Abstract
Oak powdery mildew caused by Erysiphe alphitoides (Griffon and Maubl.; U. Braun & S. Takam.) is a common disease in European forests. One of the most susceptible species is the pedunculate oak (Quercus robur L.). Presently, a few methods are available to control powdery mildew, e.g., the use of fungicides (e.g., based on citric acid), antagonistic fungi or bacteria, chemical treatments (e.g., sulphur, potassium bicarbonate) or genetic resistance. In our study, we aimed to check the effects of using chitosan derivatives and novel active substances inducing the plants’ natural resistance: benzodiathiadiazole (both in neutral and salt form). 84 pedunculate oak seedlings were subjected to the experiment in three treatment variants (plus positive and negative controls). The plants were treated with active substances and inoculated with E. alphitoides. Although the powdery mildew symptoms appeared in all variants, they were manifested mainly by the mycelium in the form of small spots. The experiment indicated that the highest limitation of powdery mildew mycelium was achieved by applying N-methyl-N-methoxyamide-7-carboxybenzo(1,2,3)thiadiazole (BTHWA). The application of BTHWA reduced disease development by 88.9% when compared to the effects of the other variants.
1. Introduction
Oak powdery mildew (PM) caused by Erysiphe alphitoides (Griffon & Maubl.) U. Braun and S. Takam., an exotic pathogen first described in Europe in 1907 [1], is one of the most common diseases of pedunculate oak (Quercus robur L.) and sessile oak (Q. petraea (Matt.) Liebl.) [2,3]. The disease can be very severe, particularly in young plants resulting in growth reduction and seedling losses [4,5,6,7,8]. The leaves affected by E. alphitoides suffer from decreased net photosynthesis [9,10] and changes in assimilation management that can affect tree growth and survival rate [11]. Even though PM is a primary foliage disease, the knowledge of the defence responses of the tree to infection by this pathogen is limited.
Protection against oak PM is implemented primarily in forest nurseries. So far, a few methods are available to control the disease, including some conventional fungicides, e.g., based on citric acid, antagonistic bacteria, e.g., Streptomyces [12] or fungi, e.g., Trichoderma asperellum IZR D-11 [13], commercial chemical treatments, e.g., with sulphur, potassium bicarbonate, potassium phosphite, salicylic acid [14] or by developing genetic resistance.
Systemic acquired resistance (SAR) of plants is a form of induced resistance that is activated throughout a plant after exposure to pathogens or chemical substances such as nicotinic acid, salicylic acid, benzothiadiazole or chitosan [15,16]. Induction of SAR is characterized by the accumulation of salicylic acid to stimulate defence mechanisms, often resulting in a localized hypersensitive response [17].
The signalling agents contributing to SAR are salicylic acid (SA) and several components of the SA pathway, including the methylated derivative of SA (methyl SA, MeSA) [18,19]. The precise mode of SA action is not yet described. It has become increasingly evident that SA is part of a complex network associated with plant response [20]. Treatment of plants with salicylic acid and functional analogues results in a plant response with increased expression of PR family genes, including NPR1. PR genes code for proteins such as β-1,3-glucanases and chitinases and play a role in either preventing or slowing the colonization of pathogens in the host [21].
One of the most studied active substances capable of inducing plant resistance is benzo(1,2,3)thiadiazole-7-carboxylic acid S-methyl ester (ASM, BTH), which is commercially available as Bion 50 WG and Actigard 50 WG marketed by Syngenta [22,23,24].
Our earlier studies were aimed at obtaining new derivatives of benzothiadiazoles that could act as effective plant resistance inducers [25,26,27,28,29,30]. Moreover, a group of investigated new derivatives showed lower (eco)toxicity and higher biodegradability than the commercially available BTH [31].
A substance of natural origin that has also been reported to induce plant resistance is chitosan, which belongs to the group of polysaccharides [32]. Chitosan is insoluble in most solvents, but it is possible to derivatize it using e.g., acetic acid, formic acid, succinic acid, lactic acid, and malic acid to obtain the salt form of chitosan which improves its solubility [33]. Chitosan has also shown antimicrobial activity against different groups of microorganisms, such as bacteria and fungi, including the fungi causing PM [34]. However, its antimicrobial action is affected by intrinsic factors such as the type of chitosan, degree of chitosan polymerization, the type of host, natural nutrient constituency, chemical or nutrient composition of the substrates or both, and the environmental conditions [35].
Induction of plant resistance has a positive effect on general plant health. A reliable plant health assessment might be performed using the Vegetation indices (VIs) [36,37]. VIs are a type of mathematical transformation of spectral bands characterizing the spectral properties of plants. The expressed values of different VIs, including the Normalized Difference Vegetation Index (NDVI) and Simple Ration Index (SR), help to analyze crop vigour and several other vegetation properties, including biomass and chlorophyll content [38]. Vegetation indices have also been shown to be reliable indicators of abiotic stress in plants [39,40].
