First Report on Development of Genome-Wide Microsatellite Markers for Stock (Matthiola incana L.)
Abstract
1. Introduction
2. Result
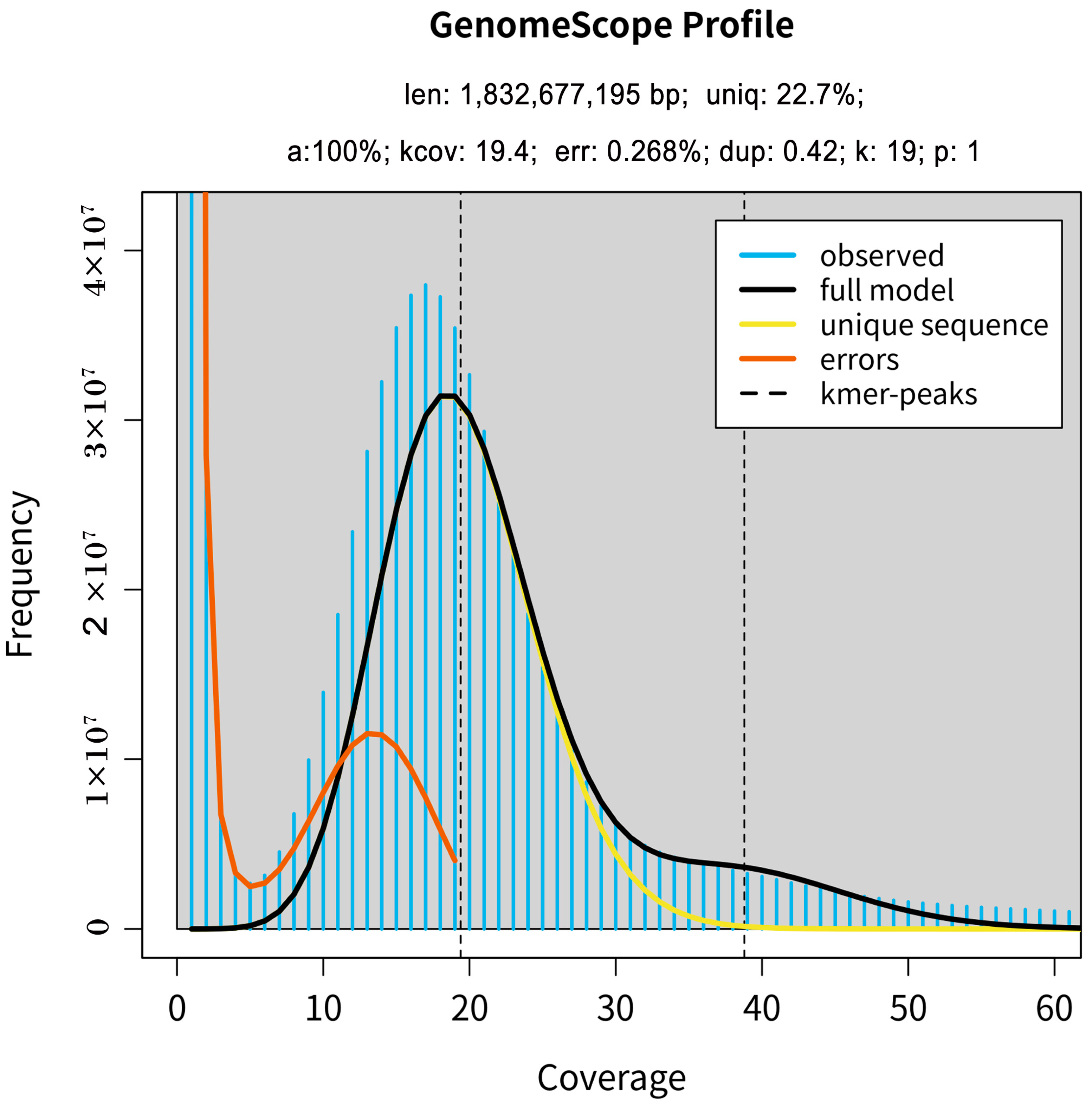
2.1. Sequencing Data Statistics and Estimates of Genome Size and Heterozygosity
2.2. Preliminary Genome Assembly Results for M. incana
2.3. SSR Loci Identification of M. incana
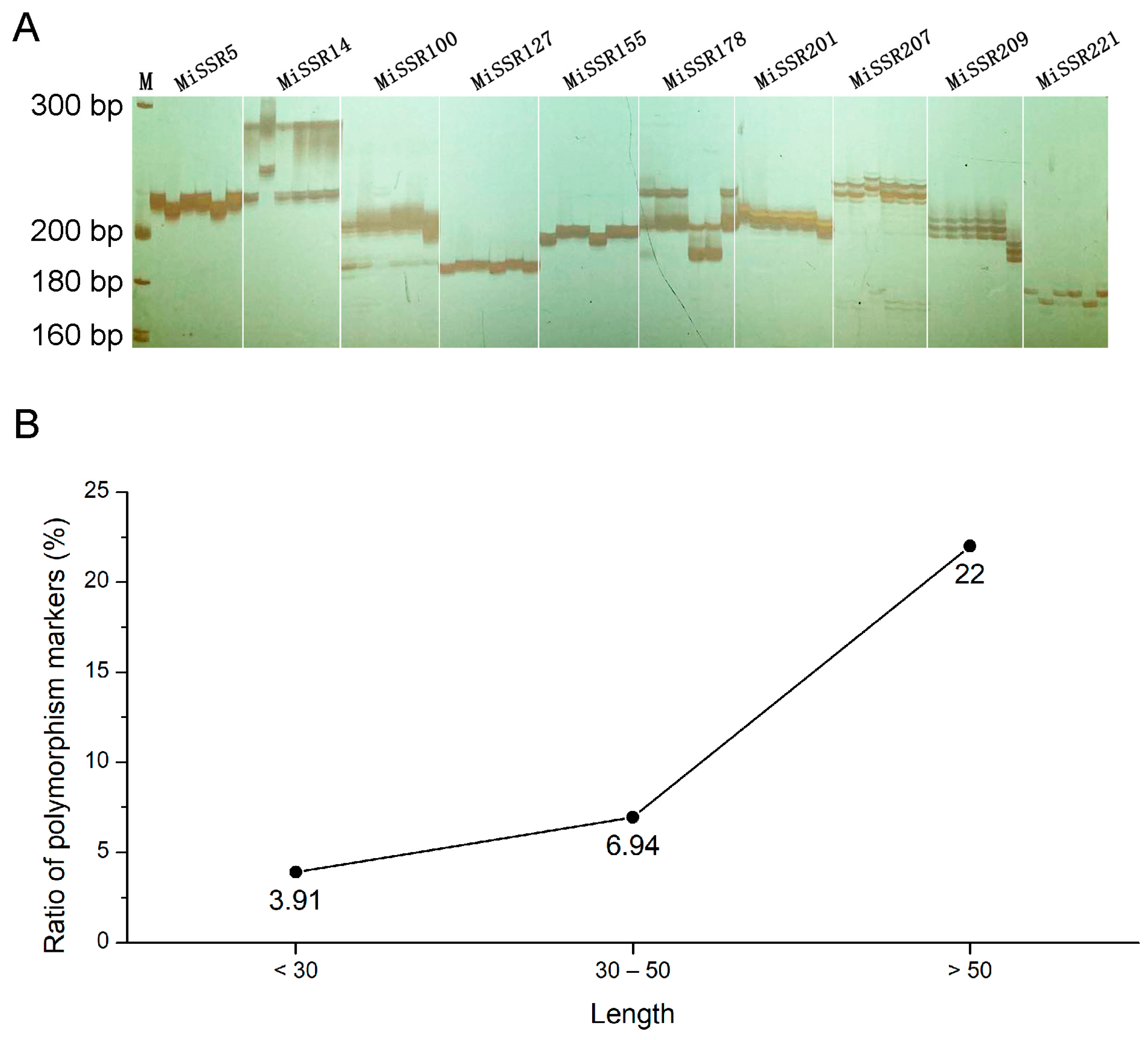
2.4. Characterization of SSRs
2.5. The Distribution of SSR Loci on Chromosomes
2.6. Genome-Wide SSR Primers Design and PCR Validation
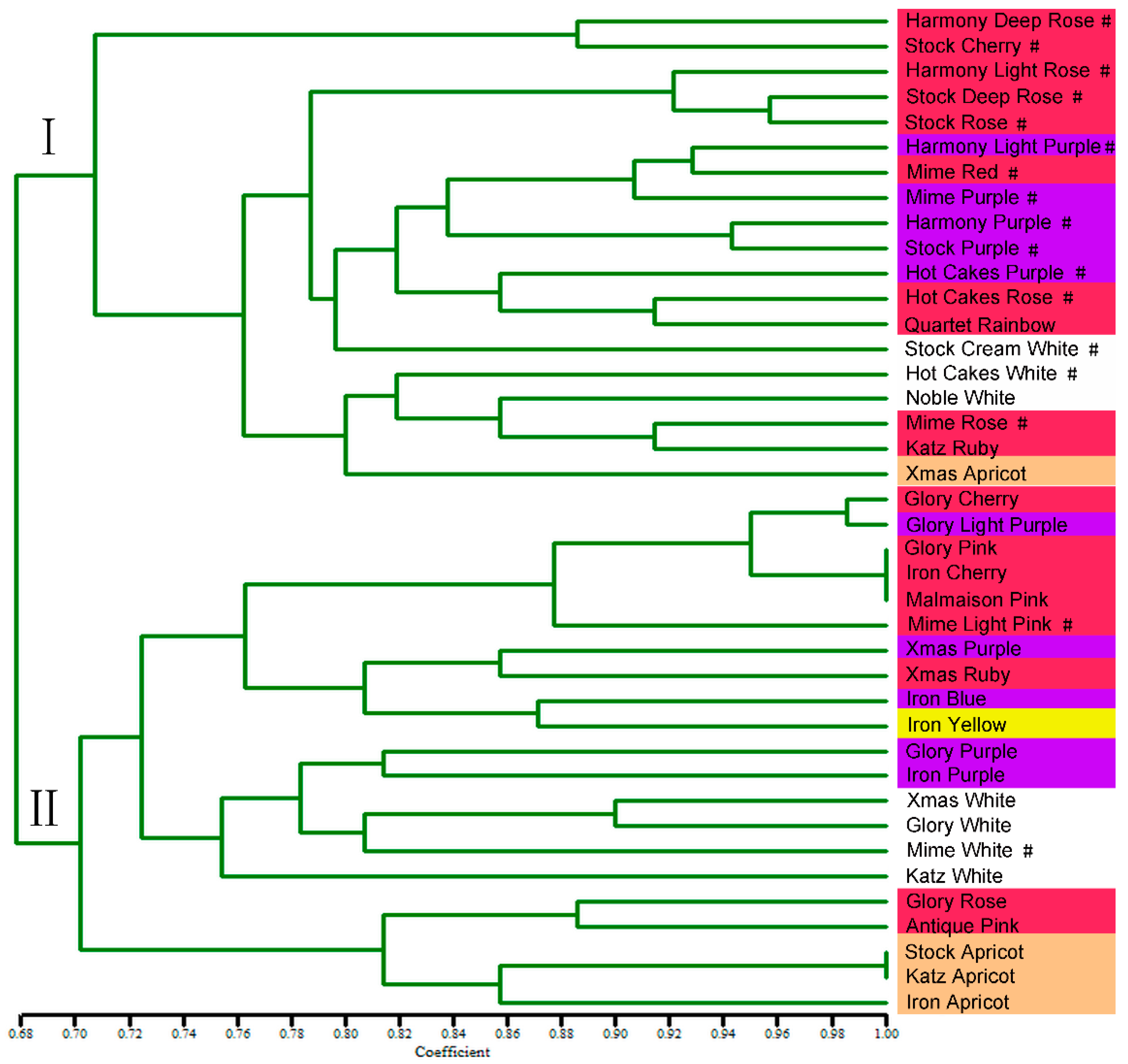
2.7. Genetic Diversity Analysis of Stock Genotypes
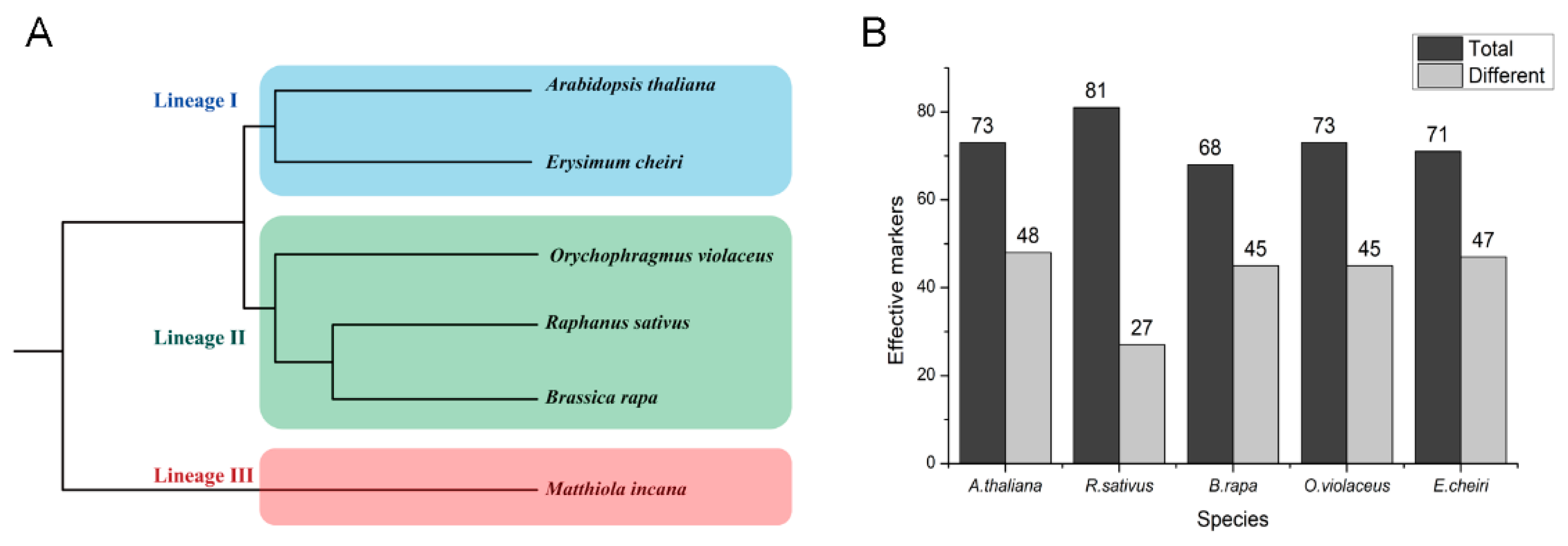
2.8. Transferability and Effectiveness of SSR Primers in Other Species of Brassicaceae Family
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials
5.2. Library Preparation and Genome Assembly
5.3. Identification of Genome-Wide SSR Loci
5.4. SSR Primer Design and Marker Validation
5.5. Genetic Diversity and Structure Assessment of Stock Varieties
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tatsuzawa, F.; Saito, N.; Toki, K.; Shinoda, K.; Honda, T. Flower colors and their anthocyanins in Matthiola incana cultivars (Brassicaceae). J. Jpn. Soc. Hortic. Sci. 2012, 81, 91–100. [Google Scholar] [CrossRef]
- Lim, T.K. Matthiola incana. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Nuraini, L.; Tatsuzawa, F.; Ochiai, M.; Suzuki, K.; Nakatsuka, T. Two independent spontaneous mutations related to anthocyanin-less flower coloration in Matthiola incana cultivars. Hortic. J. 2021, 90, 85–96. [Google Scholar] [CrossRef]
- Rasool, N.; Afzal, S.; Riaz, M.; Rashid, U.; Rizwan, K.; Zubair, M.; Ali, S.; Shahid, M. Evaluation of antioxidant activity, cytotoxic studies and GC-MS profiling of Matthiola Incana (Stock Flower). Legume Res. 2013, 36, 21–32. [Google Scholar]
- Taviano, M.F.; Miceli, N.; Acquaviva, R.; Malfa, G.A.; Ragusa, S.; Giordano, D.; Casedas, G.; Les, F.; Lopez, V. Cytotoxic, Antioxidant, and Enzyme Inhibitory Properties of the Traditional Medicinal Plant Matthiola incana (L.) R. Br. Biol. 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Garmdareh, S.E.H. Effects of Salinity on Morpho-Physiological, and Biochemical Characteristics of Stock Plant (Matthiola Incana L.). Sci. Hortic. 2019, 257, 108731. [Google Scholar] [CrossRef]
