Current Insights into m6A RNA Methylation and Its Emerging Role in Plant Circadian Clock
Abstract
1. Introduction
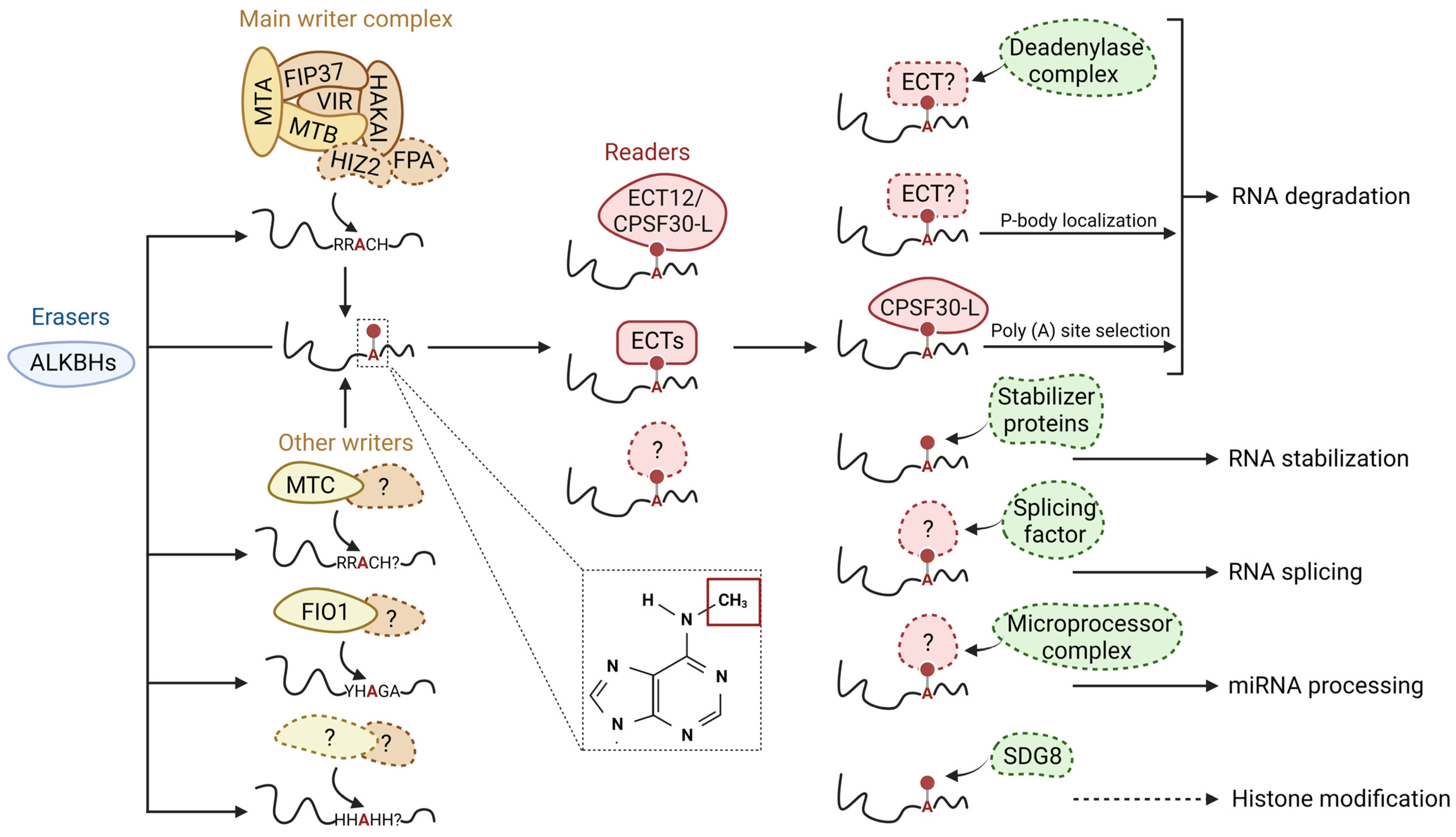
2. m6A in Plants
2.1. General Mechanisms
2.2. m6A Writers
2.3. m6A Erasers
2.4. m6A Readers
2.5. m6A Regulates RNA Activity
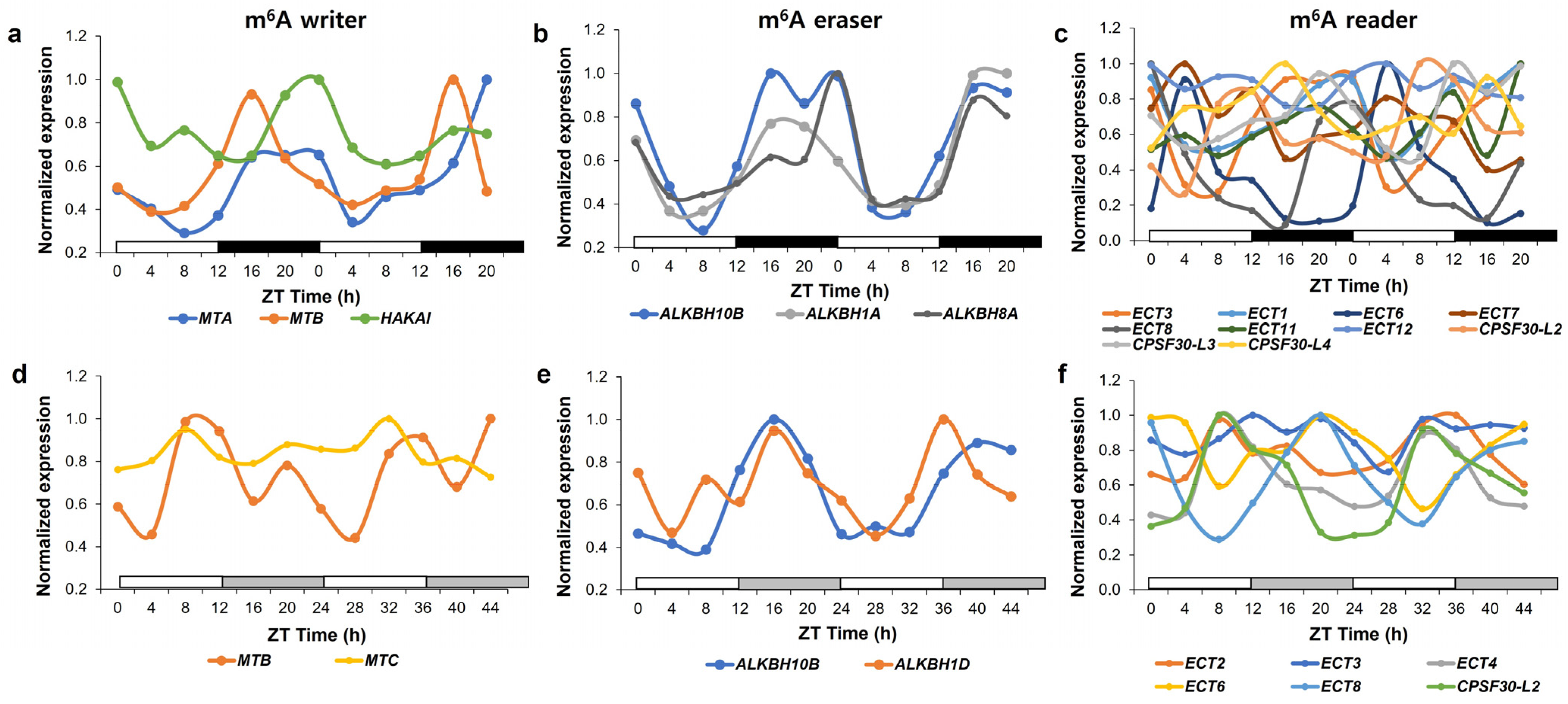
3. m6A in the Plant Circadian Rhythm
3.1. Plant Circadian Clock and m6A Methylation
3.2. Circadian Clock Regulation through m6A Methylation by a General m6A Writer Complex
3.3. FIO1, a Core Clock Component as m6A Methylase
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phillips, T. Regulation of transcription and gene expression in eukaryotes. Nat. Educ. 2008, 1, 199. [Google Scholar]
- Frye, M.; Harada, B.T.; Behm, M.; He, C. RNA modifications modulate gene expression during development. Science 2018, 361, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; Purta, E.; Kurkowska, M.; Shirvanizadeh, N.; Destefanis, E.; Groza, P.; et al. MODOMICS: A database of RNA modification pathways. 2021 update. Nucleic. Acids. Res. 2022, 50, D231–D235. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernandez, L.; Bressendorff, S.; Hansen, M.H.; Poulsen, C.; Erdmann, S.; Brodersen, P. An m6A-YTH module controls developmental timing and morphogenesis in Arabidopsis. Plant Cell 2018, 30, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.A.; Liang, Z.; Shen, L.S.; Zhang, Q.; Bao, S.J.; Geng, Y.K.; Zhang, B.; Leo, V.; Vardy, L.A.; Lu, T.G.; et al. 5-methylcytosine RNA methylation in Arabidopsis thaliana. Mol. Plant 2017, 10, 1387–1399. [Google Scholar] [CrossRef]
- Duan, H.C.; Wei, L.H.; Zhang, C.; Wang, Y.; Chen, L.; Lu, Z.K.; Chen, P.R.; He, C.; Jia, G.F. ALKBH10B is an RNA N-6-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell 2017, 29, 2995–3011. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Aparicio, F.; Lopez-Gresa, M.P.; Belles, J.M.; Sanchez-Navarro, J.A.; Pallas, V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m6A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef]
- Ruzicka, K.; Zhang, M.; Campilho, A.; Bodi, Z.; Kashif, M.; Saleh, M.; Eeckhout, D.; El-Showk, S.; Li, H.Y.; Zhong, S.L.; et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017, 215, 157–172. [Google Scholar] [CrossRef]
- Scutenaire, J.; Deragon, J.M.; Jean, V.; Benhamed, M.; Raynaud, C.; Favory, J.J.; Merret, R.; Bousquet-Antonelli, C. The YTH domain protein ECT2 is an m6A reader required for normal trichome branching in Arabidopsis. Plant Cell 2018, 30, 986–1005. [Google Scholar] [CrossRef]
- Shen, L.S.; Liang, Z.; Gu, X.F.; Chen, Y.; Teo, Z.W.N.; Hou, X.L.; Cai, W.M.; Dedon, P.C.; Liu, L.; Yu, H. N-6-methyladenosine RNA modification regulates shoot stem cell fate in Arabidopsis. Dev. Cell 2016, 38, 186–200. [Google Scholar] [CrossRef]
- Wei, L.H.; Song, P.Z.; Wang, Y.; Lu, Z.K.; Tang, Q.; Yu, Q.; Xiao, Y.; Zhang, X.; Duan, H.C.; Jia, G.F. The m6A reader ECT2 controls trichome morphology by affecting mRNA stability in Arabidopsis. Plant Cell 2018, 30, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Zuber, H.; Scheer, H.; Ferier, E.; Sement, F.M.; Mercier, P.; Stupfler, B.; Gagliardi, D. Uridylation and PABP cooperate to repair mRNA deadenylated ends in Arabidopsis. Cell Rep. 2016, 14, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.T.; Knop, K.; Sherwood, A.V.; Schurch, N.J.; Mackinnon, K.; Gould, P.D.; Hall, A.J.W.; Barton, G.J.; Simpson, G.G. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. Elife 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Z.; Tang, K.; Zhang, D.Y.; Xie, S.J.; Zhu, X.H.; Wang, Z.G.; Lang, Z.B. Transcriptome-wide high-throughput deep m6A-seq reveals unique differential m6A methylation patterns between three organs in Arabidopsis thaliana. Genome Biol. 2015, 16, 26. [Google Scholar] [CrossRef]
- Shi, H.L.; Wei, J.B.; He, C. Where, when, and how: Context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 2019, 74, 640–650. [Google Scholar] [CrossRef]
- Yue, H.; Nie, X.J.; Yan, Z.G.; Song, W.N. N6-methyladenosine regulatory machinery in plants: Composition, function and evolution. Plant Biotechnol. J. 2019, 17, 1194–1208. [Google Scholar] [CrossRef]
