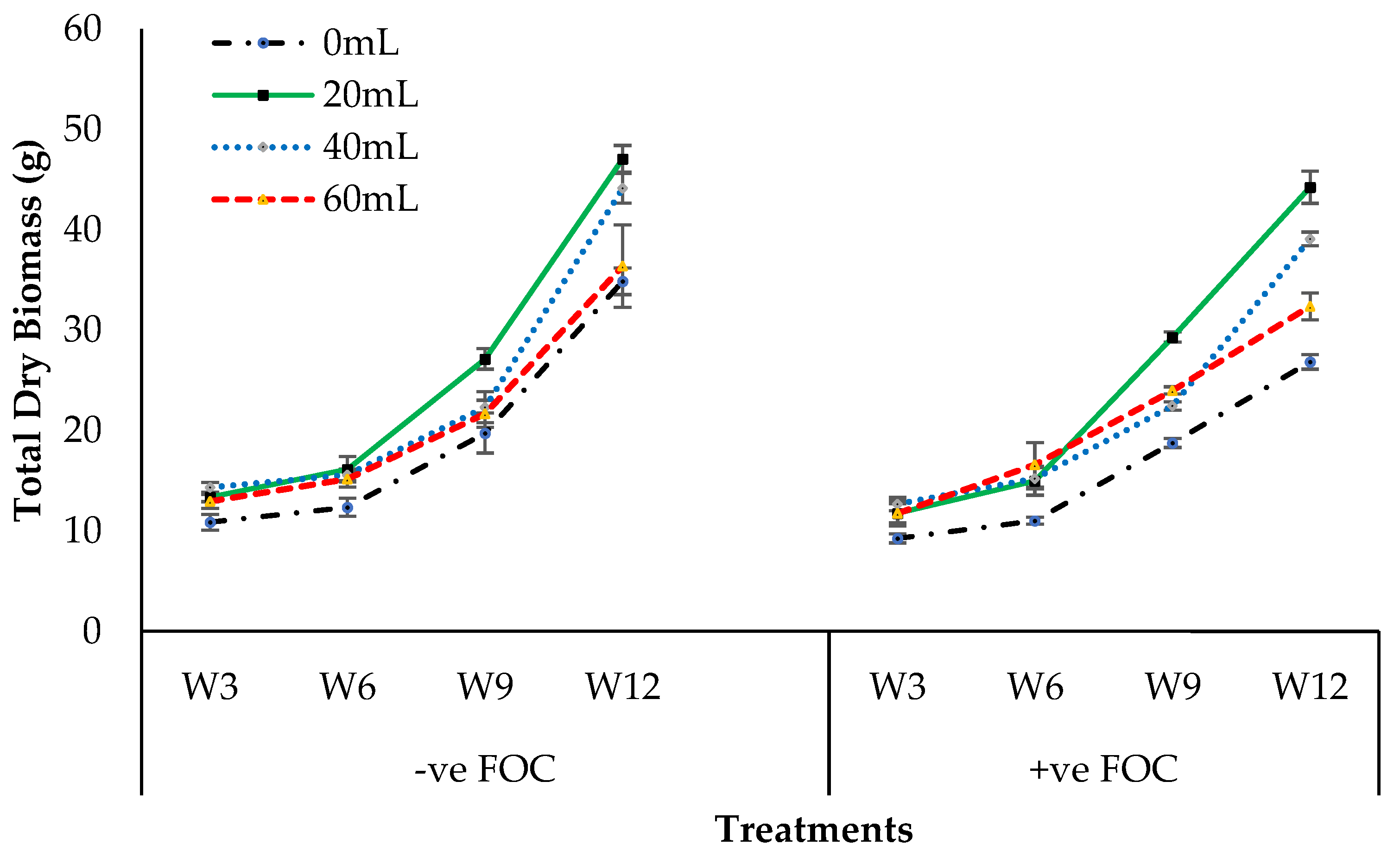
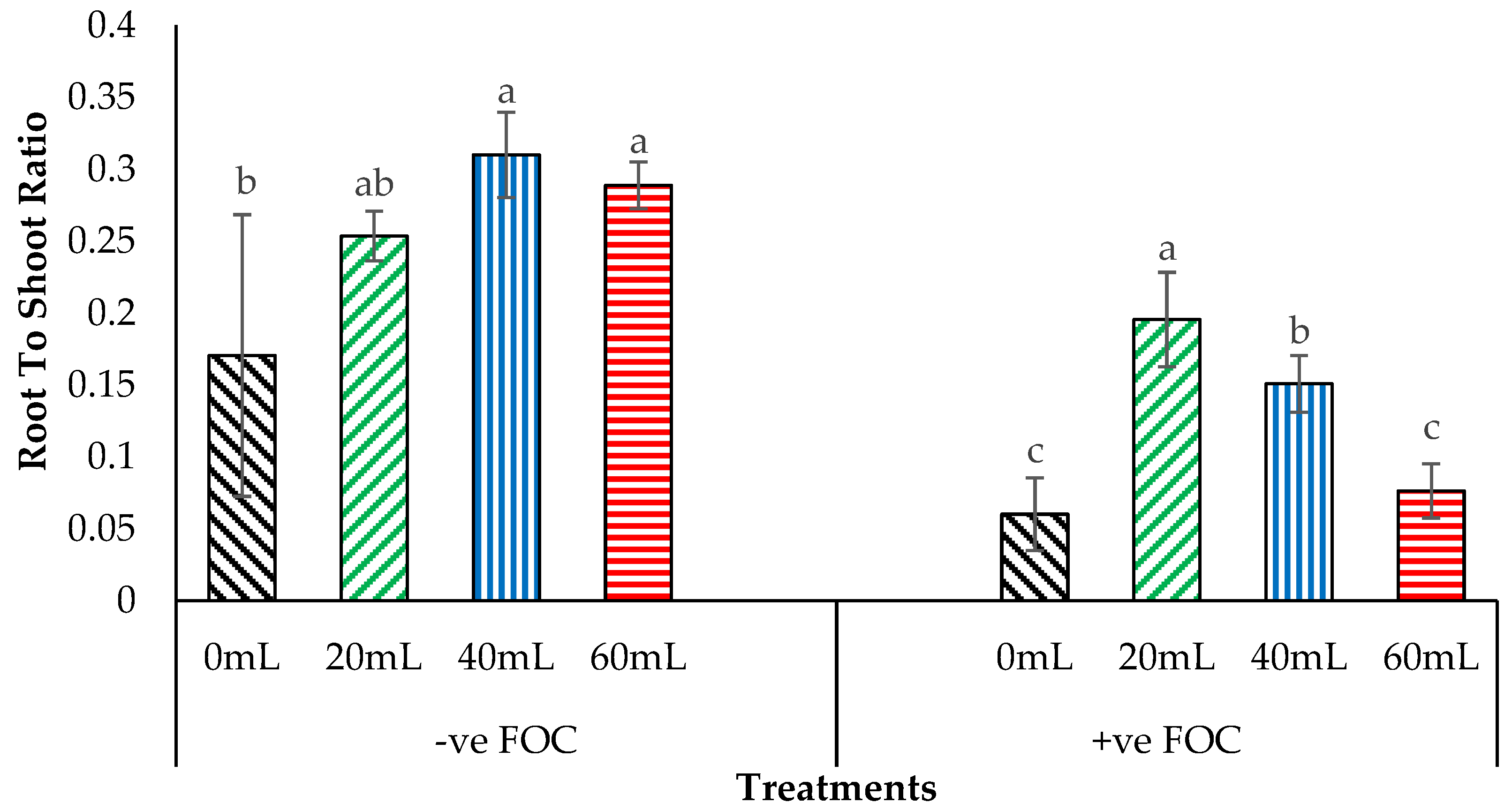
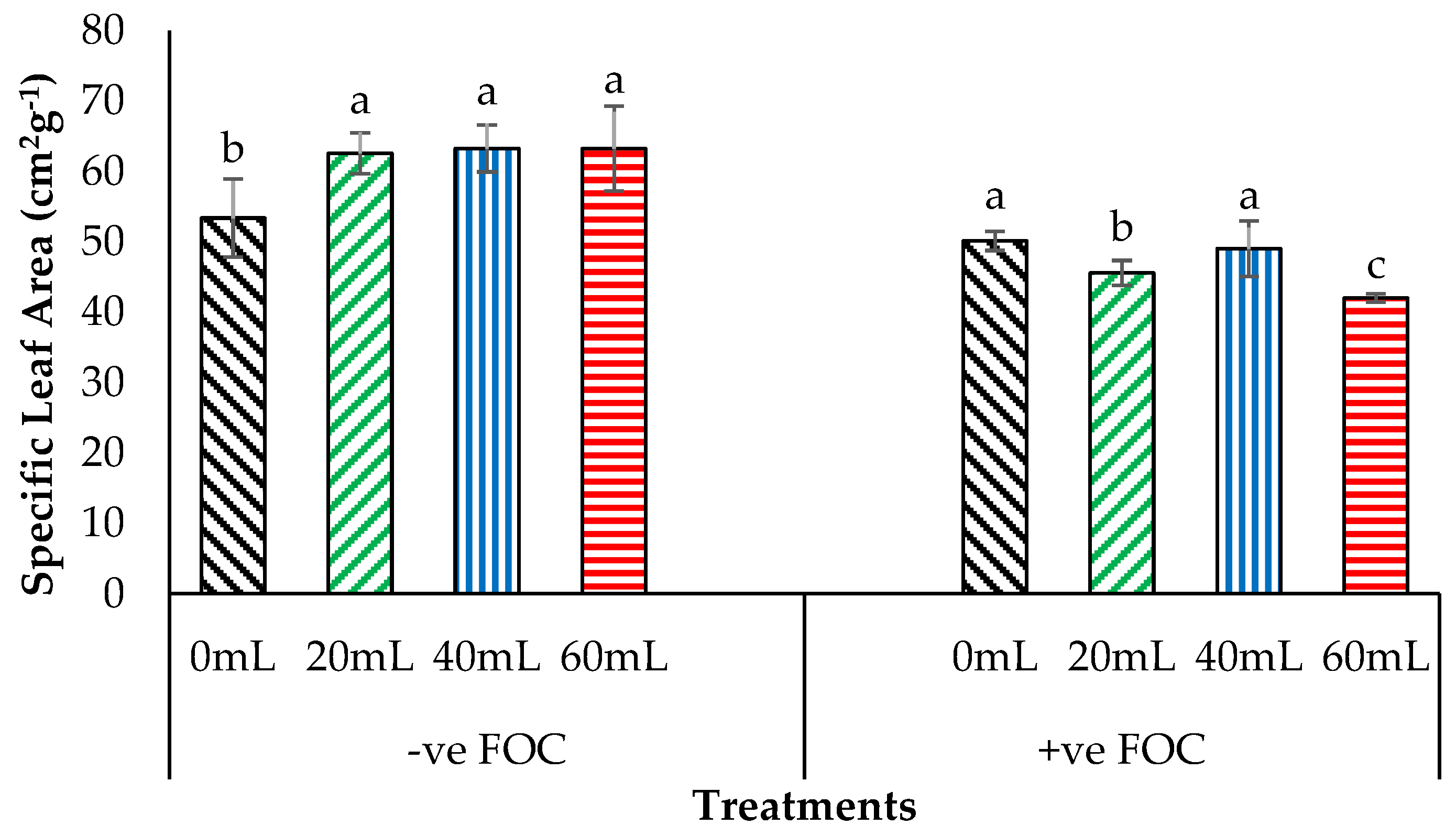
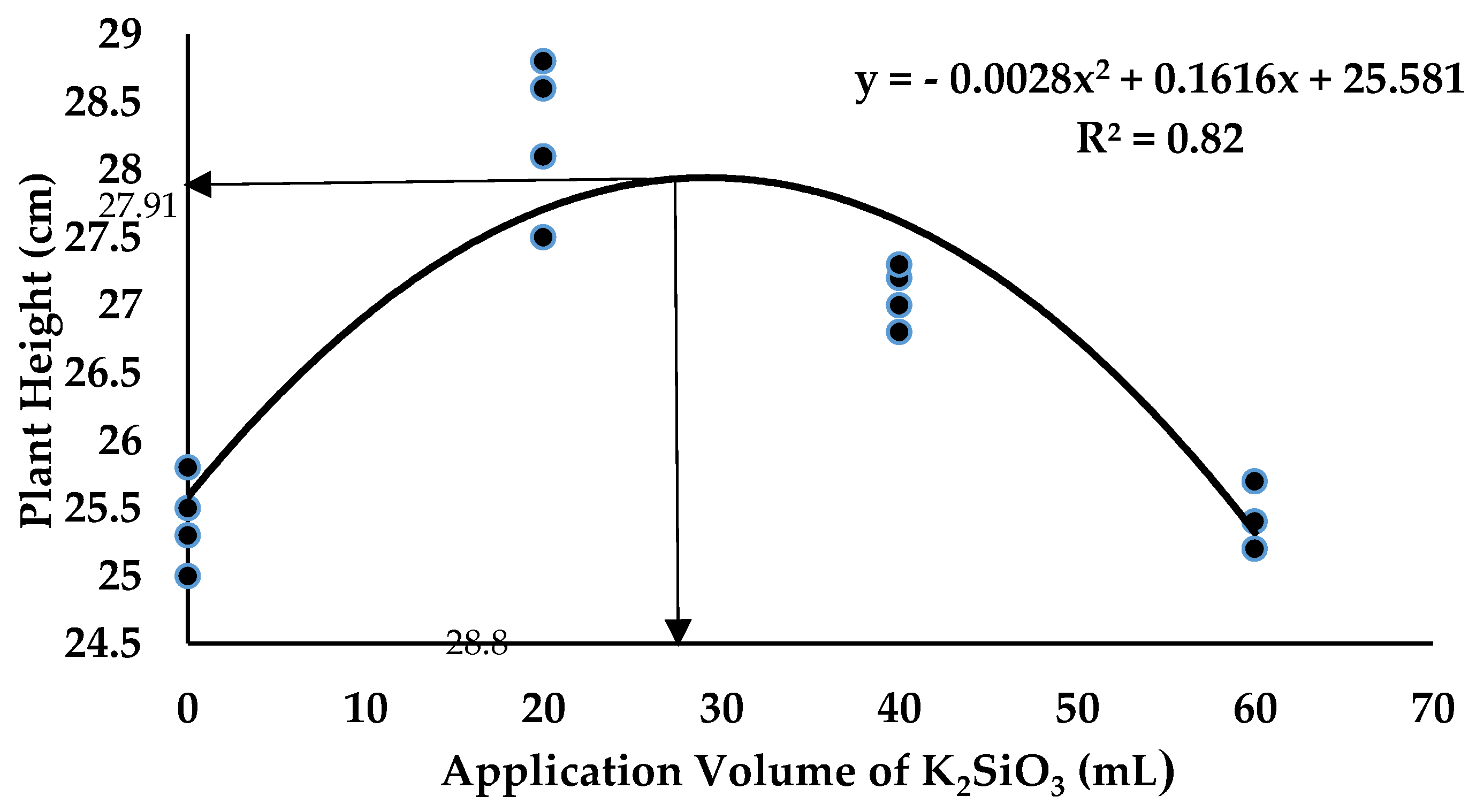
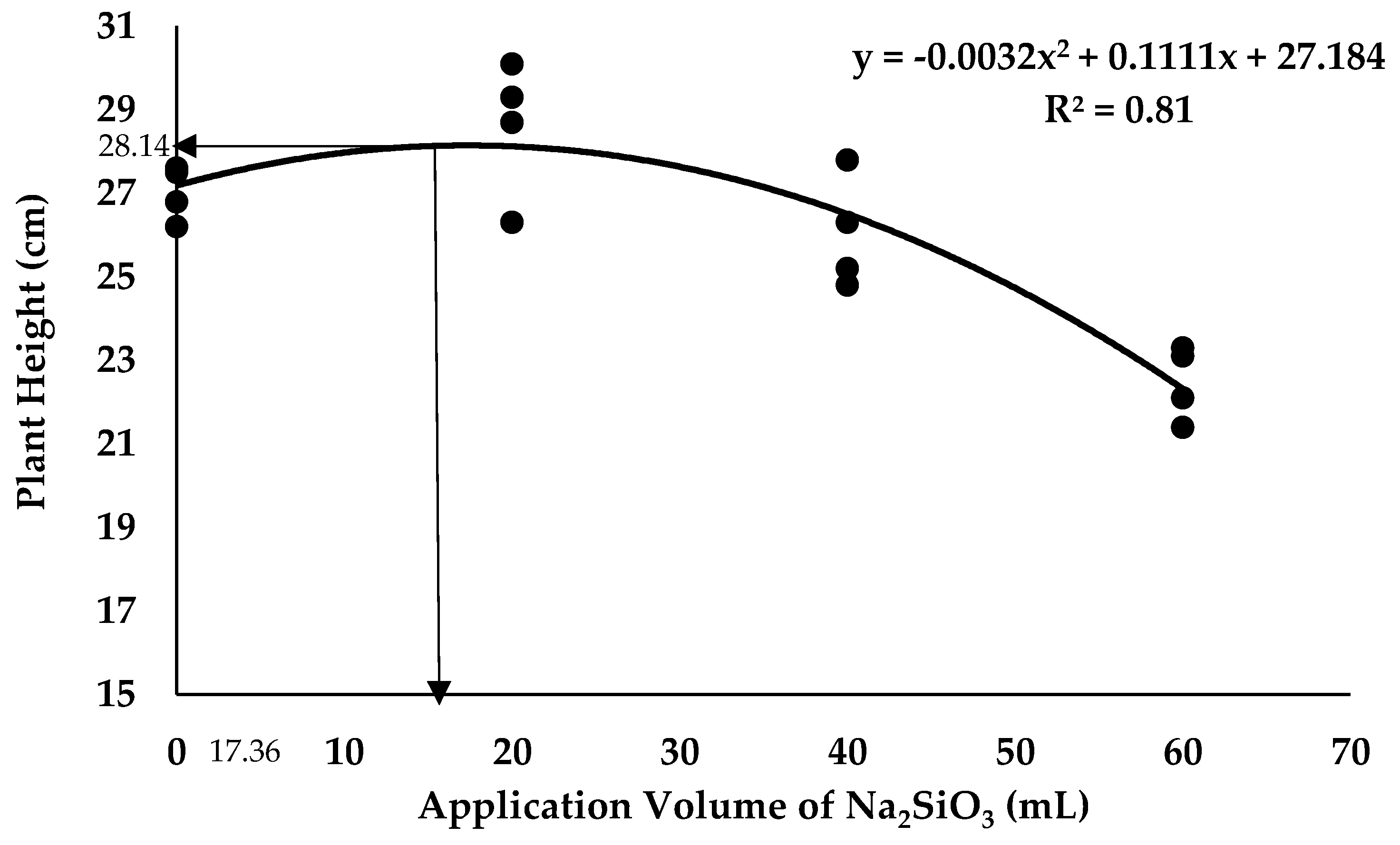
2.4. Crop Growth Performance
3. Discussion
4. Materials and Methods
4.1. Experimental Materials Preparation and Treatment
4.2. Data Collection
4.2.1. Determination of Total Chlorophyll Content and Leaf Gas Exchange
4.2.2. Physiological Attributes
4.2.3. Biochemical Assay
4.2.4. Disease Assessment and Microbial Population
4.2.5. Crop Growth Performance
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Agriculture, Malaysia (DOA). Booklet Statistik Tanaman (Sub-sektor Tanaman Makanan 2021); First Country Report; Department of Agriculture: Putrajaya, Malaysia, 2022; p. 55.
