Transcriptome Analysis Reveals the Genes Related to Water-Melon Fruit Expansion under Low-Light Stress
Abstract
1. Introduction
2. Results and Discussion
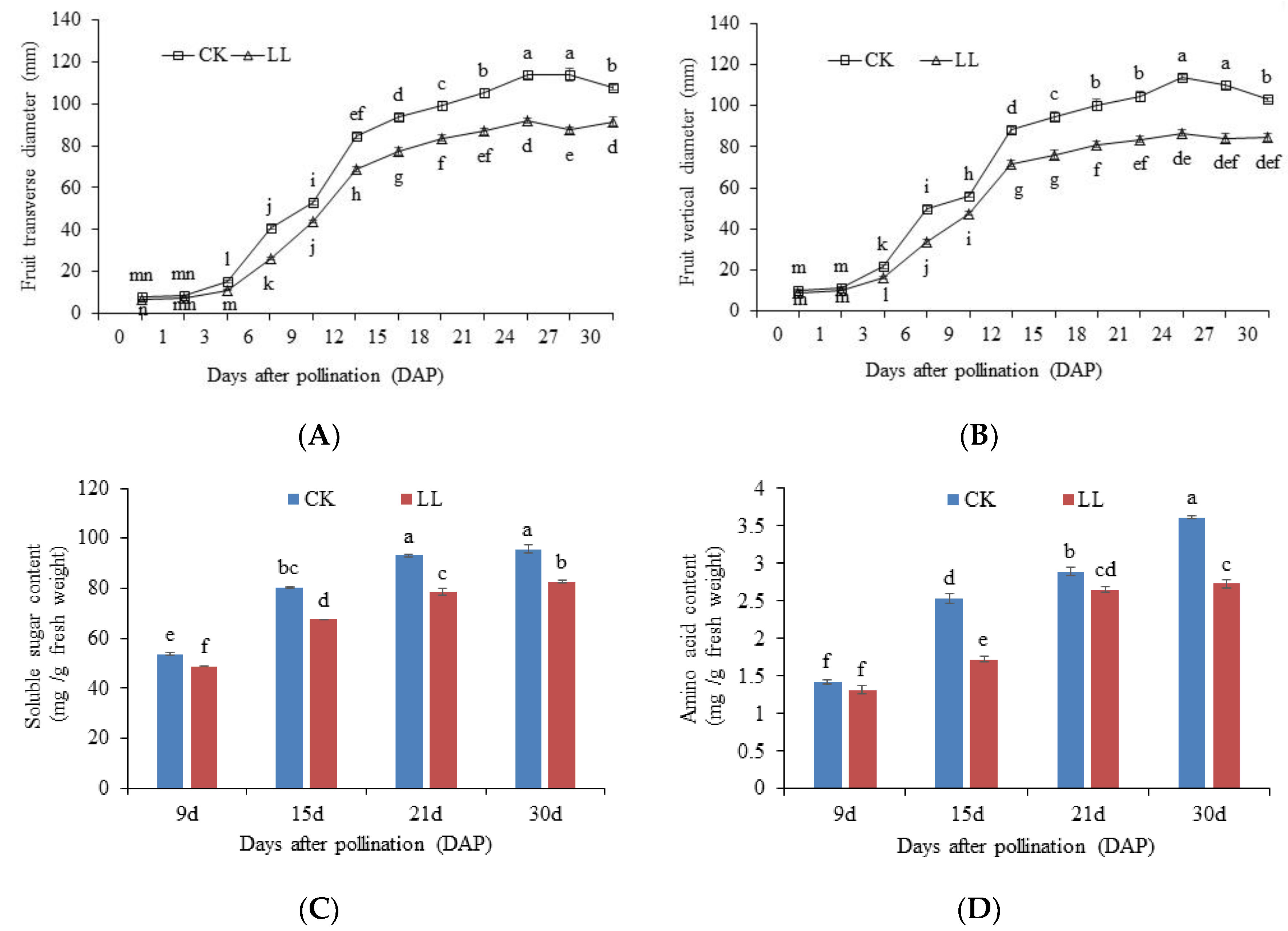
2.1. Response of Watermelon Fruits Morphological and Physiological Dynamic Changes to Low-Light Stress
2.2. Overview of Sequencing and Transcriptome Assembly
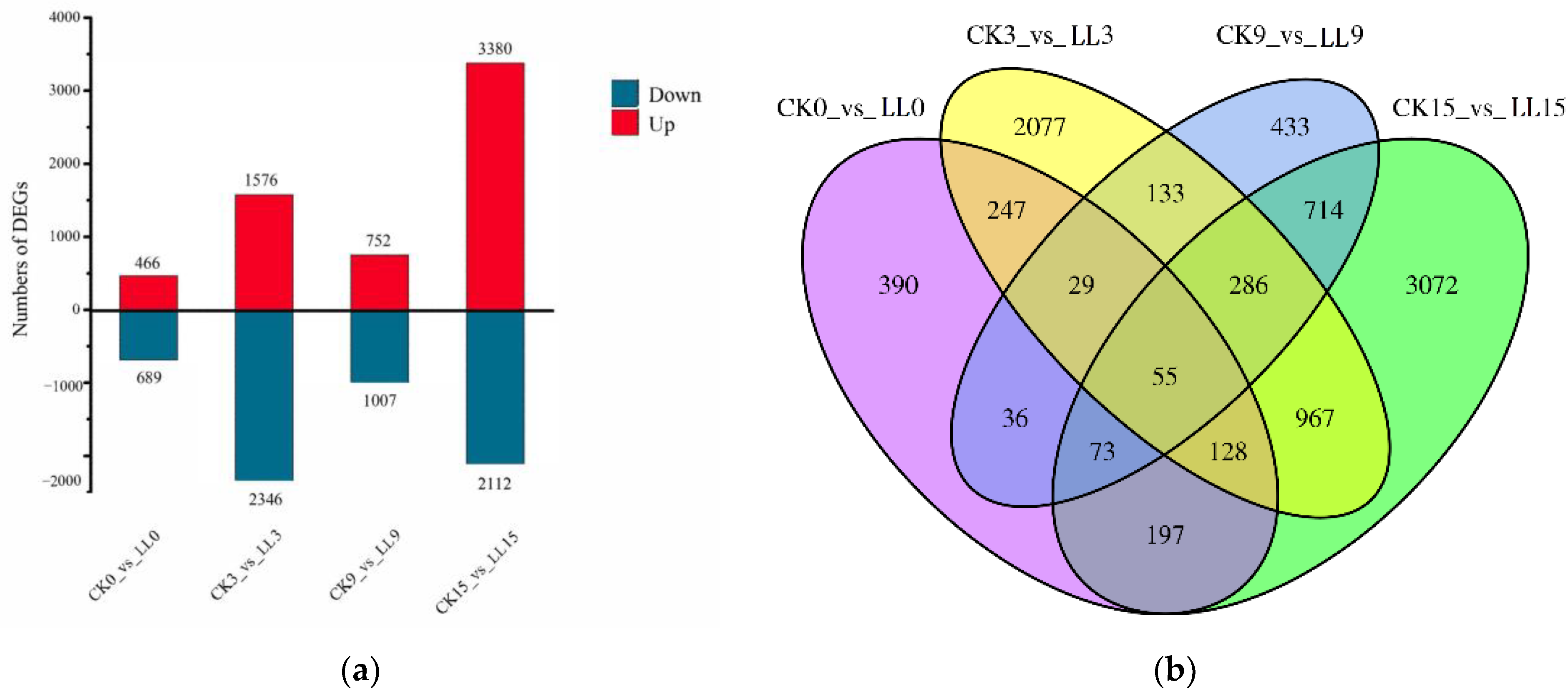
2.3. Identification of Differentially Expressed Genes (DEGs) in Watermelon Fruit
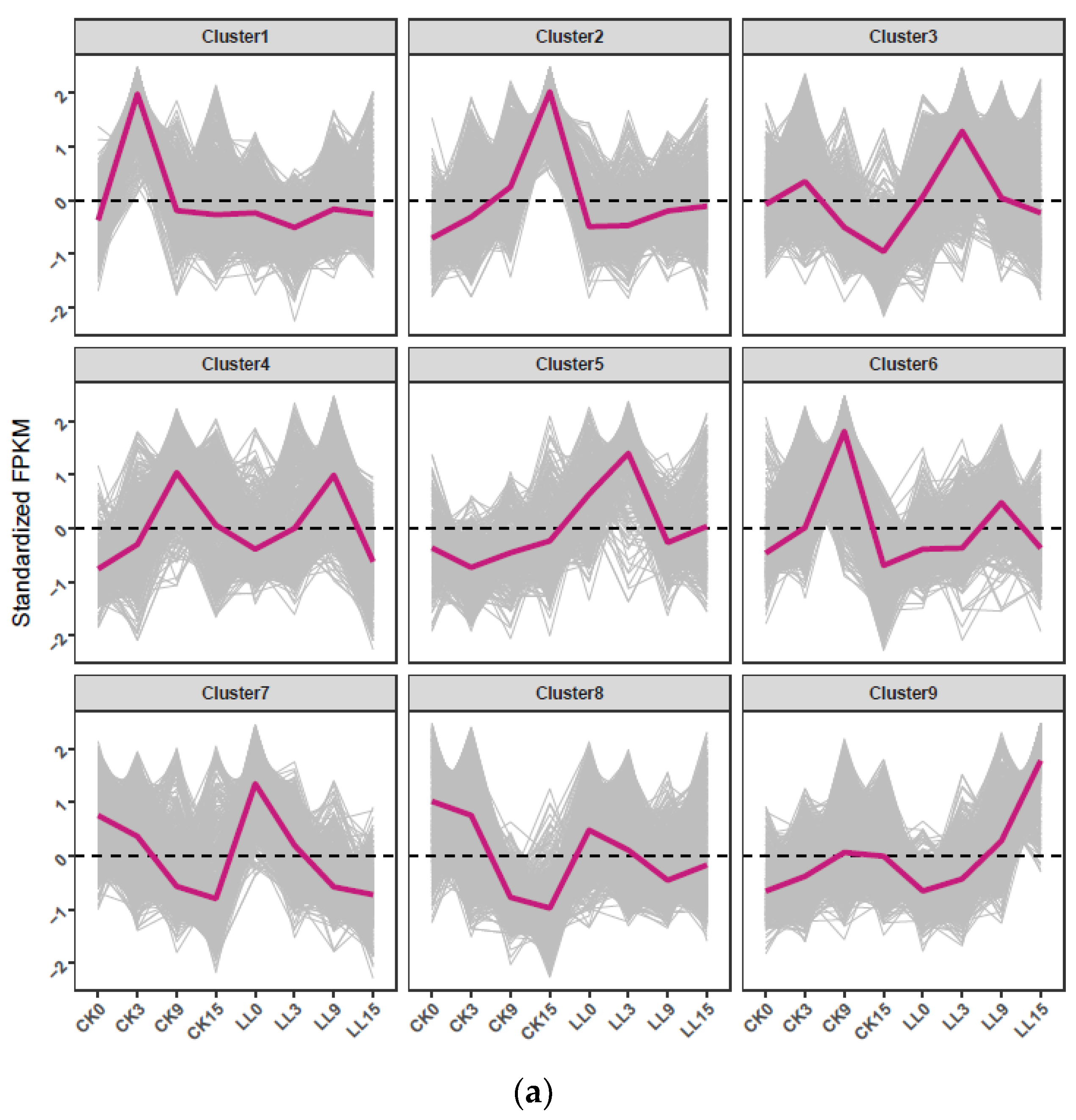
2.4. Hierachical Clustering Analysis
2.5. Weighted Correlation Network Analysis of Low-Light Stress Responsive Genes Related to Watermelon Fruit Expansion
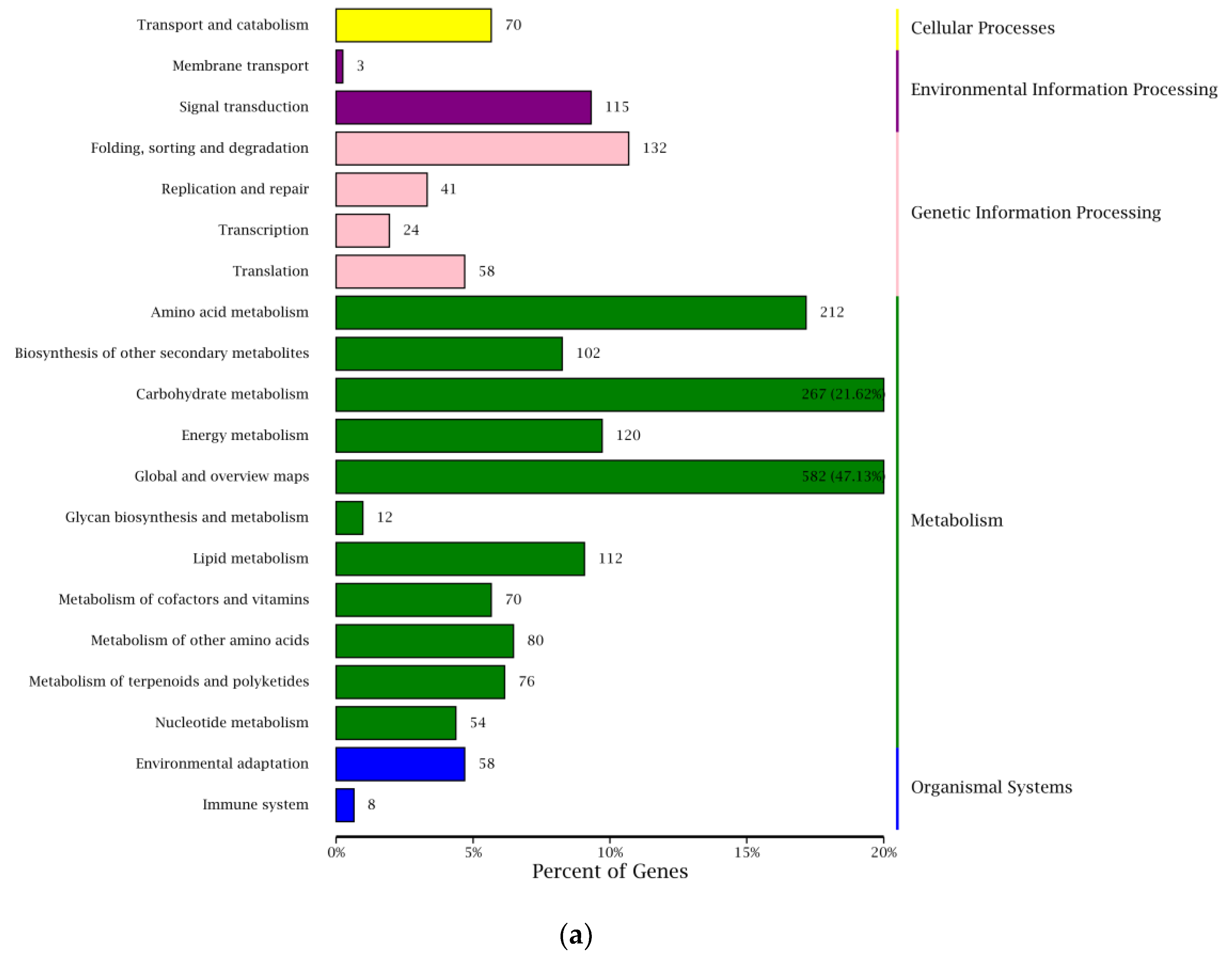
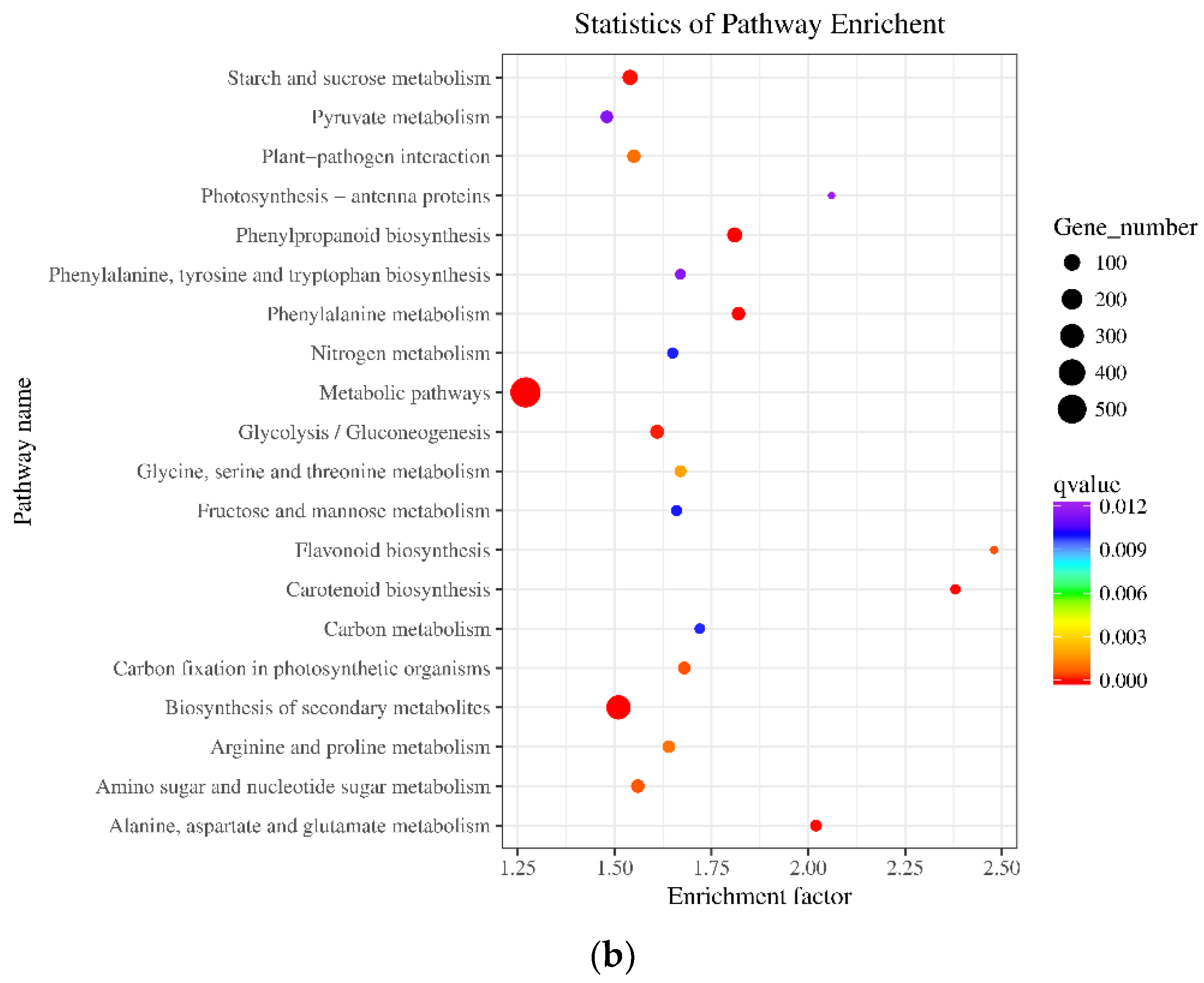
2.6. Functional Classification of DEGs
2.7. Analysis of Transcription Factors (TFs) Involved in Watermelon Fruit Development and Response to Low-Light Stress
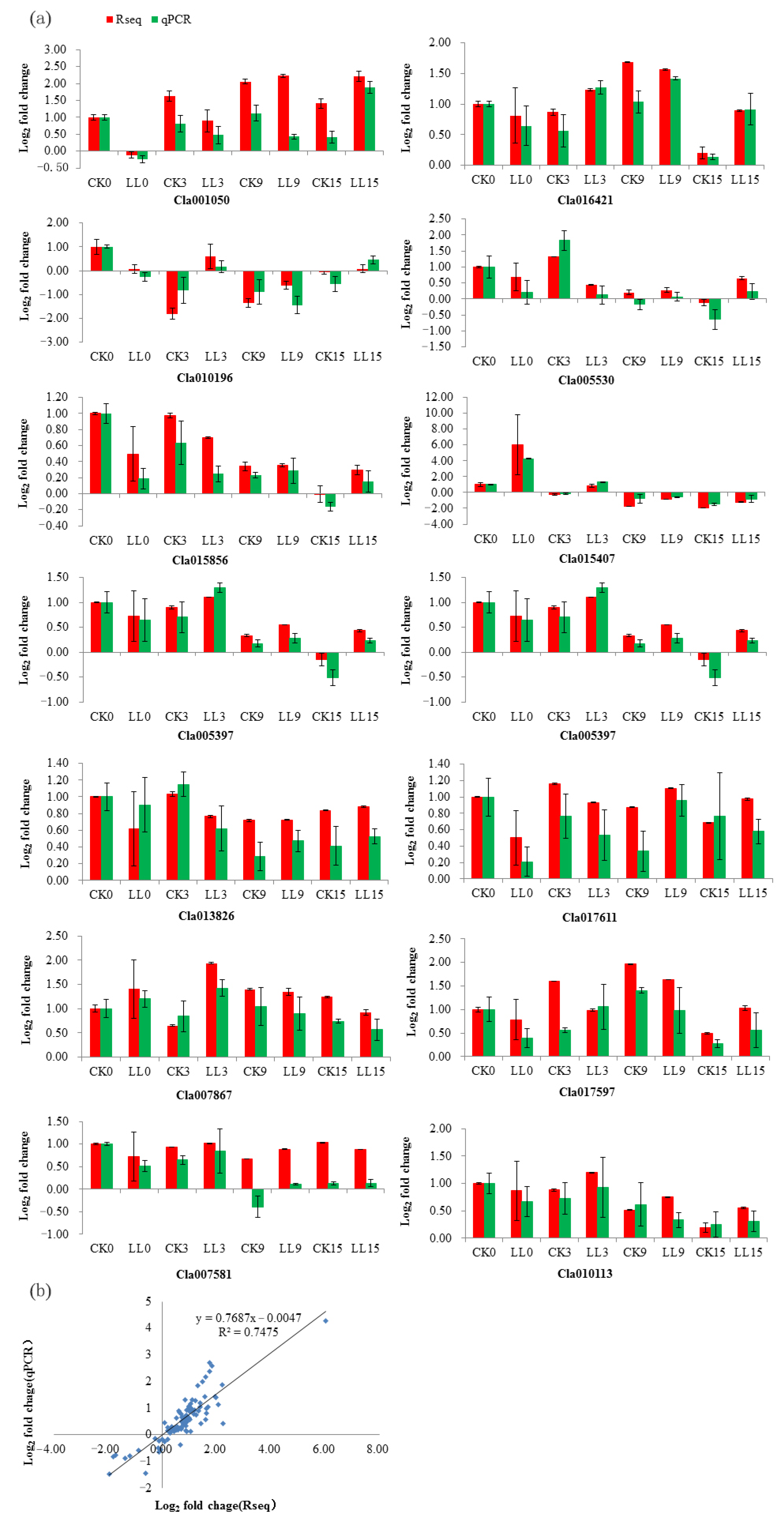
2.8. Validation of RNA-Seq Data by qRT-PCR Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Morphological and Physiological Index Measurements and Statistical Analysis
3.3. cDNA Preparation and RNA Sequencing
3.4. Transcriptomic Analysis
3.5. Digital Gene Expression Tag Profiling and Identification of Differentially Expressed Genes (DEGs)
3.6. Gene Annotation and Functional Annotation and Analysis
3.7. Weighted Correlation Network Analysis (WGCNA)
3.8. qRT-PCR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musseau, C.; Just, D.; Jorly, J.; Gevaudant, F.; Moing, A.; Chevalier, C.; Lemaire-Chamley, M.; Rothan, C.; Fernandez, L. Identification of two new mechanisms that regulate fruit growth by cell expansion in tomato. Front. Plant Sci. 2017, 8, 988. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.G.; Sun, H.H.; Zhang, H.Y.; Liu, J.A.; Ren, Y.; Gong, G.Y.; Jiao, C.; Zheng, Y.; Yang, W.C.; Fei, Z.J.; et al. Comparative transcriptome analysis of cultivated and wild watermelon during fruit development. PLoS ONE 2015, 10, e0130267. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Fan, Y.; Ma, Y.; Zhang, Z.; Yue, H.B.; Wang, L.J.; Li, J.; Jiao, Y. Effects of Exogenous gamma-Aminobutyric Acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul. 2017, 36, 436–449. [Google Scholar] [CrossRef]
