QTL Mapping for Important Agronomic Traits Using a Wheat55K SNP Array-Based Genetic Map in Tetraploid Wheat
Abstract
1. Introduction
2. Results
2.1. Phenotypic Variation
2.2. Correlation Analyses among Different Traits
2.3. Genetic Map Construction
2.4. Analysis of Segregation Distortions in the Genetic Map
2.5. QTL Mapping Analysis
2.6. QTL Cluster Analysis
2.7. Combined QTL–Environment Interaction Analysis
2.8. Epistatic Analysis
2.9. Effects of Stable Quantitative Trait Loci on Related Traits
2.10. Genetic Analysis of QPH.nwafu-2B.2, QAC.nwafu-3A.1, QAC.nwafu-4A.1, and QLWR.nwafu-7A.1
3. Discussion
3.1. Analysis of the Relation of Mapped QTLs to Those Found in Previous Studies
| Near Locus in Previous Studies | ||||||
|---|---|---|---|---|---|---|
| Traits | QTL | PVE (%) | Physical Position (MB) | Marker Interval | Physical Position (MB) | Reference |
| PH | QPH.nwafu-6A.1 | 51.10(Y19)/61.76(Y20)/66.27(Y21) /38.24(Y22)/63.87(BLUE) | 97.83–454.64 | IWA3230-IWB62878 (Rht18) | 412–445 | [48] |
| HD | QHD.nwafu-5A.1 | 14.88(Y21)/16.49(BLUE) | 493.27–661.33 | IWB4912-IWB38386 | 465.95–535.43 | [54] |
| IWA7665-IWB59530 | 512.12–527.66 | [46] | ||||
| IWB42031-IWB38885 | 587.19–612.37 | [46] | ||||
| IWA7162-wmc727 | 590.23–661.71 | [45] | ||||
| IWB51362-IWB68625 | 625.60–640.51 | [46] | ||||
| IWB14680-IWB11691 | 639.17–653.03 | [46] | ||||
| wmc727-IWB28907 | 645.16–661.37 | [46] | ||||
| SL | QSL.nwafu-2A | 14.13(Y22)/10.25(BLUE) | 319.88–532.72 | IWB51736-IWB66316 | 522.21–605.33 | WheatOmics 1.0 |
| QSL.nwafu-3A | 23.02(Y21)/12.06(Y22)/16.93(BLUE) | 714.71–736.11 | IWB12017-IWB67819 | 727.73–735.81 | [57] | |
| QSL.nwafu-4A.2 | 20.47(Y21) | 621.84–626.80 | IWB57472-IWB71581 | 625.84–641.73 | WheatOmics 1.0 | |
| QSL.nwafu-4A.4 | 5.76(Y22)/4.94(BLUE) | 530.42–536.90 | IWA7521-IWA2585 | 59.48–536.90 | WheatOmics 1.0 | |
| KPS | QKPS.nwafu-2B.3 | 10.64(Y21)/5.51(BLUE) | 100.91–128.12 | IWB26450-IWB44316 | 99.22–172.75 | [39] |
| QKPS.nwafu-4B.3 | 12.09(BLUE) | 487.64–658.68 | IWB53931-IWB73383 | 616.76–632.99 | [58] | |
| IWA1861-wPt-5265 | 651.91–668.84 | [39] | ||||
| IWB72179-IWB4448 | 651.91–658.51 | WheatOmics 1.0 | ||||
| QKPS.nwafu-5B.3 | 15.75(Y20) | 490.91–504.27 | IWB56759-IWB35093 | 475.37–510.43 | [39] | |
| TKW | QTKW.nwafu-1B.1 | 17.05(Y22)/8.53(BLUE) | 314.29–319.37 | gwm264-GWM274 | 33.99–594.22 | [54] |
| IWB9645-IWA3502 | 53.20–464.24 | [59] | ||||
| IWB9083-IWB6898 | 88.74–396.68 | [45] | ||||
| QTKW.nwafu-2B.3 | 11.45(BLUE) | 30.17–772.62 | IWA7916-IWB44381 | 53.45–63.97 | R. Patil et al. Euphytica 190 117–129 (2013) | |
| IWB26450-IWB44316 | 99.22–172.75 | [39] | ||||
| IWA5436-IWA7019 | 196.55–537.61 | [60] | ||||
| IWB49384-IWB7671 | 696.91–749.58 | [61] | ||||
| wPt-3651-IWB29332 | 702.45–733.56 | Z. Peleg et al. J. Exp. Bot. 62 5051–5061 (2011) | ||||
| IWA3474-IWB55786 | 765.31–789.41 | [45] | ||||
| QTKW.nwafu-5B.2 | 11.11(Y21) | 512.72–529.97 | IWB66909-wPt-3030 | 509.55–537.85 | [45] | |
| IWB72334-IWB65694 | 512.00–537.85 | [54] |
3.2. Segregation Distortion Regions in the Genetic Map
3.3. Candidate Gene Analysis of QPH.nwafu-2B.2, QAC.nwafu-3A.1, QAC.nwafu-4A.1, and QLWR.nwafu-7A.1
4. Materials and Methods
4.1. Plant Materials and Field Trials
4.2. Phenotypic Evaluation and Statistical Analysis
4.3. Genotyping and Genetic Map Construction
4.4. Quantitative Trait Loci Mapping
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mayer, K.F.X.; Rogers, J.; Doležel, J.; Pozniak, C.; Eversole, K.; Feuillet, C.; Gill, B.; Friebe, B.; Lukaszewski, A.J.; Sourdille, P.; et al. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef]
- Cao, S.; Xu, D.; Hanif, M.; Xia, X.; He, Z.H. Genetic architecture underpinning yield component traits in wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Rajaram, S.; Sayre, K.D. Physiological and Genetic Changes of Irrigated Wheat in the Post–Green Revolution Period and Approaches for Meeting Projected Global Demand. Crop Sci. 1999, 39, 1611–1621. [Google Scholar] [CrossRef]
- Ren, T.; Fan, T.; Chen, S.; Chen, Y.; Ou, X.; Jiang, Q.; Peng, W.; Ren, Z.; Tan, F.; Luo, P.; et al. Identification and validation of quantitative trait loci for the functional stay green trait in common wheat (Triticum aestivum L.) via high-density SNP-based genotyping. Theor. Appl. Genet. 2022, 135, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, K.; Song, W.; Zhong, N.; Wu, Y.; Fu, X. Improving Crop Nitrogen Use Efficiency Toward Sustainable Green Revolution. Annu. Rev. Plant Biol. 2022, 73, 523–551. [Google Scholar] [CrossRef]
- Guo, J.; Shi, W.; Zhang, Z.; Cheng, J.; Sun, D.; Yu, J.; Li, X.; Guo, P.; Hao, C. Association of yield-related traits in founder genotypes and derivatives of common wheat (Triticum aestivum L.). BMC Plant Biol. 2018, 18, 38. [Google Scholar] [CrossRef]
- Guo, H.; Xiong, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. Functional mutation allele mining of plant architecture and yield-related agronomic traits and characterization of their effects in wheat. BMC Genet. 2019, 20, 102. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Q.; Hao, C.; Hou, J.; Wang, L.; Zhang, H.; Zhang, S.; Chen, X.; Zhang, X. A yield-associated gene TaCWI, in wheat: Its function, selection and evolution in global breeding revealed by haplotype analysis. Theor. Appl. Genet. 2015, 128, 131–143. [Google Scholar] [CrossRef]
- Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; Poland, J.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Gupta, P.K.; Mir, R.R.; Mohan, A.; Kumar, J. Wheat Genomics: Present Status and Future Prospects. Int. J. Plant Genom. 2008, 2008, 896451. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, X.; Zhang, W.; Zhang, N.; Song, L.; Liu, L.; Xue, X.; Liu, G.; Liu, J.; Meng, D.; et al. QTL Detection for Kernel Size and Weight in Bread Wheat (Triticum aestivum L.) Using a High-Density SNP and SSR-Based Linkage Map. Front. Plant Sci. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Sriram, P.; Peng, Z.; Hare, R.A.; Sutherland, M.W.; Anke, M. Pentaploid Wheat Hybrids: Applications, Characterisation, and Challenges. Front. Plant Sci. 2017, 8, 358. [Google Scholar] [CrossRef]
- Padmanaban, S.; Sutherland, M.W.; Knight, N.L.; Martin, A. Genome inheritance in populations derived from hexaploid/tetraploid and tetraploid/hexaploid wheat crosses. Mol. Breed. 2017, 37, 48. [Google Scholar] [CrossRef]
- NÁTR, L. Crop Evolution, Adaptation and Yield. Photosynthetica 1998, 34, 56. [Google Scholar] [CrossRef]
- Cui, C.; Lu, Q.; Zhao, Z.; Lu, S.; Duan, S.; Yang, Y.; Qiao, Y.; Chen, L.; Hu, Y.G. The fine mapping of dwarf gene Rht5 in bread wheat and its effects on plant height and main agronomic traits. Planta 2022, 255, 114. [Google Scholar] [CrossRef]
- Wilhelm, E.P.; Howells, R.M.; Al-Kaff, N.; Jia, J.; Baker, C.; Leverington-Waite, M.A.; Griffiths, S.; Greenland, A.J.; Boulton, M.I.; Powell, W. Genetic characterization and mapping of the Rht-1 homoeologs and flanking sequences in wheat. Theor. Appl. Genet. 2013, 126, 1321–1336. [Google Scholar] [CrossRef]
- Jung, C.; Müller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Y.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. Genetic Mapping by Integration of 55K SNP Array and KASP Markers Reveals Candidate Genes for Important Agronomic Traits in Hexaploid Wheat. Front. Plant Sci. 2021, 12, 628478. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, G.; Zhang, L.; Xia, C.; Zhao, T.; Jia, J.; Liu, X.; Kong, X. Fine Mapping of a Novel Heading Date Gene, TaHdm605, in Hexaploid Wheat. Front. Plant Sci. 2018, 9, 1059. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Fu, D.; Szucs, P.; Yan, L.; Helguera, M.; Skinner, J.S.; von Zitzewitz, J.; Hayes, P.M.; Dubcovsky, J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 273, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nishida, H.; Zhu, J.; Nitcher, R.; Distelfeld, A.; Akashi, Y.; Kato, K.; Dubcovsky, J. Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat. Theor. Appl. Genet. 2010, 120, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kippes, N.; Zhu, J.; Chen, A.; Vanzetti, L.; Lukaszewski, A.; Nishida, H.; Kato, K.; Dvorak, J.; Dubcovsky, J. Fine mapping and epistatic interactions of the vernalization gene VRN-D4 in hexaploid wheat. Mol. Genet. Genom. 2014, 289, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.; Miyamae, M.; Kato, H.; Takumi, S.; Ogihara, Y. WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant Cell Physiol. 2003, 44, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Bullrich, L.; Appendino, L.; Tranquilli, G.; Lewis, S.; Dubcovsky, J. Mapping of a thermo-sensitive earliness per se gene on Triticum monococcum chromosome 1Am. Theor. Appl. Genet. 2002, 105, 585–593. [Google Scholar] [CrossRef]
- Wolde, G.M.; Schreiber, M.; Trautewig, C.; Himmelbach, A.; Sakuma, S.; Mascher, M.; Schnurbusch, T. Genome-wide identification of loci modifying spike-branching in tetraploid wheat. Theor. Appl. Genet. 2021, 134, 1925–1943. [Google Scholar] [CrossRef]
- Zhou, Y.; Conway, B.; Miller, D.; Marshall, D.; Cooper, A.; Murphy, P.; Chao, S.; Brown-Guedira, G.; Costa, J. Quantitative Trait Loci Mapping for Spike Characteristics in Hexaploid Wheat. Plant Genome 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Faris, J.D.; Zhang, Z.; Garvin, D.F.; Xu, S.S. Molecular and comparative mapping of genes governing spike compactness from wild emmer wheat. Mol. Genet. Genom. 2014, 289, 641–651. [Google Scholar] [CrossRef]
- Xu, B.J.; Chen, Q.; Zheng, T.; Jiang, Y.F.; Qiao, Y.Y.; Guo, Z.R.; Cao, Y.L.; Wang, Y.; Zhang, Y.Z.; Zong, L.J.; et al. An Overexpressed Q Allele Leads to Increased Spike Density and Improved Processing Quality in Common Wheat (Triticum aestivum). G3 Genes Genomes Genet. 2018, 8, 771–778. [Google Scholar] [CrossRef]
- Johnson, E.B.; Nalam, V.J.; Zemetra, R.S.; Riera-Lizarazu, O. Mapping the compactum locus in wheat (Triticum aestivum L.) and its relationship to other spike morphology genes of the Triticeae. Euphytica 2008, 163, 193–201. [Google Scholar] [CrossRef]
- Faris, J.D.; Fellers, J.P.; Brooks, S.A.; Gill, B.S. A bacterial artificial chromosome contig spanning the major domestication locus Q in wheat and identification of a candidate gene. Genetics 2003, 164, 311–321. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, H.; Wang, S.; Luo, W.; Gou, L.; Tang, H.; Mu, Y.; Deng, M.; Jiang, Q.; Chen, G.; et al. Spike Density Quantitative Trait Loci Detection and Analysis in Tetraploid and Hexaploid Wheat Recombinant Inbred Line Populations. Front. Plant Sci. 2021, 12, 796397. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.A.; Ding, A.; Jun, L.I.; Zhao, C.; Xingfeng, L.I.; Feng, D.; Wang, X.; Wang, L.; Gao, J.; Wang, H. Wheat kernel dimensions: How do they contribute to kernel weight at an individual QTL level? J. Genet. 2011, 90, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Ficco, D.; Laidò, G.; Marone, D.; Papa, R.; Blanco, A.; Gadaleta, A.; Vita, P.D.; Mastrangelo, A.M. A dense durum wheat × T. dicoccum linkage map based on SNP markers for the study of seed morphology. Mol. Breed. 2014, 34, 1579–1597. [Google Scholar] [CrossRef]