- Hunjan, M.S.; Lore, J.S. Climate Change: Impact on Plant Pathogens, Diseases, and Their Management. In Crop Protection under Changing Climate; Springer: Cham, Switzerland, 2020; pp. 85–100. [Google Scholar]
- Wong, C.K.F.; Zulperi, D.; Vadamalai, G.; Saidi, N.B.; Teh, C.Y. Phylogenetic Analysis of Fusarium oxysporum f. sp. cubense Associated with Fusarium Wilt of Bananas from Peninsular Malaysia. Sains Malays. 2019, 48, 1593–1600. [Google Scholar] [CrossRef]
- Staver, C.; Pemsl, D.E.; Scheerer, L.; Vicente, L.P.; Dita, M. Ex Ante Assessment of Returns on Research Investments to Address the Impact of Fusarium Wilt Tropical Race 4 on Global Banana Production. Front. Plant Sci. 2020, 11, 844. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The Epidemiology of Fusarium Wilt of Banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Attia, M.F.; Reyad, N.E.H.A.; Baiumy, M.A.M.; Mohamad, A.B. Comparative efficacy of fungicides, commercial bioproducts, essential oils and plant defense inducers in controlling pothos root rot. Plant Arch. 2020, 20, 919–925. [Google Scholar]
- Gbongue, L.-R.; Lalaymia, I.; Zeze, A.; Delvaux, B.; Declerck, S. Increased Silicon Acquisition in Bananas Colonized by Rhizophagus irregularis MUCL 41833 Reduces the Incidence of Pseudocercospora fijiensis. Front. Plant Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Jayanti, R.M.; Joko, T. Research article plant growth promoting and antagonistic potential of endophytic bacteria isolated from melon in Indonesia. Plant Pathol. J. 2020, 19, 200–210. [Google Scholar]
- Matichenkov, V.; Bocharnikova, E.; Romanova, A.; Doullet, P. Growth of Bacillus amyloliquefaciens as influence by Si nutrition. Arch. Microbiol. 2021, 203, 4329–4336. [Google Scholar] [CrossRef]
- Putrie, R.F.W.; Aryantha, I.N.P.; Iriawati; Antonius, S. The structure characteristic of IAA n-acetyl-transferase enzyme produced by two species of bacteria (Bacillus subtilis and Bacillus amyloliquefaciens). In Proceedings of the 7th International Symposium of Innovative Bio-Production Indonesia on Biotechnology and Bioengineering 2020, Cibinong, West Java, Indonesia, 18–20 November 2020; 762, p. 012054. [Google Scholar] [CrossRef]
- Ezrari, S.; Radouane, N.; Tahiri, A.; El Housni, Z.; Mokrini, F.; Özer, G.; Lazraq, A.; Belabess, Z.; Amiri, S.; Lahlali, R. Dry root rot disease, an emerging threat to citrus industry worldwide under climate change: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101753. [Google Scholar] [CrossRef]
- Farhat, M.G.; Haggag, W.M.; Thabet, M.S.; Mosa, A.A. Efficacy of silicon and titanium nanoparticles biosynthesis by some antagonistic fungi and bacteria for controlling powdery mildew disease of wheat plants. J. Technol. 2018, 14, 661–674. [Google Scholar]
- Ahmad, A.; Yasin, N.A.; Khan, W.U.; Akram, W.; Wang, R.; Shah, A.A.; Akbar, M.; Ali, A.; Wu, T. Silicon assisted ameliorative effects of iron nanoparticles against cadmium stress: Attaining new equilibrium among physiochemical parameters, antioxidative machinery, and osmoregulators of Phaseolus lunatus. Plant Physiol. Biochem. 2021, 166, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Cagua, D.; Rodríguez-Yzquierdo, G. Effect of biostimulants on dry matter accumulation and gas exchange in plantain plants (Musa AAB). Rev. Colomb. Cienc. Hortícolas 2019, 13, 151–160. [Google Scholar] [CrossRef]
- Din, S.N.M.; Sakimin, S.Z.; Sijam, K.; Ramlan, M.F.; Baghdadi, A.; Zakaria, M.A.T. Potential of Bacillus subtilis inoculation in BioricharTM amended soil for suppression of Fusarium wilt of banana (Musa acuminata cv. Berangan) under water stress condition. Fundam. Appl. Agric. 2018, 3, 515–524. [Google Scholar] [CrossRef]
- Zellener, W.; Tubaña, B.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Plant Stress Reduction and Why This Element Is Not Used Routinely for Managing Plant Health. Plant Dis. 2021, 105, 2033–2049. [Google Scholar] [CrossRef]
- Shah, A.A.; Yasin, N.A.; Akram, K.; Ahmad, A.; Khan, W.U.; Akram, W.; Akbar, M. Ameliorative role of Bacillus subtilis FBL-10 and silicon against lead induced stress in Solanum melongena. Plant Physiol. Biochem. 2021, 158, 486–496. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, S.-H.; Khaine, I.; Kwak, M.J.; Lee, H.K.; Lee, T.Y.; Lee, W.Y.; Woo, S.Y. Physiological changes and growth promotion induced in poplar seedlings by the plant growth-promoting rhizobacteria Bacillus subtilis JS. Photosynthetica 2018, 56, 1188–1203. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; El-Nagdi, W.M.A.; Youssef, M.M.A.; Elgawad, M.M.M.A.; Dawood, M.G. Protective effect of Bacillus subtilis, B. pumilus, and Pseudomonas fluorescens isolates against root knot nematode Meloidogyne incognita on cowpea. Bull. Natl. Res. Cent. 2019, 43, 64. [Google Scholar] [CrossRef]