- Akat, H. Effects of mycorrhizal inoculation on growth and some quality parameters of Matthiola incana (L.) cultivation under salt stress. J. Environ. Biol. 2020, 41, 375–381. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kadam, U.S.; Chacko, P.; Karibasappa, G.S. Microsatellite and RAPD analysis of grape (Vitis spp.) accessions and identification of duplicates/misnomers in germplasm collection. Indian J. Hortic. 2010, 67, 8. [Google Scholar]
- Ganie, S.H.; Upadhyay, P.; Das, S.; Prasad Sharma, M. Authentication of medicinal plants by DNA markers. Plant Gene 2015, 4, 83–99. [Google Scholar] [CrossRef]
- Sharp, P.J.; Desai, S.; Gale, M.D. Isozyme variation and RFLPs at the β-amylase loci in wheat. Theor. Appl. Genet. 1988, 76, 691–699. [Google Scholar] [CrossRef]
- Ho, U.H.; Ri, J.H.; Ri, C.J. Morphological and molecular identification of double flowered stock (Matthiola Incana R. Br) cultivars with high fertility. PREPRINT (Version 1) 2021. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ge, X.; Zhou, Y.; Li, M.; Li, Z. Cytoplasmic and genomic effects on non-meiosis-driven genetic changes in Brassica hybrids and allotetraploids from pairwise crosses of three cultivated diploids. PLoS ONE 2013, 8, e65078. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.; Çelik, M.; Ünal, M.; Sefali, A.; Martine, E.; Kaya, A. Study of phylogenetic relationship of Turkish species of Matthiola (Brassicaceae) based on ISSR amplification. Turk. J. Bot. 2016, 40, 130–136. [Google Scholar] [CrossRef]
- Agarwal, M.; Shrivastava, N.; Padh, H. Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep. 2008, 27, 617–631. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Koishi, K. Molecular characterization of a double-flower mutation in Matthiola Incana. Plant Sci. 2018, 268, 39–46. [Google Scholar] [CrossRef]
- Xu, Y.; Xing, M.; Song, L.; Yan, J.; Lu, W.; Zeng, A. Genome-wide analysis of simple sequence repeats in cabbage (Brassica oleracea L.). Front. Plant Sci. 2021, 12, 726084. [Google Scholar] [CrossRef]
- Zhu, J.F.; Zhang, J.Y.; Jiang, M.Y.; Wang, W.R.; Jiang, J.X.; Li, Y.L.; Yang, L.Y.; Zhou, X.R. Development of genome-wide SSR markers in rapeseed by next generation sequencing. Gene 2021, 798, 145798. [Google Scholar] [CrossRef]