- Yu, M.; Mao, S.L.; Hou, D.B.; Chen, G.Y.; Pu, Z.E.; Li, W.; Lan, X.J.; Jiang, Q.T.; Liu, Y.X.; Deng, M. Analysis of contributors to grain yield in wheat at the individual quantitative trait locus level. Plant Breed. 2018, 137, 35–49. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xu, Y.; Ma, F.; Zhang, J.; Cao, Y.; Li, L.; An, D. Identification and validation of quantitative trait loci for kernel traits in common wheat (Triticum aestivum L.). BMC Plant Biol. 2020, 20, 529. [Google Scholar] [CrossRef]
- Ren, T.; Fan, T.; Chen, S.; Li, C.; Chen, Y.; Ou, X.; Jiang, Q.; Ren, Z.; Tan, F.; Luo, P.; et al. Utilization of a Wheat55K SNP array-derived high-density genetic map for high-resolution mapping of quantitative trait loci for important kernel-related traits in common wheat. Theor. Appl. Genet. 2021, 134, 807–821. [Google Scholar] [CrossRef]
- Fatiukha, A.; Filler, N.; Lupo, I.; Lidzbarsky, G.; Klymiuk, V.; Korol, A.B.; Pozniak, C.; Fahima, T.; Krugman, T. Grain protein content and thousand kernel weight QTLs identified in a durum × wild emmer wheat mapping population tested in five environments. Theor. Appl. Genet. 2020, 133, 119–131. [Google Scholar] [CrossRef]
- Mangini, G.; Gadaleta, A.; Colasuonno, P.; Marcotuli, I.; Signorile, A.M.; Simeone, R.; De Vita, P.; Mastrangelo, A.M.; Laidò, G.; Pecchioni, N.; et al. Genetic dissection of the relationships between grain yield components by genome-wide association mapping in a collection of tetraploid wheats. PLoS ONE 2018, 13, e0190162. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Meng, Y.; Hao, Y.; Xu, H.; Hao, M.; Lan, S.; Zhang, Y.; Lv, L.; Zhang, K.; et al. Dissection of Genetic Basis Underpinning Kernel Weight-Related Traits in Common Wheat. Plants 2021, 10, 713. [Google Scholar] [CrossRef]
- Ellis, M.H.; Rebetzke, G.J.; Azanza, F.; Richards, R.A.; Spielmeyer, W. Molecular mapping of gibberellin-responsive dwarfing genes in bread wheat. Theor. Appl. Genet. 2005, 111, 423–430. [Google Scholar] [CrossRef]
- Fu, Q.; Meng, X.; Luan, S.; Chen, B.; Cao, J.; Li, X.; Kong, J. Segregation distortion: High genetic load suggested by a Chinese shrimp family under high-intensity selection. Sci. Rep. 2020, 10, 21820. [Google Scholar] [CrossRef] [PubMed]
- Sa, K.J.; Park, J.Y.; Woo, S.Y.; Ramekar, R.V.; Jang, C.S.; Lee, J.K. Mapping of QTL traits in corn using a RIL population derived from a cross of dent corn × waxy corn. Genes Genom. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Hina, A.; Cao, Y.; Song, S.; Li, S.; Sharmin, R.A.; Elattar, M.A.; Bhat, J.A.; Zhao, T. High-Resolution Mapping in Two RIL Populations Refines Major “QTL Hotspot” Regions for Seed Size and Shape in Soybean (Glycine max L.). Int. J. Mol. Sci 2020, 21, 1040. [Google Scholar] [CrossRef] [PubMed]
- Roncallo, P.F.; Akkiraju, P.C.; Cervigni, G.L.; Echenique, V.C. QTL mapping and analysis of epistatic interactions for grain yield and yield-related traits in Triticum turgidum L. var. durum. Euphytica 2017, 213, 277. [Google Scholar] [CrossRef]
- Maccaferri, M.; Cane, M.A.; Sanguineti, M.C.; Salvi, S.; Colalongo, M.C.; Massi, A.; Clarke, F.; Knox, R.; Pozniak, C.J.; Clarke, J.M.; et al. A consensus framework map of durum wheat (Triticum durum Desf.) suitable for linkage disequilibrium analysis and genome-wide association mapping. BMC Genom. 2014, 15, 873. [Google Scholar] [CrossRef]
- Maccaferri, M.; Sanguineti, M.C.; Demontis, A.; El-Ahmed, A.; Garcia del Moral, L.; Maalouf, F.; Nachit, M.; Nserallah, N.; Ouabbou, H.; Rhouma, S.; et al. Association mapping in durum wheat grown across a broad range of water regimes. J. Exp. Bot. 2011, 62, 409–438. [Google Scholar] [CrossRef]
- Tang, T. Physiological and Genetic Studies of an Alternative Semi-Dwarfing Gene Rht18 in Wheat. Ph.D. Thesis, University of Tasmania, Tasmania, Australia, 2016. [Google Scholar]
- Tang, T.; Botwright Acuña, T.; Spielmeyer, W.; Richards, R.A. Effect of gibberellin-sensitive Rht18 and gibberellin-insensitive Rht-D1b dwarfing genes on vegetative and reproductive growth in bread wheat. J. Exp. Bot. 2021, 72, 445–458. [Google Scholar] [CrossRef]
- Ford, B.A.; Foo, E.; Sharwood, R.; Karafiatova, M.; Vrána, J.; MacMillan, C.; Nichols, D.S.; Steuernagel, B.; Uauy, C.; Doležel, J.; et al. Rht18 Semidwarfism in Wheat Is Due to Increased GA 2-oxidaseA9 Expression and Reduced GA Content. Plant Physiol. 2018, 177, 168–180. [Google Scholar] [CrossRef]
- Vikhe, P.; Patil, R.; Chavan, A.; Oak, M.; Tamhankar, S. Mapping gibberellin-sensitive dwarfing locus Rht18 in durum wheat and development of SSR and SNP markers for selection in breeding. Mol. Breed. 2017, 37, 28. [Google Scholar] [CrossRef]