- Ilic, Z.S.; Milenkovic, L.; Stanojevic, L.; Cvetkovic, D.; Fallik, E. Effects of the modification of light intensity by color shade nets on yield and quality of tomato fruits. Sci. Hortic. 2012, 139, 90–95. [Google Scholar] [CrossRef]
- Tinyane, P.P.; Soundy, P.; Sivakumar, D. Growing ‘Hass’ avocado fruit under different coloured shade netting improves the marketable yield and affects fruit ripening. Sci. Hortic. 2018, 230, 43–49. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J.; Kwon, J.K.; Bekhzod, K.; Park, K.S.; Lee, S.Y. Yield loss and quality degradation of strawberry fruits cultivated under the deficient insolation conditions by shading. Hortic. Environ. Biotechnol. 2014, 55, 263–270. [Google Scholar] [CrossRef]
- Dash, M.; Johnson, L.K.; Malladi, A. Severe shading reduces early fruit growth in apple by decreasing cell production and expansion. J. Am. Soc. Hortic. Sci. 2012, 137, 275–282. [Google Scholar] [CrossRef]
- Wang, X.Q.; Huang, W.D.; Zhan, J.C. Effects of low light on phloem ultrastructure and subcellular localization of sucrose synthase in Prunus persica L. var. nectarina Ait. fruit. Russ. J. Plant Physiol. 2009, 56, 462–469. [Google Scholar] [CrossRef]
- Yang, L.Y.; Chen, J.J.; Sun, X.M.; Li, J.X.; Chen, N.L. Inhibition of sucrose and galactosyl-sucrose oligosaccharide metabolism in leaves and fruits of melon (Cucumis melo L.) under low light stress. Sci. Hortic. 2019, 244, 343–351. [Google Scholar] [CrossRef]
- Lu, Y.F.; Bu, Y.F.; Hao, S.X.; Wang, Y.R.; Zhang, J.; Tian, J.; Yao, Y.C. MYBs affect the variation in the ratio of anthocyanin and flavanol in fruit peel and flesh in response to shade. J. Photochem. Photobiol. B Biol. 2017, 168, 40–49. [Google Scholar] [CrossRef]
- Shanely, R.A.; Nieman, D.C.; Perkins-Veazie, P.; Henson, D.A.; Meaney, M.P.; Knab, A.M.; Cialdell-Kam, L. Comparison of watermelon and carbohydrate beverage on exercise-induced alterations in systemic inflammation, immune dysfunction, and plasma antioxidant capacity. Nutrients 2016, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhang, Z.P.; Wang, L.J. Promotion of 5-aminolevulinic acid treatment on leaf photosynthesis is related with increase of antioxidant enzyme activity in watermelon seedlings grown under shade condition. Photosynthetica 2009, 47, 347–354. [Google Scholar] [CrossRef]
