Identification and Comparative Expression Profiles of Candidate Olfactory Receptors in the Transcriptomes of the Important Egg Parasitoid Wasp Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Tissue Collection
2.2. cDNA Library Construction and Sequencing
2.3. Transcriptome Assembly and Functional Annotation
2.4. Identification of Olfactory Receptor Genes
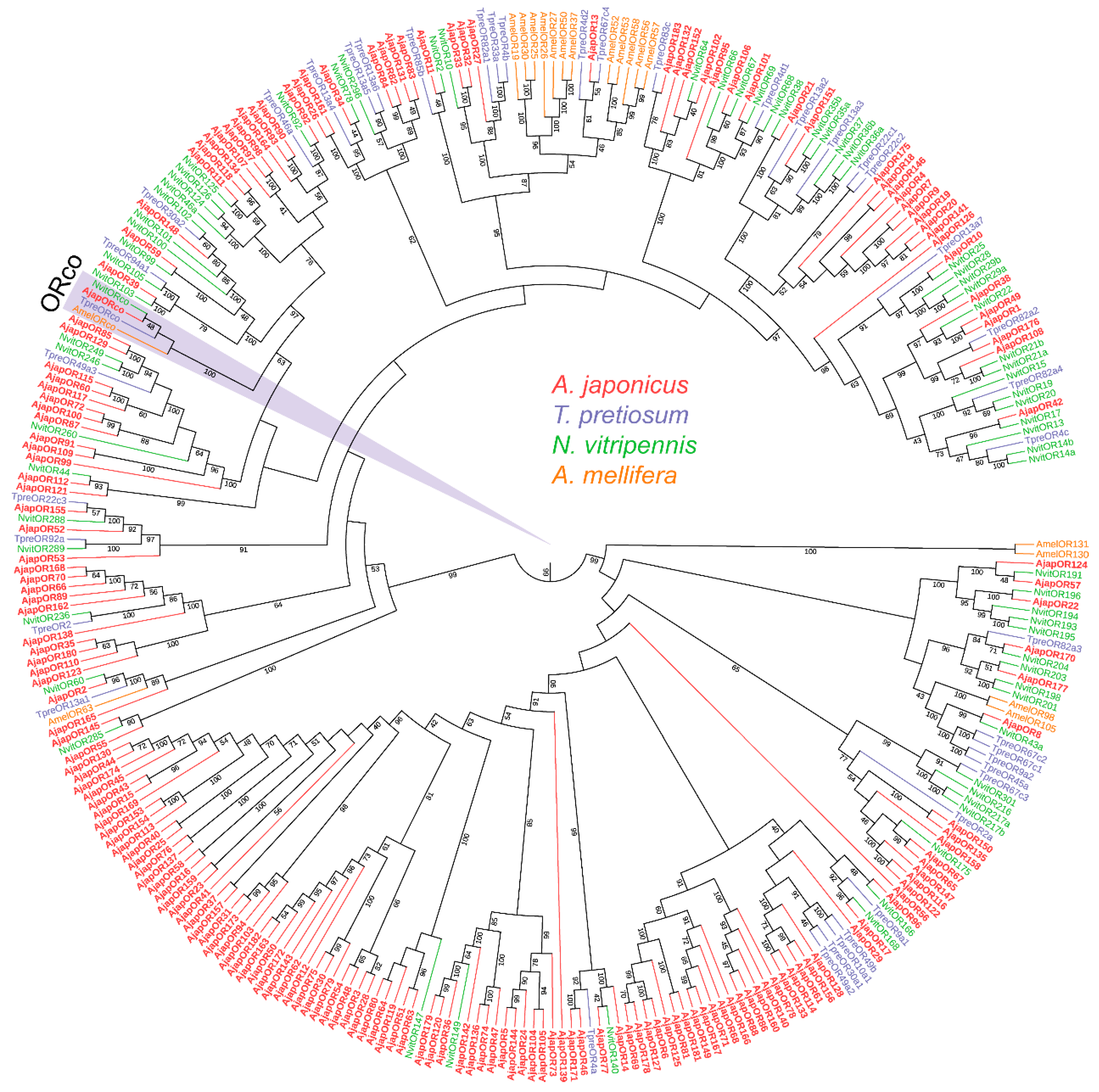
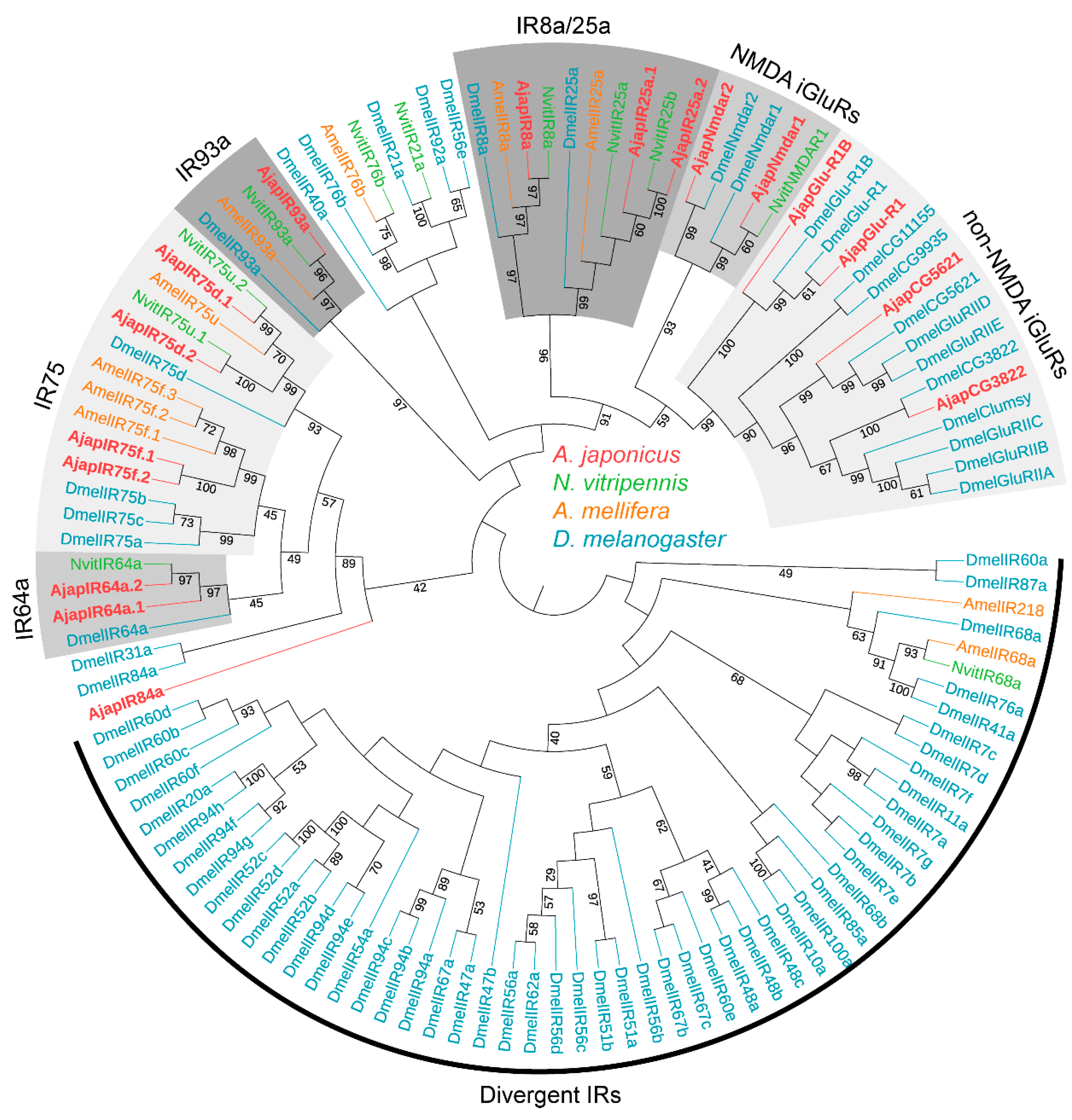
2.5. Phylogenetic Analysis
2.6. Expression Abundance Analysis of Olfactory Receptor Genes
3. Results
3.1. Overview of the Anastatus japonicus Transcriptome
3.2. Identification of Putative Odorant Receptors
3.3. Identification of Putative Ionotropic Receptors
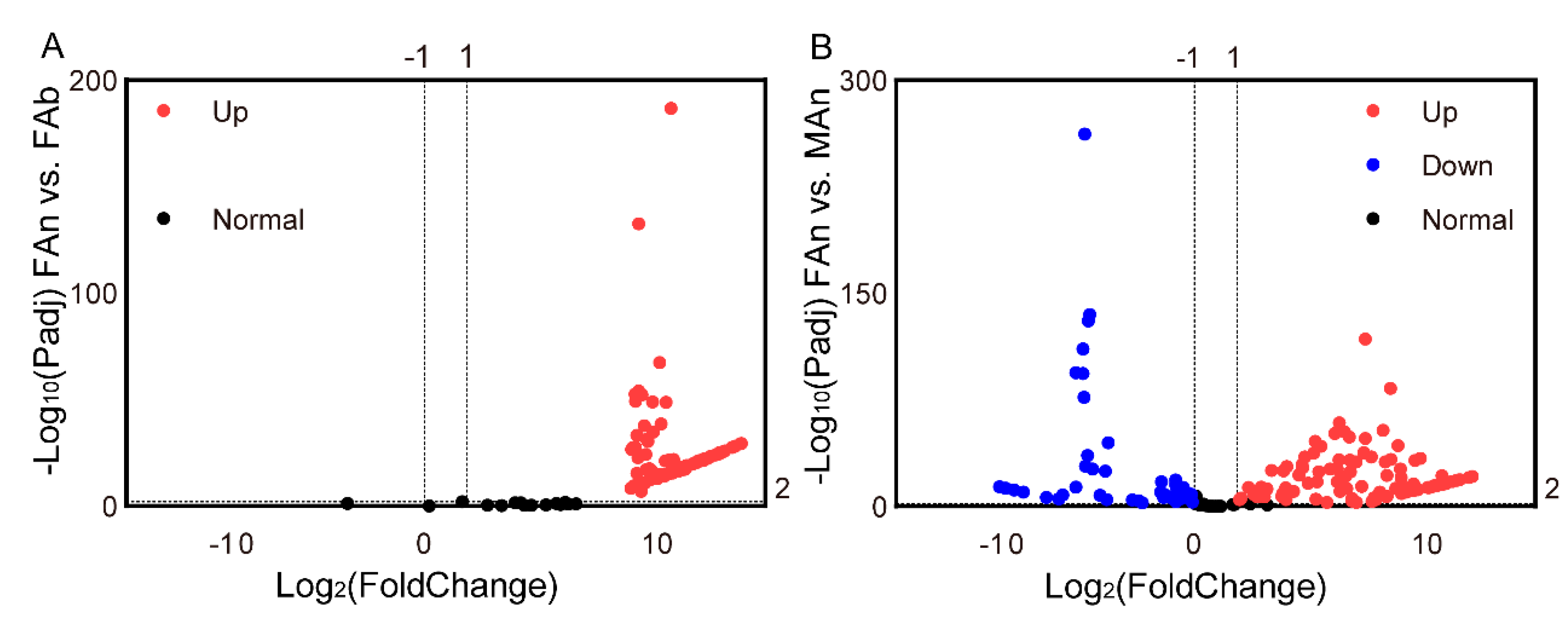
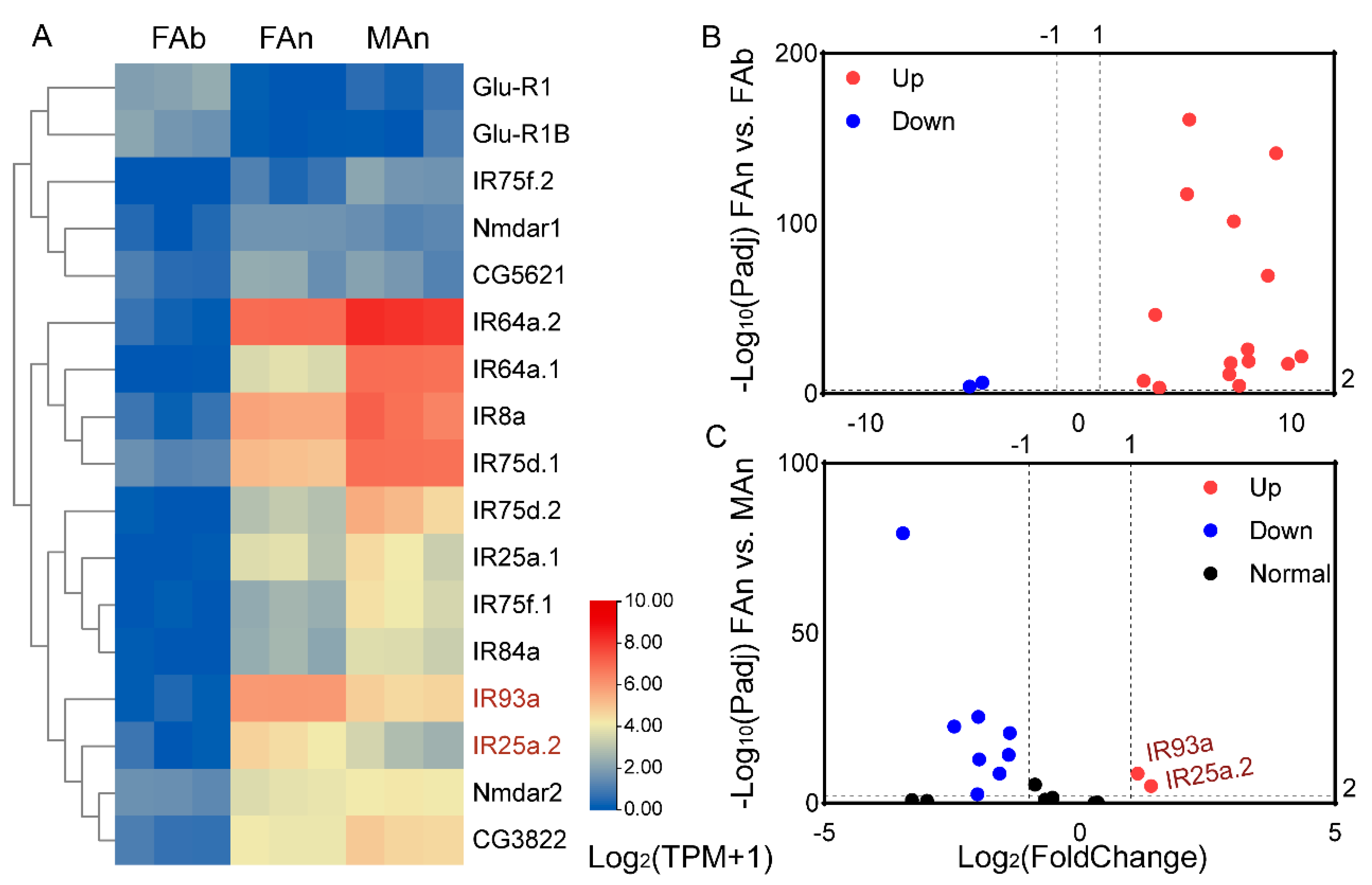
3.4. Transcription Profiling of the Olfactory Receptor Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, L.; Gibson, G.A.P.; Tang, L.U.; Xiang, J. Review of the species of Anastatus (Hymenoptera: Eupelmidae) known from China, with description of two new species with brachypterous females. Zootaxa 2020, 4767, 351–401. [Google Scholar] [CrossRef] [PubMed]
- Vetek, G.; Karolyi, B.; Meszaros, A.; Horvath, D.; Koranyi, D. The invasive brown marmorated stink bug (Halyomorpha halys) is now widespread in Hungary. Entomol. Gen. 2018, 38, 3–14. [Google Scholar] [CrossRef]
- Musolin, D.L.; Konjevic, A.; Karpun, N.N.; Protsenko, V.Y.; Ayba, L.Y.; Saulich, A.K. Invasive brown marmorated stink bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: History of invasion, range expansion, early stages of establishment, and first records of damage to local crops. Arthropod-Plant Interact. 2018, 12, 517–529. [Google Scholar] [CrossRef]
- Chartois, M.; Streito, J.-C.; Pierre, E.; Armand, J.-M.; Gaudin, J.; Rossi, J.-P. A crowdsourcing approach to track the expansion of the brown marmorated stinkbug Halyomorpha halys (Stål, 1855) in France. Biodivers Data J. 2021, 9, e66335. [Google Scholar] [CrossRef] [PubMed]
