Identification and Characterization of Fusarium Species Causing Watermelon Fruit Rot in Northern Thailand
Abstract
1. Introduction
2. Results
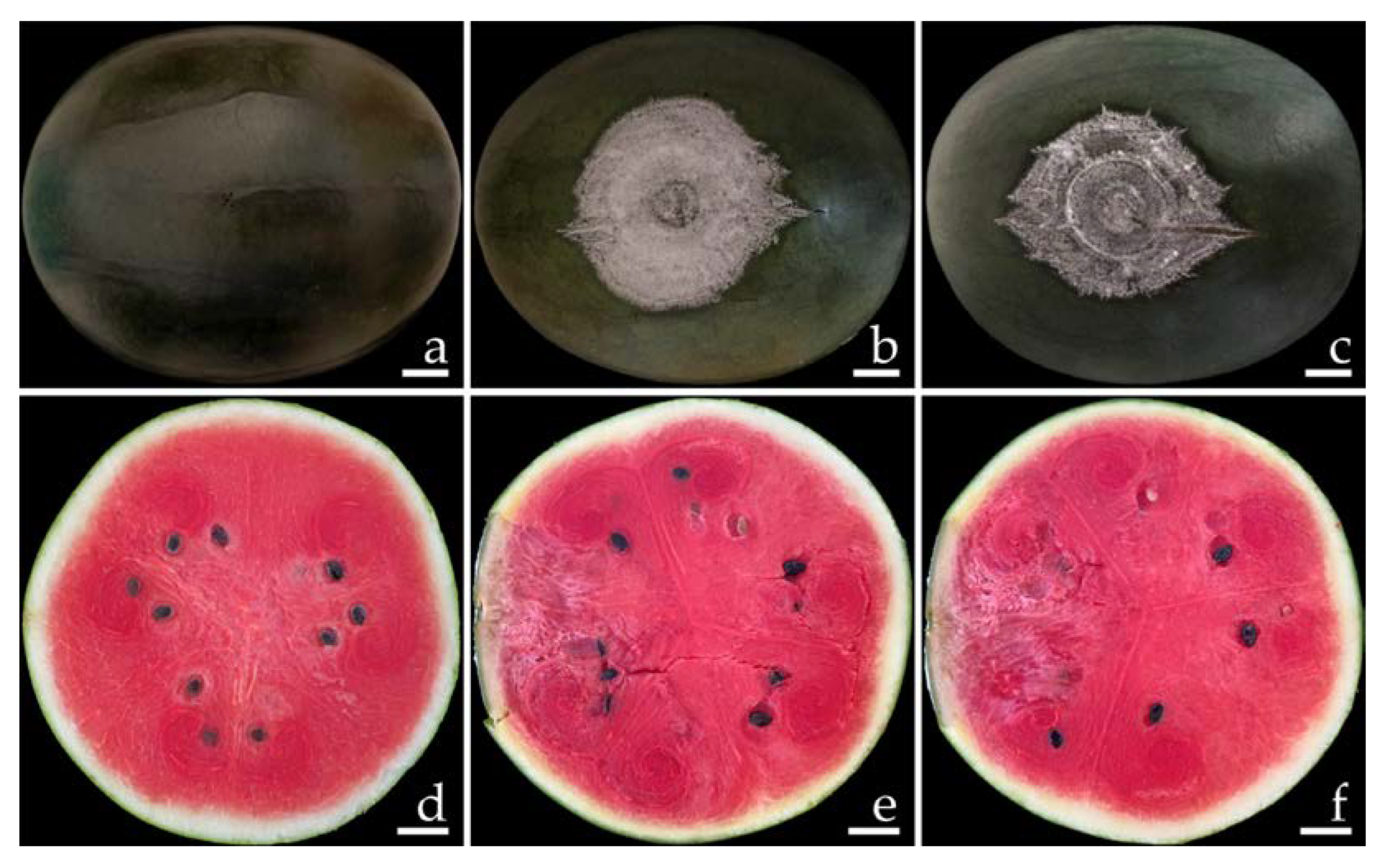
2.1. Disease Symptoms
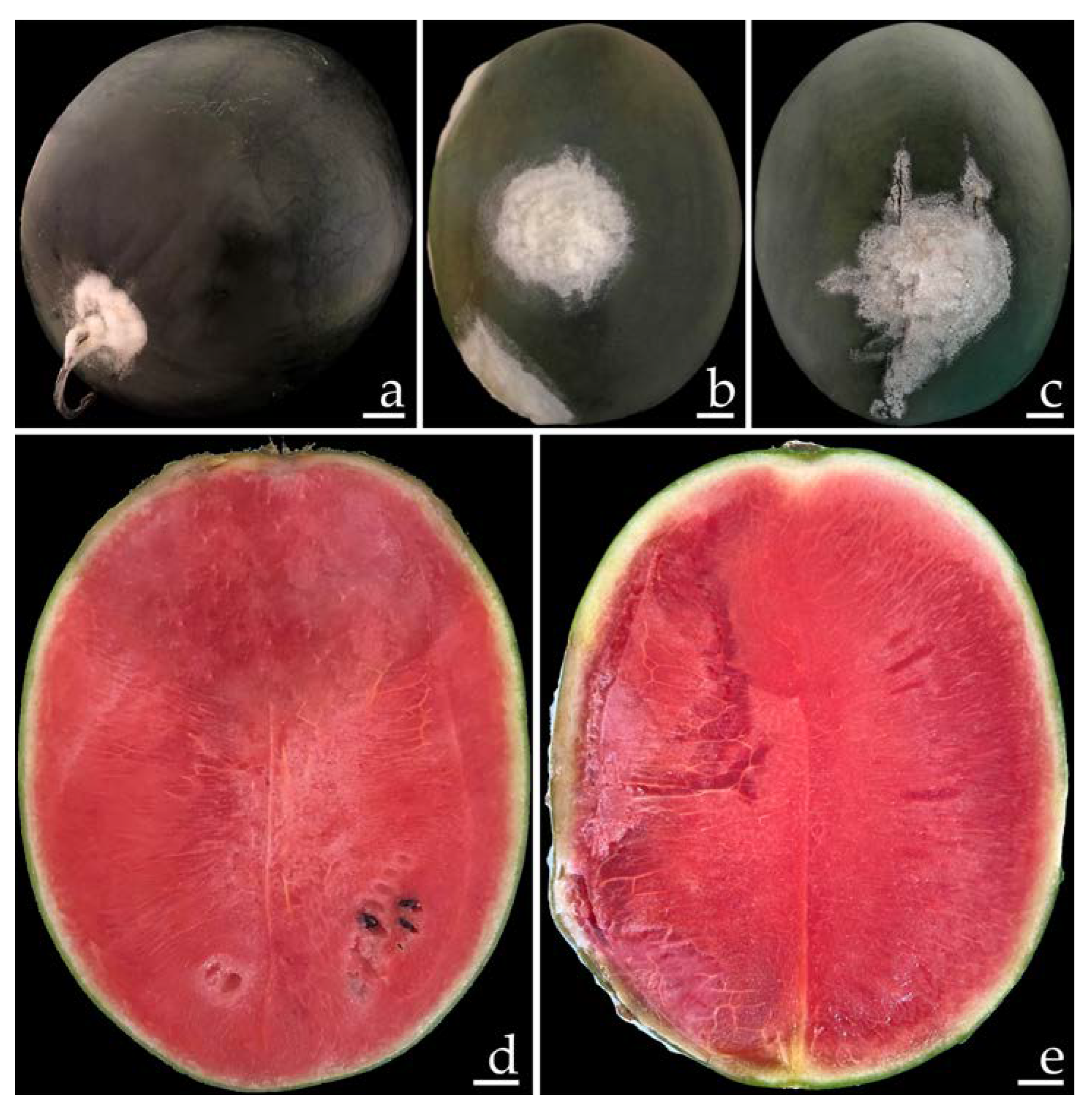
2.2. Fungal Isolation
2.3. Morphological Observations
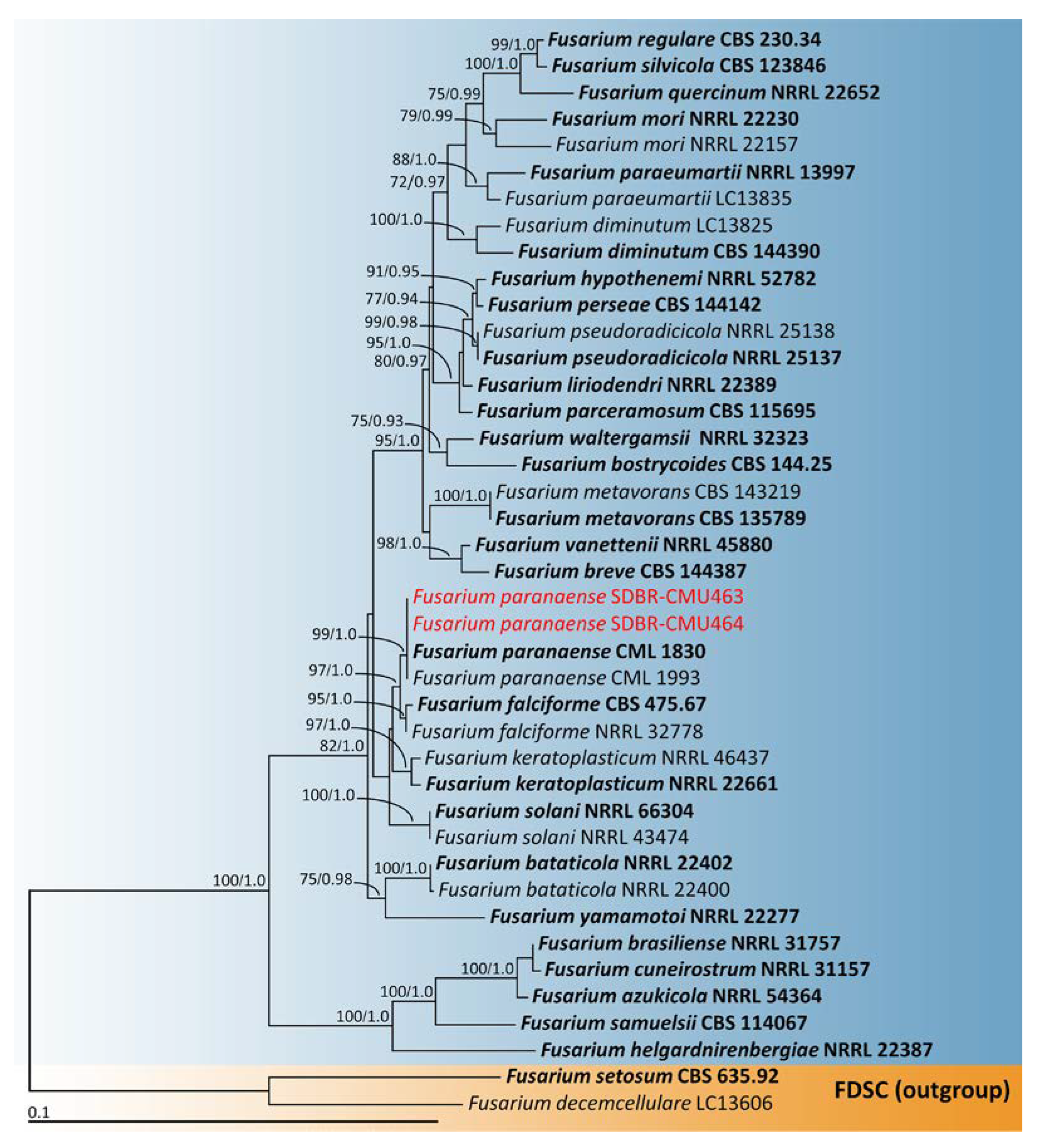
2.4. Phylogenetic Analysis
2.5. Morphological Descriptions
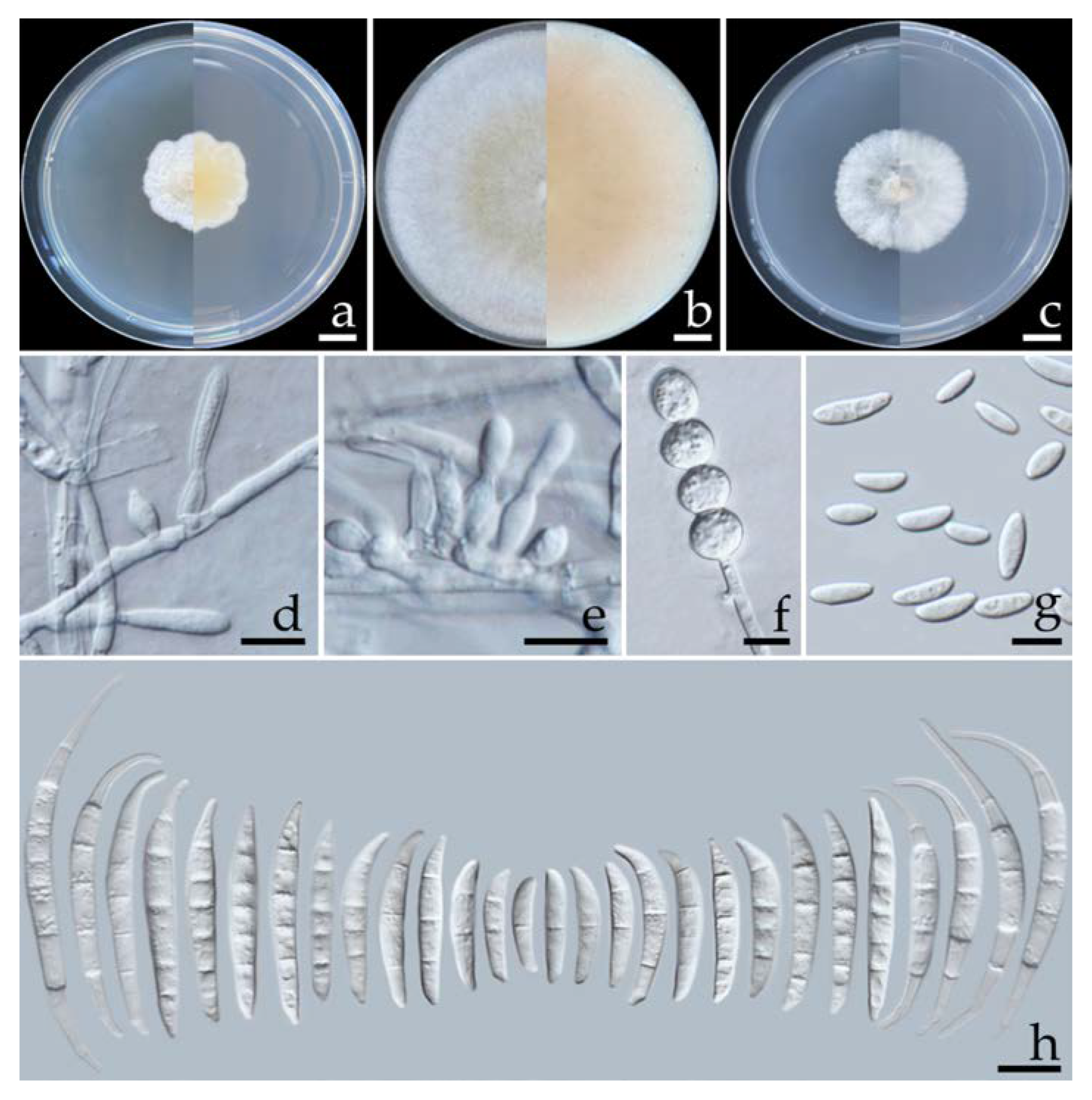
2.5.1. Fusarium compactum (Wollenw.) Raillo, Fungi of the Genus Fusarium: 180 (1950) (Figure 4)
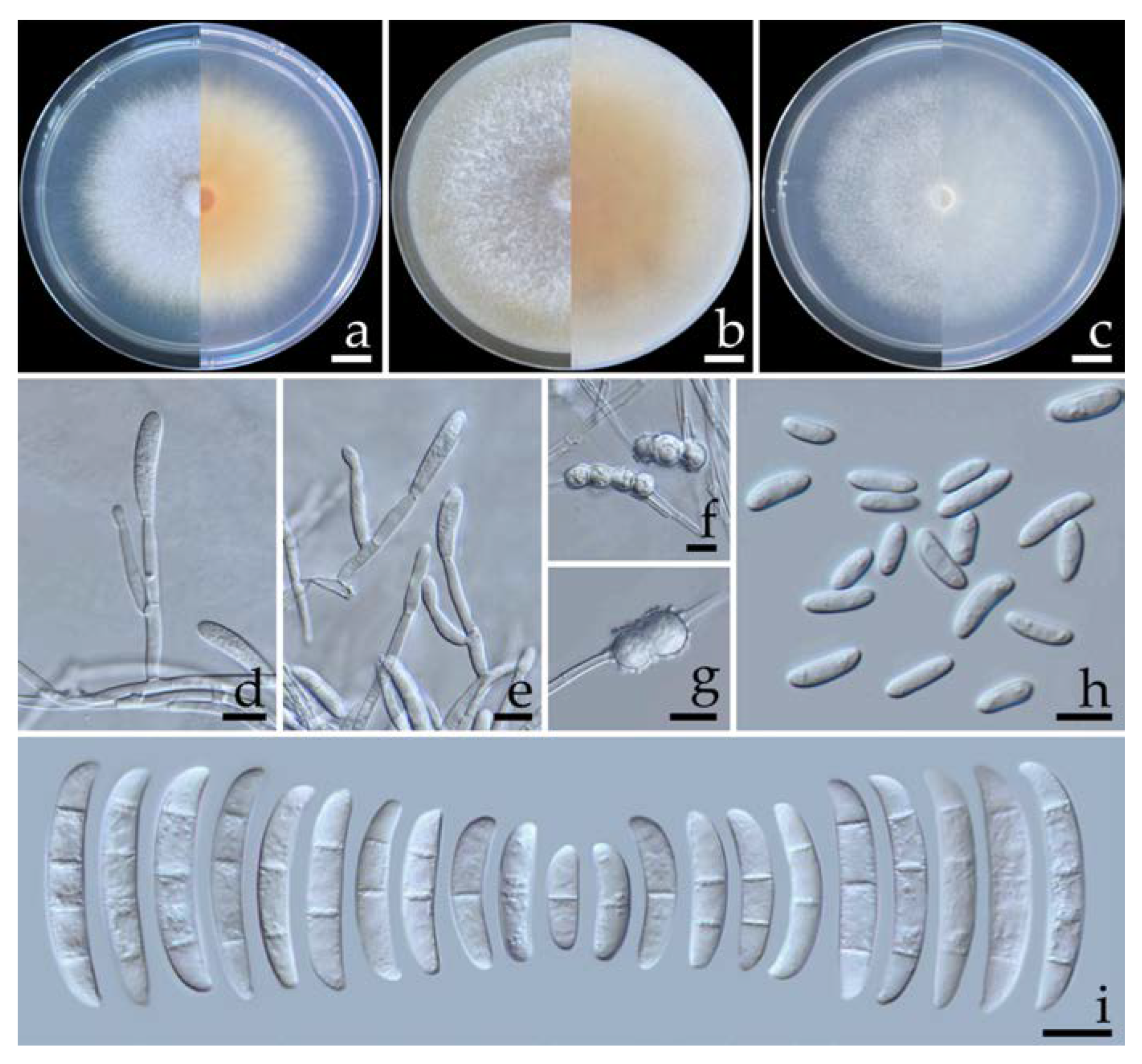
2.5.2. Fusarium paranaense Costa, Matos & Pfenning, Fungal Biology 120: 55 (2015) (Figure 5)
2.6. Pathogenicity Test
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Fungal Isolation
4.3. Pathogenicity Tests
4.4. Fungal Identification
4.4.1. Morphological Studies
4.4.2. DNA Extraction, Amplification, and Sequencing
4.4.3. Sequence Alignment and Phylogenetic Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olayinka, B.U.; Etejere, E.O. Proximate and chemical compositions of watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai cv red and cucumber (Cucumis sativus L. cv Pipino). Int. Food Res. J. 2018, 25, 1060–1066. [Google Scholar]
- Saediman, H.; Alwi, L.O.; Rianse, I.S.; Taridala, S.A.A.; Salahuddin, S.; Indarsyih, Y.; Astuti, R.W. Comparative profitability of melon and watermelon production in South Konawe District of Southeast Sulawesi. WSEAS Trans. Bus. Econ. 2020, 17, 933–939. [Google Scholar] [CrossRef]
- Masika, F.B.; Alicai, T.; Shimelis, H.; Ddamulira, G.; Athman, S.Y.; Ipulet, P.; Andama, A.; Tugume, A.K. Pumpkin and watermelon production constraints and management practices in Uganda. CABI Agric. Biosci. 2022, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Available online: http://faostat.fao.org (accessed on 23 August 2022).
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid content of 50 watermelon cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.D.; Hur, O.S.; Ro, N.Y.; Lee, J.E.; Hwang, A.J.; Kim, B.S.; Rhee, J.H.; Yi, J.Y.; Kim, J.H.; Lee, H.S.; et al. Fruit morphology, citrulline, and arginine levels in diverse watermelon (Citrullus lanatus) germplasm collections. Plants 2020, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Malik, A.U.; Azam, M.; Liu, W. High-throughput LC-ESI-MS/MS metabolomics approach reveals regulation of metabolites related to diverse functions in mature fruit of grafted watermelon. Biomolecules 2021, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, C.D.; Jain, A.; Tambe, M.S. Phytochemical and pharmacological profile of Citrullus lanatus (THUNB). Biolife 2015, 3, 483–488. [Google Scholar]
- Manivannan, A.; Lee, E.S.; Han, K.; Lee, H.E.; Kim, D.S. Versatile nutraceutical potentials of watermelon—A modest fruit loaded with pharmaceutically valuable phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef]