- Watanabe, S.; Nakano, Y.; Okano, K. Effect of planting density on fruit size, light-interception and photosynthetic activity of vertically trained watermelon (Citrullus lanatus (Thunb.) Matsum. et Nakai) plants. J. Jpn. Soc. Hortic. Sci. 2003, 72, 497–503. [Google Scholar] [CrossRef]
- Guo, S.G.; Liu, J.A.; Zheng, Y.; Huang, M.Y.; Zhang, H.Y.; Gong, G.Y.; He, H.J.; Ren, Y.; Zhong, S.L.; Fei, Z.J.; et al. Characterization of transcriptome dynamics during watermelon fruit development: Sequencing, assembly, annotation and gene expression profiles. BMC Genom. 2011, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Beffa, R.S.; Neuhaus, J.M.; Meins, F. Physiological compensation in antisense transformants- Specific induction of an eresatz Gluan Endo-1,3-Beta-Glucosidase in plants infected with necrotizing viruses. Proc. Natl. Acad. Sci. USA 1993, 90, 8792–8796. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Li, X.L.; Luo, X.; Deng, Q.X. Shading inhibits sugar accumulation in leaf and fruit of jujube (Ziziphus jujuba Mill.). Horticulturae 2022, 8, 592. [Google Scholar] [CrossRef]
- Wang, J.; Shi, K.; Lu, W.P.; Lu, D.L. Post-silking shading stress affects leaf nitrogen metabolism of spring maize in Southern China. Plants 2020, 9, 210. [Google Scholar] [CrossRef]
- Long, X.Y.; He, B.; Wang, C.; Fang, Y.J.; Qi, J.Y.; Tang, C.R. Molecular identification and characterization of the pyruvate decarboxylase gene family associated with latex regeneration and stress response in rubber tree. Plant Physiol. Biochem. 2015, 87, 35–44. [Google Scholar] [CrossRef]
- Duan, E.; Wang, Y.; Liu, L.; Zhu, J.; Zhong, M.; Zhang, H.; Li, S.; Ding, B.; Zhang, X.; Guo, X.; et al. Pyrophosphate: Fructose-6-phosphate 1-phosphotransferase (PFP) regulates carbon metabolism during grain filling in rice. Plant Cell Rep. 2016, 35, 1321–1331. [Google Scholar] [CrossRef]
- Yi, S.Y.; Ku, S.S.; Sim, H.J.; Kim, S.K.; Park, J.H.; Lyu, J.I.; So, E.J.; Choi, S.Y.; Kim, J.; Ahn, M.S.; et al. An alcohol dehydrogenase gene from Synechocystis sp Confers salt tolerance in transgenic tobacco. Front. Plant Sci. 2017, 8, 1965. [Google Scholar] [CrossRef] [PubMed]