- Mi, Q.Q.; Zhang, J.P.; Haye, T.; Zhang, B.X.; Zhao, C.; Lei, Y.M.; Li, D.S.; Zhang, F. Fitness and interspecific competition of Trissolcus japonicus and Anastatus japonicus, egg parasitoids of Halyomorpha halys. Biol. Control 2021, 152, 1004461. [Google Scholar] [CrossRef]
- Chen, Y.M.; Gibson, G.A.P.; Peng, L.F.; Iqbal, A.; Zang, L.S. Anastatus Motschulsky (Hymenoptera, Eupelmidae): Egg parasitoids of Caligula japonica Moore (Lepidoptera, Saturniidae) in China. Zookeys 2019, 881, 109–134. [Google Scholar] [CrossRef]
- Chen, Y.M.; Qu, X.R.; Li, T.H.; Iqbal, A.; Wang, X.; Ren, Z.Y.; Desneux, N.; Zang, L.S. Performances of six eupelmid egg parasitoids from China on Japanese giant silkworm Caligula japonica with different host age regimes. J. Pest Sci. 2020, 94, 309–319. [Google Scholar] [CrossRef]
- Chen, Y.M.; Iqbal, A.; Lv, R.E.; Wang, X.; Desneux, N.; Zang, L.S. Chinese oak silkworm Antherae pernyi egg, a suitable factitious host for rearing eupelmid egg parasitoids. Pest Manag. Sci. 2022, 78, 1789–1799. [Google Scholar] [CrossRef]
- Wei, X.Y.; Chen, Y.M.; Wang, X.; Lv, R.E.; Zang, L.S. Demography and fitness of Anastatus japonicus reared from Antheraea pernyi as a biological control agent of Caligula japonica. Insects 2022, 13, 349. [Google Scholar] [CrossRef]
- Vinson, S.B. The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 1998, 11, 79–96. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Doutt, R.L. The biology of parasitic Hymenoptera. Annu. Rev. Entomol. 1959, 4, 161–182. [Google Scholar] [CrossRef]
- Buck, J.C.; Weinstein, S.B.; Young, H.S. Ecological and evolutionary consequences of parasite avoidance. Trends Ecol. Evol. 2018, 33, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.M.; Ruther, J. Chemical ecology of the parasitoid wasp Genus Nasonia (Hymenoptera, Pteromalidae). Front. Ecol. Evol. 2019, 7, 22. [Google Scholar] [CrossRef]