Among the tested compounds, N-methyl-N-methoxyamide-7-carboxybenzo(1,2,3)thiadiazole (BTHWA), which is a derivative of benzothiadiazole in a neutral form, has shown the highest effectiveness of SAR induction [41]. The second substance with similar activity is a benzothiadiazole derivative in ionic form, cholinium benzo(1,2,3) thiadiazole-7-carboxylate ([CHOL][BTHCOO]). Both substances have been subjected to molecular biology studies to verify their action as SAR inducers [41]. After the treatment, the plants treated either with BTHWA or [CHOL][BTHCOO] solution showed a higher level of SAR marker genes NPR1, PAL, and PR-1b at 4 h than the untreated control. Moreover, 7 days after infection of plants treated with both above-mentioned compounds, the level of viral RNA accumulation was lower compared to this of untreated control plants, which leads to the conclusion that the replication of the virus was less efficient. These experiments showed that both BTHWA and [CHOL][BTHCOO] act only through plant metabolism and do not possess antifungal properties [41].
The general aim of the study was to check the effect of using novel substances representing benzothiadiazoles, a BTH derivative, namely: N-methyl-N-methoxyamide-7-carboxybenzo(1,2,3)thiadiazole (BTHWA); cholinium benzo(1,2,3)thiadiazole-7-carboxylate ([CHOL][BTHCOO]) and chitosan lactate (CHL) being SAR elicitor of natural origin, on the protection of pedunculate oak against the PM. We hypothesized that the above-mentioned substances would strengthen the oak’s natural defence mechanisms in response to fungal inoculation and, consequently, trigger the oak’s response against the PM.
2. Results
Plants subjected to the study were treated with the test substances five times in total, including two treatments before fungal inoculation, as described in Table 1. After the inoculation, the number of PM symptoms started to appear and increase in time. The first visible symptoms of PM, mostly manifested by small mycelium patches up to 7 mm in diameter, appeared seven days after the inoculation. Interestingly, they were present only on the plants subjected to both controls and were located mostly on top leaves. On the 35th day after the fungal inoculation, the symptoms appeared in all treatments (Table 1) and started to vary. Mycelium was limited to small spots in the seedlings treated by [CHOL][BTHCOO] and BTHWA. On the plants subjected to other treatments, a higher presence of more scattered (diffuse) mycelium of size over 7 mm was observed (Figure 1, Figure 2 and Figure 3). Furthermore, severe tissue necrosis and leaf defoliation were not observed.

Table 1.
The percentage of seedlings with visible symptoms of powdery mildew on the following days after inoculation.
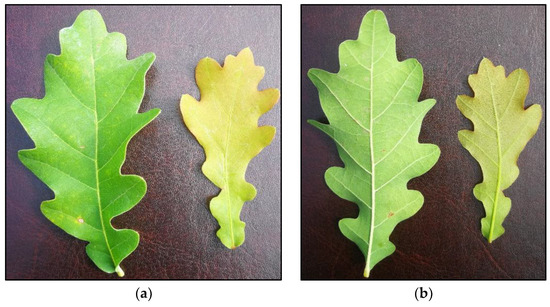
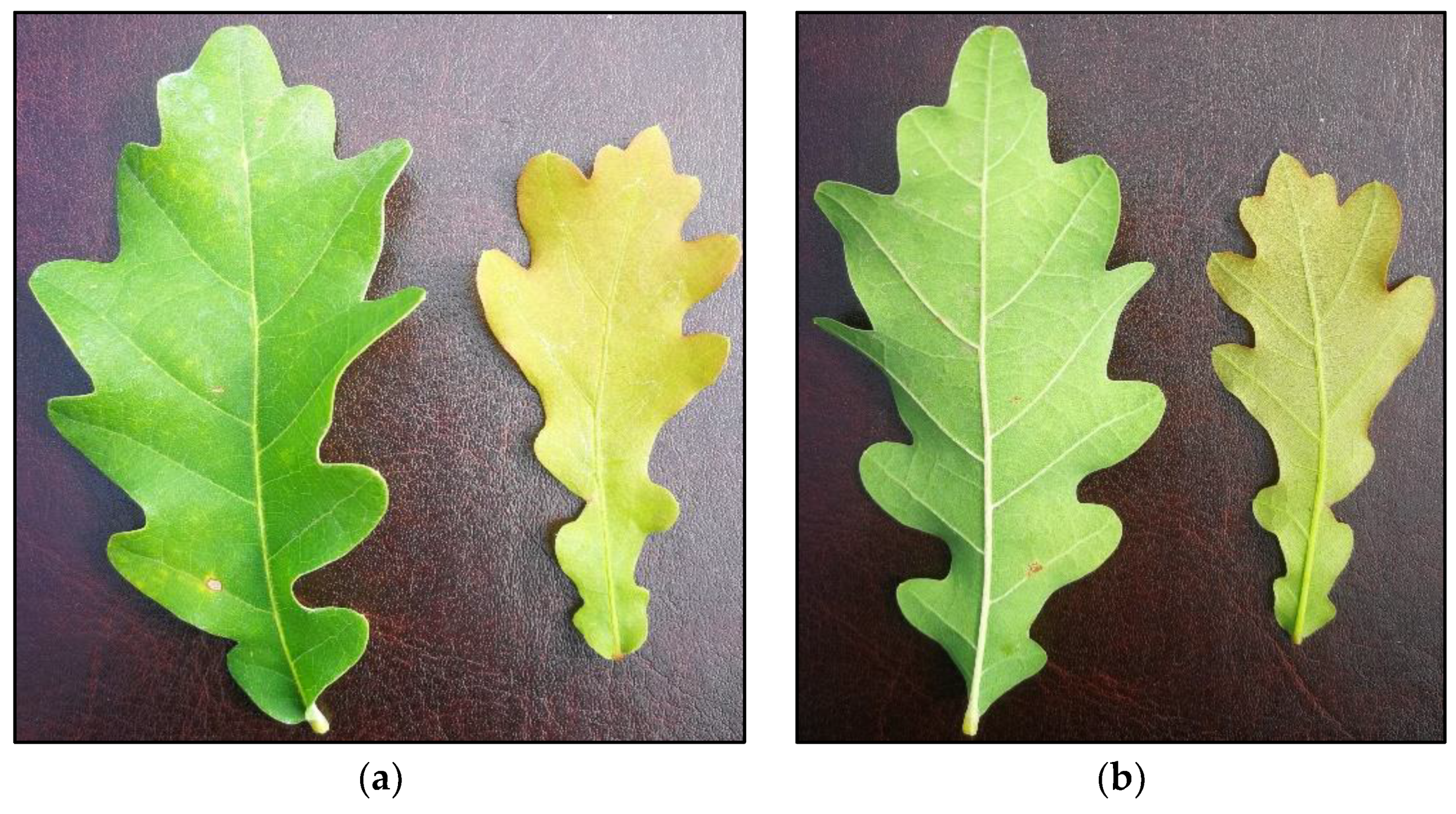
Figure 1.