- Dyck, J.A.; Matus-Cádiz, M.A.; Hucl, P.; Talbert, L.; Hunt, T.; Dubuc, J.P.; Nass, H.; Clayton, G.; Dobb, J.; Quick, J. Agronomic Performance of Hard Red Spring Wheat Isolines Sensitive and Insensitive to Photoperiod. Crop Sci. 2004, 44, 1976–1981. [Google Scholar] [CrossRef]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ronin, Y.; Fahima, T.; Röder, M.S.; Li, Y.; Nevo, E.; Korol, A. Domestication quantitative trait loci in Triticum dicoccoides, the progenitor of wheat. Proc. Natl. Acad. Sci. USA 2003, 100, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef] [PubMed]
- Shitsukawa, N.; Ikari, C.; Mitsuya, T.; Sakiyama, T.; Ishikawa, A.; Takumi, S.; Murai, K. Wheat SOC1 functions independently of WAP1/VRN1, an integrator of vernalization and photoperiod flowering promotion pathways. Physiol. Plant. 2007, 130, 627–636. [Google Scholar] [CrossRef]
- Giraldo, P.; Royo, C.; González, M.; Carrillo, J.M.; Ruiz, M. Genetic Diversity and Association Mapping for Agromorphological and Grain Quality Traits of a Structured Collection of Durum Wheat Landraces Including subsp. durum, turgidum and diccocon. PLoS ONE 2016, 11, e0166577. [Google Scholar] [CrossRef]
- Kidane, Y.G.; Hailemariam, B.N.; Mengistu, D.K.; Fadda, C.; Pè, M.E.; Dell’Acqua, M. Genome-Wide Association Study of Septoria tritici Blotch Resistance in Ethiopian Durum Wheat Landraces. Front. Plant Sci. 2017, 8, 1586. [Google Scholar] [CrossRef]
- Graziani, M.; Maccaferri, M.; Royo, C.; Salvatorelli, F.; Tuberosa, R. QTL dissection of yield components and morpho-physiological traits in a durum wheat elite population tested in contrasting thermo-pluviometric conditions. Crop Pasture Sci. 2014, 65, 80–95. [Google Scholar] [CrossRef]
- Faris, J.D.; Zhang, Q.; Chao, S.; Zhang, Z.; Xu, S.S. Analysis of agronomic and domestication traits in a durum × cultivated emmer wheat population using a high-density single nucleotide polymorphism-based linkage map. Theor. Appl. Genet. 2014, 127, 2333–2348. [Google Scholar] [CrossRef]
- Soriano, J.M.; Malosetti, M.; Roselló, M.; Sorrells, M.E.; Royo, C. Dissecting the old Mediterranean durum wheat genetic architecture for phenology, biomass and yield formation by association mapping and QTL meta-analysis. PLoS ONE 2017, 12, e0178290. [Google Scholar] [CrossRef]
- Ma, L.; Li, T.; Hao, C.; Wang, Y.; Chen, X.; Zhang, X. TaGS5-3A, a grain size gene selected during wheat improvement for larger kernel and yield. Plant Biotechnol. J. 2016, 14, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, J.; Xie, X.; Xu, Q.; Tang, H.; Mu, Y.; Pu, Z.; Li, Y.; Ma, J.; Gao, Y.; et al. Genetic Mapping and Validation of Loci for Kernel-Related Traits in Wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 667493. [Google Scholar] [CrossRef] [PubMed]
- Poursarebani, N.; Seidensticker, T.; Koppolu, R.; Trautewig, C.; Gawroński, P.; Bini, F.; Govind, G.; Rutten, T.; Sakuma, S.; Tagiri, A.; et al. The Genetic Basis of Composite Spike Form in Barley and ‘Miracle-Wheat’. Genetics 2015, 201, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Hou, F.; Chen, J.; Chen, S.L.; Xing, L.P.; Feng, Y.G.; Cao, A.Z. Agronomic characterization and genetic analysis of the supernumerary spikelet in tetraploid wheat (Triticum turgidum L.). J. Integr. Agric. 2017, 16, 1304–1311. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.B.; Pont, C.; Orlov, Y.L.; Salse, J. Development of new SSR markers for homoeologous WFZP gene loci based on the study of the structure and location of microsatellites in gene-rich regions of chromosomes 2AS, 2BS, and 2DS in bread wheat. Russ. J. Genet. Appl. Res. 2016, 6, 330–337. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.B.; Amagai, Y.; Popova, K.I.; Dresvyannikova, A.E.; Martinek, P.; Krasnikov, A.A.; Watanabe, N. Genes WHEAT FRIZZY PANICLE and SHAM RAMIFICATION 2 independently regulate differentiation of floral meristems in wheat. BMC Plant Biol. 2017, 17, 252. [Google Scholar] [CrossRef]
- Wang, Z. Distorted segregation in plant hybrids and its implication for evolution. Yi Chuan. 2016, 38, 801–810. [Google Scholar] [CrossRef]
- Lyttle, T.W. Segregation distorters. Annu. Rev. Genet. 1991, 25, 511–557. [Google Scholar] [CrossRef]
- Zuo, J.F.; Niu, Y.; Cheng, P.; Feng, J.Y.; Han, S.F.; Zhang, Y.H.; Shu, G.; Wang, Y.; Zhang, Y.M. Effect of marker segregation distortion on high density linkage map construction and QTL mapping in Soybean (Glycine max L.). Heredity 2019, 123, 579–592. [Google Scholar] [CrossRef]
- Hackett, C.A.; Broadfoot, L.B. Effects of genotyping errors, missing values and segregation distortion in molecular marker data on the construction of linkage maps. Heredity 2003, 90, 33–38. [Google Scholar] [CrossRef]
- Ellstrand, N.C. Is gene flow the most important evolutionary force in plants? Am. J. Bot. 2014, 101, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Ingvarsson, P.K. Common features of segregation distortion in plants and animals. Genetica 2003, 117, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xiao, J.; Gu, T.; Yu, X.; Zhang, Y.; Chang, J.; Yang, G.; He, G. Genome-wide identification and analysis of WD40 proteins in wheat (Triticum aestivum L.). BMC Genom. 2018, 19, 803. [Google Scholar] [CrossRef]
- Feng, C.; Andreasson, E.; Maslak, A.; Mock, H.P.; Mundy, J. Arabidopsis MYB68 in development and responses to environmental cues. Plant Sci. 2004, 167, 1099–1107. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, H.J.; Ryu, J.S.; Choi, H.; Jeong, S.; Shin, J.; Choi, G.; Nam, H.G. CRY1 inhibits COP1-mediated degradation of BIT1, a MYB transcription factor, to activate blue light-dependent gene expression in Arabidopsis. Plant J. 2008, 55, 361–371. [Google Scholar] [CrossRef]