- Luo, G.Z.; MacQueen, A.; Zheng, G.Q.; Duan, H.C.; Dore, L.C.; Lu, Z.K.; Liu, J.; Chen, K.; Jia, G.F.; Bergelson, J.; et al. Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 2014, 5, 8. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, U201–U284. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.D.; Alemu, E.A.; Mertens, C.; Gantman, E.C.; Fak, J.J.; Mele, A.; Haripal, B.; Zucker-Scharff, I.; Moore, M.J.; Park, C.Y.; et al. A majority of m6A residues are in the last exons, allowing the potential for 3′UTR regulation. Genes Dev. 2015, 29, 2037–2053. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Slobodin, B.; Han, R.Q.; Calderone, V.; Vrielink, J.; Loayza-Puch, F.; Elkon, R.; Agami, R. Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell 2017, 169, 326–337. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, X.L.; Li, C.P.; Hu, S.N.; Yu, J.; Song, S.H. Transcriptome-wide N-6-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in selective mRNA modification. RNA Biol. 2014, 11, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.F.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.Q.; Yang, Y.; Yi, C.Q.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Grozhik, A.V.; Olarerin-George, A.O.; Meydan, C.; Mason, C.E.; Jaffrey, S.R. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 2015, 12, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Chang, J.W.; Li, Y.S.; Gao, Z.Y.; Qiu, Q.; Wang, Q.F.; Han, G.Q.; Chai, J.H.; Feng, M.D.; Wang, P.P.; et al. Differential m6A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell 2022, 29, 149–159. [Google Scholar] [CrossRef]
- Meyer, K.D. DART-seq: An antibody-free method for global m6A detection. Nat. Methods 2019, 16, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Dong, S.Q.; Yu, Q.; Jia, G.F. Antibody-free enzyme-assisted chemical approach for detection of N-6-methyladenosine. Nat. Chem. Biol. 2020, 16, 896–903. [Google Scholar] [CrossRef]
- Hu, L.L.; Liu, S.; Peng, Y.; Ge, R.Q.; Su, R.; Senevirathne, C.; Harada, B.T.; Dai, Q.; Wei, J.B.; Zhang, L.S.; et al. m6A RNA modifications are measured at single-base resolution across the mammalian transcriptome. Nat. Biotechnol. 2022, 40, 1210–1219. [Google Scholar] [CrossRef]
- Garalde, D.R.; Snell, E.A.; Jachimowicz, D.; Sipos, B.; Lloyd, J.H.; Bruce, M.; Pantic, N.; Admassu, T.; James, P.; Warland, A.; et al. Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 2018, 15, 201–206. [Google Scholar] [CrossRef]
- Yin, R.; Li, Y.; Tian, W.; Zhou, F.; Zhang, H. RNA m6A modification: Mapping methods, roles, and mechanisms in acute myeloid leukemia. Blood Sci. 2022, 4, 116–124. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Hang, D.; Meng, J.; Wei, Z. Detecting RNA modification using direct RNA sequencing: A systematic review. Comput. Struct. Biotechnol. J. 2022, 20, 5740–5749. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.J.; Kramer, M.C.; Gosai, S.J.; Yu, X.; Vandivier, L.E.; Nelson, A.D.L.; Anderson, Z.D.; Beilstein, M.A.; Fray, R.G.; Lyons, E.; et al. N-6-methyladenosine inhibits local ribonucleolytic cleavage to stabilize mRNAs in Arabidopsis. Cell Rep. 2018, 25, 1146–1157. [Google Scholar] [CrossRef]
- Chen, M.J.; Urs, M.J.; Sanchez-Gonzalez, I.; Olayioye, M.A.; Herde, M.; Witte, C.P. m6A RNA degradation products are catabolized by an evolutionarily conserved N-6-methyl-AMP deaminase in plant and mammalian Cells. Plant Cell 2018, 30, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.C.; Liao, J.Y.; Yu, Y.; Zhou, Y.F.; Feng, Y.Z.; Yang, Y.W.; Lei, M.Q.; Bai, M.; Wu, H.; et al. The subunit of RNA N6-methyladenosine methyltransferase OsFIP regulates early degeneration of microspores in rice. PLoS Genet. 2019, 15, 19. [Google Scholar] [CrossRef]
- Zhou, L.L.; Tian, S.P.; Qin, G.Z. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019, 20, 23. [Google Scholar] [CrossRef]
- Li, Z.R.; Shi, J.; Yu, L.; Zhao, X.Z.; Ran, L.L.; Hu, D.Y.; Song, B.A. N-6-methyl-adenosine level in Nicotiana tabacum is associated with tobacco mosaic virus. Virol. J. 2018, 15, 10. [Google Scholar] [CrossRef]