Oak leaves (mature—Left; juvenile—Right) with no symptoms of powdery mildew after 69 days of fungal inoculation and five treatments with BTHWA: (a) upper side; (b) bottom side.
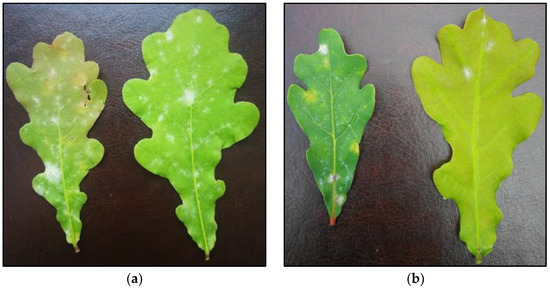
Figure 2.
Powdery mildew mycelium in the form of small spots in oak leaves after 69 days of fungal inoculation and five treatments with (a) [CHOL][BTHCOO]; (b) BTHWA.

Figure 3.
Powdery mildew mycelium scattered over a larger area after 69 days of fungal inoculation: (a) C+; (b) after five treatments with CHL.
Among the groups of seedlings treated with the tested substances (all variants), the highest number of plants with symptoms on the 49th and 69th day after inoculation was observed among those treated with CHL. Interestingly, the percentage of seedlings with symptoms on the second and third dates of assessments was lower when compared to those treated with [CHOL][BTHCOO]. However, the development of the disease accelerated considerably in the subsequent days. The substance producing the greatest reduction in the number of plants with disease symptoms was BTHWA. Its application resulted in 11% of seedlings with symptoms, compared to 73% of plants with symptoms among the seedlings of C+ and 56%—for plants of C− (Table 1). An interesting but not surprising result was a high infection of plants in C−. It shows that the pathogen was present in the environment and, therefore, naturally infected the uninoculated plants in the experiment (See also DSI index; Table 2).

Table 2.
The Disease Severity Index of seedlings on the 49th day and the 69th day after inoculation.
On the fourth measurement date, the PM infection of leaves was evaluated in more detail. A similar evaluation was repeated on the 69th day after inoculation with the fungus to determine whether the disease was still developing. The results are expressed in terms of the DSI index in Table 2. The most effective protection was provided by the BTHWA (DSI index−3%). Furthermore, it should be emphasized that only on the plants treated with this substance no further development of the pathogen was observed. The second lowest value of DSI index was recorded for the plants treated with [CHOL][BTHCOO]. The seedlings treated with CHL, despite initially good values in terms of the number of infected plants (Table 1), showed the poorest treatment effects at the end of the experiment. Moreover, the assessment made on the 69th day provided practically the same result as that of the C−.
Additionally, the plants subjected to all treatments were analysed in terms of their growth (Table 3). As indicated before, infection with PM might lead to a reduction in biomass increase. Although no statistically significant differences were observed between the plants representing all treatments, the plants sprayed with the tested substances showed a higher growth rate than the plants from inoculated control variant.

Table 3.
Plants’ height at the beginning and end of the experiment.
In the presented study, no significant differences were observed between VIs measured for plants before and after treatment and inoculation (Table 4). The NDVI values for the plants subjected to different treatments before infection were in the range of 0.71–0.73, while after infection, they were in the range of 0.67–0.73. The SR values ranged from 5.94 to 6.70 and 5.15 to 6.37 for the plants subjected to all treatment variants before and after infection, respectively.

Table 4.
Measured Vegetation indices (VIs) in the study.
3. Discussion
There is a plethora of reports on the investigation of plant defence mechanisms through studies on herbaceous annuals or short-lived perennials which include model plant species such as Arabidopsis thaliana (mouse-ear cress), Cucumis sativus (cucumber), and Nicotiana tabacum (tobacco). However, not much attention has been paid to investigating these phenomena for trees, both angiosperms and gymnosperms [42].
Trees have some unique features when compared with herbaceous plants: they are usually much larger, have much longer life spans (sometimes of millennia), are characterized by life histories that have no equals among herbaceous model plants, and exhibit different architectural forms linked to secondary growth. Trees may be subject to different patterns of herbivore and pathogen pressure and require different protection modes. Hence, findings from reports on herbaceous studies might not always apply to forest trees [43].