- Strommer, J. The plant ADH gene family. Plant J. 2011, 66, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Shimizu, T.; Miyakawa, S.; Kanno, Y.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015, 128, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Marmagne, A.; Talbotec, J.; Krupinska, K.; Masclaux-Daubresse, C. The identification of new cytosolic glutamine synthetase and asparagine synthetase genes in barley (Hordeum vulgare L.), and their expression during leaf senescence. J. Exp. Bot. 2015, 66, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Melida, H.; Lopez, G.; Dabos, P.; Tremousaygue, D.; Denance, N.; Miedes, E.; Bulone, V.; Goffner, D.; Molina, A. Arabidopsis response regulator 6 (ARR6) modulates plant cell-wall composition and disease resistance. Mol. Plant-Microbe Interact. 2020, 33, 767–780. [Google Scholar] [CrossRef]
- Wong, M.M.; Bhaskara, G.B.; Wen, T.N.; Lin, W.D.; Nguyen, T.T.; Chong, G.L.; Verslues, P.E. Phosphoproteomics of Arabidopsis Highly ABA-Induced1 identifies AT-Hook-Like10 phosphorylation required for stress growth regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 2354–2363. [Google Scholar] [CrossRef]
- Zhao, L.J.; Lu, Y.J.; Chen, W.; Yao, J.B.; Li, Y.; Li, Q.L.; Pan, J.W.; Fang, S.T.; Sun, J.; Zhang, Y.S. Genome-wide identification and analyses of the AHL gene family in cotton (Gossypium). BMC Genom. 2020, 21, 69. [Google Scholar] [CrossRef]
- Denance, N.; Szurek, B.; Noel, L.D. Emerging functions of nodulin-like proteins in non-nodulating plant species. Plant Cell Physiol. 2014, 55, 469–474. [Google Scholar] [CrossRef]
- Gong, S.Y.; Huang, G.Q.; Sun, X.; Li, P.; Zhao, L.L.; Zhang, D.J.; Li, X.B. GhAGP31, a cotton non-classical arabinogalactan protein, is involved in response to cold stress during early seedling development. Plant Biol. 2012, 14, 447–457. [Google Scholar] [CrossRef]
- Bao, F.; Du, D.L.; An, Y.; Yang, W.R.; Wang, J.; Cheng, T.R.; Zhang, Q.X. Overexpression of Prunus mume dehydrin genes in tobacco enhances tolerance to cold and drought. Front. Plant Sci. 2017, 8, 151. [Google Scholar] [CrossRef]
- Lu, J.; Sun, M.-H.; Ma, Q.-J.; Kang, H.; Liu, Y.-J.; Hao, Y.-J.; You, C.-X. MdSWEET17, a sugar transporter in apple, enhances drought tolerance in tomato. J. Integr. Agric. 2019, 18, 2041–2051. [Google Scholar] [CrossRef]