- Miao, Z.Y.; Zhang, T.; Qi, Y.H.; Song, J.; Han, Z.X.; Ma, C. Evolution of the RNA N-6-methyladenosine methylome mediated by genomic duplication. Plant Physiol. 2020, 182, 345–360. [Google Scholar] [CrossRef]
- Reichel, M.; Koster, T.; Staiger, D. Marking RNA: m6A writers, readers, and functions in Arabidopsis. J. Mol. Cell. Biol. 2019, 11, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.M.; Wilusz, C.J. Small changes, big implications: The impact of m6A RNA methylation on gene expression in pluripotency and development. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 194402. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pan, T. N-6-methyladenosine-encoded epitranscriptomics. Nat. Struct. Mol. Biol. 2016, 23, 98–102. [Google Scholar] [CrossRef]
- Liao, S.; Sun, H.; Xu, C. YTH domain: A family of N6-methyladenosine (m6A) readers. Genom. Proteom. Bioinf. 2018, 16, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nishat, Z.S.; Hasan, M.S.; Islam, M.S.; Hossain, T.; Ghosh, A. Identification of epitranscriptomic methylation marker genes in Arabidopsis and their expression profiling in response to developmental, anatomical, and environmental modulations. Curr. Plant Biol. 2022, 30, 100247. [Google Scholar] [CrossRef]
- Bujnicki, J.M.; Feder, M.; Radlinska, M.; Blumenthal, R.M. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA: m6 a methyltransferase. J. Mol. Evol. 2002, 55, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Lence, T.; Paolantoni, C.; Worpenberg, L.; Roignant, J.Y. Mechanistic insights into m6A RNA enzymes. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 222–229. [Google Scholar] [CrossRef]
- Knuckles, P.; Lence, T.; Haussmann, I.U.; Jacob, D.; Kreim, N.; Carl, S.H.; Masiello, I.; Hares, T.; Villasenor, R.; Hess, D.; et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018, 32, 415–429. [Google Scholar] [CrossRef]
- Bokar, J.A.; Shambaugh, M.E.; Polayes, D.; Matera, A.G.; Rottman, F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N-6-adenosine)-methyltransferase. RNA 1997, 3, 1233–1247. [Google Scholar]
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell 2016, 63, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Yue, Y.N.; Han, D.L.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.F.; Yu, M.; Lu, Z.K.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N-6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Xue, Y.; Guan, Z.Y.; Zhang, D.L.; Liu, Z.; Gong, Z.; Wang, Q.; Huang, J.B.; Tang, C.; et al. Structural basis of N-6-adenosine methylation by the METTL3-METTL14 complex. Nature 2016, 534, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.L.; Sun, B.F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef]
- Patil, D.P.; Chen, C.K.; Pickering, B.F.; Chow, A.; Jackson, C.; Guttman, M.; Jaffrey, S.R. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016, 537, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, J.; Cui, X.; Cao, J.; Luo, G.; Zhang, Z.; Cheng, T.; Gao, M.; Shu, X.; Ma, H.; et al. VIRMA mediates preferential m6A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018, 4, 10. [Google Scholar] [CrossRef]
- Wen, J.; Lv, R.T.; Ma, H.H.; Shen, H.J.; He, C.X.; Wang, J.H.; Jiao, F.F.; Liu, H.; Yang, P.Y.; Tan, L.; et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell 2018, 69, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Bawankar, P.; Lence, T.; Paolantoni, C.; Haussmann, I.U.; Kazlauskiene, M.; Jacob, D.; Heidelberger, J.B.; Richter, F.M.; Nallasivan, M.P.; Morin, V.; et al. Hakai is required for stabilization of core components of the m6A mRNA methylation machinery. Nat. Commun. 2021, 12, 3778. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.L.; Li, H.Y.; Bodi, Z.; Button, J.; Vespa, L.; Herzog, M.; Fray, R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 2008, 20, 1278–1288. [Google Scholar] [CrossRef]
- Parker, M.T.; Knop, K.; Zacharaki, V.; Sherwood, A.V.; Tome, D.; Yu, X.H.; Martin, P.G.; Beynon, J.; Michaels, S.D.; Barton, G.J.; et al. Widespread premature transcription termination of Arabidopsis thaliana NLR genes by the spen protein FPA. Elife 2021, 10, e65537. [Google Scholar] [CrossRef]