The activity of benzothiadiazoles related to the induction of plant resistance against PM has been reported on various crops such as cucumber [44], strawberry [45] or wheat [46]. In addition, the activity of the BTHWA substance was confirmed in our previous studies performed on ash seedlings (Fraxinus excelsior L.). After the application of this substance, the development of the fungus Hymenoscyphus fraxineus was significantly slowed down or even completely stopped [47]. Moreover, the treatment with BTHWA limited the size of necrotic phloem lesions [47]. According to preliminary unpublished data, BTHWA and [CHOL][BTHCOO] have no direct effect on the fungi species such as Alternaria alternate, Trichoderma viride and Oidium neolycopersici causing PM on tomato.
In the case of PM, we decided to check the efficiency of the tested substances in reducing disease symptoms. It is crucial, especially considering that E. alphitoides affects oaks of all ages, but primarily young plants, both in the renewals and forest nurseries, by reducing their growth and causing seedling losses [5,6,7,8]. Our study showed that the first visible symptoms of PM, mostly manifested by small mycelium, appeared seven days after inoculation in both controls. During the experiment, the symptoms occurred in the plants subjected to the other variants of treatment. However, the appearance of PM in uninoculated control was interesting but not surprising. It shows that the PM spores were present in the open air and could naturally infect seedlings stored in the open area. Moreover, this indicates that both artificially and “naturally” infected controls resulted in a higher number of seedlings with PM symptoms, i.e., 73% (C+) and 56% (C−), than plants sprayed with BTHWA and [CHOL][BTHCOO].
Comparing the plants with the induced resistance against PM, the lowest number of symptoms was noted in the plants treated with BTHWA (11%) and [CHOL][BTHCOO] (31%). In these treatments, we observed that the mycelium was limited only to small spots, whilst they were more scattered in the controls and plants treated with CHL. These observations are supported by the values of DSI index which were the lowest for the plants treated with BTHWA (3%). Moreover, the DSI index for the seedlings treated with BTHWA took equally low values after the 49th and 69th days after inoculation. In other words, we can assume that the natural defence mechanisms triggered by the applied substance were still active.
As for the results obtained for the plants treated with CHL, we assume that the initially low percentage of seedlings showing symptoms of PM resulted from CHL showing both plant resistance-inducing activity and antifungal properties. The observations made on the 21st and 35th day after inoculation suggest that such a double action could provide better protection than the chemical inducer [CHOL][BTHCOO]. However, during the experiment, it turned out that the protection provided by CHL was broken, and the development of the pathogen significantly accelerated. Our results are in line with literature data describing chitosan as plant resistance inducers with lower efficacy than benzothiadiazoles [48].
During the experiment, we also observed that the seedlings sprayed with the tested substances showed higher mean growth than plants subjected to untreated control. Most likely, this resulted from a lower degree of PM infection, which is known to have a significant effect on the growth of young trees [4,5,6,7,8].
It has been reported in the literature that NDVI is a reliable indicator of plant health [36,37] and VIs are sensitive to abiotic stress responses in plants [39,40]. Thus, we checked the plant’s response to PM using selected spectral indices, i.e., NDVI and SR. However, the results showed no statistically significant differences (Table 4). The NDVI values indicated that before and after inoculation, the tested treatment variants maintained plant health, and the applied substances did not induce abiotic stress on plants. We assume that the lack of VIs response may be connected with the slight PM symptoms, i.e., without severe defoliation or leaf necrosis observed in all treatments. Additionally, both NDVI and SR values after inoculation in C− group tended to decrease slightly. In order to substantiate this, more frequent measurements are needed. Moreover, more sensitive approaches, including Chlorophyll fluorescence [49] and gas exchange measurements, can be considered for future studies.
This preliminary study showed that the tested substances, especially BTHWA, have a high potential among resistance inducers to protect oak seedlings against the PM. However, we still need to conduct more research to elucidate the protection’s mechanisms in more detail. Perhaps the modification of the measurement methodology and its extension to include other parameters, i.e., related to the fast kinetics of chlorophyll, will allow a better understanding of the plant’s response. Moreover, in the future study, we plan to analyse physiological features that can be affected by PM [50,51,52].
Moreover, to prove that such a plant response was caused by SAR induction, it would be necessary to supplement these results with detailed biochemical studies on enzymes or molecular studies on genes characteristic of this phenomenon. For instance, monitoring of changes in the level of expression of SAR marker genes in subsequent days after treatment will allow for a more accurate demonstration of how long the plant remains in a stimulated state. In addition, such a comparative analysis performed for the tested substances will allow for a more accurate comparison of their effectiveness on a given experimental model.
Detailed analyses may also concern a more accurate estimation of the level of infection, which can be performed several times after infection. This will allow for monitoring of the level of disease development. Such a study should also be supplemented with histo-cytochemical analysis of leaf symptoms, as their source might be an abiotic factor and not a pathogen attack [53]. Similar studies on plant response to treatment with different concentrations of BTH have already been conducted and the presented methodology can be applied to our further tests [54,55].
Nevertheless, our study is a “first step” in this area, and we believe that the testing of the activity of new substances capable of providing effective protection to trees will be of key importance for forestry.
Considering the results obtained, we conclude that among administered substances used to strengthen the oak’s natural defence mechanisms in response to E. alphitoides inoculation, the highest efficiency in reducing the disease development had BTHWA. The study is the first report of using benzothiadiazoles to protect oak against PM.