- Sun, Y.L.; Dong, J.F.; Ning, C.; Ding, P.P.; Huang, L.Q.; Sun, J.G.; Wang, C.Z. An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera. Insect Mol. Biol. 2019, 28, 23–34. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Xie, S.; Chen, J.N.; Wang, Z.H.; Yang, P.; Zhou, S.C.; Pang, L.; Li, F.; Shi, M.; Huang, J.H.; et al. Expression and functional characterization of odorant-binding protein genes in the endoparasitic wasp Cotesia vestalis. Insect Sci. 2021, 28, 1354–1368. [Google Scholar] [CrossRef]
- Wang, S.N.; Peng, Y.; Lu, Z.Y.; Dhiloo, K.H.; Gu, S.H.; Li, R.J.; Zhou, J.J.; Zhang, Y.J.; Guo, Y.Y. Identification and expression analysis of putative chemosensory receptor genes in Microplitis mediator by antennal transcriptome screening. Int. J. Biol. Sci. 2015, 11, 737–751. [Google Scholar] [CrossRef]
- Li, S.S.; Yan, Z.C.; Zhao, J.J.; Li, Y.X. Transcriptomic analyses of chemosensory genes in Trichogramma japonicum (Hymenoptera: Trichogrammatidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100755. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: Peceptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Rimal, S.; Lee, Y. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect Mol. Biol. 2018, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L.B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef]
- Touhara, K.; Vosshall, L.B. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Clyne, P.J.; Warr, C.G.; Freeman, M.R.; Lessing, D.; Kim, J.; Carlson, J.R. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron 1999, 22, 327–338. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Benton, R. On the origin of smell: Odorant receptors in insects. Cell. Mol. Life Sci. 2006, 63, 1579–1585. [Google Scholar] [CrossRef]
- Wicher, D. Tuning insect odorant receptors. Front. Cell. Neurosci. 2018, 12, 94. [Google Scholar] [CrossRef]
- Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064. [Google Scholar] [CrossRef]
