Arabinogalactan Structures of Repetitive Serine-Hydroxyproline Glycomodule Expressed by Arabidopsis Cell Suspension Cultures
Abstract
1. Introduction
2. Results
2.1. Hyp-O-Polysaccharides from the Arabidopsis Fusion Glycoprotein (Ser-Hyp)32-EGFP
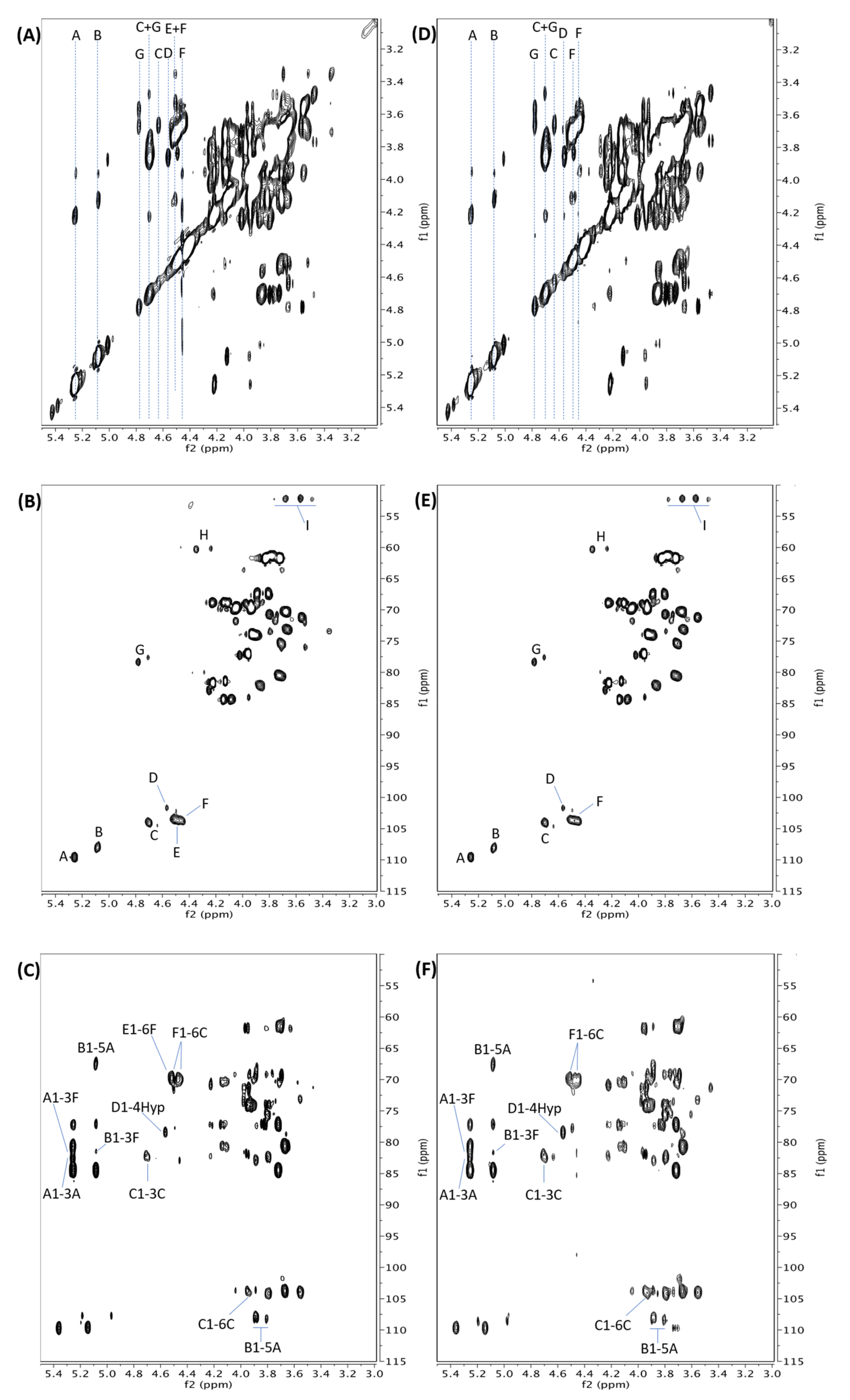
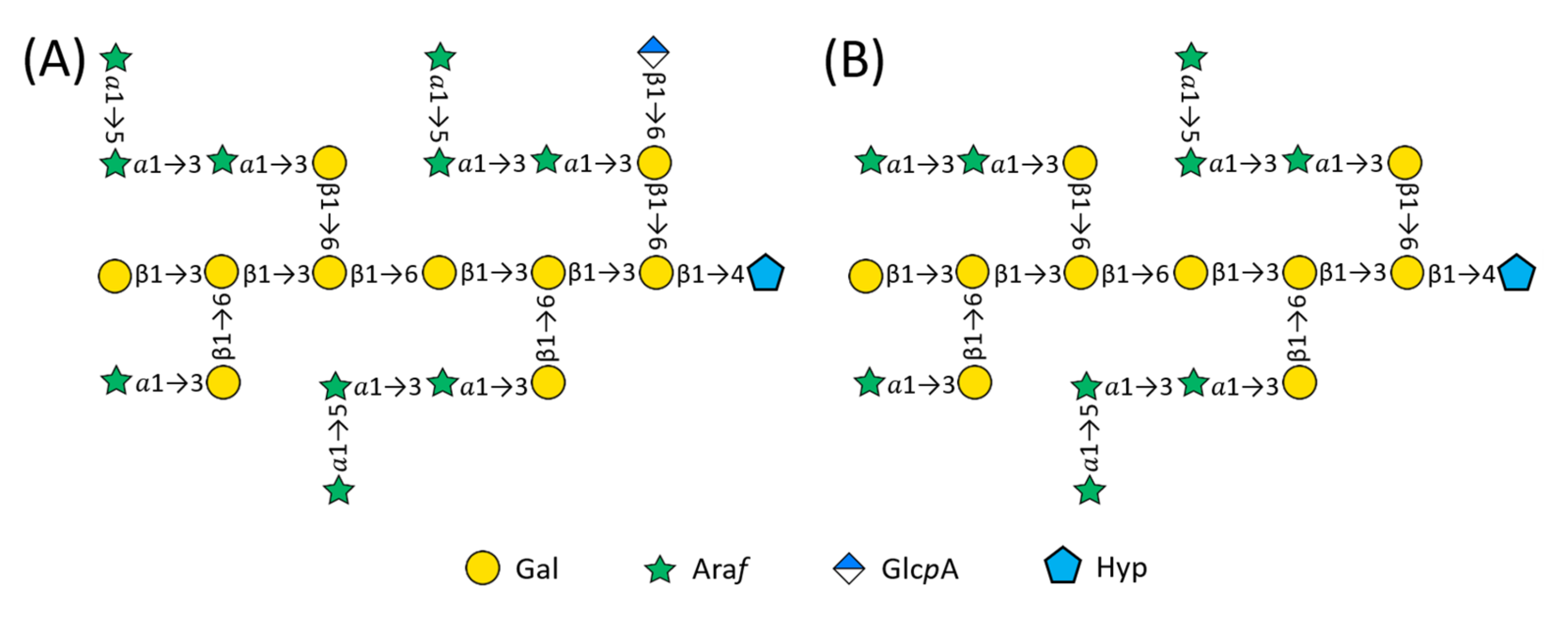
2.2. Structure of AtSPHP-1
2.3. Structure of AtSPHP-2
3. Discussion
AtSPHP-1 and AtSPHP-2 Are Variations on a Conserved Theme
4. Materials and Methods
4.1. Isolation of (Ser-Hyp)32-EGFP from Arabidopsis Suspension Cultured Cells
4.2. Co-Precipitation with Yariv Reagent
4.3. Isolation of Hyp-O-Glycans
4.4. Sugar Analyses and Hyp Assay
4.5. NMR Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Showalter, A.M.; Egelund, J.; Hernandez-Sanchez, A.; Doblin, M.S.; Bacic, A. Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Front. Plant Sci. 2012, 3, 140. [Google Scholar] [CrossRef]
- Nguema-Ona, E.; Vicre-Gibouin, M.; Cannesan, M.-A.; Driouich, A. Arabinogalactan proteins in root-microbe interactions. Trends Plant Sci. 2013, 18, 440–449. [Google Scholar] [CrossRef]
- Ma, Y.; Zeng, W.; Bacic, A.; Johnson, K. AGPs through time and space. Annu. Plant Rev. 2018, 1, 1–38. [Google Scholar]
- Classen, B.; Baumann, A.; Utermoehlen, J. Arabinogalactan-proteins in spore-producing land plants. Carbohydr. Polym. 2019, 210, 215–224. [Google Scholar] [CrossRef]
- Lopez-Hernandez, F.; Tryfona, T.; Rizza, A.; Yu, X.L.; Harris, M.O.B.; Webb, A.A.R.; Kotake, T.; Dupree, P. Calcium binding by Arabinogalactan polysaccharides is important for normal plant development. Plant Cell 2020, 32, 3346–3369. [Google Scholar] [CrossRef]
- Showalter, A.M.; Keppler, B.; Lichtenberg, J.; Gu, D.; Welch, L.R. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiol. 2010, 153, 485–513. [Google Scholar] [CrossRef]
- Baldwin, T.C.; McCann, M.; Roberts, K. A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota. Plant Physiol. 1993, 103, 115–123. [Google Scholar] [CrossRef]
