The Effect of Short-Term Heating on Photosynthetic Activity, Pigment Content, and Pro-/Antioxidant Balance of A. thaliana Phytochrome Mutants
Abstract
1. Introduction
2. Results
2.1. Plant Morphological Features
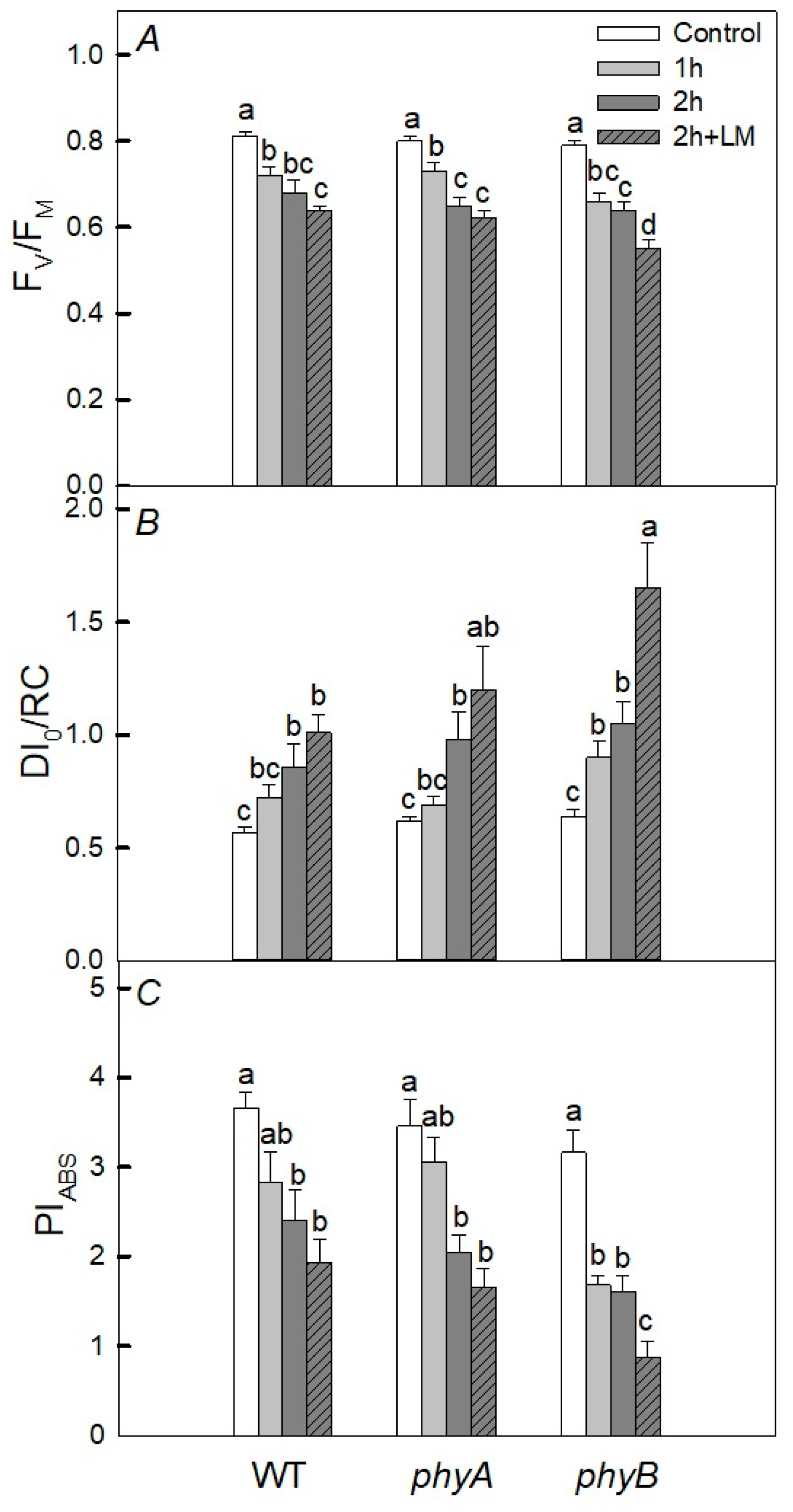
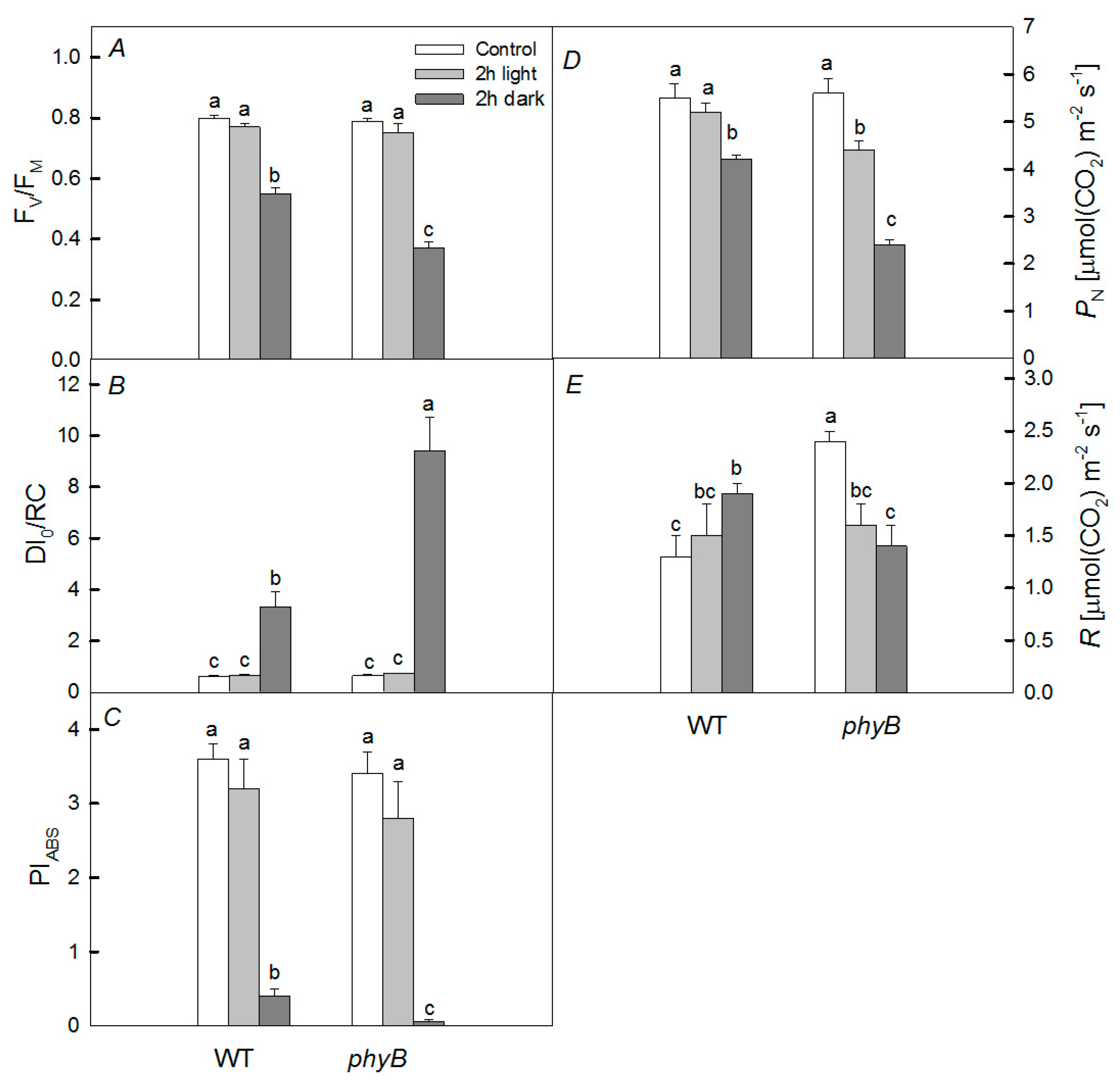
2.2. Photosynthetic Activity
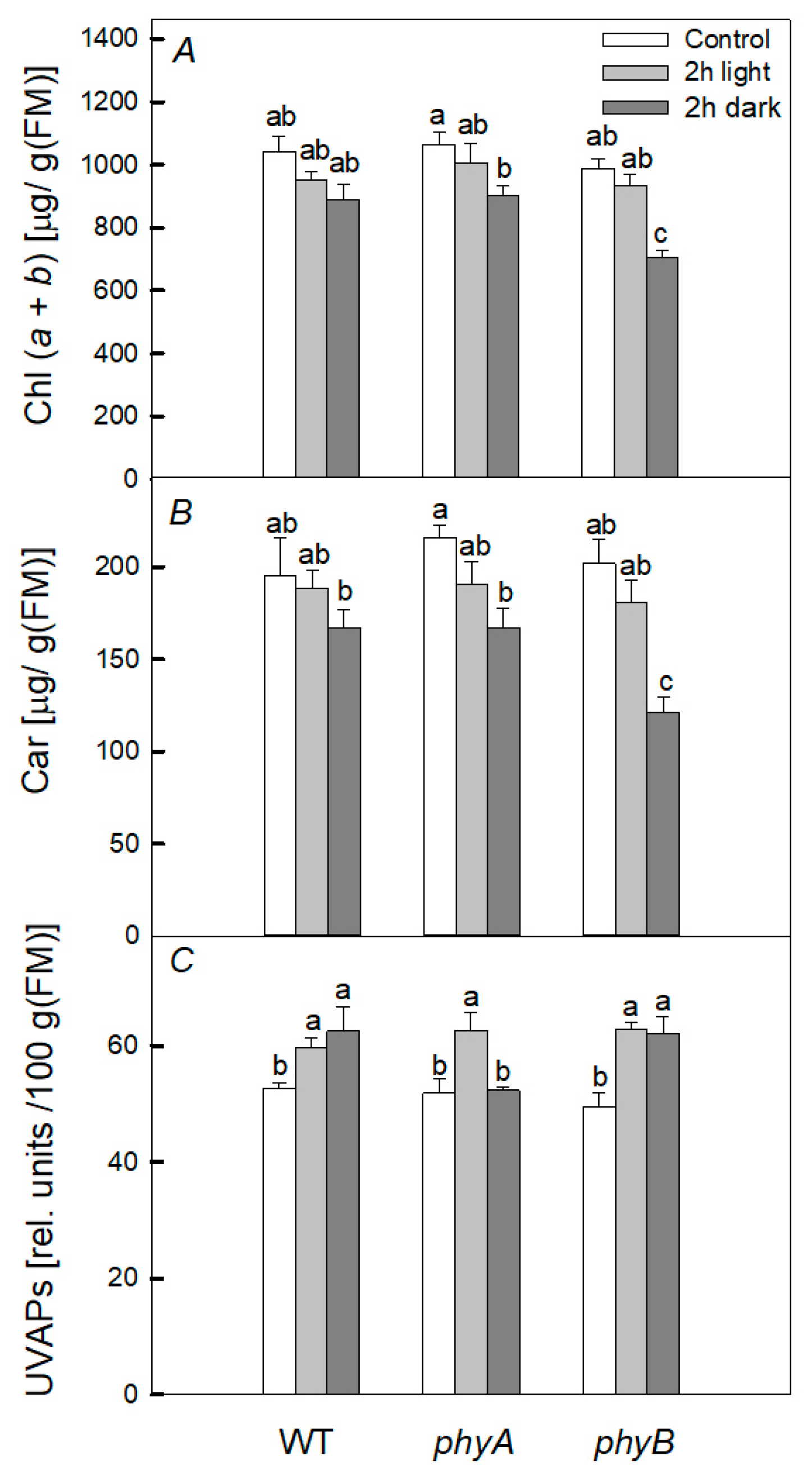
2.3. Pigments
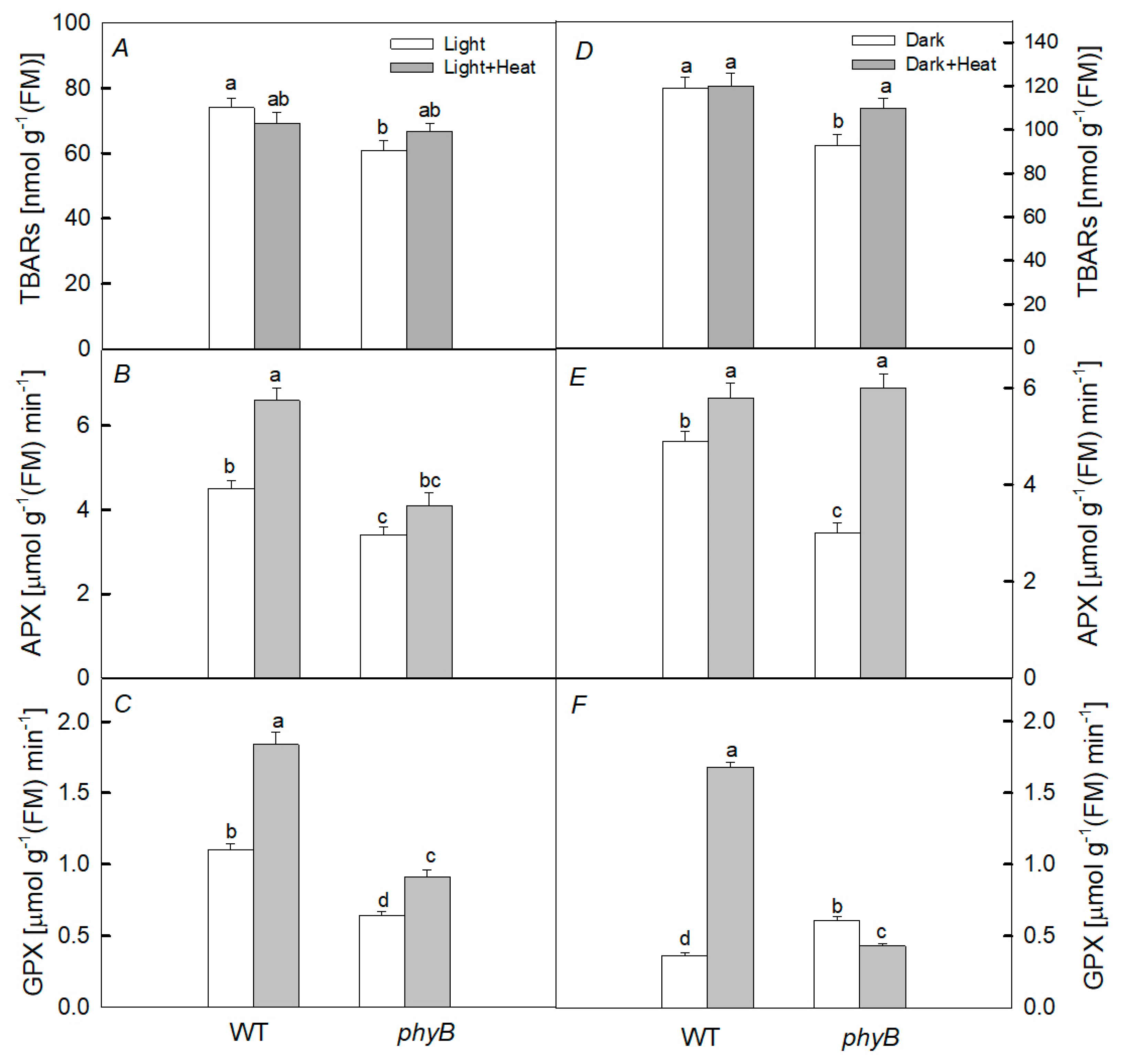
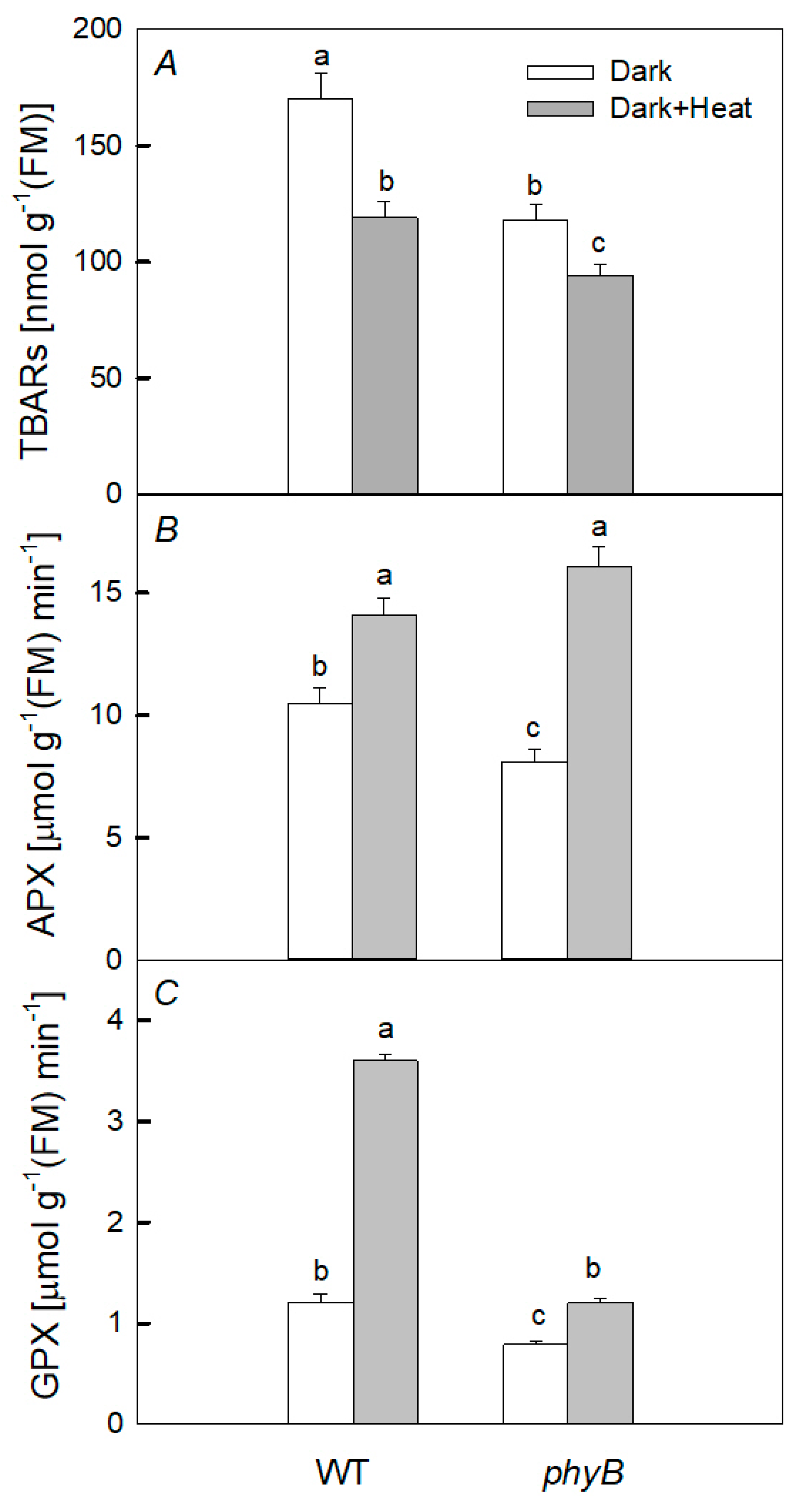
2.4. Pro-/Antioxidant Balance
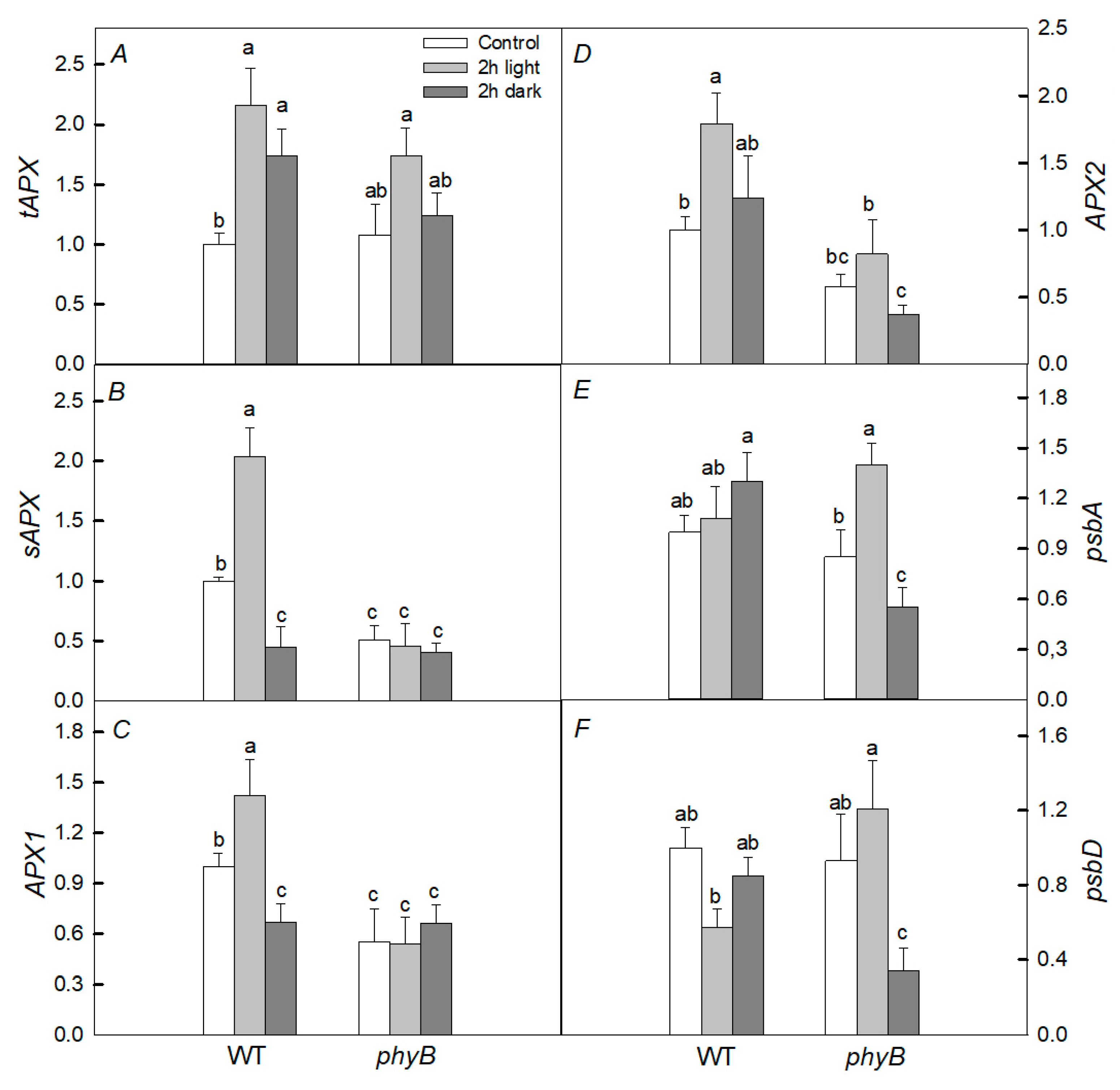
2.5. Expression of Genes
3. Discussion
3.1. Heating under Dark Conditions
3.2. Heating under Light Conditions
4. Materials and Methods
4.1. Cultivation of Plants and Scheme of the Experiment
4.2. Photochemical Activity
4.3. CO2 Gas Exchange and Transpiration
4.4. Photosynthetic and UV-Absorbing Pigments
4.5. Thiobarbituric Acid Reactive Substances and Enzyme Activity
4.6. Quantitative Real-Time PCR
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | Locus | Forward Primer | Reverse Primer |
|---|---|---|---|
| UBQ5 | AT3G62250 | GACGCTTCATCTCGTCC | CCACAGGTTGCGTTAG |
| tAPX | AT1G77490 | CTCCCGAGGGCATAGTCATTGAAA | AGTGGACAAGAGGACCAAAACG |
| sAPX | AT4G08390 | CTCCCGAGGGCATAGTCATTGAAA | TTCAAGGATTACGCTGTAGCCCATG |