- Kumar, M.; Rakesh Sharma, V.; Kumar, V.; Sirohi, U.; Chaudhary, V.; Sharma, S.; Saripalli, G.; Naresh, R.K.; Yadav, H.K.; Sharma, S. Genetic diversity and population structure analysis of Indian garlic (Allium sativum L.) collection using SSR markers. Physiol. Mol. Biol. Plants 2019, 25, 377–386. [Google Scholar] [CrossRef]
- Li, Z.; Yun, L.; Gao, Z.Q.; Wang, T.; Ren, X.M.; Zhao, Y. EST-SSR primer development and genetic structure analysis of psathyrostachys juncea Nevski. Front. Plant Sci. 2022, 13, 837787. [Google Scholar] [CrossRef]
- Yin, H.; Fang, X.; Li, P.; Yang, Y.; Hao, Y.; Liang, X.; Bo, C.; Ni, F.; Ma, X.; Du, X.; et al. Genetic mapping of a novel powdery mildew resistance gene in wild emmer wheat from “Evolution Canyon” in Mt. Carmel Israel. Theor. Appl. Genet. 2021, 134, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhardwaj, S.C.; Gangwar, O.P.; Sharma, A.; Qureshi, N.; Kumaran, V.V.; Khan, H.; Prasad, P.; Miah, H.; Singh, G.P.; et al. Lr80: A new and widely effective source of leaf rust resistance of wheat for enhancing diversity of resistance among modern cultivars. Theor. Appl. Genet. 2021, 134, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Matsunaga, K.; Shirasawa, K. Quantitative trait loci analysis based on high-density mapping of single-nucleotide polymorphisms by genotyping-by-sequencing against pine wilt disease in Japanese black pine (Pinus thunbergii). Front. Plant Sci. 2022, 13, 850660. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, J.; Wu, P.; Li, Y.; Qiu, D.; Qu, Y.; Xie, J.; Zhang, H.; Yang, L.; Fu, T.; et al. Development of SNP, KASP, and SSR markers by BSR-Seq technology for saturation of genetic linkage map and efficient detection of wheat powdery mildew resistance gene Pm61. Int. J. Mol. Sci. 2019, 20, 750. [Google Scholar] [CrossRef]