- Rytz, R.; Croset, V.; Benton, R. Ionotropic receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 2013, 43, 888–897. [Google Scholar] [CrossRef]
- Chen, C.; Buhl, E.; Xu, M.; Croset, V.; Rees, J.S.; Lilley, K.S.; Benton, R.; Hodge, J.J.L.; Stanewsky, R. Drosophila ionotropic receptor 25a mediates circadian clock resetting by temperature. Nature 2015, 527, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory coreceptor IR8a governs larval feces-mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Liu, Z.; Xia, Y.; Li, Y.; Song, Z.; Zhang, B.; Li, D. Temperature and photoperiodic response of diapause induction in Anastatus japonicus, an egg parasitoid of stink bugs. Insects 2021, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Mi, Q.Q.; Zhang, J.P.; Yasir, A.M.; Zhong, Y.Z.; Mills, N.J.; Li, D.S.; Lei, Y.M.; Zhang, F. Reproductive attributes and functional response of Anastatus japonicus on eggs of Antheraea pernyi, a factitious host. Pest Manag. Sci. 2022, 78, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiao, J.; Xia, Y.; Wu, Q.; Zhao, C.; Li, D. Selection and validation of reference genes for RT-qPCR-based analyses of Anastatus japonicus Ashmead (Hymenoptera: Helicopteridae). Front. Physiol. 2022, 13, 1046204. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.J.; Wang, Y.L.; Ren, B.Z. Identification and expression analysis of antennal gustatory receptor genes of Anastatus japonicus. J. Jilin Agr. Univ. 2017, 39, 292–298+312. [Google Scholar]
- Wang, Y.; Chen, Q.; Guo, J.; Li, J.; Wang, J.; Wen, M.; Zhao, H.; Ren, B. Molecular basis of peripheral olfactory sensing during oviposition in the behavior of the parasitic wasp Anastatus japonicus. Insect Biochem. Mol. Biol. 2017, 89, 58–70. [Google Scholar] [CrossRef]
- Ye, X.; Yang, Y.; Zhao, C.; Xiao, S.; Sun, Y.H.; He, C.; Xiong, S.; Zhao, X.; Zhang, B.; Lin, H.; et al. Genomic signatures associated with maintenance of genome stability and venom turnover in two parasitoid wasps. Nat. Commun. 2022, 13, 6417. [Google Scholar] [CrossRef]