- Xu, J.; Tan, L.; Lamport, D.T.A.; Showalter, A.M.; Kieliszewski, M.J. The O-Hyp glycosylation code in tobacco and Arabidopsis and a proposed role of Hyp-glycans in secretion. Phytochemistry 2008, 69, 1631–1640. [Google Scholar] [CrossRef]
- Johnson, K.L.; Cassin, A.M.; Lonsdale, A.; Wong, G.K.-S.; Soltis, D.E.; Miles, N.W.; Melkonian, M.; Melkonian, B.; Deyholos, M.K.; Leebens-Mack, J.; et al. Insights into the evolution of hydroxyproline-rich glycoproteins from 1000 plant transcriptomes. Plant Physiol. 2017, 174, 904–921. [Google Scholar] [CrossRef]
- Leonard, R.; Peterson, B.O.; Himly, M.; Kaar, W.; Wopfner, N.; Kolarich, D.; van Ree, R.; Ebner, C.; Duus, J.O.; Ferreira, F.; et al. Two novel types of O-glycans on the Mugwort pollen allergen Art v 1 and their role in antibody binding. J. Biol. Chem. 2005, 280, 7932–7940. [Google Scholar] [CrossRef]
- Baumann, A.; Pfeifer, L.; Classen, B. Arabinogalactan-proteins from non-coniferous gymnosperms have unusual structural features. Carbohydr. Polym. 2021, 261, 117831. [Google Scholar] [CrossRef]
- Tan, L.; Qiu, F.; Lamport, D.T.A.; Kieliszewski, M.J. Structure of a hydroxyproline (Hyp)-arabinogalactan polysaccharide from repetitive Ala-Hyp expressed in transgenic Nicotiana tabacum. J. Biol. Chem. 2004, 279, 13156–13165. [Google Scholar] [CrossRef]
- Shpak, E.; Barbar, E.; Leykam, J.F.; Kieliszewski, M.J. Contiguous hydroxyproline residues direct hydroxyproline arabinosylation in Nicotiana tabacum. J. Biol. Chem. 2001, 276, 11272–11278. [Google Scholar] [CrossRef]
- Tan, L.; Leykam, J.F.; Kieliszewski, M.J. Glycosylation motifs that direct arabinogalactan addition to arabinogalactan-proteins. Plant Physiol. 2003, 132, 1362–1369. [Google Scholar] [CrossRef]
- Shpak, E.; Leykam, J.F.; Kieliszewski, M.J. Synthetic genes for glycoprotein design and the elucidation of hydroxyproline-O-glycosylation codes. Proc. Natl. Acad. Sci. USA 1999, 96, 14736–14741. [Google Scholar] [CrossRef]