4. Materials and Methods
4.1. Material Collection
In early June 2022, we collected 100 two-year-old oak seedlings (Q. robur) from a forest nursery in the Lipka Forest District, NW Poland. However, we have used only 84 plants of approximately 10–15 cm in height. The rest of seedlings were removed as we wanted to have a similar starting height point. The seedlings were collected together with soil and placed directly into pots with a capacity of four litres. The plants were stored in an open place and watered once or twice a week to keep the soil moist. All collected oaks showed no visible symptoms of PM or other diseases. The inspection was repeated twice, i.e., during the collecting of the plants from the forest nursery and on the same day when the fungal inoculation was performed.
4.2. Tested Substances
The substances studied were two benzothiadiazole derivatives obtained in our group: N-methoxy-N-methylbenzo(1,2,3)thiadiazole-7-carboxamide (BTHWA) [56,57], cholinium benzo(1,2,3)thiadiazole-7-carboxylate ([CHOL][BTHCOO]) [30], and a Chitosan lactate (CHL). BTHWA, [CHOL][BTHCOO] and CHL were provided by company Innosil Ltd. (Poznan, Poland). The tested substances were dissolved in water in the following concentrations: BTHWA—20 mg/L, [CHOL][BTHCOO]—25 mg/L, and CHL—400 mg/L.
4.3. Experimental Design
Two weeks after putting the seedlings into pots, we applied the tested substances for the first time. The experiment included five variants of treatment (Table 5). The tested substances were applied to plants by foliar application every 14 days. Each plant was sprayed with 10 mL of a working solution containing a specified concentration of the tested substance. The plants were sprayed in the evenings. After two treatments, the seedlings, except those representing C−, were inoculated with fungus (seven days after the second treatment). Subsequently, the third application of the tested substances was made after the next seven days. Altogether, five applications were performed between the end of June and August 2022.

Table 5.
The variants of treatment in the experiment and simplified schedule of treatment.
4.4. Seedling Inoculation
Inoculation was performed on the 7th day after the second application of the tested substances. To obtain sufficient inoculum of the E. alphitoides isolates, 30 min before inoculation, fresh leaves with visible symptoms of PM were collected from an infected Q. robur (52°42′6051″ N, 16°90′0498″ E). Subsequently, the leaves were placed in paper bags and transported to the experiment site. The seedlings were inoculated late in the evening to allow the fungi to germinate in the morning dew and to protect them from the hot air during the day. The leaves were inoculated by gently rubbing the adaxial epidermis of three expanded leaves with sporulating colonies on the surface of the source leaves. Inoculation was performed once.
4.5. Disease Severity Assessment
The pathogen infection with visible symptoms on leaves was assessed every 14 days, on the same dates as the five treatments, before the tested substances were applied. Moreover, two additional observations were made when the application of substances was stopped (on the 49th and 69th day). The percentage of seedlings with visible symptoms of mycelium on the plants subjected to each variant was determined to check the progress of the disease development. More detailed analyses were performed when the application of substances was stopped, i.e., on the 49th and 69th day after the fungus inoculation. The plants were rated for infection on a scale of 0–4: 0, no infection; 1, less than 10% leaves affected by mycelium; 2, 11–25% leaves affected; 3, 26–50% leaves affected; 4, more than 50% leaves affected. Plant pathologists, breeders, and professionals in other disciplines often simplify the ordinal scale rating data by transforming these numbers into a disease severity index (DSI) [58,59]. The DSI is a single index number for summarising the total effect of the disease on a single plant or a small sample of plants. The calculation was made according to the equation:
The simplification of disease severity resulted from the observed symptoms of PM in our experiment—mostly small mycelium in the form of spots up to 7 mm in diameter or less frequent—mycelium which scattered on a larger area of leaves (over 7 mm). Furthermore, we did not note severe leaf defoliation or tissue necrosis.
4.6. Vegetation Indices Collection
In the presented study, spectral vegetation indices such as NDVI and SR were measured (Table 4). The NDVI was calculated based on the equation NDVI = (RNIR − RRED)/(RNIR + RRED) [30], and the SR index was based on the equation SR = RNIR/RRED [60,61]. Two repetitions were made during the experiment. The first measurement was carried out on the 10th day of the experiment (before treatment and inoculation) to access the initial spectral properties of the plants not treated either with active substances or infected with PM. The second measurement was carried out on the 69th day after fungal inoculation (after treatment and inoculation measurements). The selected and marked fully matured leaves (one leaf in an individual plant) were measured using the handheld device PolyPen RP 410 (Photon Systems Instruments Ltd., Drásov, Czech Republic). The leaf (held in place with a RP 410 mechanical leaf holder) was exposed to an internal light source of RP 410 (Xenon incandescent lamp with a spectral range of 380–1050 nm) with a UVIS sensor (380–790 nm). Five plants representing each variant of treatment were measured, including controls. The recorded data were downloaded and exported using integrated Spectrapen software from the device provider (Photon Systems Instruments Ltd., Drásov, Czech Republic).
4.7. Statistical Analysis
Morphological parameters of oak seedlings and VIs are means ± Standard Deviation (SD). Data were evaluated by analysis of variance (ANOVA), and the mean differences were compared by post hoc test at a p < 0.05 level, according to Tukey’s HSD. Statistical analyses were performed using OriginLab 2022 software for Windows (OriginLab Corp., Northampton, MA, USA). The Disease Severity Index (DSI%) and the data of seedlings with visible symptoms of PM are given as percentages per treatment. Such an approach comes from the visual assessment of symptoms based on the sum of visual scoring data but not mean values. Hence, no statistical analysis was performed for this scoring data set.