- Yang, S.W.; Jang, I.C.; Henriques, R.; Chua, N.H. FAR-RED ELONGATED HYPOCOTYL1 and FHY1-LIKE associate with the Arabidopsis transcription factors LAF1 and HFR1 to transmit phytochrome A signals for inhibition of hypocotyl elongation. Plant Cell 2009, 21, 1341–1359. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.; Bednarek, P.; Weisshaar, B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhu, H.; Huang, C.; Liu, C.; Chang, Y.; Kong, Z.; Zhou, Z.; Wang, G.; Lin, Y.; et al. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol. 2019, 223, 705–721. [Google Scholar] [CrossRef]
- van den Burg, H.A.; Tsitsigiannis, D.I.; Rowland, O.; Lo, J.; Rallapalli, G.; Maclean, D.; Takken, F.L.; Jones, J.D. The F-box protein ACRE189/ACIF1 regulates cell death and defense responses activated during pathogen recognition in tobacco and tomato. Plant Cell 2008, 20, 697–719. [Google Scholar] [CrossRef]
- Chandler, J.W. Auxin response factors. Plant Cell Environ. 2016, 39, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, L.; Shen, C.; Ji, Z.; Zhang, H.; Zhang, T.; Li, Y.; Yu, J.; Yang, N.; He, Y.; et al. Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. Plant Cell 2021, 33, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.D.; Punyarani, K.; Singh, N.S.; Devi, H.S. An efficient protocol for total DNA extraction from the members of order Zingiberales-suitable for diverse PCR based downstream applications. SpringerPlus 2013, 2, 669. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Zhu, J.; Wei, J.; Zhou, J.; Xu, Q.; Tang, H.; Mu, Y.; Deng, M.; Jiang, Q.; Liu, Y.; et al. The 55K SNP-Based Exploration of QTLs for Spikelet Number Per Spike in a Tetraploid Wheat (Triticum turgidum L.) Population: Chinese Landrace “Ailanmai” × Wild Emmer. Front. Plant Sci. 2021, 12, 732837. [Google Scholar] [CrossRef]
- Qu, X.; Li, C.; Liu, H.; Liu, J.; Luo, W.; Xu, Q.; Tang, H.; Mu, Y.; Deng, M.; Pu, Z.; et al. Quick mapping and characterization of a co-located kernel length and thousand-kernel weight-related QTL in wheat. Theor. Appl. Genet. 2022, 135, 2849–2860. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, L. Inclusive Composite Interval Mapping of QTL by Environment Interactions in Biparental Populations. PLoS ONE 2015, 10, e0132414. [Google Scholar] [CrossRef]




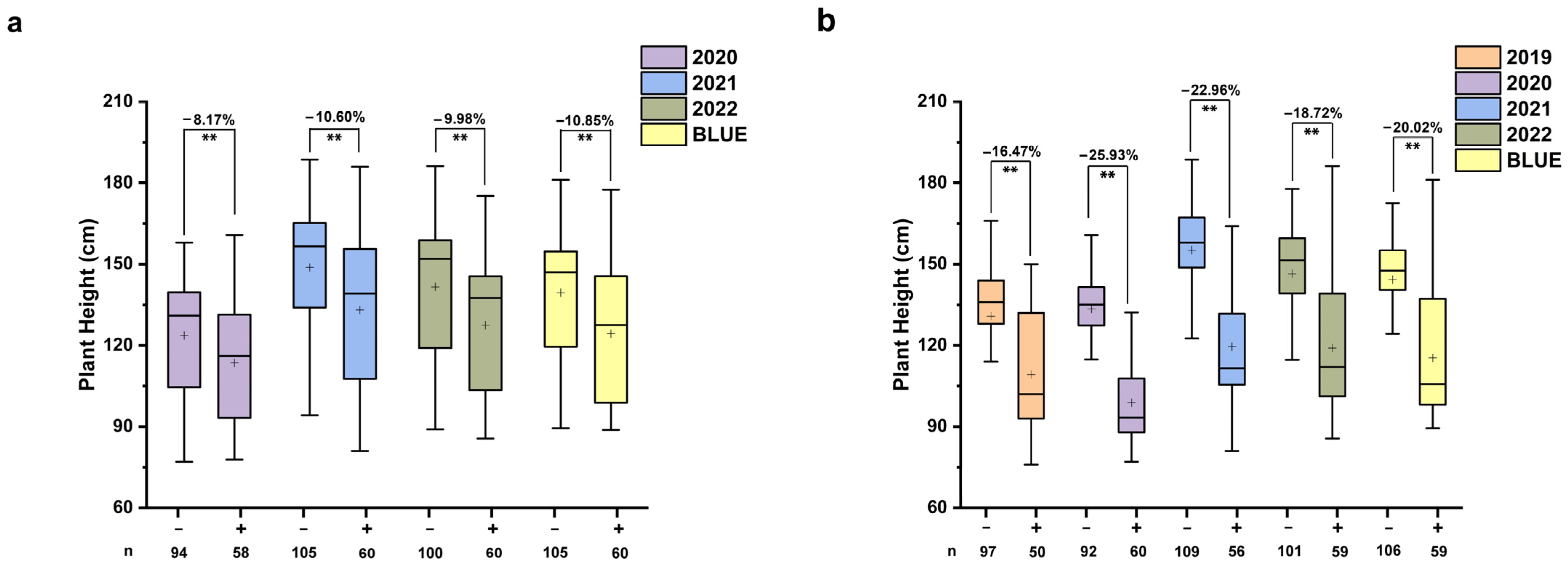
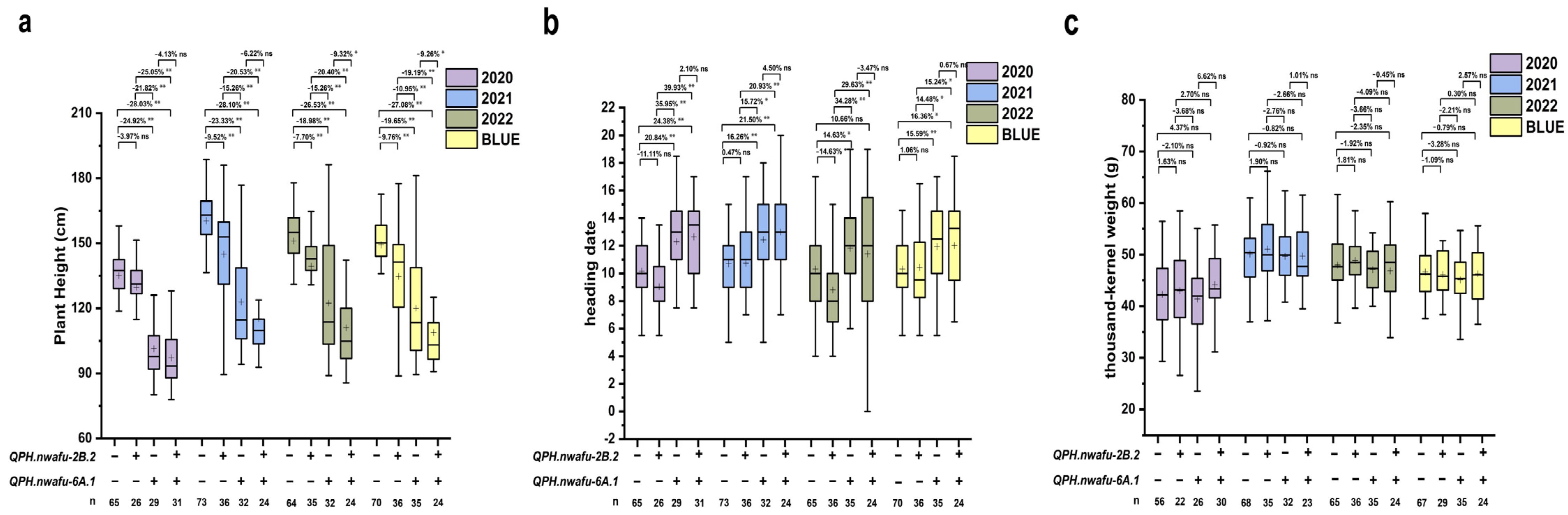

| Traits | Year | Parental Lines | RIL Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Icaro | Y4 | Min | Max | Mean | SD | CV% | Skewness | Kurtosis | H2 | ||
| PH | Y19 | 78.6 | 158.5 | 70.0 | 171.0 | 123.48 | 24.39 | 19.8 | −0.425 | −0.979 | 0.96 |
| Y20 | 80.6 | 156.8 | 77.0 | 160.8 | 119.83 | 22.65 | 18.9 | −0.366 | −1.182 | ||
| Y21 | 81.4 | 168.4 | 81.0 | 188.6 | 143.11 | 26.75 | 18.7 | −0.441 | −1.091 | ||
| Y22 | 83.0 | 159.8 | 85.6 | 186.3 | 136.33 | 24.96 | 18.3 | −0.390 | −1.100 | ||
| BLUE | 76.1 | 157.3 | 88.8 | 181.2 | 133.97 | 24.39 | 18.2 | −0.433 | −1.143 | ||
| HD | Y20 | 6 | 10 | 0 | 19 | 10.89 | 2.75 | 25.2 | −0.241 | 0.713 | 0.83 |
| Y21 | 5 | 10 | 0 | 20 | 11.38 | 2.90 | 25.4 | −0.320 | 0.768 | ||
| Y22 | 3 | 8 | 0 | 19 | 10.48 | 3.38 | 32.2 | 0.022 | −0.225 | ||
| BLUE | 4 | 9 | 0 | 18.5 | 10.94 | 2.90 | 26.5 | −0.180 | 0.173 | ||
| SL | Y20 | 6.2 | 9.9 | 5.5 | 12.4 | 8.95 | 1.15 | 12.8 | −0.105 | 0.142 | 0.76 |
| Y21 | 6.6 | 10.7 | 5.8 | 12.8 | 9.33 | 1.42 | 15.2 | −0.053 | −0.137 | ||
| Y22 | 6.5 | 10.4 | 5.6 | 12.2 | 8.32 | 1.23 | 14.7 | 0.227 | −0.081 | ||
| BLUE | 6.6 | 10.3 | 6.0 | 11.7 | 8.81 | 1.20 | 13.6 | −0.114 | −0.408 | ||
| KPS | Y20 | 45 | 60 | 18 | 64 | 42.25 | 8.27 | 19.6 | 0.179 | 0.391 | 0.50 |
| Y21 | 56 | 78 | 26 | 134 | 74.35 | 17.80 | 23.9 | 0.439 | 0.451 | ||
| Y22 | 58 | 84 | 33 | 100 | 60.14 | 12.35 | 20.5 | 0.236 | 0.191 | ||
| BLUE | 56 | 81 | 36 | 92 | 58.15 | 9.04 | 15.5 | 0.246 | 0.751 | ||
| TKW | Y20 | 43.16 | 49.27 | 19.00 | 60.97 | 42.72 | 7.48 | 17.5 | −0.286 | 0.094 | 0.64 |
| Y21 | 46.06 | 51.89 | 31.98 | 75.58 | 50.19 | 6.56 | 13.1 | 0.711 | 1.797 | ||
| Y22 | 41.20 | 46.09 | 25.94 | 64.17 | 47.82 | 5.38 | 11.2 | −0.247 | 1.423 | ||
| BLUE | 42.74 | 49.89 | 33.56 | 67.01 | 46.21 | 5.06 | 10.9 | 0.541 | 1.007 | ||
| KL | Y20 | 5.79 | 7.38 | 5.69 | 7.65 | 6.36 | 0.89 | 14.0 | −1.344 | 0.931 | 0.41 |
| Y21 | 5.84 | 7.04 | 5.09 | 7.36 | 6.72 | 0.22 | 3.3 | −0.033 | 0.493 | ||
| Y22 | 6.40 | 7.57 | 6.19 | 8.21 | 7.28 | 0.42 | 5.8 | −0.222 | −0.258 | ||
| BLUE | 5.93 | 7.48 | 5.49 | 7.55 | 6.79 | 0.39 | 5.7 | −0.803 | 0.725 | ||
| KW | Y20 | 2.69 | 3.37 | 2.63 | 3.43 | 2.99 | 0.39 | 13.1 | −1.107 | 0.469 | 0.27 |
| Y21 | 2.71 | 3.26 | 2.41 | 3.32 | 2.84 | 0.12 | 4.1 | 0.323 | 3.112 | ||
| Y22 | 3.10 | 3.44 | 2.83 | 3.96 | 3.41 | 0.20 | 6.0 | −0.270 | 0.271 | ||
| BLUE | 2.87 | 3.41 | 2.65 | 3.40 | 2.98 | 0.16 | 5.5 | −0.316 | −0.023 | ||
| LWR | Y20 | 2.15 | 2.19 | 1.92 | 3.01 | 2.31 | 0.17 | 7.6 | 0.711 | 1.165 | 0.86 |
| Y21 | 2.15 | 2.16 | 1.76 | 2.48 | 2.04 | 0.14 | 6.8 | 0.348 | −0.351 | ||
| Y22 | 2.07 | 2.20 | 1.82 | 2.57 | 2.15 | 0.16 | 7.2 | 0.066 | −0.610 | ||
| BLUE | 2.11 | 2.20 | 1.83 | 2.56 | 2.17 | 0.14 | 6.3 | 0.231 | −0.400 | ||
