Customized Technological Designs to Improve the Traditional Use of Rosa canina Fruits in Foods and Ingredients
Abstract
:1. Introduction
2. Results and Discussion
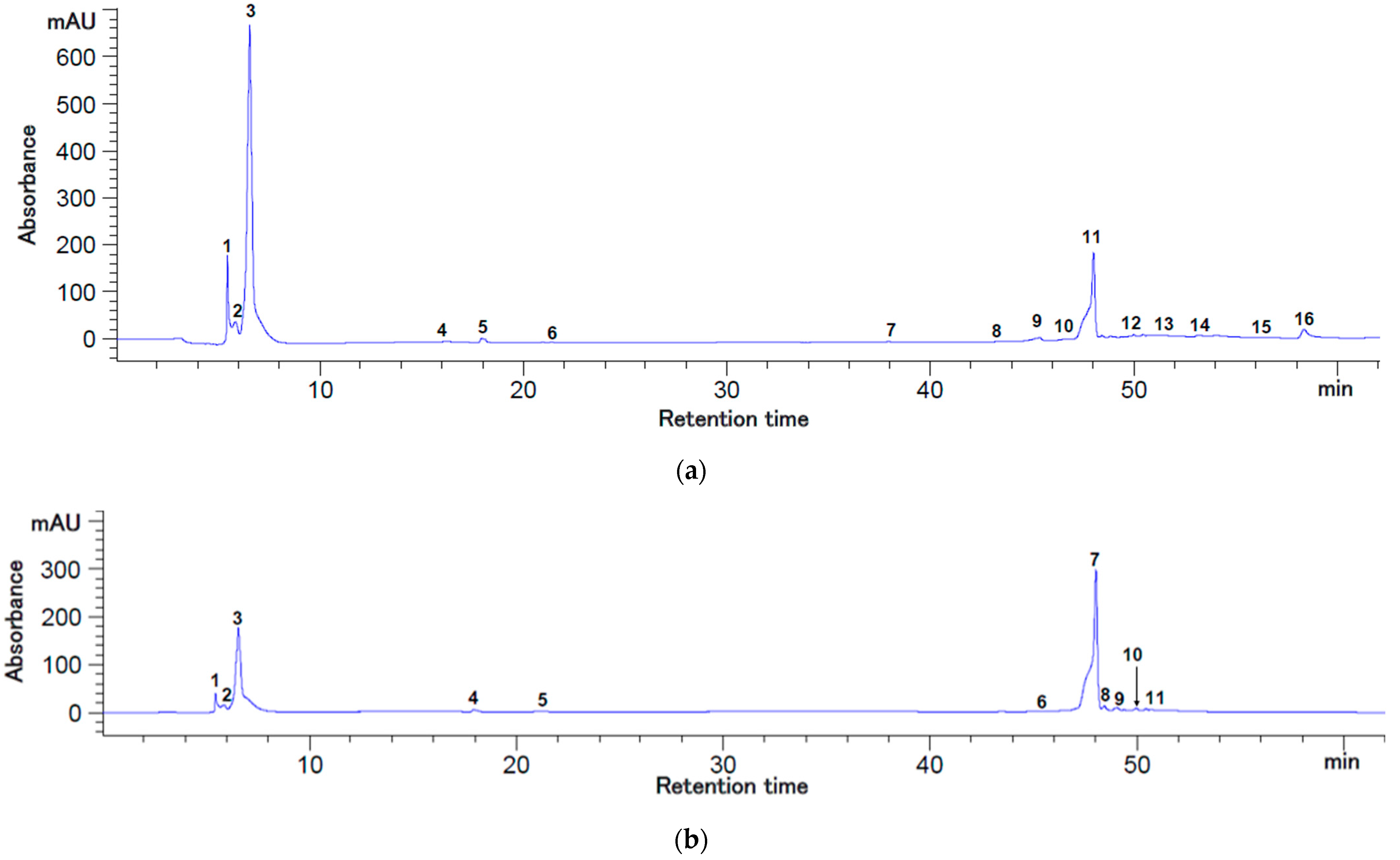
2.1. The Polyphenolic Content of the Extracts
2.2. The Carotenoid Profile of the Jellified Products
2.3. The Texture Analysis of the Jellified Products
2.4. Color Parameters of the Jellified Products
2.5. Proximate Chemical Composition of the Jellified Products
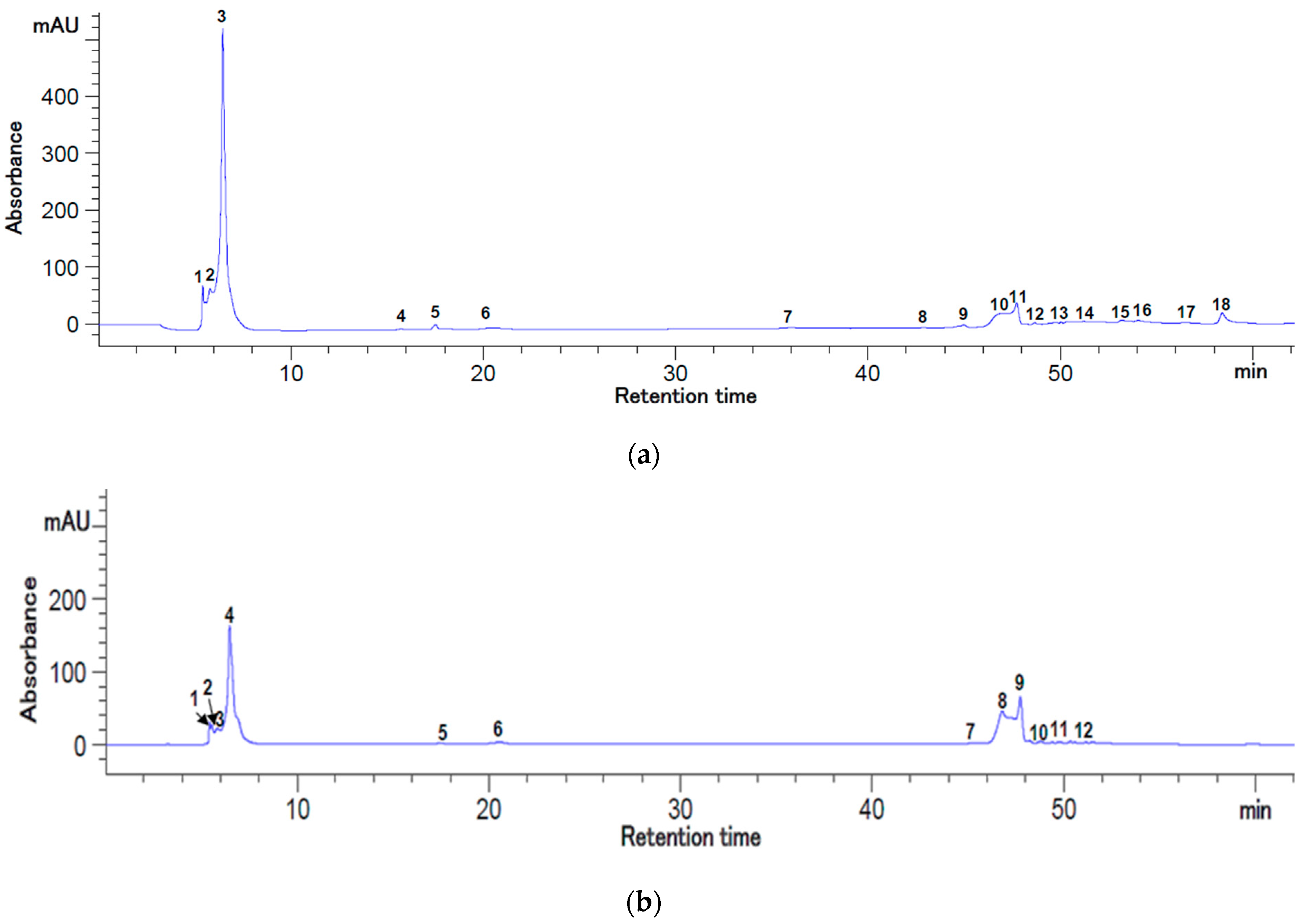
2.6. The Carotenoids and Polyphenolic Profile of the Rosehip Juices
2.7. The Acceptance Test for Jellified Products and Juices
2.8. The Phytochemical Characterization and Cell Viability of the Inoculated Powder
2.9. The Inhibitory Activity of the Powder
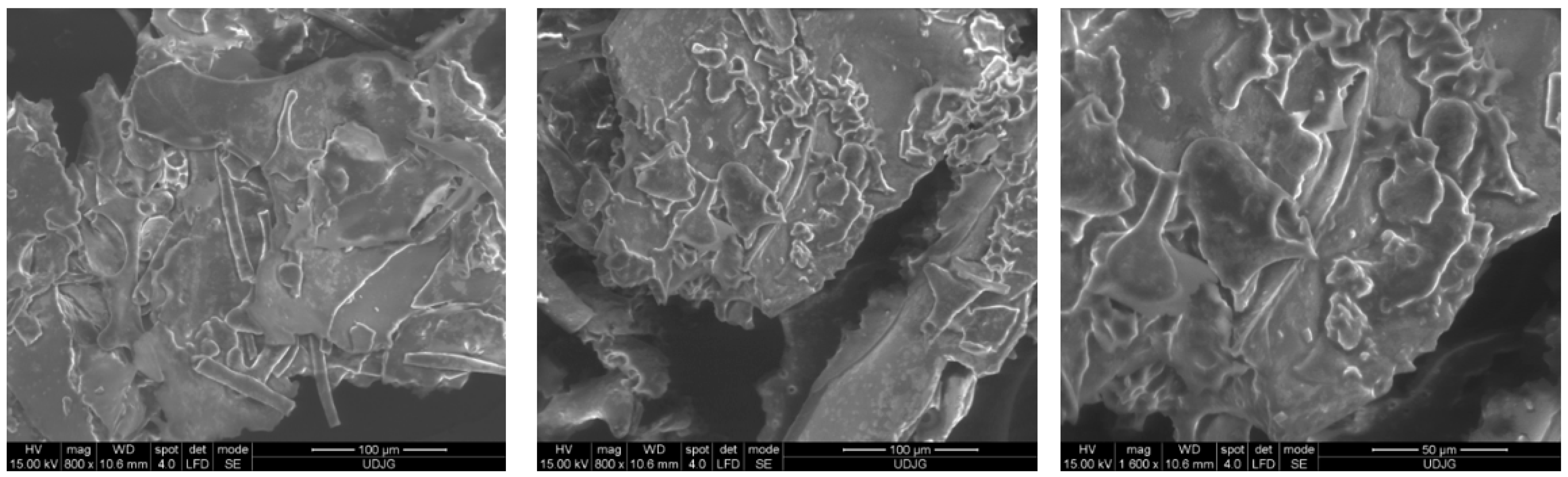
2.10. Microscopic Structure of the Powder
3. Materials and Methods
3.1. Chemicals
3.2. Fruits Processing
3.3. HPLC Analysis
3.4. Jellified Products Manufacturing
3.5. Juices Manufacturing
3.6. Inoculation of Rosehip Pulp with Lactobacillus acidophilus
3.7. Global Phytochemical Characterization of Rosehip Products
3.8. Total Polyphenols (TP) and Total Flavonoids (TF) Analysis
3.9. Carotenoids Content Evaluation
3.10. Antiradical Scavenging Activity
3.11. Texture Analysis of the Jellified Products
3.12. CIEL*a*b* Analysis of the Jellified Products
3.13. Nutritional Evaluation of the Jellified Products
3.14. Sensorial Analysis of the Jellified Products and Juices
3.15. Viability of Lb. acidophilus
3.16. Inhibitory Activity on Metabolic Syndrome Associated Enzymes
3.17. Powder Structure and Morphology
3.18. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT 2022, 154, 112716. [Google Scholar] [CrossRef]