Author Contributions
Conceptualization, M.B., K.T. and R.K.; methodology, K.T., M.B., R.K., R.P., M.S. (Maciej Spychalski) and M.S. (Marcin Smiglak); investigation, K.T., M.B. and R.P.; formal analysis and data curation and validation, K.T., R.P. and M.S. (Maciej Spychalski); writing—original draft preparation, K.T., R.K., M.S. (Maciej Spychalski) and M.B.; writing—review and editing, K.T., M.B., R.K., M.S. (Maciej Spychalski), R.P. and M.S. (Marcin Smiglak). All authors have read and agreed to the published version of the manuscript.
Funding
The “New plant resistance inducers and their application as innovative approach to plant protection against pathogens” project is carried out within the TEAMTECH (POIR.04/04.00-00-5BD9/17-00) programme of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the staff of the Lipka Forest District for providing oak seedlings for our experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marçais, B.; Desprez-Loustau, M.L. European oak powdery mildew: Impact on trees, effects of environmental factors, and potential effects of climate change. Ann. For. Sci. 2014, 71, 633–642. [Google Scholar] [CrossRef]
- Mougou, A.; Dutech, C.; Desprez-Loustau, M.L. New insights into the identity and origin of the causal agent of oak powdery mildew in Europe. For. Pathol. 2008, 38, 275–287. [Google Scholar] [CrossRef]
- Foex, M.E. L’invasion des chênes d’Europe par le blanc ou oidium. Rev. Eaux For. 1941, 79, 338–349. [Google Scholar]
- Desprez-Loustau, M.L.; Feau, N.; Mougou-Hamdane, A.; Dutech, C. Interspecific and intraspecific diversity in oak powdery mildew in Europe: Coevolution history and adaptation to their hosts. Mycoscience 2011, 52, 165–173. [Google Scholar] [CrossRef]
- Soutrenon, A. Une experimentation pluri-annuelle confirme l’impact de l’oïdium sur de jeunes sujets. In Les Cahiers du DSF, 1–2000 (la Santé des Forets [France] en 1997); Ministère de l’Agriculture et de la Pêche: Paris, France, 1998; pp. 93–94. [Google Scholar]
- Roszak, R.; Baranowska, M.; Bełka, M.; Behnke-Borowczyk, J. Zastosowanie technik biologii molekularnej do detekcji Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. w organach roślinnych [Applying the molecular biology techniques to the detection of Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. in plant parts]. Sylwan 2019, 163, 740–745. [Google Scholar]
- Marçais, B.; Bréda, N. Role of an opportunistic pathogen in the decline of stressed oak trees. J. Ecol. 2006, 94, 1214–1223. [Google Scholar] [CrossRef]
- Marçais, B.; Kavkova, M.; Desprez-Loustau, M.L. Phenotypic variation in the phenology of ascospore production between European populations of oak powdery mildew. Ann. For. Sci. 2009, 66, 814. [Google Scholar] [CrossRef]
- Hewitt, H.G.; Ayres, P.G. Effect of infection by Microsphaera alphitoides (powdery mildew) on carbohydrate levels and translocation in seedlings of Quercus robur. New Phytol. 1976, 77, 379–390. [Google Scholar] [CrossRef]
- Hajji, M.; Dreyer, E.; Benoit, M. Impact of Erysiphe alphitoides on transpiration and photosynthesis in Quercus robur leaves. Eur. J. Plant Pathol. 2009, 125, 63–67. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Saint-Jean, G.; Barrès, B.; Dantec, C.F.; Dutech, C. Biochemical changes in Quercus robur L. leaves after Erysiphe alphitoides infection. For. Pathol. 2022, 52, e12756. [Google Scholar]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Große, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-Induced Resistance against Oak Powdery Mildew Involves Host Plant Responses in Defense, Photosynthesis, and Secondary Metabolism Pathways. Mol. Plant Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Oszako, T.; Voitka, D.; Stocki, M.; Stocka, N.; Nowakowska, J.A.; Linkiewicz, A.; Hsiang, T.; Belbahri, L.; Berezovska, D.; Malewski, T. Trichoderma asperellum efficiently protects Quercus robur leaves against Erysiphe alphitoides. Eur. J. Plant. Pathol. 2021, 159, 295–308. [Google Scholar] [CrossRef]
- Percival, G.; Haynes, I. The Influence of Systemic Inducing Resistance Chemicals for the Control of Oak Powdery Mildew (Microsphaera alphitoides) Applied as a Therapeutic Treatment. Arboric. Urban For. 2008, 34, 271–279. [Google Scholar] [CrossRef]
- Gozzo, F.; Faoro, F. Systemic acquired resistance (50 years afte discovery): Moving from the lab to the field. J. Agric. Food Chem. 2013, 61, 12473–12491. [Google Scholar] [CrossRef]
- Hartman, G.L.; Pawlowski, M.L.; Chang, H.X.; Hill, C.B. Successful technologies and approaches used to develop and manage resistance against crop disease and pests. Emerg. Technol. Promot. Food Sect. 2016, 16, 43–66. [Google Scholar]