- Brady, J.D.; Sadler, I.H.; Fry, S.C. Di-isodityrosine, a novel tetrameric derivative of tyrosine in plant cell wall proteins: A new potential cross-link. Biochem. J. 1996, 315, 323–327. [Google Scholar] [CrossRef]
- Held, M.A.; Tan, L.; Kamyab, A.; Hare, M.; Shpak, E.; Kieliszewski, M.J. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extension analogs cross-linked in vitro. J. Biol. Chem. 2004, 279, 55474–55482. [Google Scholar] [CrossRef]
- Serpe, M.D.; Nothenagel, E.A. Arabinogalactan-proteins in the multiple domains of the plant cell surface. Adv. Bot. Res. 1999, 30, 207–289. [Google Scholar]
- Schultz, C.J.; Johnson, K.L.; Currie, G.; Bacic, A. The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell 2000, 12, 1751–1768. [Google Scholar] [CrossRef]
- Tan, L.; Eberhard, S.; Pattathil, S.; Warder, C.; Glushka, J.; Yuan, C.; Hao, Z.; Zhu, X.; Avci, U.; Miller, J.S.; et al. An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 2013, 25, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhang, L.; Black, I.; Glushka, J.; Urbanowicz, B.; Heiss, C.; Azadi, P. Most of the rhamnogalacturonan-I from cultured Arabidopsis cell walls is covalently linked to arabinogalactan-protein. Carbohydr. Polym. 2023, 301, 120340. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, L.; Goodrum, K.J.; Kieliszewski, M.J. High-yields and extended serum half-life of human interferon α2b expressed in tobacco cells as arabinogalactan-protein fusions. Biotechnol. Bioeng. 2007, 97, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Defaye, J.; Wong, N.K. Structural studies of gum Arabic, the exudate polysaccharide from Acacia senegal. Carbohydr. Res. 1986, 150, 221–231. [Google Scholar] [CrossRef]
- Gane, A.M.; Craik, D.; Munro, S.L.; Howlett, G.J.; Clarke, A.E.; Bacic, A. Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydr. Res. 1995, 277, 67–85. [Google Scholar] [CrossRef]