- Zhang, M.; Bodi, Z.; Mackinnon, K.; Zhong, S.L.; Archer, N.; Mongan, N.P.; Simpson, G.G.; Fray, R.G. Two zinc finger proteins with functions in m6A writing interact with HAKAI. Nat. Commun. 2022, 13, 1227. [Google Scholar] [CrossRef]
- Pendleton, K.E.; Chen, B.B.; Liu, K.Q.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 2017, 169, 824–835. [Google Scholar] [CrossRef]
- Tran, N.V.; Ernst, F.G.M.; Hawley, B.R.; Zorbas, C.; Ulryck, N.; Hackert, P.; Bohnsack, K.E.; Bohnsack, M.T.; Jaffrey, S.R.; Graille, M.; et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019, 47, 7719–7733. [Google Scholar] [CrossRef]
- Ma, H.H.; Wang, X.Y.; Cai, J.B.; Dai, Q.; Natchiar, S.K.; Lv, R.T.; Chen, K.; Lu, Z.K.; Chen, H.; Shi, Y.G.; et al. N-6-methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 2019, 15, 88–94. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Yeom, M.; Kim, J.H.; Nam, H.G. FIONA1 is essential for regulating period length in the Arabidopsis circadian clock. Plant Cell 2008, 20, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Yang, J.B.; Song, P.Z.; Zhang, W.; Lu, Q.; Yu, Q.; Jia, G.F. FIONA1 is an RNA N-6-methyladenosine methyltransferase affecting Arabidopsis photomorphogenesis and flowering. Genome Biol. 2022, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Mo, J.Z.; Chen, H.; Hu, Z.T.; Wang, B.H.; Wu, J.B.; Liang, Z.Y.; Xie, W.H.; Du, K.X.; Peng, M.L.; et al. Structural insights into molecular mechanism for N-6-adenosine methylation by MT-A70 family methyltransferase METTL4. Nat. Commun. 2022, 13, 5636. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell. Bio. 2019, 20, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.J.; Molinie, B.; Wang, J.K.; Qu, K.; Zhang, J.J.; Li, L.J.; Bouley, D.M.; Lujan, E.; Haddad, B.; Daneshvar, K.; et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 2014, 15, 707–719. [Google Scholar] [CrossRef]
- Engel, M.; Eggert, C.; Kaplick, P.M.; Eder, M.; Roh, S.; Tietze, L.; Namendorf, C.; Arloth, J.; Weber, P.; Rex-Haffner, M.; et al. The role of m6A/m-RNA methylation in stress response regulation. Neuron 2018, 99, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Laurent, B.; Hsu, C.H.; Nachtergaele, S.; Lu, Z.K.; Sheng, W.Q.; Xu, C.Y.; Hen, H.C.; Jian, O.Y.; Wang, S.Q.; et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature 2017, 543, 573–576. [Google Scholar] [CrossRef]
- Yu, J.Y.; Li, Y.; Wang, T.; Zhong, X. Modification of N-6-methyladenosine RNA methylation on heat shock protein expression. PLoS ONE 2018, 13, e0198604. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, J.; Gao, X.W.; Zhang, X.Q.; Jaffrey, S.R.; Qian, S.B. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 2015, 526, 591–594. [Google Scholar] [CrossRef]
- Du, M.J.; Zhang, Y.J.; Mao, Y.S.; Mou, J.W.; Zhao, J.; Xue, Q.; Wang, D.L.; Huang, J.F.; Gao, S.G.; Gao, Y.S. MiR-33a suppresses proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem. Biophys. Res. Commun. 2017, 482, 582–589. [Google Scholar] [CrossRef]
- Du, Y.Z.; Hou, G.F.; Zhang, H.L.; Dou, J.Z.; He, J.F.; Guo, Y.M.; Li, L.; Chen, R.; Wang, Y.L.; Deng, R.; et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018, 46, 5195–5208. [Google Scholar] [CrossRef] [PubMed]
- Sorci, M.; Ianniello, Z.; Cruciani, S.; Larivera, S.; Ginistrelli, L.C.; Capuano, E.; Marchioni, M.; Fazi, F.; Fatica, A. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Scholler, E.; Weichmann, F.; Treiber, T.; Ringle, S.; Treiber, N.; Flatley, A.; Feederle, R.; Bruckmann, A.; Meister, G. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA 2018, 24, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.X.S.; Roundtree, I.A.; He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Dahl, J.A.; Niu, Y.M.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vagbo, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 Is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Scarrow, M.; Chen, N.; Sun, G.L. Insights into the N-6-methyladenosine mechanism and its functionality: Progress and questions. Crit. Rev. Biotechnol. 2020, 40, 639–652. [Google Scholar] [CrossRef]