- Elmastaş, M.; Demir, A.; Genç, N.; Dölek, U.; Güneş, M. Changes in Flavonoid and Phenolic Acid Contents in Some Rosa species During Ripening. Food Chem. 2017, 15, 154–159. [Google Scholar]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Petkova, N.; Ognyanov, M.; Kirchev, M.; Stancheva, M. Bioactive compounds in water extracts prepared from rosehip-containing herbal blends. J. Food Process. Preserv. 2021, 45, e14645. [Google Scholar] [CrossRef]
- Winther, K.; Vinther Hansen, A.S.; Campbell-Tofte, J. Bioactive ingredients of rose hips (Rosa canina L.) with special reference to antioxidative and anti inflammatory properties: In vitro studies. Botanics 2016, 6, 11–23. [Google Scholar]
- Bruneau, A.; Starr, J.R.; Joly, S. Phylogenetic relationships in the genus Rosa: New evidence from chloroplast DNA sequences and an appraisal of current knowledge. Syst. Bot. 2007, 32, 366–378. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Miguel, M.G.; Hasnaoui, B.; Sebei, H.; Ksontini, M.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G. Phenols, essential oils, and carotenoids of Rosa canina from Tunisia and their antioxidant activities. Afr. J. Biotechnol. 2010, 9, 2709–2716. [Google Scholar]
- Goztepe, B.; Kayacan, S.; Bozkurt, F.; Tomas, M.; Sagdic, O.; Karasu, S. Drying kinetics, total bioactive compounds, antioxidant activity, phenolic profile, lycopene and β-carotene content and color quality of Rosehip dehydrated by different methods. LWT 2022, 153, 112476. [Google Scholar]
- Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar]
- Ilyasoğlu, H. Characterization of Rosehip (Rosa canina L.) Seed and Seed Oil. Int. J. Food Prop. 2014, 17, 1591–1598. [Google Scholar]
- Yildiz, O.; Alpaslan, M. Properties of Rose Hip Marmalades. Food Technol. Biotechnol. 2012, 50, 98–106. [Google Scholar]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar]
- Angelov, G.; Boyadzhieva, S.; Georgieva, S. Rosehip extraction; process optimization and antioxidant capacity of extracts. Central Eur. J. Chem. 2014, 12, 502–508. [Google Scholar]