- Tsumuraya, Y.; Mochizuki, N.; Hashimoto, Y.; Kovac, P. Purification of an exo-β-(1,3)-D-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalctan-proteins. J. Biol. Chem. 1990, 265, 7207–7215. [Google Scholar] [CrossRef]
- Tryfona, T.; Liang, H.C.; Kotake, T.; Kaneko, S.; Marsh, J.; Ichinose, H.; Lovegrove, A.; Tsumuraya, Y.; Shewry, P.R.; Stephens, E.; et al. Carbohydrate structural analysis of wheat flour arabinogalactan protein. Carbohydr. Res. 2010, 345, 2648–2656. [Google Scholar] [CrossRef]
- Tryfona, T.; Liang, H.C.; Kotake, T.; Tsumuraya, Y.; Stephens, E.; Dupree, P. Structural characterization of Arabidopsis leaf arabinogalactan polysaccharides. Plant Physiol. 2012, 160, 653–666. [Google Scholar] [CrossRef]
- Geshi, N.; Johansen, J.N.; Dilokpimol, A.; Rolland, A.; Belcram, K.; Verger, S.; Kotake, T.; Tsumuraya, Y.; Kaneko, S.; Tryfona, T.; et al. A galactosyltransferase acting on arabinogalactan protein glycans is essential for embryo development in Arabidopsis. Plant J. 2013, 76, 128–137. [Google Scholar] [CrossRef]
- Pfeifer, L.; Shafee, T.; Johnson, K.L.; Bacic, A.; Classen, B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci. Rep. 2020, 10, 8232. [Google Scholar] [CrossRef]
- Silva, J.; Ferraz, R.; Dupree, P.; Showalter, A.M.; Coimbra, S. Three decades of advances in arabinogalactan-protein biosynthesis. Front. Plant Sci. 2020, 11, 610377. [Google Scholar] [CrossRef]
- Tan, L.; Varnai, P.; Lamport, D.T.A.; Yuan, C.; Xu, J.; Qiu, F.; Kieliszewski, M.J. Plant O-Hydroxyproline arabinogalactans are composed of repeating trigalactosyl subunits with short bifurcated side chains. J. Biol. Chem. 2010, 285, 24575–24583. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kato, K. An extracellular arabinogalactan-protein from Nicotiana tabacum. Phytochemistry 1981, 20, 2507–2510. [Google Scholar] [CrossRef]
- Churms, S.C.; Stephen, A.M. Structural studies of an arabinogalactan-protein from the gum exudate of Acacia robusta. Carbohydr. Res. 1984, 133, 105–123. [Google Scholar] [CrossRef]