- Jabborova, D.; Enakiev, Y.; Sulaymanov, K.; Kadirova, D.; Ali, A.; Annapurna, K. Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Sci. Today 2021, 8, 66–71. [Google Scholar] [CrossRef]
- Llorente, B.; Torres-Montilla, S.; Morelli, L.; Florez-Sarasa, I.; Matus, J.T.; Ezquerro, M.; D’Andrea, L.; Houhou, F.; Majer, E.; Picó, B.; et al. Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development. Proc. Natl. Acad. Sci. USA 2020, 117, 21796–21803. [Google Scholar] [CrossRef]
- Zhang, D.; Jiao, X.; Du, Q.; Song, X.; Li, J. Reducing the excessive evaporative demand improved photosynthesis capacity at low costs of irrigation via regulating water driving force and moderating plant water stress of two tomato cultivars. Agric. Water Manag. 2018, 199, 22–33. [Google Scholar] [CrossRef]
- Kabashnikova, L.; Abramchik, L.; Domanskaya, I.; Savchenko, G.; Shpileuski, S. β-1,3-glucan effect on the photosynthetic apparatus and oxidative stress parameters of tomato leaves under Fusarium wilt. Funct. Plant Biol. 2020, 47, 988. [Google Scholar] [CrossRef] [PubMed]
- D’addazio, V.; Silva, J.; Jardim, A.; Longue, L.; Santos, R.; Fernandes, A.; Silva, M.; Silva, D.; Santos, T.; Schmildt, E.; et al. Silicon improves the photosynthetic performance of black pepper plants inoculated with Fusarium solani f. sp. piperis. Photosynthetica 2020, 58, 692–701. [Google Scholar] [CrossRef]
- Qin, J.; Shangguan, Z. Effects of forest types on leaf functional traits and their interrelationships of Pinus massoniana coniferous and broad-leaved mixed forests in the subtropical mountain, Southeastern China. Ecol. Evol. 2019, 9, 6922–6932. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Ding, L.; Chaumont, F.; Aroca, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef]
- Shezi, S.; Magwaza, L.S.; Mashilo, J.; Tesfay, S.Z.; Mditshwa, A. Photosynthetic efficiency and relationship to mesocarp dry matter content of ‘Carmen’avocado (Persea mericana Mill.) fruit in a cool subtropical climate. Sci. Hortic. 2019, 253, 209–216. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Amitrano, C.; Rouphael, Y.; De Pascale, S.; Cirillo, C. Changes in morpho-anatomical and eco-physiological responses of Viburnum tinus l. var lucidum as modulated by sodium chloride and calcium chloride salinization. Horticulturae 2022, 8, 119. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Roy, S.; Mathur, P. Delineating the mechanisms of elevated CO2 mediated growth, stress tolerance and phytohormonal regulation in plants. Plant Cell Rep. 2021, 40, 1345–1365. [Google Scholar] [CrossRef]
- Iqbal, N.; Czékus, Z.; Ördög, A.; Poór, P. Ethylene-dependent effects of fusaric acid on the photosynthetic activity of tomato plants. Photosynthetica 2021, 59, 337–348. [Google Scholar] [CrossRef]
- Filek, M.; Sieprawska, A.; Kościelniak, J.; Oklestkova, J.; Jurczyk, B.; Telk, A.; Biesaga-Kościelniak, J.; Janeczko, A. The role of chloroplasts in the oxidative stress that is induced by zearalenone in wheat plants—The functions of 24-epibrassinolide and selenium in the protective mechanisms. Plant Physiol. Biochem. 2019, 137, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Damoo, D.; Djamei, A.; Kronstad, J. Chloroplasts and Plant Immunity: Where Are the Fungal Effectors? Pathogens 2020, 9, 19. [Google Scholar] [CrossRef]
- Kaushik, P.; Saini, D.K. Silicon as a Vegetable Crops Modulator—A Review. Plants 2019, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.; Al-Qahtani, R.M.; Ibrahim, Y.E.; Almasrahi, A.A.; Al-Saleh, M.A. Soil application of Trichoderma asperellum strains significantly improves Fusarium root and stem rot disease management and promotes growth in cucumbers in semi-arid regions. Eur. J. Plant Pathol. 2022, 162, 637–653. [Google Scholar] [CrossRef]
- Wong, C.K.F.; Zulperi, D.; Saidi, N.B.; Vadamalai, G. A Consortium of Pseudomonas aeruginosa and Trichoderma harzianum for Improving Growth and Induced Biochemical Changes in Fusarium Wilt Infected Bananas. Trop. Life Sci. Res. 2021, 32, 23–45. [Google Scholar] [CrossRef]