- Medveckienė, B.; Kulaitienė, J.; Levickienė, D.; Hallmann, E. The effect of ripening stages on the accumulation of carotenoids, polyphenols and vitamin C in rosehip species/cultivars. Appl. Sci. 2021, 11, 6761. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Genovese, D.B. Fruit jellies enriched with dietary fibre: Development and characterization of a novel functional food product. LWT 2019, 111, 423–428. [Google Scholar]
- Ben Rejeb, I.; Dhen, N.; Kassebi, S.; Gargouri, M. Quality evaluation and functional properties of reduced sugar jellies formulated from citrus fruits. J. Chem. 2020, 2020, 5476872. [Google Scholar]
- Nistor, O.-V.; Bolea, C.A.; Andronoiu, D.G.; Cotârleț, M.; Stănciuc, N. Attempts for Developing Novel Sugar-Based and Sugar-Free Sea Buckthorn Marmalades. Molecules 2021, 26, 3073. [Google Scholar]
- Bourne, M.C. Principles of objective texture measurement. In Food Texture and Viscosity. Concept and Measurement; Elsevier: Amsterdam, The Netherlands, 2002; pp. 107–188. [Google Scholar]
- Featherstone, S. Jams, jellies, and related products. In A Complete Course in Canning and Related Processes, Volume 3: Processing Procedures for Canned Food Products, 14th ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2016; pp. 313–349. [Google Scholar]
- Igual, M.; Chi, M.S.; Paucean, A.; Vodnar, D.C.; Muste, S.; Man, S.; Martínez-Monzó, J.; García-Segovia, P. Valorization of Rose Hip (Rosa canina) Puree Co-Product in Enriched Corn Extrudates. Foods 2021, 10, 2787. [Google Scholar] [CrossRef]
- Drożdż, W.; Tomaszewska-Ciosk, E.; Zdybel, E.; Boruczkowska, H.; Boruczkowski, T.; Regiec, P. Effect of apple and rosehip pomaces on colour, total phenolics and antioxidant activity of corn extruded snacks. Pol. J. Chem. Technol. 2014, 16, 7. [Google Scholar] [CrossRef]