- Bacic, A.; Churms, S.C.; Stephen, A.M.; Cohen, P.B.; Fincher, G.B. Fine structure of the arabinogalactan-protein from Lolium multiflorum. Carbohydr. Res. 1987, 162, 85–93. [Google Scholar] [CrossRef]
- Xu, J.; Okada, S.; Tan, L.; Goodrum, K.J.; Kopchick, J.J.; Kieliszewski, M.J. Human growth hormone expressed in tobacco cells as an arabinogalactan-protein fusion glycoprotein has a prolonged serum life. Transgenic Res. 2010, 19, 849–867. [Google Scholar] [CrossRef]
- Chang, S.-C.; Saldivar, R.K.; Liang, P.-H.; Hsieh, Y.S. Structures, biosynthesis, and physiological functions of (1,3:1,4)-β-D-glucans. Cells 2021, 10, 510. [Google Scholar] [CrossRef]
- Lamport, D.T.A.; Varnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef]
- Kitazawa, K.; Tryfona, T.; Yoshimi, Y.; Hayashi, Y.; Kawauchi, S.; Antonov, L.; Tanaka, H.; Takahashi, T.; Kaneko, S.; Dupree, P.; et al. β-galactosyl Yariv reagen binds to the β-1,3-galactan of arabinogalactan proteins. Plant Physiol. 2013, 161, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Santander, J.; Martin, T.; Loh, A.; Pohlenz, C.; Gatlin, D.M., III; Curtiss, R., III. Mechanisms of intrinsic resistance to antimicrobial peptides of Edwardsiella ictaluri and its influence on fish gut inflammation and virulence. Microbiology 2013, 159, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Stupak, J.; Richards, M.R.; Whitfield, C. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 7868–7873. [Google Scholar] [CrossRef] [PubMed]
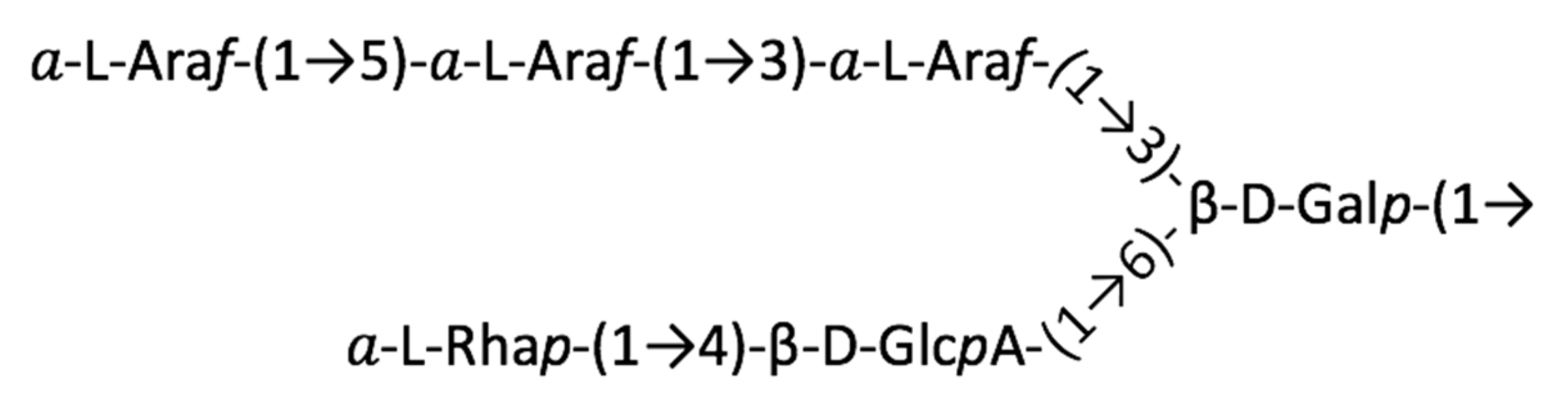
| AtSPHP-1 | AtSPHP-2 | ||||||
|---|---|---|---|---|---|---|---|
| Glycosyl Residues | Mol% | Glycosyl Linkages | Mol% | Glycosyl Residues | Mol% | Glycosyl Linkages | Mol% |
| Ara | 45.1 | t-Araf | 18.9 | Ara | 45.8 | t-Araf | 21.8 |
| 3-Araf | 13.9 | 3-Araf | 14.8 | ||||
| 5-Araf | 15.1 | 5-Araf | 9.6 | ||||
| Gal | 52.5 | t-Galp | 4.6 | Gal | 54.2 | t-Galp | 4.9 |
| 3-Galp | 13.9 | 3-Galp | 21.2 | ||||
| 6-Galp | 4.5 | 6-Galp | 5.5 | ||||
| 3,6-Galp | 24.1 | 3,6-Galp | 22.2 | ||||