- Dong, H.; Ye, Y.; Guo, Y.; Li, H. Comparative transcriptome analysis revealed resistance differences of Cavendish bananas to Fusarium oxysporum f. sp. cubense race1 and race4. BMC Genet. 2020, 21, 122. [Google Scholar] [CrossRef]
- Ahmed, M.; Qadeer, U.; Hassan, F.U.; Fahad, S.; Naseem, W.; Duangpan, S.; Ahmad, S. Abiotic Stress Tolerance in Wheat and the Role of Silicon: An Experimental Evidence. In Agronomic Crops; Springer: Singapore, 2020; pp. 443–479. [Google Scholar]
- Fung, S.M.; Razali, Z.; Somasundram, C. Reactive oxygen species scavenging enzyme activities in Berangan banana plant infected by Fusarium oxysporum f. sp. cubense. Chiang Mai J. Sci. 2019, 46, 1084–1095. [Google Scholar]
- Yang, W.; Ma, X.; Ma, D.; Shi, J.; Hussain, S.; Han, M.; Costes, E.; Zhang, D. Modeling canopy photosynthesis and light interception partitioning among shoots in bi-axis and single-axis apple trees (Malus domestica Borkh.). Trees 2021, 35, 845–861. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Artyszak, A.; Kondracka, M.; Gozdowski, D.; Siuda, A.; Litwińczuk-Bis, M. Impact of Foliar Application of Various Forms of Silicon on the Chemical Composition of Sugar Beet Plants. Sugar Tech. 2021, 23, 546–559. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2020, 185, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Mofunanya, A.; Towolabi, A.; Nkang, A. Comparative Study of the Effect of Telfairia Mosaic Virus (TEMV) on the Growth Characteristics of Two Ecotypes of Telfairia occidentalis (Hooker Fil). Int. J. Virol. 2015, 11, 54–65. [Google Scholar] [CrossRef]
- Paye, W. Silicon Fertilization in Rice and Wheat: Dynamics with Trace Elements and Effect of Silicate Slag Granular Size on the Release Pattern of Monosilicic Acid in Soil. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2019. [Google Scholar]
- Brahma, R.; Ahmed, P.; Choudhury, M. Silicon nutrition for alleviation of abiotic stress in plants: A review. J. Pharmacogn. Phytochem. 2020, 9, 1374–1381. [Google Scholar]
- Zhou, J.; Tang, S.; Pan, W.; Xiao, H.; Ma, Q.; Sun, Y.; Xu, M.; Liu, M.; Wu, L. Silicon isotope fractionation dynamics during uptake and translocation by various crop species under three soil types. Plant Soil 2022, 477, 41–55. [Google Scholar] [CrossRef]
- Shabbir, I.; Samad, M.Y.A.; Othman, R.; Wong, M.-Y.; Sulaiman, Z.; Jaafar, N.M.; Bukhari, S.A.H. White root rot disease suppression in rubber plant with microbial co-inoculants and silicon addition. Rhizosphere 2020, 15, 100221. [Google Scholar] [CrossRef]
- Vashi, J.; Saravaiya, S.; Patel, A.; Chaudhari, B. Silicon—The most under-appreciated element for vegetables. Int. J. Chem. Stud. 2020, 8, 2122–2127. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Sawah, A.M. The Mode of Integration Between Azotobacter and Rhizobium Affect Plant Growth, Yield, and Physiological Responses of Pea (Pisum sativum L.). J. Soil Sci. Plant Nutr. 2022, 22, 1238–1251. [Google Scholar] [CrossRef]
- Suwignyo, B.; Arifin, L.; Umami, N.; Muhlisin, M.; Suhartanto, B. The performance and genetic variation of first and second generation tropical alfalfa (Medicago sativa). Biodiversitas J. Biol. Divers. 2021, 22, d220631. [Google Scholar] [CrossRef]
- Abro, B.A.; Memon, M.; Razaq, A.; Abro, D.A. Assessing nitrogen nutrition of banana Basra cultivar (DWARF CAVENDISH) through leaf analysis and chlorophyll determination. Pak. J. Bot. 2021, 31, 1859–1864. [Google Scholar]
- Anthony, K.K.; George, D.S.; Singh, H.K.B.; Fung, S.M.; Santhirasegaram, V.; Razali, Z.; Somasundram, C. Reactive Oxygen Species Activity and Antioxidant Properties of Fusarium Infected Bananas. J. Phytopathol. 2017, 165, 213–222. [Google Scholar] [CrossRef]
- Rana, Z.H.; Alam, M.K.; Akhtaruzzaman, M. Nutritional composition, total phenolic content, antioxidant and α-amylase inhibitory activities of different fractions of selected wild edible plants. Antioxidants 2019, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Mansora, A.M.; Lima, J.S.; Anib, F.N.; Hashima, H.; Hoa, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. 2019, 72, 79–84. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium Wilt Disease Incidence Is Influenced by Shifts of Soil Microbial Communities Under Different Monoculture Spans. Microb. Ecol. 2017, 75, 739–750. [Google Scholar] [CrossRef]