- Ramadan, M.F. Enzymes in Fruit Juice Processing. In Enzymes in Food Biotechnology Production, Applications, and Future Prospects; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–59. [Google Scholar]
- Pashazadeh, O.; Özdemir, N.; Zannou, O.; Koca, I.; Mayıs, O. Antioxidant capacity, phytochemical compounds, and volatile compounds related to aromatic property of vinegar produced from black rosehip (Rosa pimpinellifolia L.) juice. Food Biosci. 2021, 44, 101318. [Google Scholar]
- Turgut, T.; Çetin, B.; Erdoğan, A.; Gürses, M. Some microbiological characteristics of rosehip yoghurt inoculated with Lactobacillus acidophilus DSMZ 20079. Acta Hortic. 2005, 690, 299–302. [Google Scholar] [CrossRef]
- Milea, Ș.A.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.-M.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Co-Microencapsulation of flavonoids from yellow onion skins and lactic acid bacteria lead to multifunctional ingredient for nutraceutical and pharmaceutics applications. Pharmaceutics 2020, 12, 1053. [Google Scholar] [PubMed]
- Dermengiu, N.E.; Milea, Ș.A.; Păcularu Burada, B.; Stanciu, S.; Cîrciumaru, A.; Râpeanu, G.; Stănciuc, N. A Dark Purple Multifunctional Ingredient from Blueberries Pomace enhanced with Lactic acid bacteria for Various Applications. J. Food Sci. 2022, 87, 4725–4737. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, L.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Fathollahi, M.; Aminzare, M.; Mohseni, M.; Hassanzadazar, H. Antioxidant capacity, antimicrobial activities and chemical composition of Pistacia atlantica subsp. kurdica essential oil. Vet. Res. Forum 2019, 10, 299–305. [Google Scholar]
- AOAC. Official Methods of Analysis 15th Edition of the Association of Official Analytical Chemists; AOAC: Rockville, MD, USA, 1990. [Google Scholar]