- Li, R.T.; Huang, L.Q.; Dong, J.F.; Wang, C.Z. A moth odorant receptor highly expressed in the ovipositor is involved in detecting host-plant volatiles. eLife 2020, 9, e53706. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Bateman, A.; Coin, L.; Durbin, R.; Finn, R.D.; Hollich, V.; Griffiths-Jones, S.; Khanna, A.; Marshall, M.; Moxon, S.; Sonnhammer, E.L.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2004, 32, D138–D141. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Al-Jalely, B.H.; Xu, W. Olfactory sensilla and olfactory genes in the parasitoid wasp Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Insects 2021, 12, 998. [Google Scholar] [CrossRef]
- Robertson, H.M.; Gadau, J.; Wanner, K.W. The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia vitripennis. Insect Mol. Biol. 2010, 19, 121–136. [Google Scholar] [CrossRef]
- Zhou, X.; Rokas, A.; Berger, S.L.; Liebig, J.; Ray, A.; Zwiebel, L.J. Chemoreceptor evolution in Hymenoptera and its implications for the evolution of eusociality. Genome Biol. Evol. 2015, 7, 2407–2416. [Google Scholar] [CrossRef]
- Robertson, H.M.; Wanner, K.W. The chemoreceptor superfamily in the honey bee, Apis mellifera: Expansion of the odorant, but not gustatory, receptor family. Genome Res. 2006, 16, 1395–1403. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Hao, E.; Qiao, H.; Wang, J.; Wu, W.; Zhou, J.; Lu, P. Antennal transcriptome analysis of olfactory genes and characterizations of odorant binding proteins in two woodwasps, Sirex noctilio and Sirex nitobei (Hymenoptera: Siricidae). BMC Genom. 2021, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, L.; Zhu, Y.; Yang, S.; Zhou, Q.; Wang, G.; Liu, Y. Identification and sex-biased profiles of candidate olfactory genes in the antennal transcriptome of the parasitoid wasp Cotesia vestalis. Comp. Biochem. Phys. D 2020, 34, 100657. [Google Scholar] [CrossRef]