- Maoto, M.M.; Beswa, D.; Jideani, A.I.O. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- Nadeem, M.; Navida, M.; Ameer, K.; Iqbal, A.; Malik, F.; Nadeem, M.A.; Fatima, H.; Ahmed, A.; Din, A. A comprehensive review on the watermelon phytochemical profile and their bioactive and therapeutic effects. Korean J. Food Preserv. 2022, 29, 546–576. [Google Scholar] [CrossRef]
- Johnson, G.I.; Weinberger, K.; Wu, M.H. The Vegetable Industry in Tropical Asia: Thailand, an Overview of Production and Trade; AVRDC—The World Vegetable Center: Tainan, Taiwan, 2008; pp. 1–59. [Google Scholar]
- Khuna, S.; Kumla, J.; Thitla, T.; Nuangmek, W.; Lumyong, S.; Suwannarach, N. Morphology, molecular identification, and pathogenicity of two novel Fusarium species associated with postharvest fruit rot of cucurbits in Northern Thailand. J. Fungi 2022, 8, 1135. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Lumyong, S. Leaf spot on cattleya orchid caused by Neoscytalidium orchidacearum in Thailand. Can. J. Plant Pathol. 2018, 40, 109–114. [Google Scholar] [CrossRef]
- Reingold, V.; Lachman, O.; Sela, N.; Luria, N.; Dombrovsky, A. Watermelon fruit rot disease in Israel is caused by a distinct Squash vein yellowing virus (SqVYV) strain. Plant Dis. 2016, 100, 1176–1183. [Google Scholar] [CrossRef]
- Oyedeji, E.O.; Arogundade, O.; Tairu, F.M.; Elum, C.G. Identification and characterization of fungi pathogen causing fruit rot disease of watermelon (Citrullus lanatus). Arch. Phytopathol. Plant Prot. 2022, 55, 344–354. [Google Scholar] [CrossRef]
- Kousik, C.S.; Ikerd, J.L.; Turechek, W.W. Development of phytophthora fruit rot caused by Phytophthora capsici on resistant and susceptible watermelon fruit of different ages. Plant Dis. 2017, 102, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, J.; Goh, K.S.; Sani, S.F.; Alam, M.W.; Ismail, N.A.; Gleason, M.L.; Rosli, H. Fusarium falciforme and F. oxysporum causing postharvest fruit rot of watermelon (Citrullus lanatus) in Malaysia: A first report. Crop Prot. 2023, 163, 106115. [Google Scholar] [CrossRef]
- Kousik, C.S.; Ikerd, J.L.; Harrison, H.F. Development of pre- and postharvest Phytophthora fruit rot on watermelons treated with fungicides in the field. Plant Health Prog. 2014, 15, 145–151. [Google Scholar] [CrossRef]
- Wei, S.Q.; Zhong, Y.; Ma, Z.T.; Jiang, H. A survey on watermelon diseases in the northern China. China Fruits 1991, 1, 36–37. [Google Scholar]
- Kwon, J.H.; Park, C.S. Occurrence of fruit rot of watermelon (Citrullus lanatus) caused by Sclerotium rolfsii. Res. Plant Dis. 2009, 15, 51–53. [Google Scholar] [CrossRef]
- Kousik, C.S.; Ikerd, J.L.; Wechter, P.; Harrison, H.; Levi, A. Resistance to phytophthora fruit rot of watermelon caused by Phytophthora capsici in U.S. Plant Introductions. HortScience 2012, 47, 1682–1689. [Google Scholar] [CrossRef]
- Ojo, O.A.; Oladipo, S.O.; Odelade, K.A. In vitro assessment of fungicidal activity of Ageratum conyzoides and Cybopogon flexuosus weed extracts against some phytopathogenic fungi associated with fruit rot of watermelon (Citrullus lunatus (Thunb.)). Arch. Phytopathol. Plant Prot. 2014, 47, 2421–2428. [Google Scholar] [CrossRef]