- Vasile, M.A.; Milea, Ș.-A.; Enachi, E.; Barbu, V.; Cîrciumaru, A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Functional enhancement of bioactives from black beans and lactic acid bacteria into an innovative food ingredient by co-microencapsulation. Food Bioproc. Technol. 2020, 13, 978–987. [Google Scholar]
- Meziant, L.; Bachir-bey, M.; Bensouici, C.; Saci, F.; Boutiche, M.; Louaileche, H. Assessment of inhibitory properties of flavonoid-rich fig (Ficus carica L.) peel extracts against tyrosinase, α-glucosidase, urease and cholinesterases enzymes, and relationship with antioxidant activity. Eur. J. Integr. Med. 2021, 43, 101272. [Google Scholar]
| Bioactive Compound | Concentration, mg/100 g DW | ||||
|---|---|---|---|---|---|
| Extract | Extract Treated Pectinex® Ultra Color | ||||
| Calibration Equation 280 and 320 nm | 280 nm | 320 nm | 280 nm | 320 nm | |
| Gallic acid | Y = 32,492x + 1935.20 (R2 = 0.991) Y = 3650,50x + 43.06 (R2 = 0.998) | 2.84 ± 0.05 1 b | 40.32 ± 4.47 a | 9.74 ± 0.24 a | 48.75 ± 1.16 a |
| Chlorogenic acid | Y = 37,938x − 473.39 (R2 = 0.995) Y = 65,614x + 420.71 (R2 = 0.998) | 39.65 ± 9.12 a | 3.72 ± 0.14 a | 23.12 ± 1.67 a | 2.39 ± 0.03 b |
| Epicatechin | Y = 3441.80x + 188.57 (R2 = 0.995) | 2077.22 ± 45.62 a | 733.93 ± 18.25 a | 2145.04 ± 14.95 a | 658.86 ± 2.72 b |
| Quercetin | Y = 62,923x − 668.60 (R2 = 0.999) Y = 63,432x − 1092.50 (R2 = 0.999) | 17.74 ± 1.37 b | 29.55 ± 9.12 a | 40.23 ± 0.48 a | 6.24 ± 0.08 a |
| Kaempferol | Y = 12,672x + 947.22 (R2 = 0.992) Y = 51,624x + 2429.90 (R2 = 0.991) | 0.90 ± 0.06 b | 0.27 ± 0.02 b | 5.06 ± 0.05 a | 0.56 ± 0.01 a |
| Quercetin 3-β-D-glucoside | Y = 1875.80x − 6.97 (R2 = 0.999) Y = 70,182x + 1690 (R2 = 0.998) | n.d. | 798.54 ± 24.73 a | 25.74 ± 0.77 a | n.d. |
| Variants | P | V | V1 | V2 | V3 |
|---|---|---|---|---|---|