- Fu, Z.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kaiyomo, E.; Kumar, D.; Mosher, S.; Klessig, D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic Acquired Resistance and Salicylic Acid: Past, Present, and Future. Mol. Plant Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef]
- Sudisha, J.; Sharathchandra, R.G.; Amruthesh, K.N.; Kumar, A.; Shetty, H.S. Pathogenesis related proteins in plant defense response. Biol. Control 2012, 12, 379–403. [Google Scholar]
- Nakao, S.; Watanabe, H.; Yano, T.; Yamaoka, Y.; Ishii, H. Control efficacy of the systemic acquired resistance (SAR) inducer acibenzolar-S-methyl against Venturia nashicola in Japanese pear orchards. J. Gen. Plant Pathol. 2021, 87, 307–315. [Google Scholar] [CrossRef]
- Kenney, J.R.; Grandmont, M.E.; Mauck, K.E. Priming Melon Defenses with Acibenzolar-S-methyl Attenuates Infections by Phylogenetically Distinct Viruses and Diminishes Vector Preferences for Infected Hosts. Viruses 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- De Jong, H.; Reglinski, T.; Elmer, P.A.G. Integrated Use of Aureobasidium pullulans Strain CG163 and Acibenzolar-S-Methyl for Management of Bacterial Canker in Kiwifruit. Plants 2019, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Smiglak, M.; Kukawka, R.; Lewandowski, P.; Pospieszny, H. Cationic derivatives of the plant resistance inducer benzo (1,2,3)thiadiazole-7-carbothioic acid S-methyl ester (BTH) as bifunctional ionic liquids. Tetrahedron Lett. 2014, 55, 3565–3568. [Google Scholar] [CrossRef]
- Kukawka, R.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. New ionic liquids based on systemic acquired resistance inducers combined with the phytotoxicity reducing cholinium cation. New J. Chem. 2018, 42, 11984–11990. [Google Scholar] [CrossRef]
- Feder-Kubis, J.; Czerwoniec, P.; Lewandowski, P.; Pospieszny, H.; Smiglak, M. Ionic Liquids with Natural Origin Component: A Path to New Plant Protection Products. ACS Sustain. Chem. Eng. 2020, 8, 842–852. [Google Scholar] [CrossRef]
- Zajac, A.; Kukawka, R.; Pawlowska-Zygarowicz, A.; Stolarska, O.; Smiglak, M. Ionic liquids as bioactive chemical tools for use in agriculture and the preservation of agricultural products. Green Chem. 2018, 20, 4764–4789. [Google Scholar] [CrossRef]
- Smiglak, M.; Kukawka, R.; Lewandowski, P.; Budziszewska, M.; Obrępalska-Stęplowska, A.; Krawczyk, K.; Zwolińska, A.; Pospieszny, H. New Dual Functional Salts Based on Cationic Derivative of Plant Resistance Inducer Benzo [1.2.3] thiadiazole-7-carbothioic Acid, S-Methyl Ester. ACS Sustain. Chem. Eng. 2016, 4, 3344–3351. [Google Scholar] [CrossRef]
- Smiglak, M.; Lewandowski, P.; Kukawka, R.; Budziszewska, M.; Krawczyk, K.; Obrępalska-Stęplowska, A.; Pospieszny, H. Dual functional salts of benzo [1.2.3] thiadiazole-7-carboxylates as a highly efficient weapon against viral plant diseases. ACS Sustain. Chem. Eng. 2017, 5, 4197–4204. [Google Scholar] [CrossRef]
- Markiewicz, M.; Lewandowski, P.; Spychalski, M.; Kukawka, R.; Feder-Kubis, J.; Beil, S.; Smiglak, M.; Stolte, S. New bifunctional ionic liquid-based plant systemic acquired resistance (SAR) inducers with improved environmental hazard profile. Green Chem. 2021, 23, 5138–5149. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Mohammadkhani, G.; Kumar Ramamoorthy, S.; Adolfsson, K.H.; Mahboubi, A.; Hakkarainen, M.; Zamani, A. New solvent and coagulating agent for development of chitosan fibers by wet spinning. Polymers 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Ruano-Rosa, D.; Sánchez-Hernández, E.; Baquero-Foz, R.; Martín-Ramos, P.; Martín-Gil, J.; Torres-Sánchez, S.; Casanova-Gascón, J. Chitosan-Based Bioactive Formulations for the Control of Powdery Mildew in Viticulture. Agronomy 2022, 12, 495. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Peñuelas, J.; Valentini, R. Relatioships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef]
- Almeida-Cortez, J.; de Paula, M.D.; de Oliveira, M.D.; Rodrigues dos Santos, C.I. Herbivory Rate on Woody Species of the Caatinga and NDVI as Indicators of Plant Stress (Taxa de Herbivoria em Espécies Arbóreas da Caatinga e o Uso do Índice de Vegetação por Diferença Normalizada (NDVI) como Indicador de Estresse em Planta). Rev. Bras. Geogr. Fís. 2012, 4, 909. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of remote sensing in precision agriculture: A review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Narrow-band reflectance indices for mapping the combined effects of water and nitrogen stress in field grown tomato crops. Biosyst. Eng. 2020, 192, 133–143. [Google Scholar] [CrossRef]
- Zelazny, W.R.; Lukáš, J. Drought Stress Detection in Juvenile Oilseed Rape Using Hyperspectral Imaging with a Focus on Spectra Variability. Remote Sens. 2020, 12, 3462. [Google Scholar] [CrossRef]