- Liang, W.W.; Sun, F.F.; Zhao, Y.N.; Shan, L.Z.; Lou, H.Y. Identification of susceptibility modules and genes for cardiovascular disease in diabetic patients using WGCNA analysis. J. Diabetes Res. 2020, 2020, 4178639. [Google Scholar] [CrossRef] [PubMed]
- Voxeur, A.; Hofte, H. Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 2016, 26, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Adams, Z.P.; Ehlting, J.; Edwards, R. The regulatory role of shikimate in plant phenylalanine metabolism. J. Theor. Biol. 2019, 462, 158–170. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Wechter, W.P.; Levi, A.; Harris, K.R.; Davis, A.R.; Fei, Z.J.; Katzir, N.; Giovannoni, J.J.; Salman-Minkov, A.; Hernandez, A.; Thimmapuram, J.; et al. Gene expression in developing watermelon fruit. BMC Genom. 2008, 9, 275. [Google Scholar] [CrossRef]
- Wessels, B.; Seyfferth, C.; Escamez, S.; Vain, T.; Antos, K.; Vahala, J.; Delhomme, N.; Kangasjarvi, J.; Eder, M.; Felten, J.; et al. An AP2/ERF transcription factor ERF139 coordinates xylem cell expansion and secondary cell wall deposition. New Phytol. 2019, 224, 1585–1599. [Google Scholar] [CrossRef]
- Zhao, M.R.; Li, J.; Zhu, L.; Chang, P.; Li, L.L.; Zhang, L.Y. Identification and characterization of MYB-bHLH-WD40 regulatory complex members controlling anthocyanidin biosynthesis in blueberry fruits development. Genes 2019, 10, 496. [Google Scholar] [CrossRef]
- Mao, K.; Dong, Q.L.; Li, C.; Liu, C.H.; Ma, F.W. Genome wide identification and characterization of apple bHLH transcription factors and expression analysis in response to drought and salt stress. Front. Plant Sci. 2017, 8, 480. [Google Scholar] [CrossRef]
- Wu, P.P.; Peng, M.S.; Li, Z.G.; Yuan, N.; Hu, Q.; Foster, C.E.; Saski, C.; Wu, G.H.; Sun, D.F.; Luo, H. DRMY1, a Myb-like protein, regulates cell expansion and seed production in Arabidopsis thaliana. Plant Cell Physiol. 2019, 60, 285–302. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, W.Q.; Zeng, J.K.; Zhang, J.; Grierson, D.; Li, X.; Yin, X.R.; Chen, K.S. A NAC transcription factor, EjNAC1, affects lignification of loquat fruit by regulating lignin. Postharvest Biol. Technol. 2015, 102, 25–31. [Google Scholar] [CrossRef]