- Abiala, M.A.; Ayandeko, F.M.; Odebode, A.C. Antifungal effects of selected botanicals on fungal pathogens of watermelon fruit. Arch. Phytopathol. Plant Prot. 2015, 48, 569–577. [Google Scholar] [CrossRef]
- Jidda, M.B.; Adamu, M.I. Rot inducing fungi of watermelon (Citrullus lanatus) fruits in storage and fruit stalls in Maiduguri, Nigeria. Int. J. Adv. Acad. Res. 2017, 3, 11–18. [Google Scholar]
- Rahman, M.Z.; Ahmad, K.; Siddiqui, Y.; Saad, N.; Hun, T.G.; Hata, E.M.; Rashed, O.; Hossain, M.I. First report of Fusarium equiseti, causing fruit rot disease of watermelon in Malaysia. Plant Dis. 2022, 106, 326. [Google Scholar] [CrossRef] [PubMed]
- Kousik, C.S.; Ji, P.; Egel, D.S.; Quesada-Ocampo, L.M. Fungicide rotation programs for managing Phytophthora fruit rot of watermelon in Southeastern United States. Plant Health Prog. 2017, 18, 28–34. [Google Scholar] [CrossRef]
- Keinath, A.P. Minimizing yield and quality losses in watermelon with multi-site and strobilurin fungicides effective against foliar and fruit anthracnose. Crop Prot. 2018, 106, 72–78. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Kousik, C.S.; Adams, M.L.; Jester, W.R.; Hassell, R.; Harrison, H.F.; Holmes, D.J. Effect of cultural practices and fungicides on Phytophthora fruit rot of watermelon in the Carolinas. Crop Prot. 2011, 30, 888–894. [Google Scholar] [CrossRef]
- Yang, C. Fungicide: Modes of action and possible impact on nontarget microorganisms. ISRN Ecol. 2011, 8, 130289. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Wilkinson, K.; Grant, W.P.; Green, L.E.; Hunter, S.; Jeger, M.J.; Lowe, P.; Medley, G.F.; Mills, P.; Phillipson, J.; Poppy, G.M.; et al. Infectious diseases of animals and plants: An interdisciplinary approach. Phil. Trans. R. Soc. B 2011, 366, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Nuangmek, W.; Aiduang, W.; Suwannarach, N.; Kumla, J.; Kiatsiriroat, T.; Lumyong, S. First report of fruit rot on cantaloupe caused by Fusarium equiseti in Thailand. J. Gen. Plant Pathol. 2019, 85, 295–300. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names–restyling the Fusarium incarnatum-equiseti species complex. Persoonia 2019, 43, 186–221. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Gueidan, C.; Crous, P.W.; Geiser, D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum—F. equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009, 47, 3851–3861. [Google Scholar] [CrossRef]
- Santos, A.C.S.; Trindade, J.V.C.; Lima, C.S.; Barbosa, R.N.; Costa, A.F.; Tiago, P.V.; Oliveira, N.T. Morphology, phylogeny, and sexual stage of Fusarium caatingaense and Fusarium pernambucanum, new species of the Fusarium incarnatum-equiseti species complex associated with insects in Brazil. Mycologia 2019, 111, 244–259. [Google Scholar] [CrossRef]
- Lombard, L.; van Doorn, R.; Crous, P.W. Neotypification of Fusarium chlamydosporum—A reappraisal of a clinically important species complex. Fungal Syst. Evol. 2019, 4, 183–200. [Google Scholar] [CrossRef]