- Mielecki, D.; Zugaj, D.L.; Muszewska, A.; Piwowarski, J.; Chojnacka, A.; Mielecki, M.; Nieminuszczy, J.; Grynberg, M.; Grzesiuk, E. Novel AlkB dioxygenases-alternative models for in silico and in vivo studies. PLoS ONE 2012, 7, e30588. [Google Scholar] [CrossRef]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018, 28, 113–127. [Google Scholar] [CrossRef]
- Wojtas, M.N.; Pandey, R.R.; Mendel, M.; Homolka, D.; Sachidanandam, R.; Pillai, R.S. Regulation of m6 A transcripts by the 3′ -> 5′ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol. Cell 2017, 68, 374–387. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Q.; Zheng, G.Q.; He, C.; Parisien, M.; Pan, T. N-6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef]
- Liu, N.A.; Zhou, K.I.; Parisien, M.; Dai, Q.; Diatchenko, L.; Pan, T. N-6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017, 45, 6051–6063. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Weng, H.Y.; Sun, W.J.; Qin, X.; Shi, H.L.; Wu, H.Z.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N-6- methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Theler, D.; Dominguez, C.; Blatter, M.; Boudet, J.; Allain, F.H.T. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: A reader of methylated RNA. Nucleic Acids Res. 2014, 42, 13911–13919. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, P.; Rafalska, I.; Stamm, S. YTH: A new domain in nuclear proteins. Trends Biochem. Sci. 2002, 27, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Song, P.Z.; Yang, J.B.; Wang, C.L.; Lu, Q.; Shi, L.Q.; Tayier, S.; Jia, G.F. Arabidopsis N-6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol. Plant 2021, 14, 571–587. [Google Scholar] [CrossRef]
- Li, D.Y.; Zhang, H.J.; Hong, Y.B.; Huang, L.; Li, X.H.; Zhang, Y.F.; Ouyang, Z.G.; Song, F.M. Genome-wide identification, biochemical characterization, and expression analyses of the YTH domain-containing RNA-binding protein family in Arabidopsis and Rice. Plant Mol. Biol. Rep. 2014, 32, 1169–1186. [Google Scholar] [CrossRef]
- Delaney, K.J.; Xu, R.Q.; Zhang, J.X.; Li, Q.Q.; Yun, K.Y.; Falcone, D.L.; Hunt, A.G. Calmodulin interacts with and regulates the RNA-binding activity of an Arabidopsis polyadenylation factor subunit. Plant Physiol. 2006, 140, 1507–1521. [Google Scholar] [CrossRef]
- Hunt, A.G.; Xing, D.H.; Li, Q.S.Q. Plant polyadenylation factors: Conservation and variety in the polyadenylation complex in plants. BMC Genom. 2012, 13, 641. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; He, J.Q.; Zhang, Y.; Xi, H.R.; Liu, M.F.; Ma, J.B.; Wu, L.G. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 11. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.K.; Gomez, A.; Hon, G.C.; Yue, Y.N.; Han, D.L.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.F.; et al. N-6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Toth, J.I.; Petroski, M.D.; Zhang, Z.L.; Zhao, J.C. N-6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014, 16, 191–198. [Google Scholar] [CrossRef]
- Kramer, M.C.; Janssen, K.A.; Palos, K.; Nelson, A.D.L.; Vandivier, L.E.; Garcia, B.A.; Lyons, E.; Beilstein, M.A.; Gregory, B.D. N-6-methyladenosine and RNA secondary structure affect transcript stability and protein abundance during systemic salt stress in Arabidopsis. Plant Direct 2020, 4, e00239. [Google Scholar] [CrossRef] [PubMed]
- Spitale, R.C.; Flynn, R.A.; Zhang, Q.C.; Crisalli, P.; Lee, B.; Jung, J.W.; Kuchelmeister, H.Y.; Batista, P.J.; Torre, E.A.; Kool, E.T.; et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 2015, 519, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.F.; Sun, J.; Wu, B.X.; Gao, Y.Y.; Nie, H.B.; Nie, Z.T.; Quan, S.X.; Wang, Y.; Cao, X.F.; Li, S.S. CPSF30-L-mediated recognition of mRNA m6A modification controls alternative polyadenylation of nitrate signaling-related gene transcripts in Arabidopsis. Mol. Plant 2021, 14, 688–699. [Google Scholar] [CrossRef]
- Pontier, D.; Picart, C.; El Baidouri, M.; Roudier, F.; Xu, T.; Lahmy, S.; Llauro, C.; Azevedo, J.; Laudie, M.; Attina, A.; et al. The m6A pathway protects the transcriptome integrity by restricting RNA chimera formation in plants. Life Sci. Alliance 2019, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Wang, Y.; Wang, M.; Zhang, L.Y.; Peng, H.R.; Zhou, Y.Y.; Jia, G.F.; He, Y. Natural variation in RNA m6A methylation and its relationship with translational status. Plant Physiol. 2020, 182, 332–344. [Google Scholar] [CrossRef]