- Nwadibe, E.C.; Aniebonam, E.E.; Jude, O.U. Effect of crude oil pollution on soil and aquatic bacteria and fungi. J. Exp. Biol. Agric. Sci. 2020, 8, 176–184. [Google Scholar] [CrossRef]
- Zakaria, M.A.T.; Sakimin, S.Z.; Juraimi, A.S.; Ramlan, M.F.; Jaafar, H.Z.; Baghdadi, A.; Din, S.N.M. Effect of different water regimes and plant growth regulators on growth, physiology and yield of banana (Musa acuminata cv. Berangan) in tropical climate. Fundam. Appl Agric. 2018, 3, 505–514. [Google Scholar] [CrossRef]
- Demelash, H.; Tadesse, T.; Menamo, T.; Menzir, A. Determination of root system architecture variation of drought adapted sorghum genotypes using high throughput root phenotyping. Rhizosphere 2021, 19, 100370. [Google Scholar] [CrossRef]
- Marenco, R.; Antezana-Vera, S.A.; Nascimento, H. Relationship between specific leaf area, leaf thickness, leaf water content and SPAD-502 readings in six Amazonian tree species. Photosynthetica 2009, 47, 184–190. [Google Scholar] [CrossRef]
- Rugemalila, D.M.; Cory, S.T.; Smith, W.K.; Anderson, T.M. The role of microsite sunlight environment on growth, architecture, and resource allocation in dominant Acacia tree seedlings, in Serengeti, East Africa. Plant Ecol. 2020, 221, 1187–1199. [Google Scholar] [CrossRef]

| Factors | Photosynthesis Rate | Stomatal Conductance |
|---|---|---|
| (µmol CO2 m−2s−1) | (mmol m−2s−1) | |
| Main plot means: Treatments | ||
| −ve FOC | 18.43 ± 0.58 a | 0.74 ± 0.04 a |
| +ve FOC | 14.59 ± 1.89 b | 0.56 ± 0.23 a |
| LSD (p < 0.05) | 0.98 ** | NS |
| Subplot means: Antagonistic bacteria | ||
| Control (0B) | 15.41 ± 3.11 b | 0.55 ± 0.21 b |
| B. subtilis (BS) | 17.03 ± 1.75 a | 0.67 ± 0.17 ab |
| B. thuringiensis (BT) | 17.08 ± 1.99 a | 0.74 ± 0.13 a |
| LSD (p < 0.05) | 1.37 * | 0.14 * |
| Significance interaction | NS | NS |
| Factors | Leaf Proline Content | Total Flavonoid Content | Total Phenolic Content | Lignin Content |
|---|---|---|---|---|
| (µmolg−1 Fresh Weight) | (mg CE/100 mL) | (µg/g FW) | (LTGAg−1 Tissue) | |
| Main plot means: Treatments | ||||
| −ve FOC | 32.65 ± 5.29 b | 8.02 ± 1.62 b | 110.17 ± 14.53 b | 1.32 ± 0.65 a |
| +ve FOC | 40.72 ± 5.61 a | 11.20 ± 2.46 a | 142.26 ± 22.14 a | 1.76 ± 0.43 a |
| LSD (p < 0.05) | 2.01 ** | 1.97 * | 17.98 * | 0.56 * |
| Subplot means: Antagonistic bacteria | ||||
| Control (0B) | 36.82 ± 5.59 a | 11.62 ± 2.35 a | 128.48 ± 15.95 a | 1.09 ± 0.43 b |
| B. subtilis (BS) | 32.17 ± 4.89 b | 7.31 ± 1.65 c | 110.67 ± 19.03 b | 1.89 ± 0.54 a |
| B. thuringiensis (BS) | 41.07 ± 7.01 a | 9.90 ± 1.81 b | 139.5 ± 29.75 a | 1.65 ± 0.50 a |
| LSD (p < 0.05) | 4.59 ** | 0.73 *** | 12.84 ** | 0.51 * |
| Significance interaction | NS | NS | NS | NS |
| Factors | Initial Experiment (CFU log10 g−1) | Final Experiment (CFU log10 g−1) | ||
|---|---|---|---|---|
| Fungus | Bacteria | Fungus | Bacteria | |
| Main-plot means: Treatments | ||||
| −ve FOC | 4.10 ± 0.19 a | 4.04 ± 0.44 a | 3.99 ± 0.18 b | 4.03 ± 0.52 a |
| +ve FOC | 4.12 ± 0.25 a | 4.19 ± 0.09 a | 4.12 ± 0.20 a | 3.86 ± 0.61 a |
| LSD (p < 0.05) | NS | NS | 0.04 ** | NS |
| Subplot means: Antagonistic bacteria | ||||
| Control (0B) | 4.18 ± 0.14 a | 4.20 ± 0.09 a | 4.22 ± 0.13 a | 3.46 ± 0.77 b |
| B. subtilis (BS) | 4.01 ± 0.19 a | 4.16 ± 0.06 a | 3.90 ± 0.20 b | 4.24 ± 0.15 a |
| B. thuringiensis (BS) | 4.15 ± 0.28 a | 3.98 ± 0.54 a | 4.04 ± 0.14 b | 4.14 ± 0.13 a |
| LSD (p < 0.05) | NS | NS | 0.14 ** | 0.55 * |
| Significance interaction | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, M.A.T.; Sakimin, S.Z.; Ismail, M.R.; Ahmad, K.; Kasim, S.; Baghdadi, A. Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease. Plants 2023, 12, 1124. https://doi.org/10.3390/plants12051124
Zakaria MAT, Sakimin SZ, Ismail MR, Ahmad K, Kasim S, Baghdadi A. Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease. Plants. 2023; 12(5):1124. https://doi.org/10.3390/plants12051124
Chicago/Turabian StyleZakaria, Md Aiman Takrim, Siti Zaharah Sakimin, Mohd Razi Ismail, Khairulmazmi Ahmad, Susilawati Kasim, and Ali Baghdadi. 2023. "Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease" Plants 12, no. 5: 1124. https://doi.org/10.3390/plants12051124
APA StyleZakaria, M. A. T., Sakimin, S. Z., Ismail, M. R., Ahmad, K., Kasim, S., & Baghdadi, A. (2023). Biostimulant Activity of Silicate Compounds and Antagonistic Bacteria on Physiological Growth Enhancement and Resistance of Banana to Fusarium Wilt Disease. Plants, 12(5), 1124. https://doi.org/10.3390/plants12051124