- Frackowiak, P.; Pospieszny, H.; Smiglak, M.; Obrępalska-Stęplowska, A. Assessment of the efficacy and mode of action of Benzo(1,2,3)-Thiadiazole-7-Carbothioic Acids-Methyl ester (BTH) and its derivatives in plant protection against viral disease. Int. J. Mol. Sci. 2019, 20, 1598. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, R. Host–pathogen interaction in conifers: Complicated systems yield interesting possibilities for research. Physiol. Mol. Plant Pathol. 2006, 68, 93–94. [Google Scholar] [CrossRef]
- Lin, T.C.; Ishizaka, M.; Ishii, H. Acibenzolar-S-methyl-induced systemic resistance against anthracnose and powdery mildew diseases on cucumber plants without accumulation of phytoalexins. J. Phytopathol. 2009, 157, 40–50. [Google Scholar] [CrossRef]
- Meller-Harel, Y.; Kolton, M.; Elad, Y.; Rav-David, D.; Cytryn, E.; Ezra, D.; Borenstein, M.; Shulchani, R.; Graber, E.R. Induced systemic resistance in strawberry (Fragaria × ananassa) to powdery mildew using various control agents. IOBC/WPRS Bull. 2011, 71, 47–51. [Google Scholar]
- Görlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 1996, 8, 629–643. [Google Scholar]
- Turczański, K.; Bełka, M.; Kukawka, R.; Spychalski, M.; Smiglak, M. A Novel Plant Resistance Inducer for the Protection of European Ash (Fraxinus excelsior L.) against Hymenoscyphus fraxineus—Preliminary Studies. Forests 2021, 12, 1072. [Google Scholar] [CrossRef]
- Faoro, F.; Maffi, D.; Cantu, D.; Iriti, M. Chemical-induced resistance against powdery mildew in barley: The effects of chitosan and benzothiadiazole. BioControl 2008, 53, 387–401. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Goltsev, V.N.; Zuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence. In Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; p. 222. [Google Scholar]
- Vaštag, E.; Kastori, R.; Orlović, S.; Bojović, M.M.; Kesić, L.A.; Pap, P.L.; Stojnić, S.M. Effects of oak powdery mildew (Erysiphe alphitoides [Griffon and Maubl.] U. Braun and S. Takam.) on photosynthesis of pedunculate oak (Quercus robur L.). Zb. Matice Srp. Prir. Nauk. 2019, 136, 43–56. [Google Scholar] [CrossRef]
- Copolovici, L.; Väärtnõu, F.; Portillo Estrada, M.; Niinemets, Ü. Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol. 2014, 34, 1399–1410. [Google Scholar] [CrossRef]
- Pap, P.; Stojnić, S.; Nikolić, N.; Orlović, S.; Marković, M.; Vasić, V.; Stevanov, M. Impact of Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. on Leaf Physiological Parameters in Pedunculate Oak (Quercus robur L.) Saplings. Balt. For. 2014, 20, 2–9. [Google Scholar]
- Picchi, V.; Iriti, M.; Quaroni, S.; Saracchi, M.; Viola, P.; Faoro, F. Climate variations and phenological stages modulate ozone damages in field-grown wheat. a three-year study with eight modern cultivars in po valley (Northern Italy). Agric. Ecosyst. Environ. 2010, 135, 310–317. [Google Scholar] [CrossRef]
- Picchi, V.; Gobbi, S.; Fattizzo, M.; Zefelippo, M.; Faoro, F. Chitosan Nanoparticles Loaded with N-Acetyl Cysteine to Mitigate Ozone and Other Possible Oxidative Stresses in Durum Wheat. Plants 2021, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Burlini, N.; Iriti, M.; Daghetti, A.; Faoro, F.; Ruggiero, A.; Bernasconi, S. Benzothiadiazole (BTH) Activates Sterol Pathway and Affects Vitamin D 3 Metabolism in Solanum malacoxylon Cell Cultures. Plant Cell Rep. 2011, 30, 2131–2141. [Google Scholar] [CrossRef]
- Spychalski, M.; Kukawka, R.; Krzesiński, W.; Spiżewski, T.; Michalecka, M.; Poniatowska, A.; Puławska, J.; Mieszczakowska-Frąc, M.; Panasiewicz, K.; Kocira, A.; et al. Use of New BTH Derivative as Supplement or Substitute of Standard Fungicidal Program in Strawberry Cultivation. Agronomy 2021, 11, 1031. [Google Scholar] [CrossRef]
- Smiglak, M.; Pospieszny, H.; Kukawka, R.; Lewandowski, P.; Stolarska, O.; Maciejewski, H. Application of 7-Carboxybenzo(1,2,3)thiadiazoleamides as Plant Stimulants. Patent Application No. WO/2017/017626, 2 February 2017. [Google Scholar]
- Chaube, H.S.; Singh, U.S. Plant Disease Management: Principles and Practices, 1st ed.; CRC Press: Boca Raton, FL, USA, 1991; 335p. [Google Scholar]
- Bock, C.H.; Chiang, K.S.; Del Ponte, E.M. Accuracy of plant specimen disease severity estimates: Concepts, history, methods, ramifications and challenges for the future. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2016, 39, 1–13. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite 1 Symposium. Volume I: Technical Presentations; NASA SP-351; Freden, S.C., Mercanti, E.P., Becker, M., Eds.; NASA: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Jordan, C.F. Derivation of leaf-area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).