- Bhat, S.S.; Bielewicz, D.; Gulanicz, T.; Bodi, Z.; Yu, X.; Anderson, S.J.; Szewc, L.; Bajczyk, M.; Dolata, J.; Grzelak, N.; et al. mRNA adenosine methylase (MTA) deposits m6A on pri-miRNAs to modulate miRNA biogenesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2020, 117, 21785–21795. [Google Scholar] [CrossRef]
- Alarcon, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N-6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Alarcon, C.R.; Goodarzi, H.; Lee, H.; Liu, X.H.; Tavazoie, S.; Tavazoie, S.F. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 2015, 162, 1299–1308. [Google Scholar] [CrossRef]
- Chen, T.; Hao, Y.J.; Zhang, Y.; Li, M.M.; Wang, M.; Han, W.F.; Wu, Y.S.; Lv, Y.; Hao, J.; Wang, L.B.; et al. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell 2015, 16, 289–301. [Google Scholar] [CrossRef]
- Shim, S.; Lee, H.G.; Lee, H.; Seo, P.J. H3K36me2 is highly correlated with m6 A modifications in plants. J. Integr. Plant. Biol. 2020, 62, 1455–1460. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kevei, E.; Toth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Goodspeed, D.; Chehab, E.W.; Min-Venditti, A.; Braam, J.; Covington, M.F. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 4674–4677. [Google Scholar] [CrossRef] [PubMed]
- Alabadi, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Mas, P.; Kay, S.A. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Farre, E.M.; Harmer, S.L.; Harmon, F.G.; Yanovsky, M.J.; Kay, S.A. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 2005, 15, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Kolmos, E.; Bujdoso, N.; Yuan, Y.; Wang, M.; Berns, M.C.; Uhlworm, H.; Coupland, G.; Saini, R.; Jaskolski, M.; et al. EARLY FLOWERING4 recruitment of EARLY FLOWERING3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 2012, 24, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Devisetty, U.K.; Harmer, S.L. Accurate timekeeping is controlled by a cycling activator in Arabidopsis. Elife 2013, 2, e00473. [Google Scholar] [CrossRef]
- Rugnone, M.L.; Faigon Soverna, A.; Sanchez, S.E.; Schlaen, R.G.; Hernando, C.E.; Seymour, D.K.; Mancini, E.; Chernomoretz, A.; Weigel, D.; Mas, P.; et al. LNK genes integrate light and clock signaling networks at the core of the Arabidopsis oscillator. Proc. Natl. Acad. Sci. USA 2013, 110, 12120–12125. [Google Scholar] [CrossRef]
- Mas, P.; Alabadi, D.; Yanovsky, M.J.; Oyama, T.; Kay, S.A. Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell 2003, 15, 223–236. [Google Scholar] [CrossRef]
- Fujiwara, S.; Wang, L.; Han, L.; Suh, S.S.; Salome, P.A.; McClung, C.R.; Somers, D.E. Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins. J. Biol. Chem. 2008, 283, 23073–23083. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, X.; Zhong, M.; Li, X.; Qu, L.; Zhang, H.; Tang, D.; Liu, X.; He, C.; et al. The blue light receptor CRY1 interacts with FIP37 to promote N(6)-methyladenosine RNA modification and photomorphogenesis in Arabidopsis. New Phytol. 2022, 237, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Covington, M.F.; Maloof, J.N.; Straume, M.; Kay, S.A.; Harmer, S.L. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, A.; Yanovsky, M.J. Circadian rhythms and post-transcriptional regulation in higher plants. Front. Plant Sci. 2015, 6, 437. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Shingle, D.L.; Green, C.B. Post-transcriptional control of circadian rhythms. J. Cell. Sci. 2011, 124, 311–320. [Google Scholar] [CrossRef]
- Mateos, J.L.; de Leone, M.J.; Torchio, J.; Reichel, M.; Staiger, D. Beyond transcription: Fine-tuning of circadian timekeeping by post-transcriptional regulation. Genes 2018, 9, 616. [Google Scholar] [CrossRef]
- Fustin, J.M.; Doi, M.; Yamaguchi, Y.; Hida, H.; Nishimura, S.; Yoshida, M.; Isagawa, T.; Morioka, M.S.; Kakeya, H.; Manabe, I.; et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 2013, 155, 793–806. [Google Scholar] [CrossRef]