| GlcA | 2.4 | t-GlcpA | 5.0 | GlcA | trace | t-GlcpA | trace |
| Sugar/Hyp Residue | C-1/H-1 | C-2/H2 | C-3/H-3 | C-4/H-4 | C-5/H-5 | C-6/H-6 | |
|---|---|---|---|---|---|---|---|
| B | t-α-Araf | 108.0/5.09 | 81.9/4.21 | 77.5/4.00 | 85.0/4.13 | 62.0/3.82, 3.71 | |
| A | 5-α-Araf | 109.5/5.24 | 81.9/4.21 | 77.0/3.96 | 85.0/4.13 | 67.3/3.81, 3.88 | |
| A | 3-α-Araf | 109.5/5.24 | 81.8/4.11 | 83.0/4.25 | 85.0/4.09 | 62.0/3.82, 3.71 | |
| E | t-β-GlcpA | 103.8/4.51 | 73.7/3.34 | 76.2/3.74 | 71.0/3.54 | 76.0/3.52 | |
| F | 3,6-β-Galsc | 104.0/4.46 | 70.2/3.66 | 80.9/3.72 | 69.1/4.10 | 74.2/3.88 | 70.0/4.03, 3.92 |
| F | 3-β-Galsc | 104.0/4.46 | 70.2/3.66 | 80.9/3.72 | 69.1/4.10 | 75.8/3.71 | 61.4/3.76 |
| F | 3-β-Galsc | 104.0/4.46 | 70.2/3.66 | 80.9/3.70 | 69.1/4.10 | 75.8/3.71 | 61.4/3.76 |
| D | 3,6-β-Galbb | 102.0/4.56 | 70.2/3.70 | 82.8/3.82 | 69.2/4.21 | 73.9/3.79 | 70.0/4.03, 3.92 |
| C | 3,6-β-Galbb | 104.6/4.68 | 70.2/3.78 | 82.8/3.84 | 69.2/4.21 | 73.9/3.79 | 70.0/4.03, 3.92 |
| C | 6-β-Galbb | 104.3/4.63 | 72.4/3.62 | 73.1/3.67 | 69.0/3.96 | 73.9/3.79 | 70.0/4.03, 3.92 |
| C | 3,6-β-Galbb | 104.3/4.68 | 70.2/3.78 | 82.8/3.86 | 69.2/4.21 | 73.9/3.79 | 70.0/4.03, 3.92 |
| C | 3,6-β-Galbb | 104.3/4.70 | 70.2/3.79 | 82.8/3.88 | 69.2/4.21 | 73.9/3.79 | 70.0/4.03, 3.92 |
| C | t-β-Galbb | 104.3/4.68 | 72.4/3.72 | 73.1/3.66 | 69.0/3.88 | 75.8/3.70 | 61.4/3.76 |
| Hyp | set 1 | 60.2/4.32 | 35.8/2.63,2.20 | 78.3/4.78 | 52.3/3.68, 3.56 | ||
| set 2 | 60.0/4.22 | 35.2/2.55,2.45 | 77.9/4.69 | 52.3/3.78,3.48 | |||
| Sugar/Hyp Residue | C-1/H-1 | C-2/H2 | C-3/H-3 | C-4/H-4 | C-5/H-5 | C-6/H-6 | |
|---|---|---|---|---|---|---|---|
| B | t-α-Araf | 108.0/5.09 | 81.9/4.21 | 77.3/4.01 | 84.3/4.13 | 61.8/3.82, 3.71 | |
| A | 5-α-Araf | 109.5/5.25 | 81.9/4.21 | 77.0/3.95 | 84.3/4.13 | 67.9/3.79, 3.88 | |
| A | 3-α-Araf | 109.5/5.25 | 81.4/4.11 | 82.6/4.24 | 84.3/4.08 | 61.8/3.82, 3.71 | |
| F | 3-β-Galsc | 104.0/4.45 | 70.2/3.66 | 80.5/3.73 | 69.1/4.10 | 75.1/3.72 | 61.2/3.78 |