| Peri | Y20 | 17.21 | 19.43 | 15.46 | 19.42 | 16.17 | 2.34 | 14.5 | −1.481 | 1.131 | 0.26 |
| Y21 | 18.45 | 18.57 | 14.77 | 20.50 | 18.43 | 0.52 | 2.8 | 0.366 | 2.933 | ||
| Y22 | 18.75 | 19.27 | 16.47 | 21.27 | 18.92 | 0.96 | 5.1 | −0.152 | −0.145 | ||
| BLUE | 18.49 | 19.17 | 15.79 | 20.63 | 18.84 | 0.92 | 4.9 | −1.001 | 1.064 | ||
| Area | Y20 | 15.12 | 19.03 | 14.89 | 19.22 | 17.64 | 3.27 | 18.5 | −1.090 | 0.402 | 0.20 |
| Y21 | 15.20 | 16.27 | 13.55 | 17.91 | 16.40 | 0.55 | 3.4 | 0.702 | 4.428 | ||
| Y22 | 17.44 | 19.41 | 14.29 | 23.69 | 18.86 | 1.72 | 9.1 | −0.184 | 0.080 | ||
| BLUE | 16.28 | 18.06 | 14.11 | 20.59 | 17.64 | 1.30 | 7.4 | −0.5 | 0.180 | ||
| AC | Y19 | 0 | 2 | 0 | 2.00 | 0.46 | 0.84 | 0.90 | |||
| Y20 | 0 | 2 | 0 | 2.00 | 0.65 | 0.94 | |||||
| Y21 | 0 | 2 | 0 | 2.00 | 0.42 | 0.82 | |||||
| Y22 | 0 | 2 | 0 | 2.00 | 0.40 | 0.80 | |||||
| SB | Y20 | 0 | 2 | 0 | 2.00 | 0.58 | 0.94 | 0.95 | |||
| Y21 | 0 | 2 | 0 | 2.00 | 0.72 | 0.96 | |||||
| Y22 | 0 | 2 | 0 | 2.00 | 1.45 | 0.90 | |||||
| Traits | PH | HD | SL | KPS | TKW | KL | KW | LWR | Peri | Area | AC | SB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 1 | |||||||||||
| HD | −0.521 ** | 1 | ||||||||||
| SL | 0.004 | −0.011 | 1 | |||||||||
| KPS | 0.043 | 0.062 | 0.223 ** | 1 | ||||||||
| TKW | −0.061 | 0.054 | −0.063 | −0.558 ** | 1 | |||||||
| KL | −0.082 | 0.087 | −0.257 ** | −0.277 ** | 0.292 ** | 1 | ||||||
| KW | −0.148 | 0.155 | 0.025 | −0.286 ** | 0.532 ** | 0.515 ** | 1 | |||||
| LWR | 0.041 | −0.045 | −0.281 ** | −0.021 | −0.213 ** | 0.556 ** | −0.419 ** | 1 | ||||
| Peri | −0.109 | 0.116 | −0.234 ** | −0.307 ** | 0.362 ** | 0.974 ** | 0.684 ** | 0.367 ** | 1 | |||
| Area | −0.152 | 0.156 * | −0.150 | −0.349 ** | 0.529 ** | 0.864 ** | 0.854 ** | 0.084 | 0.943 ** | 1 | ||
| AC | −0.135 | 0.022 | 0.354 ** | 0.074 | −0.095 | −0.379 ** | −0.076 | −0.321 ** | −0.346 ** | −0.253 ** | 1 | |
| SB | 0.101 | 0.022 | 0.089 | 0.288 ** | −0.219 ** | −0.047 | −0.100 | 0.046 | −0.077 | −0.094 | −0.077 | 1 |
| Traits | QTL | Year | Interval (cM) | Left Marker | Right Marker | LOD | PVE (%) | Add |
|---|---|---|---|---|---|---|---|---|
| PH | QPH.nwafu-6A.1 | Y19 | 197.82–201.86 | AX-110419766 | AX-111161134 | 24.46 | 51.10 | 19.34 |
| Y20 | 197.82–201.86 | AX-110419766 | AX-111161134 | 47.89 | 61.76 | 20.65 | ||
| Y21 | 197.82–201.86 | AX-110419766 | AX-111161134 | 38.83 | 66.27 | 22.59 | ||
| Y22 | 197.82–201.86 | AX-110419766 | AX-111161134 | 19.73 | 38.24 | 13.08 | ||
| BLUE | 197.82–201.86 | AX-110419766 | AX-111161134 | 43.49 | 63.87 | 20.88 | ||
| QPH.nwafu-6A.2 | Y22 | 203.72–214.18 | AX-111634639 | AX-111800524 | 12.72 | 22.59 | 10.09 | |
| QPH.nwafu-2B.2 | Y20 | 165.67–166.99 | AX-109015706 | AX-110372053 | 3.84 | 2.40 | 4.09 | |
| Y21 | 165.67–166.99 | AX-109015706 | AX-110372053 | 4.08 | 3.96 | 5.46 | ||
| Y22 | 165.67–166.99 | AX-109015706 | AX-110372053 | 4.33 | 6.30 | 5.29 | ||
| BLUE | 165.67–166.99 | AX-109015706 | AX-110372053 | 6.81 | 5.64 | 6.17 | ||
| HD | QHD.nwafu-5A.1 | Y21 | 242.25–261.17 | AX-111126249 | AX-110368031 | 10.32 | 14.88 | 1.23 |
| BLUE | 242.25–261.17 | AX-111126249 | AX-110368031 | 7.80 | 16.49 | 1.14 | ||
| QHD.nwafu-6A.1 | Y22 | 197.82–201.86 | AX-110419766 | AX-111161134 | 11.88 | 26.54 | −1.83 | |
| QHD.nwafu-6A.2 | Y21 | 203.72–214.18 | AX-111634639 | AX-111800524 | 8.22 | 11.56 | −1.11 | |
| BLUE | 203.72–214.18 | AX-111634639 | AX-111800524 | 9.24 | 20.03 | −1.29 | ||
| SL | QSL.nwafu-2A | Y22 | 0–71.16 | AX-110162878 | AX-109313122 | 3.35 | 14.13 | −0.51 |
| BLUE | 0–71.16 | AX-110162878 | AX-109313122 | 3.04 | 10.25 | −0.41 | ||
| QSL.nwafu-3A | Y21 | 223.21–244.18 | AX-111762953 | AX-109078462 | 19.53 | 23.02 | 0.78 | |
| Y22 | 223.21–244.18 | AX-111762953 | AX-109078462 | 8.59 | 12.06 | 0.48 | ||
| BLUE | 223.21–244.18 | AX-111762953 | AX-109078462 | 11.16 | 16.93 | 0.53 | ||
| QSL.nwafu-4A.2 | Y21 | 303.85–305.14 | AX-111040045 | AX-110567219 | 16.49 | 20.47 | −0.74 | |
| QSL.nwafu-4A.3 | Y22 | 305.14–309.21 | AX-109849268 | AX-109369221 | 4.97 | 13.23 | −0.50 | |
| BLUE | 305.14–309.21 | AX-109849268 | AX-109369221 | 12.19 | 21.25 | −0.59 | ||
| QSL.nwafu-4A.4 | Y22 | 380.01–381.92 | AX-111067027 | AX-109393001 | 4.30 | 5.76 | 0.34 | |
| BLUE | 380.01–381.92 | AX-111067027 | AX-109393001 | 5.20 | 4.94 | 0.30 | ||
| QSL.nwafu-6A | Y21 | 196.86–197.82 | AX-108958326 | AX-110419766 | 7.77 | 6.03 | 0.41 | |
| BLUE | 197.82–201.86 | AX-110419766 | AX-111161134 | 4.10 | 3.78 | 0.26 | ||
| KPS | QKPS.nwafu-2B.3 | Y21 | 253.45–261.6 | AX-110430429 | AX-109272515 | 10.32 | 10.64 | −8.18 |
| BLUE | 253.45–261.6 | AX-110430429 | AX-109272515 | 4.16 | 5.51 | −2.77 | ||
| QKPS.nwafu-4B.2 | Y22 | 97.11–104.58 | AX-109617174 | AX-109533971 | 4.31 | 11.48 | 4.06 | |
| QKPS.nwafu-4B.3 | BLUE | 140.19–148.79 | AX-110070905 | AX-111539361 | 6.46 | 12.09 | −4.35 | |
| QKPS.nwafu-5B.3 | Y20 | 207.16–220.24 | AX-110502417 | AX-109487166 | 5.07 | 15.75 | 3.27 | |
| TKW | QTKW.nwafu-1B.1 | Y22 | 84.86–87.74 | AX-109890962 | AX-110926323 | 15.18 | 17.05 | −2.73 |
| BLUE | 84.86–87.74 | AX-109890962 | AX-110926323 | 7.98 | 8.53 | −1.92 | ||
| QTKW.nwafu-2B.3 | BLUE | 376.91–390.54 | AX-109281639 | AX-111105832 | 10.27 | 11.45 | 2.27 | |
| QTKW.nwafu-3B.2 | Y20 | 181.92–183.61 | AX-108884614 | AX-108894802 | 2.59 | 9.61 | −2.16 | |