- Ayadi, M.; Mzid, R.; Ben Ayed, R.; Kharrat, N.; Merchaoui, H.; Rebai, A.; Hanana, M. Genome-wide identification and molecular characterization of Citrus unshiu WRKY transcription factors in Satsuma mandarin: Clues for putative involvement in cell growth, fruit ripening, and stress response. Turk. J. Agric. For. 2019, 43, 209–231. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Annotation Database 1 | Annotated_Number | Percentage |
|---|---|---|
| COG_Annotation | 8060 | 36.02% |
| GO_Annotation | 9037 | 40.38% |
| KEGG_Annotation | 5156 | 23.04% |
| KOG_Annotation | 11,715 | 52.35% |
| Pfam_Annotation | 17,006 | 75.99% |
| Swissprot_Annotation | 16,206 | 72.42% |
| nr_Annotation | 22,343 | 99.84% |
| Annotated in all seven Database | 1550 | 5.73% |
| Annotated in at least one Database | 22,379 | 82.70% |
| All_Annotated | 27,063 | 100.00% |
| Gene ID | Gene Description | CK0_vs _LL0 | CK3_vs _LL3 | CK9_vs _LL9 | CK15_vs _LL15 |
|---|---|---|---|---|---|
| Metabolism | |||||
| Carbohydrate metabolism | |||||
| Cla005337 | glucan endo-1,3-beta-glucosidase 13 | Up | Up | Down | Up |
| Cla018009 | pyruvate decarboxylase 1 | Down | Down | Up | Down |
| Cla004587 | pyrophosphate—fructose 6-phosphate 1-phosphotransferase subunit beta-like | Up | Down | Down | Down |
| Cla017722 | fructose-bisphosphate aldolase cytoplasmic isozyme isoform X2 | Down | Down | Up | Down |
| Cla003920 | probable ribose-5-phosphate isomerase 1 | Up | Up | Down | Down |
| Cla015028 | alcohol dehydrogenase 1 | Down | Down | Up | Down |
| Amino acid metabolism | |||||
| Cla010147 | LOW QUALITY PROTEIN: protein NRT1/PTR FAMILY 2.13-like | Up | Up | Down | Down |
| Cla010146 | protein NRT1/PTR FAMILY 2.13-like | Up | Up | Down | Down |
| Cla019134 | protein NRT1/PTR FAMILY 2.11-like | Up | Up | Down | Down |
| Cla013371 | asparagine synthetase [glutamine-hydrolyzing] | Down | Up | Up | Up |
| Nucleotide metabolism | |||||
| Cla022500 | deoxyuridine 5'-triphosphate nucleotidohydrolase | Up | Down | Down | Down |
| Lipid metabolism | |||||
| Cla013862 | acyl-[acyl-carrier-protein] desaturase 6, chloroplastic | Down | Down | Up | Down |
| Cla012276 | cytochrome P450 734A1-like | Up | Up | Down | Up |
| Cla001235 | cytochrome P450 86A8-like | Up | Up | Down | Up |