- Maryani, N.; Sandoval-Denis, M.; Lombard, L.; Crous, P.W.; Kema, G.H.J. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia 2019, 43, 48–69. [Google Scholar] [CrossRef]
- Aoki, T.; Scandiani, M.M.; O’Donnell, K. Phenotypic, molecular phylogenetic, and pathogenetic characterization of Fusarium crassistipitatum sp. nov., a novel soybean sudden death syndrome pathogen from Argentina and Brazil. Mycoscience 2012, 53, 167–186. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Lombard, L.; Crous, P.W. Back to the roots: A reappraisal of Neocosmospora. Persoonia 2019, 43, 90–185. [Google Scholar] [CrossRef]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef]
- O’Donnell, K.; Humber, R.A.; Geiser, D.M.; Kang, S.; Park, B.; Robert, V.A.; Crous, P.W.; Johnston, P.R.; Aoki, T.; Rooney, A.P.; et al. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia 2012, 104, 427–445. [Google Scholar] [CrossRef]
- Short, D.P.G.; O’Donnell, K.; Zhang, N.; Juba, J.H.; Geiser, D.M. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 2011, 49, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Migheli, Q.; Balmas, V.; Harak, H.; Sanna, S.; Scherm, B.; Aoki, T.; O’Donnell, K. Molecular phylogenetic diversity of dermatologic and other human pathogenic fusarial isolates from hospitals in northern and central Italy. J. Clin. Microbiol. 2010, 48, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.S.; Matos, K.S.; Tessmann, D.J.; Seixas, C.D.; Pfenning, L.H. Fusarium paranaense sp. nov., a member of the Fusarium solani species complex causes root rot on soybean in Brazil. Fungal Biol. 2016, 120, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Debourgogne, A.; Gueidan, C.; de Hoog, S.; Lozniewski, A.; Machouart, M. Comparison of two DNA sequence-based typing schemes for the Fusarium solani species complex and proposal of a new consensus method. J. Microbiol. Methods 2012, 91, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schroers, H.J.; Samuels, G.J.; Zhang, N.; Short, D.P.; Juba, J.H.; Geiser, D.M. Epitypification of Fusisporium (Fusarium) solani and its assignment to a common phylogenetic species in the Fusarium solani species complex. Mycologia 2016, 108, 806–819. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sarver, B.A.; Brandt, M.; Chang, D.C.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Rooney, A.P.; Proctor, R.H.; Brown, D.W.; McCormick, S.P.; Ward, T.J.; Frandsen, R.J.; Lysoe, E.; Rehner, S.A.; Aoki, T.; et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [CrossRef]
- Carreras-Villaseñor, N.; Rodríguez-Haas, J.B.; Martínez-Rodríguez, L.A.; Pérez-Lira, A.J.; Ibarra-Laclette, E.; Villafán, E.; Castillo-Díaz, A.P.; Ibarra-Juárez, L.A.; Carrillo-Hernández, E.D.; Sánchez-Rangel, D. Characterization of two Fusarium solani species complex isolates from the ambrosia beetle Xylosandrus morigerus. J. Fungi 2022, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- James, J.E.; Santhanam, J.; Zakaria, L.; Mamat Rusli, N.; Abu Bakar, M.; Suetrong, S.; Sakayaroj, J.; Abdul Razak, M.F.; Lamping, E.; Cannon, R.D. Morphology, phenotype, and molecular identification of clinical and environmental Fusarium solani species complex isolates from Malaysia. J. Fungi 2022, 8, 845. [Google Scholar] [CrossRef]