- Natesan, S.; Duraisamy, T.; Pukalenthy, B.; Chandran, S.; Nallathambi, J.; Adhimoolam, K.; Manickam, D.; Sampathrajan, V.; Muniyandi, S.J.; Meitei, L.J.; et al. Enhancing β-carotene concentration in parental lines of CO6 maize hybrid through marker-assisted backcross breeding (MABB). Front. Nutr. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Darmanov, M.M.; Makamov, A.K.; Ayubov, M.S.; Khusenov, N.N.; Buriev, Z.T.; Shermatov, S.E.; Salakhutdinov, I.B.; Ubaydullaeva, K.A.; Norbekov, J.K.; Kholmuradova, M.M.; et al. Development of superior fibre quality upland cotton cultivar series ‘Ravnaq’ using marker-assisted selection. Front. Plant Sci. 2022, 13, 906472. [Google Scholar] [CrossRef]
- Escribano, P.; Viruel, M.A.; Hormaza, J.I. PERMANENT GENETIC RESOURCES: Development of 52 new polymorphic SSR markers from cherimoya (Annona cherimola Mill.): Transferability to related taxa and selection of a reduced set for DNA fingerprinting and diversity studies. Mol. Ecol. Resour. 2008, 8, 317–321. [Google Scholar] [CrossRef]
- Freixas-Coutin, J.A.; An, S.; Postman, J.; Bassil, N.V.; Yates, B.; Shukla, M.; Saxena, P.K. Development of a reliable Corylus sp. reference database through the implementation of a DNA fingerprinting test. Planta 2019, 249, 1863–1874. [Google Scholar] [CrossRef]
- Portis, E.; Lanteri, S.; Barchi, L.; Portis, F.; Valente, L.; Toppino, L.; Rotino, G.L.; Acquadro, A. Comprehensive characterization of simple sequence repeats in eggplant (Solanum melongena L.) genome and construction of a web resource. Front. Plant Sci. 2018, 9, 401. [Google Scholar] [CrossRef]
- Purwoko, D.; Cartealy, I.C.; Tajuddin, T.; Dinarti, D.; Sudarsono, S. SSR identification and marker development for sago palm based on NGS genome data. Breed. Sci. 2019, 69, 1–10. [Google Scholar] [CrossRef]
- Chen, D.Z.; Liu, Y.; Pan, Q.; Li, F.F.; Zhang, Q.H.; Ge, X.H.; Li, Z.Y. De novo transcriptome assembly, gene expressions and metabolites for flower color variation of two garden species in Brassicaceae. Sci. Hortic. 2018, 240, 592–602. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Tan, J.; Hu, S.; Yu, J.; Xue, Q. A genome-wide microsatellite polymorphism database for the indica and japonica rice. DNA Res. 2007, 14, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, L.; Xu, Y.; Chen, C.; Rong, T.; Ali, F.; Zhou, S.; Wu, F.; Liu, Y.; Wang, J.; et al. Development and characterization of simple sequence repeat markers providing genome-wide coverage and high resolution in maize. DNA Res. 2013, 20, 497–509. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Song, P.Y.; Koo, D.H.; Guo, L.Q.; Li, Y.M.; Sun, S.R.; Weng, Y.Q.; Yang, L.M. Genome wide characterization of simple sequence repeats in watermelon genome and their application in comparative mapping and genetic diversity analysis. BMC Genomics 2016, 17, 557. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, P.; Shi, T.; Yang, J.; Wang, L.; Wang, S.; Su, Y.; Zhang, H.; Qiao, Y.; Li, X. Genome-wide characterization of simple sequence repeats in Pyrus bretschneideri and their application in an analysis of genetic diversity in pear. BMC Genom. 2018, 19, 473. [Google Scholar] [CrossRef] [PubMed]
- Rassmann, K.; Schlötterer, C.; Tautz, D. Isolation of simple-sequence loci for use in polymerase chain reaction-based DNA fingerprinting. Electrophoresis 1991, 12, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.A.; Jong, P.M.; Rine, J.; Duyk, G. Construction of small-insert genomic DNA libraries highly enriched for microsatellite repeat sequences. Proc. Natl. Acad. Sci. USA 1992, 89, 3419–3423. [Google Scholar] [CrossRef]
- Karagyozov, L.; Kalcheva, I.D.; Chapman, V.M. Construction of random small-insert genomic libraries highly enriched for simple sequence repeats. Nucleic Acids Res. 1993, 21, 3911–3912. [Google Scholar] [CrossRef]
- Collevatti, R.G.; Brondani, R.V.; Grattapaglia, D. Development and characterization of microsatellite markers for genetic analysis of a Brazilian endangered tree species Caryocar Brasiliense. Heredity 1999, 83 Pt 6, 748–756. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Luo, Z.; Huang, L.; Chen, X.; Fang, B.; Li, Y.; Chen, J.; Zhang, X. Characterization and development of EST-derived SSR markers in cultivated sweetpotato (Ipomoea batatas). BMC Plant Biol. 2011, 11, 139. [Google Scholar] [CrossRef]
- Mariotti, R.; Cultrera, N.G.M.; Mousavi, S.; Baglivo, F.; Rossi, M.; Albertini, E.; Alagna, F.; Carbone, F.; Perrotta, G.; Baldoni, L. Development, evaluation, and validation of new EST-SSR markers in olive (Olea europaea L.). Tree Genet. Genomes 2016, 12, 12010. [Google Scholar] [CrossRef]