| Biosynthesis of other secondary metabolites | |||||
| Cla001815 | LOW QUALITY PROTEIN: salicylic acid-binding protein 2-like | Up | Up | Down | Up |
| Cla016032 | scopoletin glucosyltransferase-like | Up | Down | Down | Down |
| Cla017208 | cinnamoyl-CoA reductase 2-like | Up | Down | Up | Up |
| Cla017207 | cinnamoyl-CoA reductase 2-like | Up | Down | Up | Up |
| Environmental Information Procession | |||||
| Signal transduction | |||||
| Cla002572 | BTB/POZ and TAZ domain-containing protein 1 | Down | Down | Up | Up |
| Cla017611 | uncharacterized protein LOC103486691 | Down | Down | Up | Up |
| Cla016659 | two-component response regulator ARR12-like | Up | Up | Up | Up |
| Cla008326 | putative cell division cycle ATPase | Up | Up | Down | Up |
| Cla008169 | uncharacterized protein LOC101203772 | Down | Down | Up | Up |
| MSTRG.1055 | defensin-like protein 6 | Up | Up | Up | Down |
| Cla006543 | AT-hook motif nuclear-localized protein 15 | Up | Up | Up | Up |
| genetic information processing | |||||
| Folding, sorting and degradation | |||||
| Cla023118 | U-box domain-containing protein 26 | Down | Down | Up | Up |
| Mitochondrial biogenesis | |||||
| Cla009892 | mitochondrial translocator assembly and maintenance protein 41 homolog isoform X1 | Down | Down | Up | Down |
| Organismal Systems | |||||
| Environmental adaptation | |||||
| Cla007307 | WRKY70 | Down | Down | Down | Up |
| Cla007656 | probable WRKY transcription factor 31 | Up | Up | Down | Down |
| Cla018486 | probable receptor-like protein kinase At5g39030 | Up | Down | Down | Down |
| Cla007979 | probable transcription factor GLK2 | Down | Down | Up | Up |
| stress-related genes | |||||
| Cla023376 | Early nodulin-like protein 2 [Glycine soja] | Down | Down | Down | Up |
| MSTRG.17433 | hypothetical protein Csa_6G059230 | Up | Up | Down | Down |
| Cla010283 | hypothetical protein Csa_2G237730 | Down | Down | Up | Up |
| Cla002904 | hypothetical protein Csa_3G822280 | Down | Down | Up | Up |
| Cla015065 | hypothetical protein Csa_7G397010 | Down | Down | Down | Up |
| Cla008418 | Iron-sulfur binding oxidoreductase | Up | Up | Up | Up |
| Cla010875 | BAG family molecular chaperone regulator 6-like | Up | Down | Down | Down |