- Raillo, A.I. Griby Roda Fusarium; Gosudarstv. Izd. Sel’skochoz. Lit.: Moscow, Russia, 1950. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing Professional: Ames, IA, USA, 2006; pp. 8–240. [Google Scholar]
- Subrahmanyam, A. Fusarium laceratum. Mykosen 1983, 26, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Tuttle McGrath, M. Diseases of Cucurbits and their Management. In Diseases of Fruits and Vegetables Volume I; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 455–510. [Google Scholar]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for controlling the sporulation in Fusarium spp. J. Fungi 2023, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Rahjoo, V.; Zad, J.; Javan-Nikkhah, M.; Mirzadi Gohari, A.; Okhovvat, S.M.; Bihamta, M.R.; Razzaghian, J.; Klemsdal, S.S. Morphological and molecular identification of Fusarium isolated from maize ears in Iran. J. Plant Pathol. 2008, 90, 463–468. [Google Scholar]
- Geiser, D.M.; Jiménez-Gasco, M.M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Nitschke, E.; Nihlgard, M.; Varrelmann, M. Differentiation of eleven Fusarium spp. isolated from sugar beet, using restriction fragment analysis of a polymerase chain reaction-amplified translation elongation factor 1 gene fragment. Phytopathology 2009, 99, 921–929. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef]
- Jedidi, I.; Jurado, M.; Cruz, A.; Trabelsi, M.M.; Said, S.; González-Jaén, M.T. Phylogenetic analysis and growth profiles of Fusarium incarnatum-equiseti species complex strains isolated from Tunisian cereals. Int. J. Food Microbiol. 2021, 353, 109297. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Borman, A.M.; Brandt, M.E.; Cano, J.; Cuenca-Estrella, M.; Dannaoui, E.; Guarro, J.; Haase, G.; Kibbler, C.C.; Meyer, W.; et al. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: Where are we and where should we go from here? J. Clin. Microbiol. 2009, 47, 877–884. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Li, X.; Ji, S.; Chethana, K.W.T.; Hyde, K.D.; Yan, J. Fusarium species associated with cherry leaf spot in China. Plants 2022, 11, 2760. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yang, X.; Shen, G.; Wang, S.; Teng, H.; Yang, C.; Liu, X.; Wang, X.; Zhao, J.; et al. Fusarium species associated with maize leaf blight in Heilongjiang Province, China. J. Fungi 2022, 8, 1170. [Google Scholar] [CrossRef] [PubMed]
- Frisullo, S.; Logrieco, A.; Moretti, A.; Grammatikaki, G.; Bottalico, A. Banana corm and root rot by Fusarium compactum, in Crete. Phytopathol. Mediterr. 1994, 33, 78–82. [Google Scholar]
- Madar, Z.; Kimchi, M.; Solel, Z. Fusarium canker of Italian cypress. Eur. J. For. Path. 1996, 26, 107–112. [Google Scholar] [CrossRef]