- Temnykh, S.; DeClerck, G.; Lukashova, A.; Lipovich, L.; Cartinhour, S.; McCouch, S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): Frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001, 11, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Hanafey, M.; Powell, W. Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat. Genet. 2002, 30, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Tian, Y.L.; Yang, R.H.; Feng, H.P.; Ouyang, Q.J.; Tian, Y.; Tan, Z.Y.; Li, M.F.; Niu, Y.L.; Jiang, J.H.; et al. Coevolution between simple sequence repeats (SSRs) and virus genome size. BMC Genom. 2012, 13, 435. [Google Scholar] [CrossRef]
- Portis, E.; Portis, F.; Valente, L.; Moglia, A.; Barchi, L.; Lanteri, S.; Acquadro, A. A genome-wide survey of the microsatellite content of the globe artichoke genome and the development of a web-based database. PLoS ONE 2016, 11, e0162841. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Senalik, D.A.; Yang, L.; Simon, P.W.; Harkins, T.T.; Kodira, C.D.; Huang, S.; Weng, Y. Genome-wide characterization of simple sequence repeats in cucumber (Cucumis sativus L.). BMC Genom. 2010, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, S.S.; Wang, T.Y.; He, C.Y.; Luo, H.M.; Zhang, J.G.; Zeng, Y.F. Genomic SSR and EST-SSR markers for phylogenetic and pedigree reconstructions-A comparison in sea buckthorn. Plant Breed. 2021, 140, 167–183. [Google Scholar] [CrossRef]
- Hou, B.; Feng, S.; Wu, Y. Systemic Identification of Hevea brasiliensis EST-SSR Markers and Primer Screening. J. Nucleic Acids 2017, 2017, 6590902. [Google Scholar] [CrossRef]
- Shi, J.; Huang, S.; Zhan, J.; Yu, J.; Wang, X.; Hua, W.; Liu, S.; Liu, G.; Wang, H. Genome-wide microsatellite characterization and marker development in the sequenced Brassica crop species. DNA Res. 2014, 21, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dong, Y.; Zhao, J.; Huang, L.; Ren, X.; Chen, Y.; Huang, S.; Liao, B.; Lei, Y.; Yan, L.; et al. Genomic survey sequencing for development and validation of single-locus SSR markers in peanut (Arachis hypogaea L.). BMC Genomics. 2016, 17, 420. [Google Scholar] [CrossRef]
- Patil, P.G.; Singh, N.V.; Bohra, A.; Raghavendra, K.P.; Mane, R.; Mundewadikar, D.M.; Babu, K.D.; Sharma, J. Comprehensive characterization and validation of chromosome-specific highly polymorphic SSR markers from pomegranate (Punica granatum L.) cv. Tunisia genome. Front. Plant Sci. 2021, 12, 645055. [Google Scholar] [CrossRef]