| APX1 | AT1G07890 | GTACACGAAAGAAGGACCTGGAGCA | ATGTGGGCCTCAGCGTAATCA |
| APX2 | AT3G09640 | CCACAAGGAGCGTTCAGGAT | AGAAGAGCCTTGTCGGTTGG |
| psbA | ATCG00020 | GCAAGCAAGAGAGAGGAAAAAGG | TAAAAAGGGAGCCGCCGAAT |
| RPL2 | ATCG00830 | TGACCGATATGCCCTTAGGC | CACTTGTCCGACTGTTGCT |
| psbD | ATCG00270 | TTCCCAGGAAATCCGTGCAG | TTTCCACGTGGTAGAACCTCC |
References
- Grover, A.; Mittal, D.; Negi, M.; Lavania, D. Generating high temperature tolerant transgenic plants: Achievements and challenges. Plant Sci. 2013, 205, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Rashid, B.; Ali, Q.; Husnain, T. Mechanisms of heat sensing and responses in plants. It is not all about Ca2+ ions. Biol. Plant. 2018, 62, 409–420. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Tang, Y.; Wen, X.; Lu, O.; Cheng, Z.; Lu, C. Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiol. 2007, 143, 629–638. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front. Plant Sci. 2015, 17, 999. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta 2006, 1757, 742–749. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Monteiro, C.C.; Campos, M.L.; Meloc, H.C.; Carvalho, R.F. Phytochromes are key regulators of abiotic stress responses in tomato. Sci. Hortic. 2017, 222, 126–135. [Google Scholar] [CrossRef]