- Pisani, C.; Adkins, S.T.; Turechek, W.; Patel, P.C.; Rosskopf, E.N. First report of Macrophomina phaseolina, Fusarium brachygibbosum, and Lasiodiplodia theobromae causing fungal watermelon vine decline in southwest and west-central Florida. Plant Health Prog. 2021, 22, 544–551. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Ahmad, K.; Siddiqui, Y.; Saad, N.; Hun, T.G.; Hata, E.M.; Rashed, O.; Hossain, M.I.; Kutawa, A.B. First report of fusarium wilt disease on watermelon caused by Fusarium oxysporum f. sp. niveum in Malaysia. Plant Dis. 2021, 105, 4169. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.; Xu, J.; Liu, G.; Yao, X.; Ren, R. Characterization of Fusarium root rot disease in grafted watermelon. Eur. J. Plant Pathol. 2021, 159, 1–11. [Google Scholar] [CrossRef]
- Han, Y.K.; Dumin, W.; Park, M.J.; Bae, Y.S.; Park, J.H.; Back, C.G. First report of fusarium wilt disease caused by Fusarium equiseti on grafted watermelon in Korea. Plant Dis. 2022, 106, 2989. [Google Scholar] [CrossRef] [PubMed]
- Ikediugwu, F.E.O.; Ogieva, W.O. Fruit rot of Citrullus lanatus in Nigeria caused by Fusarium solani. Trans. Br. Mycol. Soc. 1978, 71, 209–213. [Google Scholar] [CrossRef]
- Li, Y.G.; Song, X.L.; Wang, X.Q.; Zhang, H.; Tian, S.; Ji, P. First report of fruit rot of watermelon caused by Fusarium equiseti in China. Plant Dis. 2018, 102, 1852. [Google Scholar] [CrossRef]
- Li, Y.; Ji, P. First report of fruit rot of watermelon caused by Fusarium equiseti in Georgia in the United States. Plant Dis. 2015, 99, 1272. [Google Scholar] [CrossRef]
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopathol. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Saepaisan, S.; Chawengphaophan, S.; Falab, S. Identification of the fungal fruit rot causal agents of Khiew Sawoey mango of production area in Loei province for export. Khon Kaen Agr. J. 2020, 48, 1360–1373. (In Thai) [Google Scholar]
- Thaenthanee, S.; Sukprasert, J.; Daosukho, S.; Rodprasert, S. The study on efficiency of Acorus calamus L. extract against fruit rot fungi isolated from lychee. Bulletin Appl. Sci. 2014, 3, 88–101. (In Thai) [Google Scholar]
- Piasai, R.; Chalmers, P.; Piasai, O.; Khewkhom, N. Postharvest fungicide dips to control fruit rot of ‘Monthong’ durian (Durio zibethinus). Eur. J. Plant Pathol. 2021, 160, 325–336. [Google Scholar] [CrossRef]
- Choi, Y.W.; Hyde, K.D.; Ho, W.H. Single spore isolation of fungi. Fungal Divers. 1999, 3, 29–38. [Google Scholar]
- De Oliveira, M.J.; Laranjeira, D.; Câmara, M.P.S.; Laranjeira, F.F.; Armengol, J.; Michereff, S.J. Effects of wounding, humidity, temperature, and inoculum concentrations on the severity of corky dry rot caused by Fusarium semitectum in melon fruits. Acta Sci. Agron. 2014, 36, 281–289. [Google Scholar] [CrossRef]
- Safari, Z.S.; Ding, P.; Nakasha, J.J.; Yuso, S.F. Combining chitosan and vanillin to retain postharvest quality of tomato fruit during ambient temperature storage. Coatings 2020, 10, 1222. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Hall, T. Bioedit Version 6.0.7. 2004. Available online: http://www.mbio.ncsu.edu/bioedit/bioedit.html (accessed on 20 August 2022).