- Nie, X.P.; Li, Q.L.; Xu, C.; Li, D.Z.; Zhang, Z.; Wang, M.Q.; Zhou, A.M.; Li, S.Q. Antennal transcriptome and odorant binding protein expression profiles of an invasive mealybug and its parasitoid. J. Appl. Entomol. 2018, 142, 149–161. [Google Scholar] [CrossRef]
- Sun, D.; Huang, Y.; Qin, Z.; Zhan, H.; Zhang, J.; Liu, Y.; Yang, S. Identification of Candidate Olfactory Genes in the Antennal Transcriptome of the Stink Bug Halyomorpha halys. Front. Physiol. 2020, 11, 876. [Google Scholar] [CrossRef]
- Andersson, M.N.; Keeling, C.I.; Mitchell, R.F. Genomic content of chemosensory genes correlates with host range in wood-boring beetles (Dendroctonus ponderosae, Agrilus planipennis, and Anoplophora glabripennis). BMC Genom. 2019, 20, 690. [Google Scholar] [CrossRef]
- Tian, J.; Dewer, Y.; Hu, H.; Li, F.; Yang, S.; Luo, C. Diversity and molecular evolution of odorant receptor in Hemipteran insects. Insects 2022, 13, 214. [Google Scholar] [CrossRef]
- Savard, J.; Tautz, D.; Richards, S.; Weinstock, G.M.; Gibbs, R.A.; Werren, J.H.; Tettelin, H.; Lercher, M.J. Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the base of the radiation of Holometabolous insects. Genome Res. 2006, 16, 1334–1338. [Google Scholar] [CrossRef]
- Johnson, B.R.; Borowiec, M.L.; Chiu, J.C.; Lee, E.K.; Atallah, J.; Ward, P.S. Phylogenomics resolves evolutionary relationships among ants, bees, and wasps. Curr. Biol. 2013, 23, 2058–2062. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, F.; Zhang, X.; Zhang, S.; Guo, S.; Zhu, G.; Liu, Q.; Li, M. Transcriptome and expression patterns of chemosensory genes in antennae of the parasitoid wasp Chouioia cunea. PLoS ONE 2016, 11, e0148159. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Si, P.; Liu, Y.; Zhou, Q.; Wang, G. Characterization of a specific odorant receptor for linalool in the Chinese citrus fly Bactrocera minax (Diptera: Tephritidae). Insect Biochem. Mol. Biol. 2020, 122, 103389. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Song, X.; Khashaveh, A.; Wang, S.N.; Lu, Z.Y.; Hussain Dhiloo, K.; Li, R.J.; Zhang, Y.J. A female-biased odorant receptor tuned to the lepidopteran sex pheromone in parasitoid Microplitis mediator guiding habitat of host insects. J. Adv. Res. 2023, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Komail Raza, S.A.; Wei, Z.; Keesey, I.W.; Parker, A.L.; Feistel, F.; Chen, J.; Cassau, S.; Fandino, R.A.; Grosse-Wilde, E.; et al. Competing beetles attract egg laying in a hawkmoth. Curr. Biol. 2022, 32, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, C.Z. The ethological significance and olfactory detection of herbivore-induced plant volatiles in interactions of plants, herbivorous insects, and parasitoids. Arthropod-Plant Interact. 2019, 13, 161–179. [Google Scholar] [CrossRef]
- Xia, Y.H.; Zhang, Y.N.; Hou, X.Q.; Li, F.; Dong, S.L. Large number of putative chemoreception and pheromone biosynthesis genes revealed by analyzing transcriptome from ovipositor-pheromone glands of Chilo suppressalis. Sci. Rep. 2015, 5, 7888. [Google Scholar] [CrossRef]
- Sheng, S.; Liao, C.W.; Zheng, Y.; Zhou, Y.; Xu, Y.; Song, W.M.; He, P.; Zhang, J.; Wu, F.A. Candidate chemosensory genes identified in the endoparasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) by antennal transcriptome analysis. Comp. Biochem. Phys. D 2017, 22, 20–31. [Google Scholar] [CrossRef]
- Du, L.X.; Liu, Y.; Zhang, J.; Gao, X.W.; Wang, B.; Wang, G.R. Identification and characterization of chemosensory genes in the antennal transcriptome of Spodoptera exigua. Comp. Biochem. Phys. D 2018, 27, 54–65. [Google Scholar] [CrossRef]
- Knecht, Z.A.; Silbering, A.F.; Ni, L.; Klein, M.; Budelli, G.; Abuin, L.; Ferrer, A.J.; Samuel, A.D.T.; Benton, R.; Garrity, P.A. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. eLife 2016, 5, e17879. [Google Scholar] [CrossRef]

| Samples | Total Reads | Clean Reads | GC% | Q20% |
|---|---|---|---|---|
| FAb_1 | 44,634,698 | 43,922,576 | 35.04 | 96.31 |
| FAb_2 | 43,084,650 | 41,862,678 | 37.68 | 95.99 |
