Vein Network and Climatic Factors Predict the Leaf Economic Spectrum of Desert Plants in Xinjiang, China
Abstract
1. Introduction
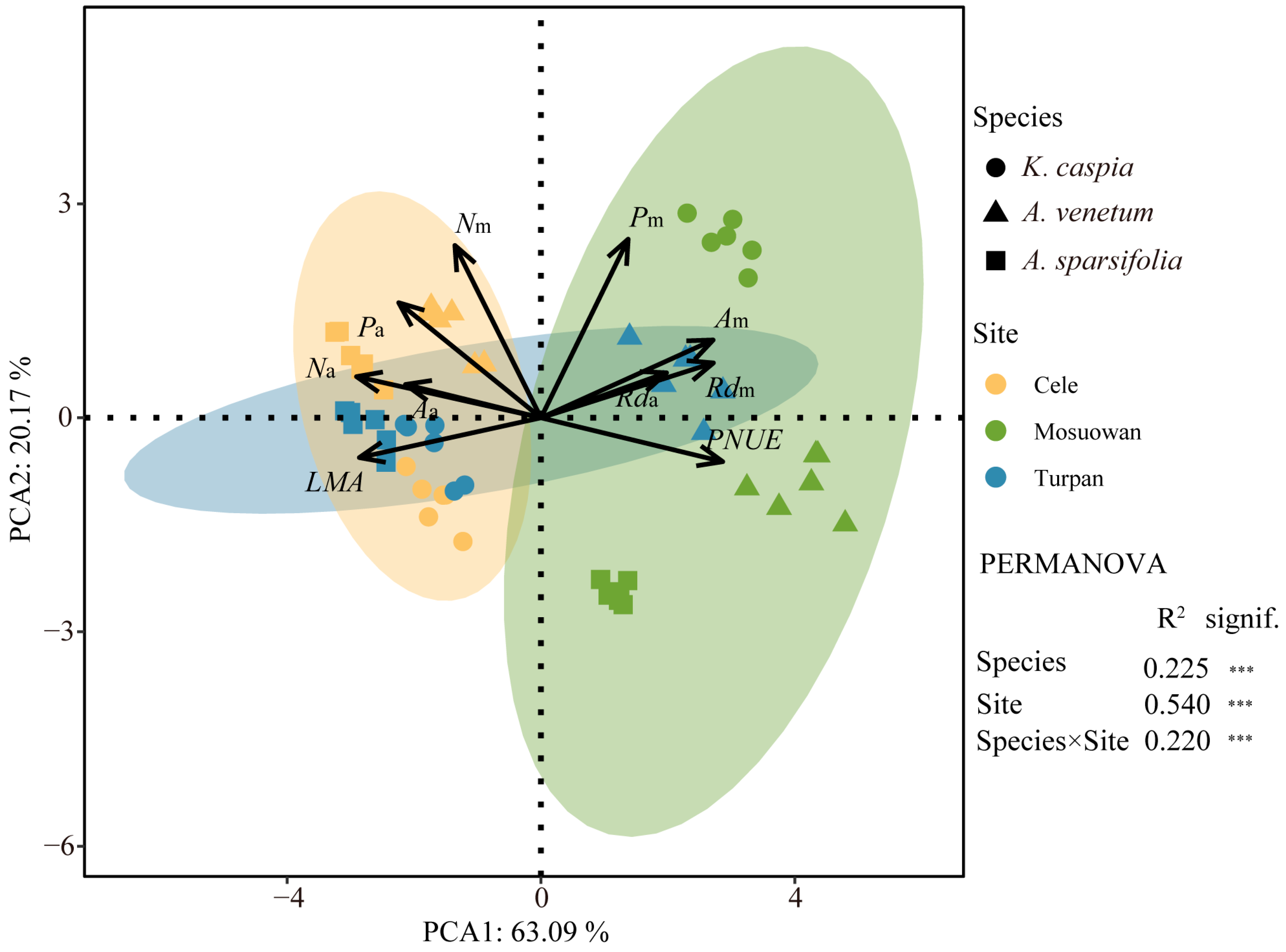
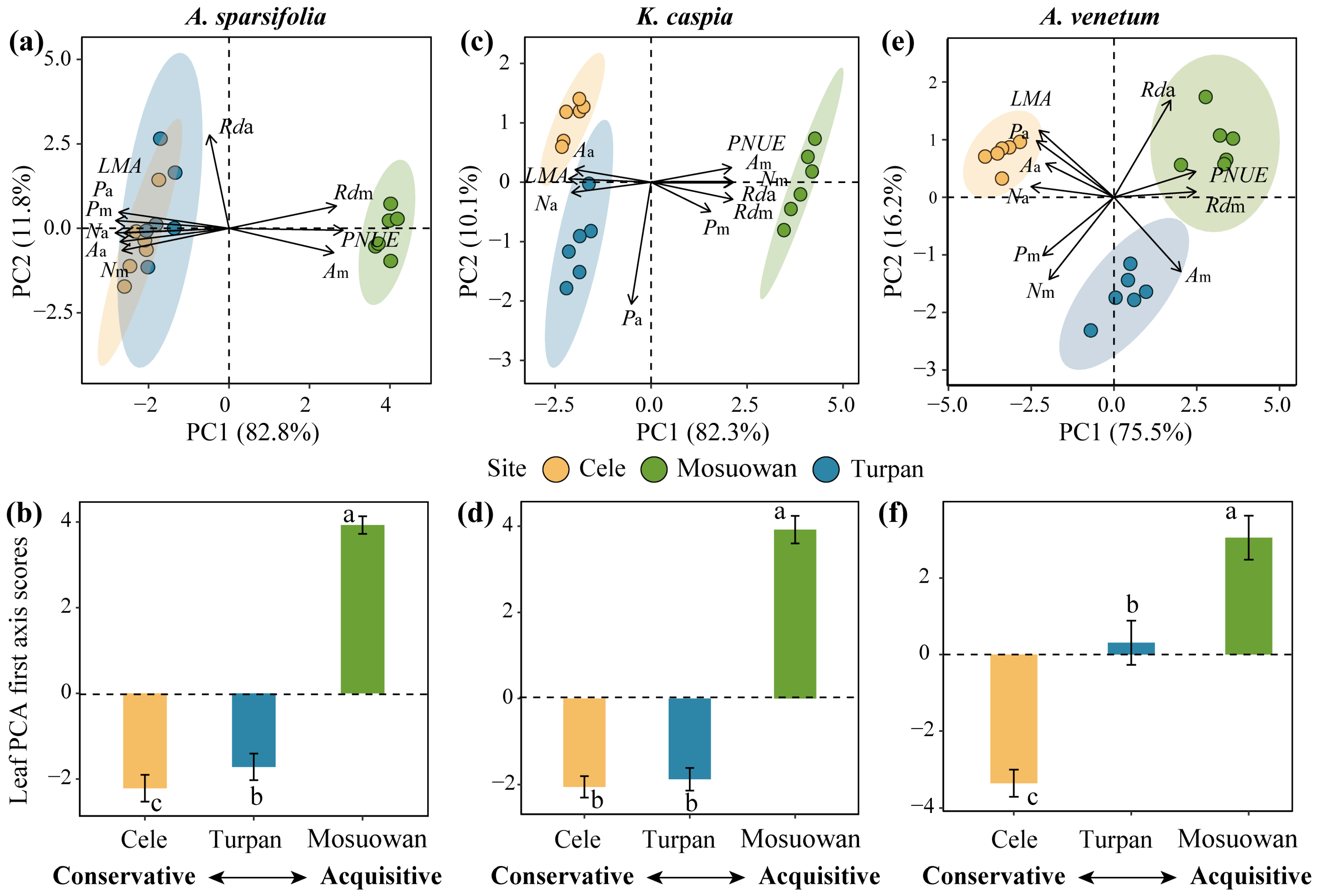
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
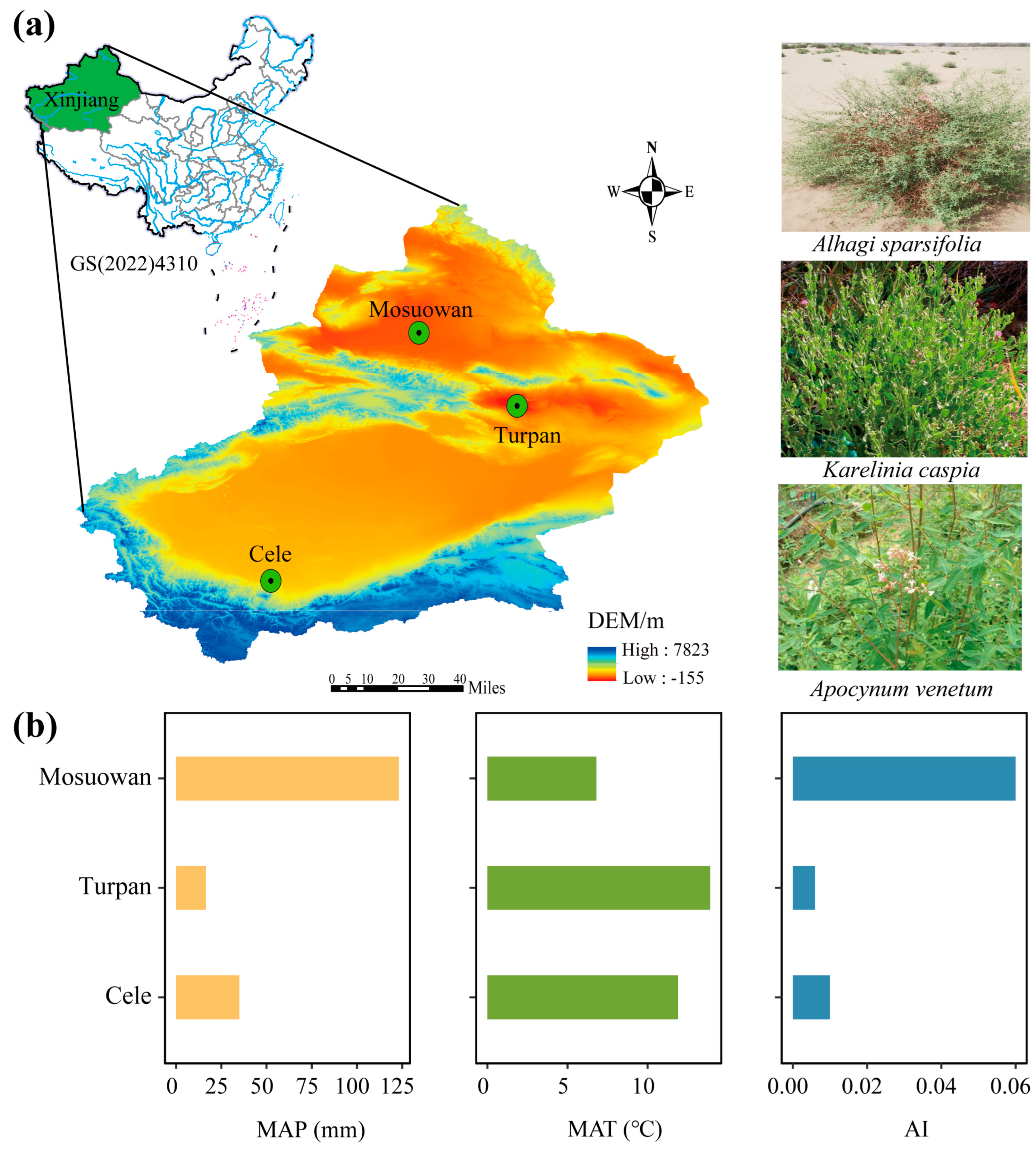
5.1. Study Site Description and Sampling Design
5.2. Determination of the Leaf Economic Traits
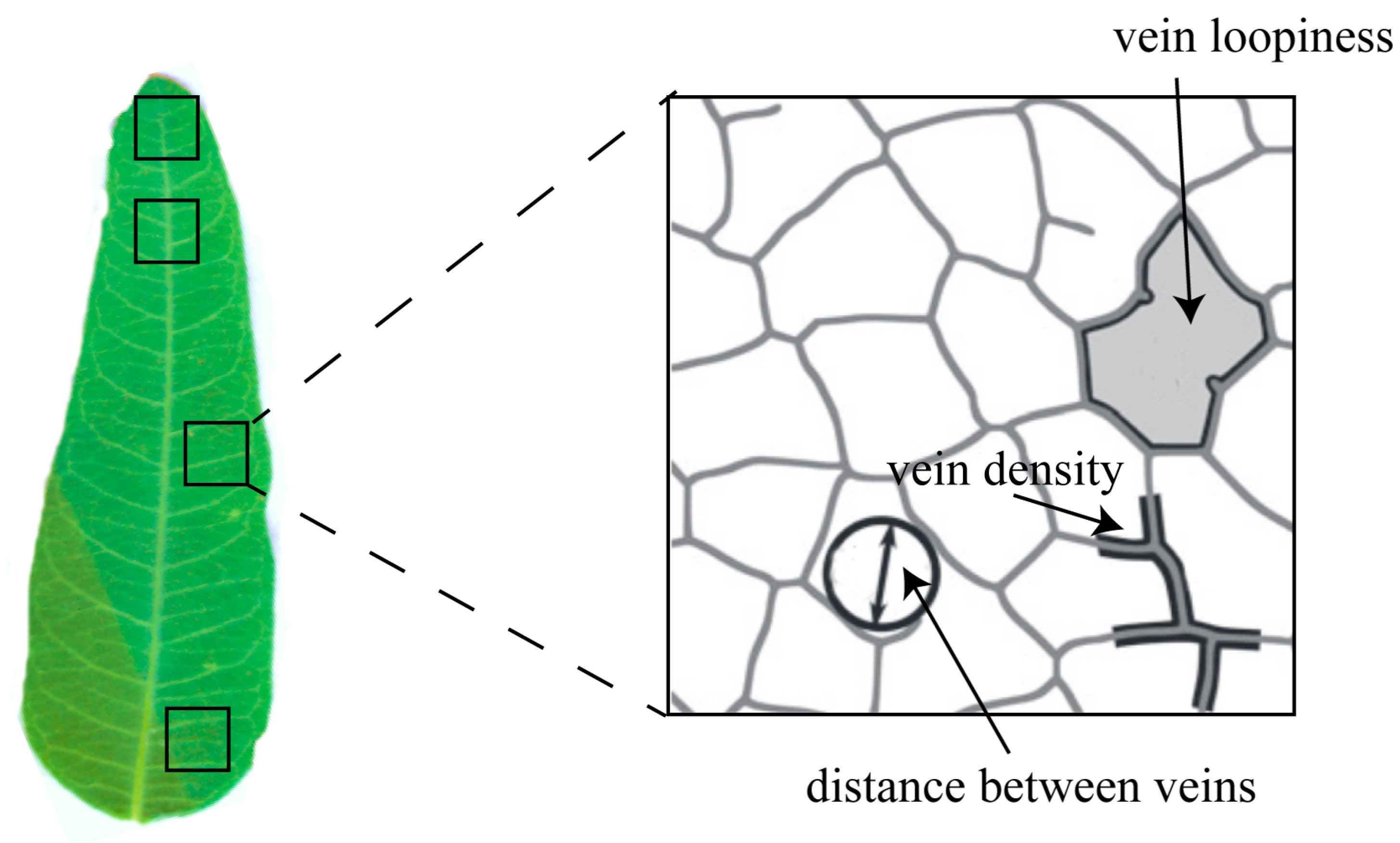
5.3. Determination of the Traits of Leaf Vein Network
5.4. Determination of the Soil Physical and Chemical Properties
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. Leaf Life-Span in Relation to Leaf, Plant, and Stand Characteristics among Diverse Ecosystems. Ecol. Monogr. 1992, 62, 365–392. [Google Scholar] [CrossRef]
- Jiang, X.; Jia, X.; Gao, S.; Jiang, Y.; Wei, N.; Han, C.; Zha, T.; Liu, P.; Tian, Y.; Qin, S. Plant Nutrient Contents Rather Than Physical Traits Are Coordinated Between Leaves and Roots in a Desert Shrubland. Front. Plant Sci. 2021, 12, 734775. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Q.; Zhang, Q.; Ding, Y.; Li, Y. Adaptation of Dominant Species to Drought in the Inner Mongolia Grassland—Species Level and Functional Type Level Analysis. Front. Plant Sci. 2019, 10, 231. [Google Scholar] [CrossRef]