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the cipres science gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; IEEE: Manhattan, NY, USA; pp. 1–8. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Tree Figure Drawing Tool Version 13; Institute of Evolutionary 623 Biology, University of Edinburgh: Edinburgh, Scotland, 2019; Available online: http://treebioedacuk/software/figtree/ (accessed on 10 August 2022).


| Fungal Taxa | Strain/Isolate | GenBank Accession Number | Reference | ||
|---|---|---|---|---|---|
| tef-1 | cam | rpb2 | |||
| Fusarium aberrans | CBS 131385 T | MN170445 | MN170311 | MN170378 | [37] |
| Fusarium arcuatisporum | LC12147 T | MK289584 | MK289697 | MK289739 | [35] |
| Fusarium arcuatisporum | LC11639 | MK289586 | MK289658 | MK289736 | [35] |
| Fusarium brevicaudatum | NRRL 43638 T | GQ505665 | GQ505576 | GQ505843 | [39] |
| Fusarium bubalinum | CBS 161.25 T | MN170448 | MN170314 | MN170381 | [37] |
| Fusarium caatingaense | URM 6779 T | LS398466 | − | LS398495 | [40] |
| Fusarium cateniforme | CBS 150.25 T | MN170451 | MN170317 | MN170384 | [37] |
| Fusarium citrullicola | SDBR-CMU422 T | OP020920 | OP020924 | OP020928 | [13] |
| Fusarium clavum | CBS 126202 T | MN170456 | MN170322 | MN170389 | [37] |
| Fusarium coffeatum | CBS 635.76 T | MN120755 | MN120696 | MN120736 | [41] |
| Fusarium coffeatum | CBS 430.81 | MN120756 | MN120697 | MN120737 | [41] |
| Fusarium compactum | CBS 186.31 ET | GQ505648 | GQ505560 | GQ505826 | [39] |
| Fusarium compactum | CBS 185.31 | GQ505646 | GQ505558 | GQ505824 | [39] |
| Fusarium compactum | SDBR-CMU461 | OQ108468 | OQ108472 | OQ108474 | This study |
| Fusarium compactum | SDBR-CMU462 | OQ108469 | OQ108473 | OQ108475 | This study |
| Fusarium duofalcatisporum | CBS 384.94 T | GQ505652 | GQ505564 | GQ505830 | [39] |
| Fusarium duofalcatisporum | CBS 264.50 | GQ505651 | GQ505563 | GQ505829 | [39] |
| Fusarium equiseti | CBS 307.94 NT | GQ505599 | GQ505511 | GQ505777 | [39] |
| Fusarium flagelliforme | CBS 162.57 T | GQ505645 | GQ505557 | GQ505823 | [39] |
| Fusarium flagelliforme | CBS 259.54 | GQ505650 | GQ505562 | GQ505828 | [39] |
| Fusarium guilinense | LC12160 T | MK289594 | MK289652 | MK289747 | [35] |
| Fusarium hainanense | LC11638 T | MK289581 | MK289657 | MK289735 | [35] |
| Fusarium incarnatum | CBS 132.73 NT | MN170476 | MN170342 | MN170409 | [37] |
| Fusarium incarnatum | NRRL 32866 | GQ505615 | GQ505527 | GQ505793 | [39] |
| Fusarium ipomoeae | LC12165 T | MK289599 | MK289704 | MK289752 | [35] |
| Fusarium ipomoeae | LC12166 | MK289600 | MK289706 | MK289753 | [35] |
| Fusarium irregulare | LC7188 T | MK289629 | MK289680 | MK289783 | [35] |
| Fusarium lacertarum | NRRL 20423 T | GQ505593 | GQ505505 | GQ505771 | [39] |
| Fusarium lacertarum | LC7942 | MK289643 | MK289696 | MK289797 | [35] |
| Fusarium longicaudatum | CBS 123.73 T | MN170481 | MN170347 | MN170414 | [37] |
| Fusarium luffae | LC12167 T | MK289601 | MK289698 | MK289754 | [35] |
| Fusarium melonis | SDBR-CMU424 T | OP020922 | OP020926 | OP020930 | [13] |
| Fusarium multiceps | CBS 130386 T | GQ505666 | GQ505577 | GQ505844 | [39] |
| Fusarium nanum | LC12168 T | MK289602 | MK289651 | MK289755 | [35] |
| Fusarium neoscirpi | CBS 610.95 T | GQ505601 | GQ505513 | GQ505779 | [39] |
| Fusarium pernambucanum | URM 7559 T | LS398489 | − | LS398519 | [40] |
| Fusarium persicinum | CBS 479.83 T | MN170495 | MN170361 | MN170428 | [37] |
| Fusarium scirpi | CBS 447.84 NT | GQ505654 | GQ505566 | GQ505832 | [39] |
| Fusarium scirpi | CBS 448.84 | GQ505592 | GQ505504 | GQ505770 | [39] |
| Fusarium serpentinum | CBS 119880 T | MN170499 | MN170365 | MN170432 | [37] |
| Fusarium sulawesiense | InaCC F940 T | LS479443 | LS479422 | LS479855 | [42] |
| Fusarium tanahbumbuense | InaCC F965 T | LS479448 | LS479432 | LS479863 | [42] |
| Fusarium toxicum | CBS 406.86 T | MN170508 | MN170374 | MN170441 | [37] |
| Fusarium camptoceras | CBS 193.65 ET | MN170450 | MN170316 | MN170383 | [37] |