| Total carotenoids (mg/g DW) | 185.17 ± 1.68 a | 89.36 ± 0.06 b | 9.66 ± 0.26 d | 5.38 ± 0.04 e | 39.22 ± 1.16 c |
| β-carotene (mg/g DW) | 148.98 ± 1.06 a | 83.52 ± 0.10 b | 7.95 ± 0.50 d | 5.24 ± 0.02 e | 38.06 ± 0.10 c |
| Lycopene (mg/g DW) | 81.16 ± 2.42 a | 42.33 ± 0.18 b | 5.11 ± 0.21 d | 2.86 ± 0.01 e | 18.47 ± 0.18 c |
| Antioxidant activity (mMol Trolox/g DW) | 36.44 ± 2.45 a | 8.19 ± 0.80 c | 6.24 ± 0.16 c | 6.05 ± 0.29 c | 20.16 ± 0.31 b |
| Variants | Firmness, N | Adhesiveness, mJ | Cohesiveness, - | Springiness, mm |
|---|---|---|---|---|
| V | 1.14 ± 0.03 a | 0.22 ± 0.02 b | 0.41 ± 0.01 b | 4.76 ± 0.11 b |
| V1 | 3.62 ± 0.08 b | 0.76 ± 0.03 a | 0.68 ± 0.02 b | 3.87 ± 0.23 b |
| V2 | 2.85 ± 0.03 b | 0.54 ± 0.02 b | 0.65 ± 0.01 b | 4.12 ± 0.12 b |
| V3 | 1.61 ± 0.02 a | 0.31 ± 0.03 b | 0.46 ± 0.01 b | 4.79 ± 0.15 b |
| Variants | L* | a* | b* | C | h |
|---|---|---|---|---|---|
| M | 32.10 ± 0.5 b | 6.39 ± 0.1 b | 20.94 ± 0.5 b | 38.28 ± 0.3 b | 33.25 ± 0.5 a |
| V | 22.39 ± 1.0 b | 9.89 ± 0.2 b | 28.44 ± 0.2 b | 27.72 ± 0.1 b | 66.65 ± 1.0 a |
| V1 | 20 ± 2.0 b | 4.86 ± 1.0 b | 21.59 ± 0.7 b | 23.12 ± 0.5 b | 77.79 ± 1.0 a |
| V2 | 18.86 ± 2.0 b | 7.02 ± 0.2 b | 21.24 ± 0.4 b | 22.40 ± 0.3 b | 71.35 ± 0.5 a |
| V3 | 29.61 ± 0.7 b | 7.01 ± 0.3 b | 17.86 ± 1.0 b | 19.18 ± 0.1 b | 22.47 ± 0.3 a |
| Characteristics (%) | V | V1 | V2 | V3 |
|---|---|---|---|---|
| Salt, of which sodium | 0.06 ± 0.001 b 0.024 ± 0.001 b | 0.08 ± 0.001 a 0.032 ± 0.001 a | 0.08 ± 0.007 a 0.032 ± 0.001 a | 0.06 ± 0.002 b 0.024 ± 0.002 b |
| Ash | 0.951 ± 0.001 a | 0.355 ± 0.001 d | 0.473 ± 0.001 c | 0.803 ± 0.003 b |
| Moisture | 78.96 ± 0.79 a | 5.21 ± 0.19 d | 33.4 ± 1.52 c | 66.92 ± 2.17 b |
| Fat | 0.12 ± 0.01 a | 0.07 ± 0.01 b | 0.04 ± 0.001 c | 0.12 ± 0.01 a |
| Proteins | 0.20 ± 0.01 a | 0.20 ± 0.01 a | 0.20 ± 0.01 a | 0.20 ± 0.01 a |