| Cla012888 | classical arabinogalactan protein 10-like | Up | Up | Down | Up |
| Cla013835 | formin-like protein 20-like | Up | Up | Down | Down |
| Cla013527 | seed biotin-containing protein SBP65 | Down | Down | Up | Up |
| Cla004456 | uncharacterized protein LOC103491021 | Down | Down | Up | Down |
| Cla007700 | uncharacterized protein LOC103495564 | Down | Down | Up | Up |
| Cla011574 | 14 kDaproline-rich protein DC2.15-like | Up | Up | Down | Down |
| Cla018055 | probably inactive leucine-rich repeat receptor-like protein kinase At3g28040 | Down | Down | Up | Up |
| Cla019959 | universal stress protein A-like protein isoform X2 | Down | Down | Up | Up |
| Cla014570 | dehydrin DHN1-like | Down | Up | Up | Up |
| Cla017570 | F-box/kelch-repeat protein At2g44130-like | Down | Down | Up | Up |
| Cla009891 | haloacid dehalogenase-like hydrolase domain-containing protein 3 | Down | Down | Up | Down |
| Cla021693 | sugar transporter ERD6-like 16 isoform X2 | Up | Down | Down | Down |
| Cla011361 | bidirectional sugar transporter SWEET4-like | Down | Up | Down | Up |
| MSTRG.15291 | no hit found | Down | Down | Up | Up |
| MSTRG.20101 | no hit found | Down | Up | Up | Up |
| MSTRG.967 | no hit found | Up | Up | Down | Down |
| MSTRG.968 | no hit found | Up | Up | Down | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; She, F.; Sun, Y.; Han, B.; Wang, X.; Xu, G. Transcriptome Analysis Reveals the Genes Related to Water-Melon Fruit Expansion under Low-Light Stress. Plants 2023, 12, 935. https://doi.org/10.3390/plants12040935
Gao W, She F, Sun Y, Han B, Wang X, Xu G. Transcriptome Analysis Reveals the Genes Related to Water-Melon Fruit Expansion under Low-Light Stress. Plants. 2023; 12(4):935. https://doi.org/10.3390/plants12040935
Chicago/Turabian StyleGao, Wenrui, Fuchun She, Yanjun Sun, Bing Han, Xiansheng Wang, and Gang Xu. 2023. "Transcriptome Analysis Reveals the Genes Related to Water-Melon Fruit Expansion under Low-Light Stress" Plants 12, no. 4: 935. https://doi.org/10.3390/plants12040935
APA StyleGao, W., She, F., Sun, Y., Han, B., Wang, X., & Xu, G. (2023). Transcriptome Analysis Reveals the Genes Related to Water-Melon Fruit Expansion under Low-Light Stress. Plants, 12(4), 935. https://doi.org/10.3390/plants12040935