| F | 3-β-Galsc | 104.0/4.51 | 71.3/3.71 | 80.5/3.73 | 69.1/4.10 | 75.1/3.72 | 61.2/3.78 |
| D | 3,6-β-Galbb | 102.0/4.56 | 70.2/3.70 | 83.0/3.86 | 69.0/4.21 | 73.5/3.79 | 69.9/4.04, 3.93 |
| C | 3,6-β-Galbb | 104.6/4.63 | 70.2/3.68 | 83.0/3.88 | 69.0/4.21 | 73.5/3.79 | 69.9/4.04, 3.93 |
| C | 6-β-Galbb | 104.2/4.70 | 71.3/3.53 | 73.4/3.65 | 69.0/3.96 | 73.5/3.79 | 70.0/4.04, 3.93 |
| C | 3,6-β-Galbb | 104.2/4.70 | 70.2/3.66 | 83.0/3.88 | 69.0/4.21 | 73.5/3.79 | 69.9/4.04, 3.93 |
| C | 3,6-β-Galbb | 104.2/4.70 | 70.2/3.65 | 83.0/3.90 | 69.0/4.21 | 73.5/3.79 | 69.9/4.04, 3.93 |
| C | t-β-Galbb | 104.2/4.70 | 71.3/3.53 | 73.4/3.65 | 69.0/3.87 | 75.5/3.69 | 61.2/3.78 |
| Hyp | set 1 | 60.4/4.33 | 35.4/2.62, 2.20 | 78.6/4.78 | 52.5/3.67, 3.57 | ||
| set 2 | 60.1/4.22 | 35.0/2.54, 2.45 | 77.9/4.69 | 52.5/3.78,3.48 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.; Xu, J.; Held, M.; Lamport, D.T.A.; Kieliszewski, M. Arabinogalactan Structures of Repetitive Serine-Hydroxyproline Glycomodule Expressed by Arabidopsis Cell Suspension Cultures. Plants 2023, 12, 1036. https://doi.org/10.3390/plants12051036
Tan L, Xu J, Held M, Lamport DTA, Kieliszewski M. Arabinogalactan Structures of Repetitive Serine-Hydroxyproline Glycomodule Expressed by Arabidopsis Cell Suspension Cultures. Plants. 2023; 12(5):1036. https://doi.org/10.3390/plants12051036
Chicago/Turabian StyleTan, Li, Jianfeng Xu, Michael Held, Derek T. A. Lamport, and Marcia Kieliszewski. 2023. "Arabinogalactan Structures of Repetitive Serine-Hydroxyproline Glycomodule Expressed by Arabidopsis Cell Suspension Cultures" Plants 12, no. 5: 1036. https://doi.org/10.3390/plants12051036
APA StyleTan, L., Xu, J., Held, M., Lamport, D. T. A., & Kieliszewski, M. (2023). Arabinogalactan Structures of Repetitive Serine-Hydroxyproline Glycomodule Expressed by Arabidopsis Cell Suspension Cultures. Plants, 12(5), 1036. https://doi.org/10.3390/plants12051036