| Carbohydrate, of which sugar | 19.9 ± 0,10 d 3.69 ± 0.02 d | 93.9 ± 0.21 a 46.48 ± 0.02 a | 65.7 ± 0.12 b 21.95 ± 0.01 b | 32.00 ± 0.01 c 5.44 ± 0.03 c |
| Energy value (kcal) (kj) | 81.48 ± 0.05 d 346.14 ± 2.36 d | 337.8 ± 1.23 a 1605.7 ± 11.05 a | 264.7 ± 5.62 b 1125.2 ± 5.46 b | 129.9 ± 2.84 c 551.8 ± 0.98 c |
| Variants | P | J | J1 | J2 |
|---|---|---|---|---|
| Total carotenoid (mg/100 mL) | 144.06 ± 0.33 a | 141.74 ± 1.16 b | 59.92 ± 0.17 c | 34.50 ± 1.22 d |
| β-carotene (mg/100 mL) | 134.61 ± 0.20 b | 140.70 ± 0.22 a | 57.68 ± 0.33 c | 32.08 ± 1.12 d |
| Lycopene (mg/100 mL) | 79.13 ± 0.41 a | 63.97 ± 0.53 b | 31.66 ± 0.12 c | 17.14 ± 0.72 d |
| Total polyphenolic content (mg GAE/100 mL) | 58.91 ± 2.89 a | 57.67 ± 3.83 a | 126.26 ± 2.38 b | 207.49 ± 1.04 c |
| Antioxidant activity (mMol trolox/100 mL) | 27.45 ± 1.68 a | 5.77 ± 0.38 d | 12.63 ± 0.64 c | 20.75 ± 1.40 b |
| Samples/Attributes | V | V1 | V2 | V3 |
|---|---|---|---|---|
| Appearance | 5.4 ± 0.84 bc | 4.5 ± 0.84 c | 6.4 ± 0.69 a | 6.1 ± 0.73 ab |
| Color | 5.4 ± 1.20 a | 5.5 ± 0.52 a | 6 ± 0.47 a | 6.3 ± 0.82 a |
| Sweet taste | 1.7 ± 0.67 b | 6.3 ± 1.05 a | 6.2 ± 1.03 a | 5.8 ± 0.78 a |
| Bitter taste | 1.7 ± 0.67 a | 1.4 ± 0.51 a | 1.3 ± 0.48 a | 1.6 ± 0.51 a |
| Flavor | 4.2 ± 1.22 b | 4.7 ± 0.67 b | 6 ± 0.47 a | 5.00 ± 1.05 ab |
| Hardness | 2.1 ± 0.56 bc | 6.9 ± 0.31 a | 2.5 ± 0.70 b | 1.7 ± 0.67 c |
| Fragility | 3.2 ± 0.63 b | 5.4 ± 0.69 a | 2.6 ± 0.84 b | 3.1 ± 0.73 b |
| Gumminess | 1.6 ± 0.69 c | 5.9 ± 0.73 a | 3.00 ± 0.66 b | 1.5 ± 0.70 c |
| Mouthfeel | 1.8 ± 0.78 b | 6.6 ± 0.78 a | 1.8 ± 0.78 b | 1.9 ± 0.73 b |
| Creaminess | 4.5 ± 1.35 a | 2.4 ± 1.05 b | 5.3 ± 1.05 a | 5.7 ± 0.94 a |
| Overall impression | 5.1 ± 1.19 a | 3.5 ± 0.78 b | 6.2 ± 0.78 a | 5.7 ± 1.25 a |
| Samples/Attributes | J | J1 | J2 |
|---|---|---|---|