- Delpiano, C.A.; Prieto, I.; Loayza, A.P.; Carvajal, D.E.; Squeo, F.A. Different Responses of Leaf and Root Traits to Changes in Soil Nutrient Availability Do Not Converge into a Community-Level Plant Economics Spectrum. Plant Soil. 2020, 450, 463–478. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which Is a Better Predictor of Plant Traits: Temperature or Precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Ren, L.; Huang, Y.; Pan, Y.; Xiang, X.; Huo, J.; Meng, D.; Wang, Y.; Yu, C. Differential Investment Strategies in Leaf Economic Traits Across Climate Regions Worldwide. Front. Plant Sci. 2022, 13, 798035. [Google Scholar] [CrossRef]
- Wang, Z.; Townsend, P.A.; Kruger, E.L. Leaf Spectroscopy Reveals Divergent Inter- and Intra-Species Foliar Trait Covariation and Trait–Environment Relationships across NEON Domains. New Phytol. 2022, 235, 923–938. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Enquist, B.J. Assessing the Causes and Scales of the Leaf Economics Spectrum Using Venation Networks in Populus Tremuloides. J. Ecol. 2013, 101, 981–989. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Bentley, L.P.; Enquist, B.J. Venation Networks and the Origin of the Leaf Economics Spectrum: Venation Networks and Leaf Economics. Ecol. Lett. 2011, 14, 91–100. [Google Scholar] [CrossRef]