- Burton, J.N.; Adey, A.; Patwardhan, R.P.; Qiu, R.; Kitzman, J.O.; Shendure, J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat. Biotechnol. 2013, 31, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Boyle, T. Population genetic analysis of codominant and dominant markers and quantitative traits. Belgjbot 1997, 129, 157–163. [Google Scholar]


| Summary of Genome Assembly | Number |
|---|---|
| No. of raw reads (Gb) | 336 |
| GC percent | 40.00% |
| % Squences with Q30 | 93.00% |
| Reads sequences (Mb) | 44,459.15 |
| Contig sequence numbers | 891 |
| Contigs length (bp) | 1,983,607,451 |
| Maximum length of contigs (bp) | 41,745,843 |
| N50 of contigs(bp) | 11,438,961 |
| Scaffold sequence numbers | 343 |
| Scaffold length (bp) | 1,983,881,451 |
| Maximum length of scaffold (bp) | 357,731,900 |
| N50 of scaffold (bp) | 258,639,456 |
| Genome assembly size (Mb) | 1977.48 |
| No. of chromosomes | 7 |
| Motifs Length | Number of Loci Identified | Cumulative Length (bp) | Average Length (bp) | Mean of Repeat Number | No. of Motifs | Density (SSR/Mb) |
|---|---|---|---|---|---|---|
| Di- | 35,279 | 725,464 | 21.76 | 10.89 | 6 | 17.84 |
| Tri- | 7319 | 150,330 | 20.7 | 6.92 | 30 | 3.70 |
| Tetra- | 1264 | 26,996 | 21.49 | 5.37 | 71 | 0.64 |
| Penta- | 992 | 28,305 | 28.56 | 5.71 | 105 | 0.50 |
| Hexa- | 758 | 26,730 | 35.63 | 5.94 | 269 | 0.38 |
| Total | 45,612 | 957,825 | . | . | 481 | 23.06 |
| Chromosome | Chromosome Length (bp) | SSR loci | Designed Primer Pairs | The Succeed Rate of Primer Designing | ||
|---|---|---|---|---|---|---|
| Number | Density (SSR Locus/Mb) | Number | Density (Primer/Mb) | |||
| chr1 | 235,669,302 | 5512 | 23.39 | 5263 | 22.33 | 95.48% |
| chr2 | 253,907,236 | 6524 | 25.69 | 6204 | 24.43 | 95.10% |
| chr3 | 301,678,771 | 6656 | 22.06 | 6355 | 21.07 | 95.48% |
| chr4 | 361,232,675 | 8368 | 23.17 | 8009 | 22.17 | 95.71% |
| chr5 | 258,445,478 | 5693 | 22.03 | 5400 | 20.89 | 94.85% |
| chr6 | 307,454,722 | 6899 | 22.44 | 6601 | 21.47 | 95.68% |
| chr7 | 259,093,400 | 5960 | 23.00 | 5708 | 22.03 | 95.77% |
| Total | 1,977,481,584 | 45,612 | 23.07 | 43,540 | 22.02 | 95.46% |