- Fustin, J.M.; Kojima, R.; Itoh, K.; Chang, H.Y.; Ye, S.; Zhuang, B.; Oji, A.; Gibo, S.; Narasimamurthy, R.; Virshup, D.; et al. Two Ck1delta transcripts regulated by m6A methylation code for two antagonistic kinases in the control of the circadian clock. Proc. Natl. Acad. Sci. USA 2018, 115, 5980–5985. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yeh, J.K.; Shie, S.S.; Hsieh, I.C.; Wen, M.S. Circadian rhythm of RNA N6-methyladenosine and the role of cryptochrome. Biochem. Biophys. Res. Commun. 2015, 465, 88–94. [Google Scholar] [CrossRef]
- Ruocco, M.; Ambrosino, L.; Jahnke, M.; Chiusano, M.L.; Barrote, I.; Procaccini, G.; Silva, J.; Dattolo, E. m6A RNA methylation in marine plants: First insights and relevance for Biological Rhythms. Int. J. Mol. Sci. 2020, 21, 7508. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, B.; Gu, L.; Chen, Y.; Mora, M.; Zhu, M.; Noory, E.; Wang, Q.; Lin, C. A photoregulatory mechanism of the circadian clock in Arabidopsis. Nat. Plants 2021, 7, 1397–1408. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Wong, C.E.; Fan, S.; Zhang, Y.; Zhang, S.; Liang, Z.; Yu, H.; Shen, L. FIONA1-mediated m6A modification regulates the floral transition in Arabidopsis. Adv. Sci. 2022, 9, e2103628. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Bhati, K.K.; Song, P.; Edwards, A.; Petri, L.; Kruusvee, V.; Blaakmeer, A.; Dolde, U.; Rodrigues, V.; Straub, D.; et al. FIONA1-mediated methylation of the 3′UTR of FLC affects FLC transcript levels and flowering in Arabidopsis. PLoS Genet. 2022, 18, e1010386. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Hu, J.; Amara, U.; Park, S.J.; Li, Y.; Jeong, D.; Lee, I.; Xu, T.; Kang, H. FIONA1 is an N6-methyladenosine methyltransferase that plays a vital role in floral transition via affecting the splicing of FLC and the stability of SPL3 and SEP3 in Arabidopsis. J. Exp. Bot. 2022, erac461. [Google Scholar] [CrossRef]
- Parker, M.T.; Soanes, B.K.; Kusakina, J.; Larrieu, A.; Knop, K.; Joy, N.; Breidenbach, F.; Sherwood, A.V.; Barton, G.J.; Fica, S.M.; et al. m6A modification of U6 snRNA modulates usage of two major classes of pre-mRNA 5′ splice site. Elife 2022, 11, e78808. [Google Scholar] [CrossRef] [PubMed]
- Bodi, Z.; Zhong, S.; Mehra, S.; Song, J.; Graham, N.; Li, H.; May, S.; Fray, R.G. Adenosine methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front. Plant. Sci. 2012, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.E.; Devlin, P.F.; Kay, S.A. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef]
- Devlin, P.F.; Kay, S.A. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 2000, 12, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Nohales, M.A.; Kay, S.A. GIGANTEA gates gibberellin signaling through stabilization of the DELLA proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 21893–21899. [Google Scholar] [CrossRef]
- Pedmale, U.V.; Huang, S.C.; Zander, M.; Cole, B.J.; Hetzel, J.; Ljung, K.; Reis, P.A.B.; Sridevi, P.; Nito, K.; Nery, J.R.; et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef]
- Quail, P.H. Photobodies reveal their secret. Nat. Plants 2021, 7, 1326–1327. [Google Scholar] [CrossRef]
- Mockler, T.C.; Michael, T.P.; Priest, H.D.; Shen, R.; Sullivan, C.M.; Givan, S.A.; McEntee, C.; Kay, S.A.; Chory, J. The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernandez, L.; Brodersen, P. Occurrence and functions of m6A and other covalent modifications in plant mRNA. Plant Physiol. 2020, 182, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dong, L.; Li, Y.; Gao, M.; He, P.C.; Liu, W.; Wei, J.; Zhao, Z.; Gao, L.; Han, L.; et al. METTL16 exerts an m6A-independent function to facilitate translation and tumorigenesis. Nat. Cell Biol. 2022, 24, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Doxtader, K.A.; Wang, P.; Scarborough, A.M.; Seo, D.; Conrad, N.K.; Nam, Y. Structural basis for regulation of METTL16, an S-adenosylmethionine homeostasis factor. Mol. Cell 2018, 71, 1001–1011.e4. [Google Scholar] [CrossRef] [PubMed]