| BLUE | 181.92–183.61 | AX-108884614 | AX-108894802 | 10.50 | 12.29 | −2.36 | ||
| QTKW.nwafu-5B.2 | Y21 | 134.2–138.55 | AX-110907796 | AX-110093465 | 7.24 | 11.11 | −2.34 | |
| KL | QKL.nwafu-6A | BLUE | 92.81–94.79 | AX-109915394 | AX-108914846 | 9.08 | 16.04 | −0.17 |
| QKL.nwafu-7A.1 | Y21 | 148.15–159.54 | AX-110077383 | AX-110632223 | 10.70 | 18.32 | −0.12 | |
| QKL.nwafu-7A.2 | Y22 | 255.14–270.77 | AX-111157442 | AX-110558104 | 5.74 | 13.14 | −0.15 | |
| QKL.nwafu-5B.1 | Y21 | 223.53–232.19 | AX-110404958 | AX-109941486 | 5.09 | 6.61 | −0.07 | |
| Y22 | 223.53–232.19 | AX-110404958 | AX-109941486 | 10.39 | 20.41 | −0.17 | ||
| QKL.nwafu-5B.2 | Y20 | 266.74–278 | AX-109040704 | AX-110503586 | 4.56 | 16.00 | −0.35 | |
| QKL.nwafu-5B.3 | BLUE | 296.97–298.76 | AX-111055910 | AX-111091795 | 10.44 | 14.47 | −0.16 | |
| QKL.nwafu-6B | Y22 | 275.64–280.48 | AX-110667505 | AX-110522948 | 4.43 | 11.76 | −0.14 | |
| KW | QKW.nwafu-3A | Y22 | 273.22–286.42 | AX-108870115 | AX-108920525 | 3.91 | 11.44 | 0.08 |
| QKW.nwafu-6A | Y22 | 310.88–360.8 | AX-108781069 | AX-108929747 | 4.11 | 10.99 | −0.08 | |
| QKW.nwafu-4B.2 | Y21 | 112.72–113.07 | AX-111221393 | AX-111254019 | 6.82 | 11.11 | 0.05 | |
| LWR | QLWR.nwafu-3A.2 | Y20 | 336.79–358.98 | AX-111806403 | AX-109925527 | 6.40 | 8.96 | −0.08 |
| BLUE | 336.79–358.98 | AX-111806403 | AX-109925527 | 8.50 | 11.19 | −0.05 | ||
| QLWR.nwafu-7A.1 | Y21 | 166.66–175.46 | AX-111545214 | AX-111513167 | 9.10 | 12.12 | 0.06 | |
| Y22 | 166.66–175.46 | AX-111545214 | AX-111513167 | 13.12 | 26.22 | 0.08 | ||
| BLUE | 166.66–175.46 | AX-111545214 | AX-111513167 | 10.05 | 12.63 | 0.06 | ||
| QLWR.nwafu-1B.4 | Y21 | 471.93–473.05 | AX-111618813 | AX-110394873 | 7.24 | 8.13 | −0.05 | |
| BLUE | 473.05–504.63 | AX-110394873 | AX-111114335 | 6.96 | 7.76 | −0.05 | ||
| QLWR.nwafu-5B.5 | Y21 | 305.92–313.88 | AX-110560995 | AX-109307903 | 2.56 | 10.99 | −0.05 | |
| QLWR.nwafu-6B | Y22 | 155.31–157.29 | AX-89438665 | AX-109417511 | 3.16 | 4.85 | −0.03 | |
| BLUE | 155.31–157.29 | AX-89438665 | AX-109417511 | 5.45 | 5.39 | −0.04 | ||
| Peri | QPeri.nwafu-7A.1 | Y21 | 148.15–159.54 | AX-110077383 | AX-110632223 | 2.95 | 15.11 | −0.17 |
| QPeri.nwafu-7A.2 | Y22 | 255.14–270.77 | AX-111157442 | AX-110558104 | 4.22 | 12.57 | −0.36 | |
| QPeri.nwafu-3B | Y22 | 191.33–199.4 | AX-110989965 | AX-109858559 | 6.98 | 13.48 | −0.35 | |
| QPeri.nwafu-5B.1 | Y22 | 220.24–222.15 | AX-109487166 | AX-111002136 | 8.59 | 16.15 | −0.38 | |
| QPeri.nwafu-5B.2 | Y20 | 278–282.23 | AX-110503586 | AX-111034200 | 4.45 | 13.82 | −0.87 | |
| Area | QArea.nwafu-1B.1 | BLUE | 52.48–62.09 | AX-111221126 | AX-109449137 | 4.36 | 12.90 | −0.48 |
| QArea.nwafu-1B.2 | Y22 | 74.29–79.16 | AX-108788663 | AX-109304925 | 7.33 | 13.51 | −0.75 | |
| QArea.nwafu-5B.2 | Y22 | 207.16–220.24 | AX-110502417 | AX-109487166 | 8.45 | 15.40 | −0.77 | |
| AC | QAC.nwafu-3A.1 | Y21 | 419.89–420.52 | AX-108885585 | AX-110531758 | 8.60 | 8.12 | 0.29 |
| Y22 | 419.89–420.52 | AX-108885585 | AX-110531758 | 7.93 | 7.90 | 0.29 | ||
| QAC.nwafu-3A.2 | Y21 | 441.43–451.72 | AX-111212311 | AX-110977533 | 6.54 | 6.05 | −0.27 | |
| Y22 | 427.82–441.43 | AX-111634183 | AX-111212311 | 5.32 | 5.50 | −0.26 | ||
| QAC.nwafu-4A.1 | Y21 | 424.31–447.4 | AX-109406677 | AX-111618007 | 6.11 | 8.92 | 0.32 | |
| Y22 | 424.31–447.4 | AX-109406677 | AX-111618007 | 4.00 | 7.01 | 0.28 | ||
| QAC.nwafu-4A.2 | Y20 | 447.4–491.27 | AX-111618007 | AX-109316763 | 4.56 | 13.72 | 0.60 | |
| QAC.nwafu-1B.3 | Y19 | 450.74–471.93 | AX-109437886 | AX-111618813 | 54.49 | 5.38 | 0.94 | |
| Y20 | 450.74–471.93 | AX-109437886 | AX-111618813 | 26.39 | 21.75 | 0.75 | ||
| QAC.nwafu-1B.4 | Y21 | 473.05–504.63 | AX-110394873 | AX-111114335 | 24.32 | 31.77 | 0.70 | |
| Y22 | 473.05–504.63 | AX-110394873 | AX-111114335 | 15.10 | 28.50 | 0.69 | ||
| SB | QSB.nwafu-2B.1 | Y21 | 253.45–261.6 | AX-110430429 | AX-109272515 | 8.11 | 10.00 | −0.39 |
| Y22 | 253.45–261.6 | AX-110430429 | AX-109272515 | 16.46 | 11.20 | −0.60 | ||
| QSB.nwafu-2B.2 | Y21 | 261.6–268.73 | AX-109272515 | AX-111680306 | 5.59 | 8.97 | 0.38 | |
| Y22 | 261.6–268.73 | AX-109272515 | AX-111680306 | 53.74 | 23.16 | −0.94 | ||
| QSB.nwafu-5B | Y21 | 125.85–134.2 | AX-111508809 | AX-110907796 | 3.33 | 15.55 | −0.49 |
| Traits | cQTL | Position | Interval (cM) | Left Marker | Right Marker | LOD | LOD (A) | LOD (A by E) | PVE | PVE (A) | PVE (A by E) | Add |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | cQPH.nwafu-6A.2 | 198 | 197.82–201.86 | AX-110419766 | AX-111161134 | 115.63 | 93.28 | 22.35 | 53.57 | 51.32 | 2.25 | 16.12 |
| cQPH.nwafu-6A.3 | 206 | 203.72–214.18 | AX-111634639 | AX-111800524 | 13.77 | 8.35 | 5.42 | 4.27 | 2.61 | 1.67 | 3.66 | |
| cQPH.nwafu-2B.2 | 166 | 165.67–166.99 | AX-109015706 | AX-110372053 | 13.31 | 11.17 | 2.13 | 3.83 | 3.55 | 0.28 | 4.25 | |
| HD | cQHD.nwafu-5A | 261 | 242.25–261.17 | AX-111126249 | AX-110368031 | 10.94 | 7.33 | 3.61 | 10.74 | 8.25 | 2.49 | 0.78 |
| cQHD.nwafu-6A.2 | 200 | 197.82–201.86 | AX-110419766 | AX-111161134 | 11.21 | 7.65 | 3.56 | 15.69 | 8.70 | 6.99 | −0.82 | |
| cQHD.nwafu-6A.3 | 204 | 203.72–214.18 | AX-111634639 | AX-111800524 | 8.53 | 5.26 | 3.27 | 8.06 | 5.89 | 2.17 | −0.68 | |
| SL | cQSL.nwafu-2A | 69 | 0–71.16 | AX-110162878 | AX-109313122 | 4.19 | 3.97 | 0.22 | 4.70 | 4.54 | 0.16 | −0.22 |