- Crispim-Filho, A.J.; Costa, A.C.; Alves, F.R.R.; Batista, P.F.; Rodrigues, A.A.; Vasconcelos Filho, S.C.; Nascimento, K.J.T. Deficiency in phytochromobilin biosynthesis enhances heat-stress-induced impairments to the photosynthetic apparatus in tomato. Biol. Plant. 2019, 63, 134–143. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V. Phytochromes and other (photo)receptors of information in plants. Russ. J. Plant Physiol. 2019, 66, 351–364. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Campos, M.L.; Azevedo, R.A. The role of phytochrome in stress tolerance. J. Integr. Plant Biol. 2011, 53, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta 2018, 1859, 400–408. [Google Scholar] [CrossRef]
- Arico, D.; Legris, M.; Castro, L.; Garcia, C.F.; Laino, A.; Casal, J.J.; Mazzella, M.A. Neighbour signals perceived by phytochrome B increase thermotolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 2554–2566. [Google Scholar] [CrossRef]
- Song, J.; Liu, Q.; Ci, D.; Hu, B.; Wu, W. Photoreceptor PhyB involved in Arabidopsis temperature perception and heat-tolerance formation. Int. J. Mol. Sci. 2017, 18, 1194. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef]
- Jung, J.H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors in Arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Rusaczonek, A.; Czarnocka, W.; Kacprzak, S.; Witoń, D.; Ślesak, I.; Szechyńska-Hebda, M.; Gawroński, P.; Karpiński, S. Role of phytochromes A and B in the regulation of cell death and acclamatory responses to UV stress in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 6679–6695. [Google Scholar] [CrossRef] [PubMed]
- Khudyakova, A.Y.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Kosobryukhov, A.A.; Allakhverdiev, S.I. Resistance of Arabidopsis thaliana L. photosynthetic apparatus to UV-B is reduced by deficit of phytochromes B and A. J. Photochem. Photobiol. B Biol. 2017, 169, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kreslavski, V.D.; Strokina, V.V.; Khudyakova, A.Y.; Shirshikova, G.N.; Kosobryukhov, A.A.; Pashkovskiy, P.P.; Alwasel, S.; Allakhverdiev, S.I. Effect of high-intensity light and UV-B on photosynthetic activity and the expression of certain light-responsive genes in A. thaliana phyA and phyB mutants. Biochim. Biophys. Acta 2021, 1862, 148445. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Asjraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Schmitt, F.-J.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Friedrich, T.; Renger, G.