- Liu, G.; Freschet, G.T.; Pan, X.; Cornelissen, J.H.C.; Li, Y.; Dong, M. Coordinated Variation in Leaf and Root Traits across Multiple Spatial Scales in Chinese Semi-arid and Arid Ecosystems. New Phytol. 2010, 188, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, D.E.; Loayza, A.P.; Rios, R.S.; Delpiano, C.A.; Squeo, F.A. A Hyper-arid Environment Shapes an Inverse Pattern of the Fast–Slow Plant Economics Spectrum for Above-, but Not Below-ground Resource Acquisition Strategies. J. Ecol. 2019, 107, 1079–1092. [Google Scholar] [CrossRef]
- Utkin, A.; Ermolova, L.; Utkina, I.; Dulepova, N.; Rosbakh, S. Utkin et al.’s Dataset on Specific Leaf Area. Ecology 2022, 103, e3714. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the Generality of Global Leaf Trait Relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant Nitrate Transporters: From Gene Function to Application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant Mineral Nutrition in Ancient Landscapes: High Plant Species Diversity on Infertile Soils Is Linked to Functional Diversity for Nutritional Strategies. Plant Soil. 2011, 348, 7. [Google Scholar] [CrossRef]
- Wakeel, A.; Ishfaq, M. Potassium Dynamics in Soils. In Potash Use and Dynamics in Agriculture; Springer: Singapore, 2022; pp. 7–17. ISBN 978-981-16-6883-8. [Google Scholar]
- Báez, S.; Homeier, J. Functional Traits Determine Tree Growth and Ecosystem Productivity of a Tropical Montane Forest: Insights from a Long-Term Nutrient Manipulation Experiment. Glob. Chang Biol. 2018, 24, 399–409. [Google Scholar] [CrossRef]