| cQSL.nwafu-3A | 244 | 223.21–244.18 | AX-111762953 | AX-109078462 | 20.28 | 20.27 | 0.01 | 26.77 | 26.42 | 0.35 | 0.53 | |
| cQSL.nwafu-4A.1 | 304 | 303.85–305.14 | AX-111040045 | AX-110567219 | 8.31 | 5.03 | 3.28 | 10.12 | 5.61 | 4.51 | −0.24 | |
| cQSL.nwafu-4A.2 | 309 | 305.14–309.21 | AX-109849268 | AX-109369221 | 3.36 | 1.81 | 1.56 | 3.82 | 2.15 | 1.67 | 0.15 | |
| cQSL.nwafu-4A.3 | 381 | 380.01–381.92 | AX-111067027 | AX-109393001 | 5.90 | 5.54 | 0.36 | 6.89 | 6.59 | 0.31 | 0.27 | |
| cQSL.nwafu-6A.2 | 197 | 196.86–197.82 | AX-108958326 | AX-110419766 | 8.09 | 7.37 | 0.72 | 10.31 | 8.60 | 1.71 | 0.30 | |
| KPS | cQKPS.nwafu-2B.3 | 261 | 253.45–261.6 | AX-110430429 | AX-109272515 | 10.77 | 8.69 | 2.08 | 21.49 | 10.10 | 11.39 | −3.08 |
| cQKPS.nwafu-4B | 103 | 97.11–104.58 | AX-109617174 | AX-109533971 | 5.28 | 3.80 | 1.48 | 6.26 | 4.26 | 2.00 | 1.99 | |
| cQKPS.nwafu-5B.1 | 140 | 138.55–140.4 | AX-110093465 | AX-109348971 | 5.17 | 3.10 | 2.07 | 9.91 | 3.58 | 6.32 | 1.88 | |
| cQKPS.nwafu-5B.2 | 211 | 207.16–220.24 | AX-110502417 | AX-109487166 | 6.23 | 1.38 | 4.85 | 3.47 | 1.33 | 2.14 | 1.16 | |
| TKW | cQTKW.nwafu-1B.1 | 85 | 84.86–87.74 | AX-109890962 | AX-110926323 | 5.61 | 3.12 | 2.49 | 6.18 | 4.15 | 2.04 | −0.90 |
| cQTKW.nwafu-3B | 183 | 181.92–183.61 | AX-108884614 | AX-108894802 | 3.08 | 2.54 | 0.54 | 5.29 | 3.22 | 2.07 | −0.81 | |
| KL | cQKL.nwafu-6A.4 | 93 | 92.81–94.79 | AX-109915394 | AX-108914846 | 3.13 | 2.54 | 0.59 | 4.02 | 2.49 | 1.54 | −0.07 |
| cQKL.nwafu-7A.3 | 149 | 148.15–159.54 | AX-110077383 | AX-110632223 | 5.33 | 1.09 | 4.25 | 1.35 | 1.20 | 0.15 | −0.05 | |
| cQKL.nwafu-7A.4 | 255 | 230.08–255.14 | AX-109561279 | AX-111157442 | 6.44 | 2.23 | 4.21 | 3.49 | 2.70 | 0.79 | −0.08 | |
| cQKL.nwafu-5B.1 | 224 | 223.53–232.19 | AX-110404958 | AX-109941486 | 13.49 | 2.81 | 10.67 | 5.65 | 3.44 | 2.20 | −0.08 | |
| cQKL.nwafu-6B | 281 | 280.48–299.16 | AX-110522948 | AX-111218215 | 4.43 | 1.33 | 3.10 | 2.45 | 1.55 | 0.90 | −0.06 | |
| KW | cQKW.nwafu-3A | 286 | 273.22–286.42 | AX-108870115 | AX-108920525 | 4.64 | 1.87 | 2.77 | 2.94 | 2.14 | 0.80 | 0.03 |
| cQKW.nwafu-6A | 312 | 310.88–360.8 | AX-108781069 | AX-108929747 | 4.83 | 1.48 | 3.35 | 3.01 | 1.70 | 1.30 | −0.03 | |
| cQKW.nwafu-4B.2 | 113 | 112.72–113.07 | AX-111221393 | AX-111254019 | 7.60 | 2.75 | 4.84 | 4.39 | 3.27 | 1.12 | 0.04 | |
| LWR | cQLWR.nwafu-7A.1 | 167 | 166.66–175.46 | AX-111545214 | AX-111513167 | 17.74 | 12.84 | 4.91 | 10.64 | 8.36 | 2.27 | 0.04 |
| cQLWR.nwafu-1B.5 | 473 | 471.93–473.05 | AX-111618813 | AX-110394873 | 8.50 | 4.28 | 4.22 | 5.50 | 2.77 | 2.74 | −0.03 | |
| cQLWR.nwafu-6B.1 | 157 | 155.31–157.29 | AX-89438665 | AX-109417511 | 7.70 | 6.33 | 1.37 | 4.50 | 4.00 | 0.50 | −0.03 | |
| Peri | cQPeri.nwafu-7A.1 | 149 | 148.15–159.54 | AX-110077383 | AX-110632223 | 7.62 | 0.95 | 6.67 | 1.64 | 1.25 | 0.39 | −0.11 |
| cQPeri.nwafu-7A.2 | 255 | 230.08–255.14 | AX-109561279 | AX-111157442 | 4.39 | 1.17 | 3.22 | 2.57 | 1.75 | 0.83 | −0.15 | |
| cQPeri.nwafu-3B | 192 | 191.33–199.4 | AX-110989965 | AX-109858559 | 7.90 | 3.02 | 4.88 | 5.57 | 4.30 | 1.27 | −0.21 | |
| cQPeri.nwafu-5B.1 | 221 | 220.24–222.15 | AX-109487166 | AX-111002136 | 9.61 | 1.33 | 8.29 | 4.60 | 1.96 | 2.64 | −0.14 | |
| cQPeri.nwafu-5B.2 | 278 | 278–282.23 | AX-110503586 | AX-111034200 | 4.82 | 3.03 | 1.79 | 18.30 | 4.72 | 13.58 | −0.21 | |
| Area | cQArea.nwafu-1B | 77 | 74.29–79.16 | AX-108788663 | AX-109304925 | 7.93 | 2.21 | 5.72 | 5.25 | 2.46 | 2.79 | −0.25 |
| cQArea.nwafu-5B.2 | 220 | 207.16–220.24 | AX-110502417 | AX-109487166 | 8.83 | 1.79 | 7.03 | 7.12 | 2.33 | 4.80 | −0.24 | |
| AC | cQAC.nwafu-3A.1 | 420 | 419.89–420.52 | AX-108885585 | AX-110531758 | 20.51 | 14.29 | 6.21 | 10.04 | 8.20 | 1.84 | 0.21 |
| cQAC.nwafu-3A.2 | 442 | 441.43–451.72 | AX-111212311 | AX-110977533 | 13.68 | 6.91 | 6.76 | 6.28 | 3.88 | 2.39 | −0.15 | |
| cQAC.nwafu-4A.2 | 445 | 424.31–447.4 | AX-109406677 | AX-111618007 | 11.35 | 10.25 | 1.10 | 5.86 | 5.53 | 0.32 | 0.18 | |
| SB | cQSB.nwafu-2B.1 | 260 | 253.45–261.6 | AX-110430429 | AX-109272515 | 23.25 | 22.77 | 0.48 | 22.52 | 21.91 | 0.62 | −0.43 |
| cQSB.nwafu-2B.2 | 268 | 261.6–268.73 | AX-109272515 | AX-111680306 | 11.65 | 11.65 | 0.00 | 12.67 | 12.62 | 0.05 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Liu, L.; Liu, T.; Jia, Y.; Jiang, Q.; Bai, H.; Ma, S.; Li, S.; Wang, Z. QTL Mapping for Important Agronomic Traits Using a Wheat55K SNP Array-Based Genetic Map in Tetraploid Wheat. Plants 2023, 12, 847. https://doi.org/10.3390/plants12040847
Ma C, Liu L, Liu T, Jia Y, Jiang Q, Bai H, Ma S, Li S, Wang Z. QTL Mapping for Important Agronomic Traits Using a Wheat55K SNP Array-Based Genetic Map in Tetraploid Wheat. Plants. 2023; 12(4):847. https://doi.org/10.3390/plants12040847
Chicago/Turabian StyleMa, Chao, Le Liu, Tianxiang Liu, Yatao Jia, Qinqin Jiang, Haibo Bai, Sishuang Ma, Shuhua Li, and Zhonghua Wang. 2023. "QTL Mapping for Important Agronomic Traits Using a Wheat55K SNP Array-Based Genetic Map in Tetraploid Wheat" Plants 12, no. 4: 847. https://doi.org/10.3390/plants12040847
APA StyleMa, C., Liu, L., Liu, T., Jia, Y., Jiang, Q., Bai, H., Ma, S., Li, S., & Wang, Z. (2023). QTL Mapping for Important Agronomic Traits Using a Wheat55K SNP Array-Based Genetic Map in Tetraploid Wheat. Plants, 12(4), 847. https://doi.org/10.3390/plants12040847