- Madhani, H.D.; Bordonne, R.; Guthrie, C. Multiple roles for U6 snRNA in the splicing pathway. Genes Dev. 1990, 4, 2264–2277. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, Y.; Ohira, T.; Isokawa, Y.; Suzuki, Y.; Suzuki, T. A single m6A modification in U6 snRNA diversifies exon sequence at the 5′ splice site. Nat. Commun. 2021, 12, 3244. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.; Chen, K.M.; Homolka, D.; Gos, P.; Pandey, R.R.; McCarthy, A.A.; Pillai, R.S. Methylation of structured RNA by the m6A writer METTL16 Is essential for mouse embryonic development. Mol. Cell 2018, 71, 986–1000.e11. [Google Scholar] [CrossRef]
- Malapeira, J.; Khaitova, L.C.; Mas, P. Ordered changes in histone modifications at the core of the Arabidopsis circadian clock. Proc. Natl. Acad. Sci. USA 2012, 109, 21540–21545. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.B.; Choe, J.; Du, P.; Triboulet, R.; Gregory, R.I. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell 2016, 62, 335–345. [Google Scholar] [CrossRef]
- Blomen, V.A.; Majek, P.; Jae, L.T.; Bigenzahn, J.W.; Nieuwenhuis, J.; Staring, J.; Sacco, R.; van Diemen, F.R.; Olk, N.; Stukalov, A.; et al. Gene essentiality and synthetic lethality in haploid human cells. Science 2015, 350, 1092–1096. [Google Scholar] [CrossRef]
- Guruharsha, K.G.; Rual, J.F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A protein complex network of Drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Stixova, L.; Komurkova, D.; Svobodova Kovarikova, A.; Fagherazzi, P.; Bartova, E. Localization of METTL16 at the nuclear periphery and the nucleolus is cell cycle-specific and METTL16 interacts with several nucleolar proteins. Life 2021, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Nance, D.J.; Satterwhite, E.R.; Bhaskar, B.; Misra, S.; Carraway, K.R.; Mansfield, K.D. Characterization of METTL16 as a cytoplasmic RNA binding protein. PLoS ONE 2020, 15, e0227647. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, A.; Valverde, F.; Pineiro, M.; Jarillo, J.A. The Arabidopsis E3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Plant Cell 2012, 24, 982–999. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Huang, P.; Huang, G.; Zhu, J.; Chen, F.; Miao, Y.; Liu, L.; Fu, Y.F.; Wang, X. Nup96 and HOS1 are mutually stabilized and gate CONSTANS protein level, conferring long-day photoperiodic flowering regulation in Arabidopsis. Plant Cell 2020, 32, 374–391. [Google Scholar] [CrossRef]
- MacGregor, D.R.; Gould, P.; Foreman, J.; Griffiths, J.; Bird, S.; Page, R.; Stewart, K.; Steel, G.; Young, J.; Paszkiewicz, K.; et al. High expression of osmotically responsive genes1 is required for circadian periodicity through the promotion of nucleo-cytoplasmic mRNA export in Arabidopsis. Plant Cell 2013, 25, 4391–4404. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Petrillo, E.; Beckwith, E.J.; Zhang, X.; Rugnone, M.L.; Hernando, C.E.; Cuevas, J.C.; Godoy Herz, M.A.; Depetris-Chauvin, A.; Simpson, C.G.; et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature 2010, 468, 112–116. [Google Scholar] [CrossRef]
- Hong, S.; Song, H.R.; Lutz, K.; Kerstetter, R.A.; Michael, T.P.; McClung, C.R. Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 21211–21216. [Google Scholar] [CrossRef]
- Jones, M.A.; Williams, B.A.; McNicol, J.; Simpson, C.G.; Brown, J.W.; Harmer, S.L. Mutation of Arabidopsis spliceosomal timekeeper locus1 causes circadian clock defects. Plant Cell 2012, 24, 4066–4082. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuong, N.N.; Doan, P.P.T.; Wang, L.; Kim, J.H.; Kim, J. Current Insights into m6A RNA Methylation and Its Emerging Role in Plant Circadian Clock. Plants 2023, 12, 624. https://doi.org/10.3390/plants12030624
Chuong NN, Doan PPT, Wang L, Kim JH, Kim J. Current Insights into m6A RNA Methylation and Its Emerging Role in Plant Circadian Clock. Plants. 2023; 12(3):624. https://doi.org/10.3390/plants12030624
Chicago/Turabian StyleChuong, Nguyen Nguyen, Phan Phuong Thao Doan, Lanshuo Wang, Jin Hee Kim, and Jeongsik Kim. 2023. "Current Insights into m6A RNA Methylation and Its Emerging Role in Plant Circadian Clock" Plants 12, no. 3: 624. https://doi.org/10.3390/plants12030624
APA StyleChuong, N. N., Doan, P. P. T., Wang, L., Kim, J. H., & Kim, J. (2023). Current Insights into m6A RNA Methylation and Its Emerging Role in Plant Circadian Clock. Plants, 12(3), 624. https://doi.org/10.3390/plants12030624