| Appearance | 6.4 ± 1.07 a | 6.1 ± 0.87 a | 5.3 ± 1.05 a |
| Color | 6.4 ± 0.96 a | 6.3 ± 0.67 a | 5.6 ± 1.42 a |
| Sweet taste | 2 ± 1.49 b | 3.9 ± 1.52 a | 4.9 ± 1.37 a |
| Bitter taste | 2.4 ± 1.89 a | 1.3 ± 0.67 a | 1.6 ± 0.69 a |
| Flavor | 4.8 ± 1.75 a | 5.1 ± 1.37 a | 5.6 ± 1.17 a |
| Overall impression | 4.8 ± 1.75 a | 5.6 ± 1.07 a | 5.9 ± 1.10 a |
| Enzyme | α-Glucosidase | Lipase | Tyrosinase |
|---|---|---|---|
| Powder | 2.92 ± 0.83 * | 1.21 ± 0.08 | 0.23 ± 0.05 |
| Acarbose | 3.97 ± 0.62 | - | - |
| Orlistat | - | 1.23 ± 0.09 | - |
| Kojic acid | - | - | 1.12 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodorescu, A.A.; Milea, Ș.A.; Păcularu-Burada, B.; Nistor, O.V.; Andronoiu, D.G.; Râpeanu, G.; Stănciuc, N. Customized Technological Designs to Improve the Traditional Use of Rosa canina Fruits in Foods and Ingredients. Plants 2023, 12, 754. https://doi.org/10.3390/plants12040754
Teodorescu AA, Milea ȘA, Păcularu-Burada B, Nistor OV, Andronoiu DG, Râpeanu G, Stănciuc N. Customized Technological Designs to Improve the Traditional Use of Rosa canina Fruits in Foods and Ingredients. Plants. 2023; 12(4):754. https://doi.org/10.3390/plants12040754
Chicago/Turabian StyleTeodorescu, Adina Andreea, Ștefania Adelina Milea, Bogdan Păcularu-Burada, Oana Viorela Nistor, Doina Georgeta Andronoiu, Gabriela Râpeanu, and Nicoleta Stănciuc. 2023. "Customized Technological Designs to Improve the Traditional Use of Rosa canina Fruits in Foods and Ingredients" Plants 12, no. 4: 754. https://doi.org/10.3390/plants12040754
APA StyleTeodorescu, A. A., Milea, Ș. A., Păcularu-Burada, B., Nistor, O. V., Andronoiu, D. G., Râpeanu, G., & Stănciuc, N. (2023). Customized Technological Designs to Improve the Traditional Use of Rosa canina Fruits in Foods and Ingredients. Plants, 12(4), 754. https://doi.org/10.3390/plants12040754