| FAb_3 | 46,109,628 | 44,842,282 | 35.8 | 96.38 |
| FAn_1 | 44,678,972 | 44,048,062 | 33.66 | 96.16 |
| FAn_2 | 55,743,476 | 54,930,122 | 32.71 | 96.81 |
| FAn_3 | 53,767,246 | 52,808,490 | 33.14 | 96.74 |
| MAn_1 | 45,106,298 | 44,630,216 | 33.51 | 96.42 |
| MAn_2 | 45,411,562 | 44,667,556 | 36.15 | 96.68 |
| MAn_3 | 45,529,476 | 44,891,754 | 33.72 | 96.31 |
| All | 424,066,006 | 416,603,736 |
| De Novo Assembly | Total Number | Total Length (bp) | Mean Length (bp) | N50 |
|---|---|---|---|---|
| Transcripts | 144,436 | 111,172,508 | 770 | 1544 |
| Unigenes | 132,646 | 82,711,657 | 624 | 905 |
| Coding genes | 17,474 | 34,875,641 | 1996 | 2796 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, H.-X.; Li, L.; Li, F.-Q.; Zang, L.-S. Identification and Comparative Expression Profiles of Candidate Olfactory Receptors in the Transcriptomes of the Important Egg Parasitoid Wasp Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae). Plants 2023, 12, 915. https://doi.org/10.3390/plants12040915
Zhan H-X, Li L, Li F-Q, Zang L-S. Identification and Comparative Expression Profiles of Candidate Olfactory Receptors in the Transcriptomes of the Important Egg Parasitoid Wasp Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae). Plants. 2023; 12(4):915. https://doi.org/10.3390/plants12040915
Chicago/Turabian StyleZhan, Hai-Xia, Lan Li, Feng-Qi Li, and Lian-Sheng Zang. 2023. "Identification and Comparative Expression Profiles of Candidate Olfactory Receptors in the Transcriptomes of the Important Egg Parasitoid Wasp Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae)" Plants 12, no. 4: 915. https://doi.org/10.3390/plants12040915
APA StyleZhan, H.-X., Li, L., Li, F.-Q., & Zang, L.-S. (2023). Identification and Comparative Expression Profiles of Candidate Olfactory Receptors in the Transcriptomes of the Important Egg Parasitoid Wasp Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae). Plants, 12(4), 915. https://doi.org/10.3390/plants12040915