- Minden, V.; Olde Venterink, H. Plant Traits and Species Interactions along Gradients of N, P and K Availabilities. Funct. Ecol. 2019, 33, 1611–1626. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. How Do Leaf Veins Influence the Worldwide Leaf Economic Spectrum? Review and Synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.T.; Hoeting, J.A.; Ames, G.M.; Pyne, M.I.; LeRoy Poff, N. A Structured and Dynamic Framework to Advance Traits-Based Theory and Prediction in Ecology. Ecol. Lett. 2010, 13, 267–283. [Google Scholar] [CrossRef]
- Bohn, S.; Magnasco, M.O. Structure, Scaling, and Phase Transition in the Optimal Transport Network. Phys. Rev. Let. 2007, 98, 088702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, H.; Guo, D.-L. Leaf venation functional traits and their ecological significance: Leaf venation functional traits and their ecological significance. Chin. J. Plant Ecol. 2013, 37, 691–698. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; McKown, A.D.; Frole, K.; Rawls, M.; Havran, J.C.; Tran, H.; Tran, T. Developmentally Based Scaling of Leaf Venation Architecture Explains Global Ecological Patterns. Nat. Commun. 2012, 3, 837. [Google Scholar] [CrossRef] [PubMed]
- Heilmeier, H. Functional Traits Explaining Plant Responses to Past and Future Climate Changes. Flora 2019, 254, 1–11. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global Patterns of Plant Leaf N and P in Relation to Temperature and Latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Shiraiwa, T. Stem Growth Habit Affects Leaf Morphology and Gas Exchange Traits in Soybean. Ann. Bot. 2009, 104, 1293–1299. [Google Scholar] [CrossRef]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf Size, Specific Leaf Area and Microhabitat Distribution of Chaparral Woody Plants: Contrasting Patterns in Species Level and Community Level Analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the Global Aridity Index and Potential Evapotranspiration Database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef]
- Zhang, Z.; Chai, X.; Tariq, A.; Zeng, F.; Graciano, C.; Li, X.; Ullah, A. Coordinated Patterns in the Allocation, Composition, and Variability of Multiple Elements Among Organs of Two Desert Shrubs Under Nitrogen Addition and Drought. J. Soil. Sci. Plant Nutr. 2022, 22, 47–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, B. Nitrogen Application Mitigates Drought-Induced Metabolic Changes in Alhagi Sparsifolia Seedlings by Regulating Nutrient and Biomass Allocation Patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]



| Soil Properties | Cele | Turpan | Mosuowan |
|---|---|---|---|
| SOC (g/kg) | 1.70 ± 0.20 b | 4.41 ± 1.23 a | 3.65 ± 0.58 a |