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. The multiple roles of various reactive oxygen species (ROS) in photosynthetic organisms. In Photosynthesis: A New Approach to the Molecular, Cellular, and Organismal Levels; Allakhverdiev, S.I., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 4–82. [Google Scholar] [CrossRef]
- Strzałka, K.; Kostecka-Gugała, A.; Latowski, D. Carotenoids and environmental stress in plants: Significance of carotenoid-mediated modulation of membrane physical properties. Russ. J. Plant Physiol. 2003, 50, 168–173. [Google Scholar] [CrossRef]
- Stetsenko, L.; Pashkovsky, P.; Voloshin, R.; Kreslavski, V.D. Role of anthocyanin and carotenoids in the adaptation of the photosynthetic apparatus of purple-and green-leaved cultivars of sweet basil (Ocimum basilicum) to high-intensity light. Photosynthetica 2020, 58, 890–901. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhur, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Khristin, M.S.; Shabnova, N.I.; Lyubimov, V.Y. Preillumination of excised spinach leaves with red light increases resistance of photosynthetic apparatus to UV radiation. Russ. J. Plant Physiol. 2012, 59, 717–723. [Google Scholar] [CrossRef]
- Minibaeva, F.V.; Gordon, L.K. Superoxide production and the activity of extracellular peroxidase in plant tissues under stress conditions. Russ. J. Plant Physiol. 2003, 50, 411–416. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldha, U.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Dutta, S.; Mohanty, S.; Tripathy, B.C. Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Inoue, Y.; Satoh, K.; Katoh, S. Effects of light on degradation of chlorophyll and proteins during senescence of detached rice leaves. Plant Cell Physiol. 1992, 33, 1183–1191. [Google Scholar] [CrossRef]
- Salvucci, M.E.; DeRidder, B.P.; Portis, A.R., Jr. R. Effect of activase level and isoform on the thermotolerance of photosynthesis in Arabidopsis. J. Exp. Bot. 2006, 57, 3793–3799. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Lankin, A.V.; Vasilyeva, G.K.; Luybimov, V.Y.; Semenova, G.N.; Schmitt, F.J.; Friedrich, T.; Allakhverdiev, S.I. Effects of polyaromatic hydrocarbons on photosystem ii activity in pea leaves. Int. J. Plant Physiol. Biochem. 2014, 81, 135–142. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 36–57. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Mirecki, R.M.; Teramura, A.H. Effect of ultraviolet B irradiance on soybean, V. The dependence of plant sensitivity on photosynthesis flux density during and after leaf expansion. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Nadezhkina, E.S. Effect of selenium on growth and antioxidant capacity of Triticum aestivum L. during development of lead-induced oxidative stress. Russ. J. Plant Physiol. 2017, 64, 215–223. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mauriat, M.; Guénin, S.; Pelloux, J.; Lefebvre, J.F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C.; et al. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plant. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef] [PubMed]
| Parameters | DI0/RC | PIABS | Fv/Fm |
|---|---|---|---|
| WT | 0.57 ± 0.03 b | 3.66 ± 0.33 a | 0.78 ± 0.01 a |
| WT, light | 0.57 ± 0.03 b | 3.78 ± 0.39 a | 0.76 ± 0.01 a |
| WT, dark | 12.90 ± 3.00 a | 0.01 ± 0.01 b | 0.21 ± 0.03 b |
| phyB | 0.53 ± 0.03 b | 3.98 ± 0.39 a | 0.79 ± 0.01 a |
| phyB, light | 0.56 ± 0.04 b | 3.56 ± 0.31 a | 0.77 ± 0.01 a |
| phyB, dark | 12.50 ± 3.20 a | 0.01 ± 0.01 b | 0.21 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreslavski, V.D.; Khudyakova, A.Y.; Kosobryukhov, A.A.; Balakhnina, T.I.; Shirshikova, G.N.; Alharby, H.F.; Allakhverdiev, S.I. The Effect of Short-Term Heating on Photosynthetic Activity, Pigment Content, and Pro-/Antioxidant Balance of A. thaliana Phytochrome Mutants. Plants 2023, 12, 867. https://doi.org/10.3390/plants12040867