| Fusarium neosemitectum | CBS 189.60 T | MN170489 | MN170355 | MN170422 | [37] |
| Fungal Taxa | Strain/Isolate | GenBank Accession Number | Reference | |
|---|---|---|---|---|
| tef-1 | rpb2 | |||
| Fusarium azukicola | NRRL 54364 T | JQ670137 | KJ511287 | [43] |
| Fusarium bataticola | NRRL 22402 T | AF178344 | FJ240381 | [44] |
| Fusarium bataticola | NRRL 22400 | AF178343 | EU329509 | [44] |
| Fusarium bostrycoides | CBS 144.25 NT | LR583597 | LR583818 | [45] |
| Fusarium brasiliense | NRRL 31757 T | EF408409 | EU329565 | [44] |
| Fusarium breve | CBS 144387 T | LR583601 | LR583822 | [45] |
| Fusarium cuneirostrum | NRRL 31157 T | EF408414 | FJ240389 | [44] |
| Fusarium diminutum | CBS 144390 T | LR583607 | LR583828 | [45] |
| Fusarium diminutum | LC13825 | MW620164 | MW474689 | [36] |
| Fusarium falciforme | CBS 475.67 T | LT906669 | LT960558 | [45] |
| Fusarium falciforme | NRRL 32778 | DQ247088 | EU329616 | [44,46] |
| Fusarium helgardnirenbergiae | NRRL 22387 T | AF178339 | EU329505 | [44] |
| Fusarium hypothenemi | NRRL 52782 T | JF740850 | JF741176 | [47] |
| Fusarium keratoplasticum | NRRL 22661 T | JN235712 | JN235897 | [48] |
| Fusarium keratoplasticum | NRRL 46437 | GU170623 | GU170588 | [49] |
| Fusarium liriodendri | NRRL 22389 T | AF178340 | EU329506 | [44] |
| Fusarium metavorans | CBS 135789 T | LR583627 | LR583849 | [45] |
| Fusarium metavorans | CBS 143219 | LR583629 | LR583851 | [45] |
| Fusarium mori | NRRL 22230 T | AF178358 | EU329499 | [44] |
| Fusarium mori | NRRL 22157 | AF178359 | EU329493 | [44] |
| Fusarium paraeumartii | NRRL 13997 T | DQ247549 | LR583855 | [45,46] |
| Fusarium paraeumartii | LC13835 | MW620180 | MW474705 | [36] |
| Fusarium paranaense | CML 1830 T | KF597797 | KF680011 | [50] |
| Fusarium paranaense | CML 1993 | KF597800 | KF680004 | [50] |
| Fusarium paranaense | SDBR-CMU463 | OQ108470 | OQ108476 | This study |
| Fusarium paranaense | SDBR-CMU464 | OQ108471 | OQ108477 | This study |
| Fusarium parceramosum | CBS 115695 T | JX435149 | JX435249 | [51] |
| Fusarium perseae | CBS 144142 T | LT991902 | LT991909 | [45] |
| Fusarium pseudoradicicola | NRRL 25137 T | JF740757 | JF741084 | [47] |
| Fusarium pseudoradicicola | NRRL 25138 | JF740758 | JF741085 | [47] |
| Fusarium quercinum | NRRL 22652 T | DQ247634 | LR583869 | [38,46] |
| Fusarium regulare | CBS 230.34 T | LR583643 | MW834029 | [38,45] |
| Fusarium samuelsii | CBS 114067 T | LR583644 | LR583874 | [45] |
| Fusarium silvicola | CBS 123846 T | LR583646 | LR583876 | [45] |
| Fusarium solani | NRRL 66304 ET | KT313611 | KT313623 | [52] |
| Fusarium solani | NRRL 43474 | EF452945 | EF469984 | [53] |
| Fusarium vanettenii | NRRL 45880 ET | FJ240352 | EU329640 | [44] |
| Fusarium waltergamsii | NRRL 32323 T | DQ246951 | EU329576 | [44,46] |
| Fusarium yamamotoi | NRRL 22277 ET | AF178336 | FJ240380 | [44] |
| Fusarium decemcellulare | LC13606 | MW580428 | MW474374 | [36] |
| Fusarium setosum | CBS 635.92 ET | MW834294 | JX171651 | [38,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identification and Characterization of Fusarium Species Causing Watermelon Fruit Rot in Northern Thailand. Plants 2023, 12, 956. https://doi.org/10.3390/plants12040956
Nuangmek W, Kumla J, Khuna S, Lumyong S, Suwannarach N. Identification and Characterization of Fusarium Species Causing Watermelon Fruit Rot in Northern Thailand. Plants. 2023; 12(4):956. https://doi.org/10.3390/plants12040956
Chicago/Turabian StyleNuangmek, Wipornpan, Jaturong Kumla, Surapong Khuna, Saisamorn Lumyong, and Nakarin Suwannarach. 2023. "Identification and Characterization of Fusarium Species Causing Watermelon Fruit Rot in Northern Thailand" Plants 12, no. 4: 956. https://doi.org/10.3390/plants12040956
APA StyleNuangmek, W., Kumla, J., Khuna, S., Lumyong, S., & Suwannarach, N. (2023). Identification and Characterization of Fusarium Species Causing Watermelon Fruit Rot in Northern Thailand. Plants, 12(4), 956. https://doi.org/10.3390/plants12040956