| TN (g/kg) | 0.13 ± 0.01 b | 0.38 ± 0.10 a | 0.28 ± 0.05 a |
| TP (g/kg) | 0.65 ± 0.04 b | 0.72 ± 0.07 a | 0.53 ± 0.02 c |
| N:P | 0.21 ± 0.01 b | 0.53 ± 0.13 a | 0.53 ± 0.07 a |
| TK (g/kg) | 17.23 ± 0.17 b | 17.14 ± 0.40 b | 19.31 ± 0.27 a |
| K:P | 26.65 ± 1.25 b | 24.04 ± 2.32 c | 36.16 ± 1.24 a |
| pH | 8.87 ± 0.10 a | 8.26 ± 0.11 b | 8.89 ± 0.13 a |
| EC (μS/cm) | 403.13 ± 76.40 b | 2239.33 ± 727.55 a | 141.57 ± 20.63 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhang, Y.; Guo, Z.; Zhang, Z.; Zeng, F. Vein Network and Climatic Factors Predict the Leaf Economic Spectrum of Desert Plants in Xinjiang, China. Plants 2023, 12, 581. https://doi.org/10.3390/plants12030581
Du Y, Zhang Y, Guo Z, Zhang Z, Zeng F. Vein Network and Climatic Factors Predict the Leaf Economic Spectrum of Desert Plants in Xinjiang, China. Plants. 2023; 12(3):581. https://doi.org/10.3390/plants12030581
Chicago/Turabian StyleDu, Yi, Yulin Zhang, Zichun Guo, Zhihao Zhang, and Fanjiang Zeng. 2023. "Vein Network and Climatic Factors Predict the Leaf Economic Spectrum of Desert Plants in Xinjiang, China" Plants 12, no. 3: 581. https://doi.org/10.3390/plants12030581
APA StyleDu, Y., Zhang, Y., Guo, Z., Zhang, Z., & Zeng, F. (2023). Vein Network and Climatic Factors Predict the Leaf Economic Spectrum of Desert Plants in Xinjiang, China. Plants, 12(3), 581. https://doi.org/10.3390/plants12030581