Kreslavski VD, Khudyakova AY, Kosobryukhov AA, Balakhnina TI, Shirshikova GN, Alharby HF, Allakhverdiev SI. The Effect of Short-Term Heating on Photosynthetic Activity, Pigment Content, and Pro-/Antioxidant Balance of A. thaliana Phytochrome Mutants. Plants. 2023; 12(4):867. https://doi.org/10.3390/plants12040867
Chicago/Turabian StyleKreslavski, Vladimir D., Alexandra Y. Khudyakova, Anatoly A. Kosobryukhov, Tamara I. Balakhnina, Galina N. Shirshikova, Hesham F. Alharby, and Suleyman I. Allakhverdiev. 2023. "The Effect of Short-Term Heating on Photosynthetic Activity, Pigment Content, and Pro-/Antioxidant Balance of A. thaliana Phytochrome Mutants" Plants 12, no. 4: 867. https://doi.org/10.3390/plants12040867
APA StyleKreslavski, V. D., Khudyakova, A. Y., Kosobryukhov, A. A., Balakhnina, T. I., Shirshikova, G. N., Alharby, H. F., & Allakhverdiev, S. I. (2023). The Effect of Short-Term Heating on Photosynthetic Activity, Pigment Content, and Pro-/Antioxidant Balance of A. thaliana Phytochrome Mutants. Plants, 12(4), 867. https://doi.org/10.3390/plants12040867