| Marker | Na * | Ne * | I * | PIC | Chromosome |
|---|---|---|---|---|---|
| MISSR99 | 2 | 2.000 | 0.693 | 0.500 | Chr1 |
| MISSR207 | 3 | 2.149 | 0.826 | 0.535 | Chr1 |
| MISSR209 | 3 | 1.256 | 0.411 | 0.204 | Chr1 |
| MISSR210 | 3 | 2.558 | 1.011 | 0.609 | Chr1 |
| MISSR127 | 4 | 3.912 | 1.375 | 0.744 | Chr2 |
| MISSR221 | 2 | 1.663 | 0.588 | 0.399 | Chr2 |
| MISSR14 | 4 | 3.564 | 1.324 | 0.719 | Chr3 |
| MISSR244 | 3 | 2.198 | 0.856 | 0.545 | Chr3 |
| MISSR227 | 2 | 1.190 | 0.297 | 0.160 | Chr3 |
| MISSR243 | 2 | 1.835 | 0.647 | 0.455 | Chr3 |
| MISSR152 | 4 | 3.236 | 1.260 | 0.691 | Chr4 |
| MISSR155 | 2 | 1.724 | 0.611 | 0.420 | Chr4 |
| MISSR253 | 4 | 2.159 | 0.854 | 0.537 | Chr4 |
| MISSR24 | 4 | 2.660 | 1.108 | 0.624 | Chr5 |
| MISSR264 | 2 | 1.882 | 0.662 | 0.469 | Chr5 |
| MISSR265 | 3 | 2.612 | 1.024 | 0.617 | Chr5 |
| MISSR178 | 4 | 3.756 | 1.353 | 0.734 | Chr6 |
| MISSR271 | 3 | 2.632 | 1.030 | 0.620 | Chr6 |
| MISSR289 | 5 | 3.493 | 1.366 | 0.714 | Chr6 |
| MISSR291 | 2 | 1.220 | 0.325 | 0.180 | Chr6 |
| MISSR5 | 2 | 1.551 | 0.540 | 0.355 | Chr7 |
| MISSR192 | 4 | 3.556 | 1.322 | 0.719 | Chr7 |
| Mean | 3.0 | 2.400 | 0.886 | 0.525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.; Zhang, H.; Chen, H.; Guan, M.; Zhu, Z.; Cao, X.; Ge, X.; Zhu, B.; Chen, D. First Report on Development of Genome-Wide Microsatellite Markers for Stock (Matthiola incana L.). Plants 2023, 12, 748. https://doi.org/10.3390/plants12040748
Tan C, Zhang H, Chen H, Guan M, Zhu Z, Cao X, Ge X, Zhu B, Chen D. First Report on Development of Genome-Wide Microsatellite Markers for Stock (Matthiola incana L.). Plants. 2023; 12(4):748. https://doi.org/10.3390/plants12040748
Chicago/Turabian StyleTan, Chen, Haimei Zhang, Haidong Chen, Miaotian Guan, Zhenzhi Zhu, Xueying Cao, Xianhong Ge, Bo Zhu, and Daozong Chen. 2023. "First Report on Development of Genome-Wide Microsatellite Markers for Stock (Matthiola incana L.)" Plants 12, no. 4: 748. https://doi.org/10.3390/plants12040748
APA StyleTan, C., Zhang, H., Chen, H., Guan, M., Zhu, Z., Cao, X., Ge, X., Zhu, B., & Chen, D. (2023). First Report on Development of Genome-Wide Microsatellite Markers for Stock (Matthiola incana L.). Plants, 12(4), 748. https://doi.org/10.3390/plants12040748






