Influence of Nitrogen Supply on Growth, Antioxidant Capacity and Cadmium Absorption of Kenaf (Hibiscus cannabinus L.) Seedlings
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Traits
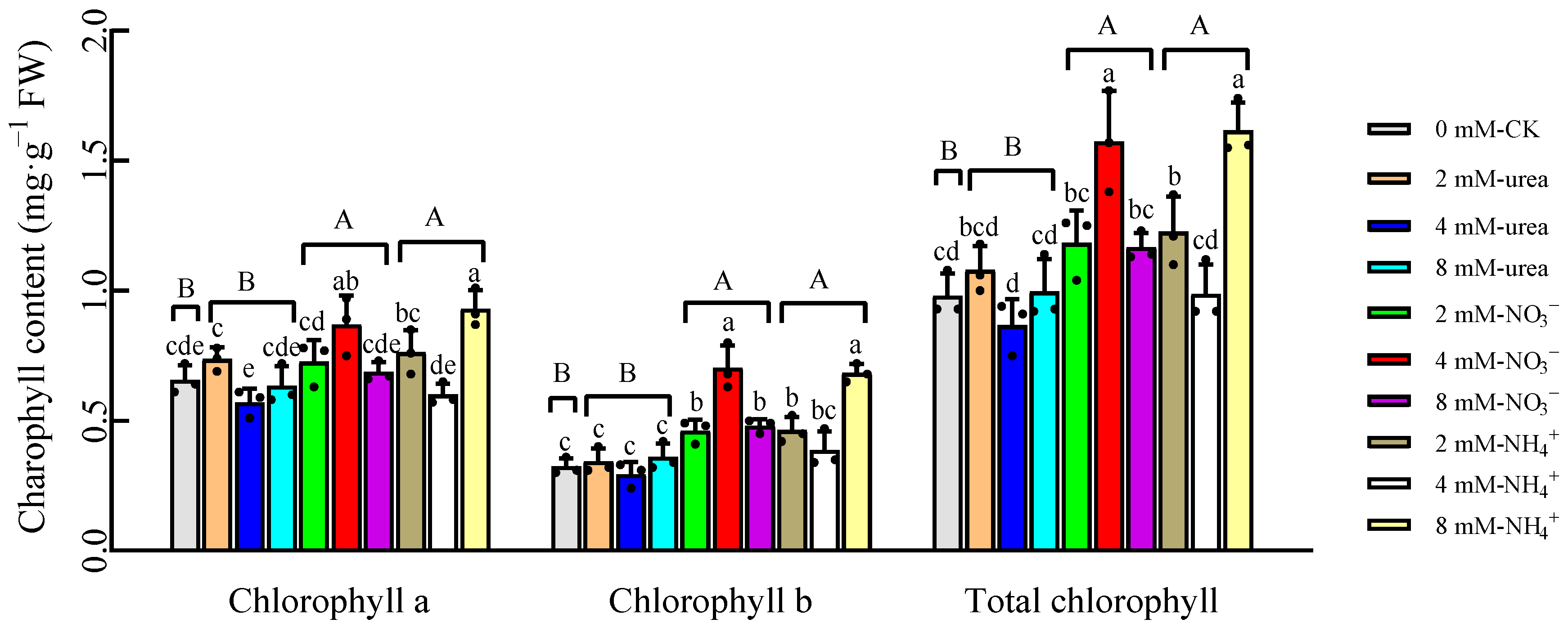
2.2. Chlorophyll Content
2.3. Uptake, Transfer and Accumulation of Cd in Different Parts of Kenaf
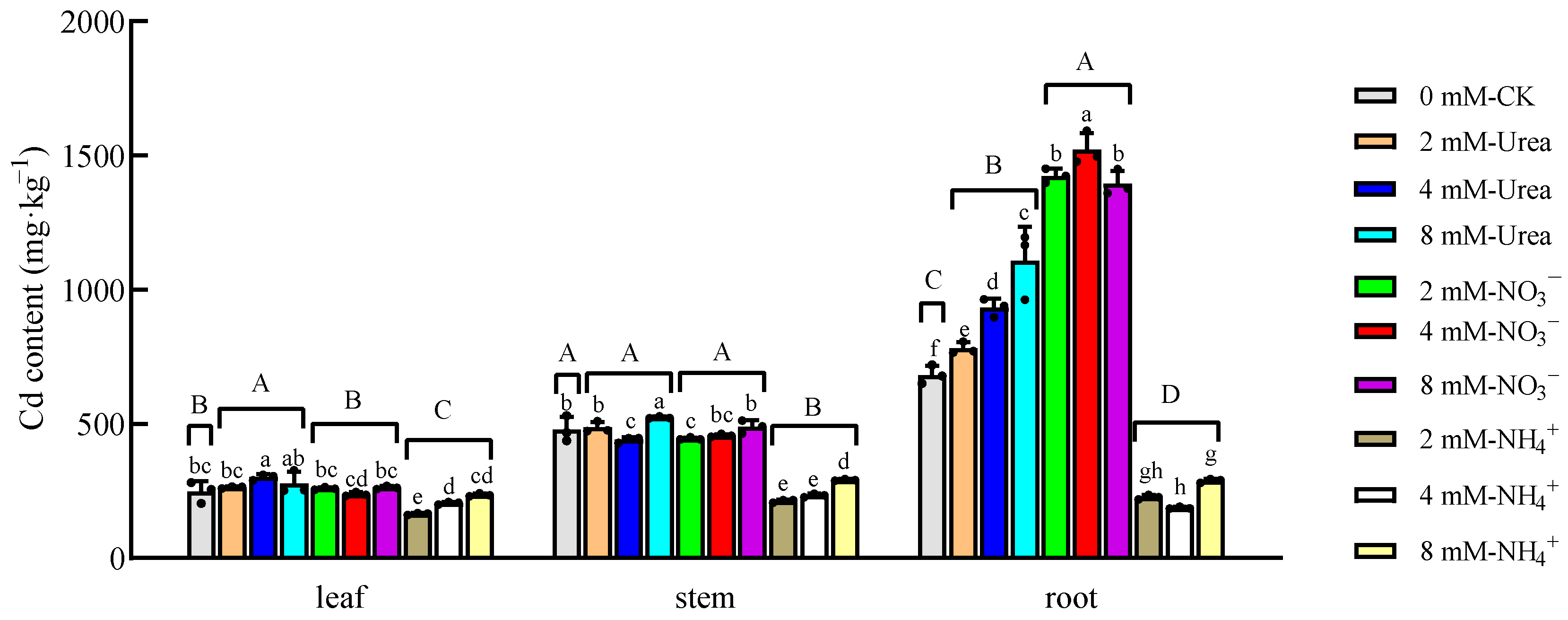
2.3.1. Cd Content
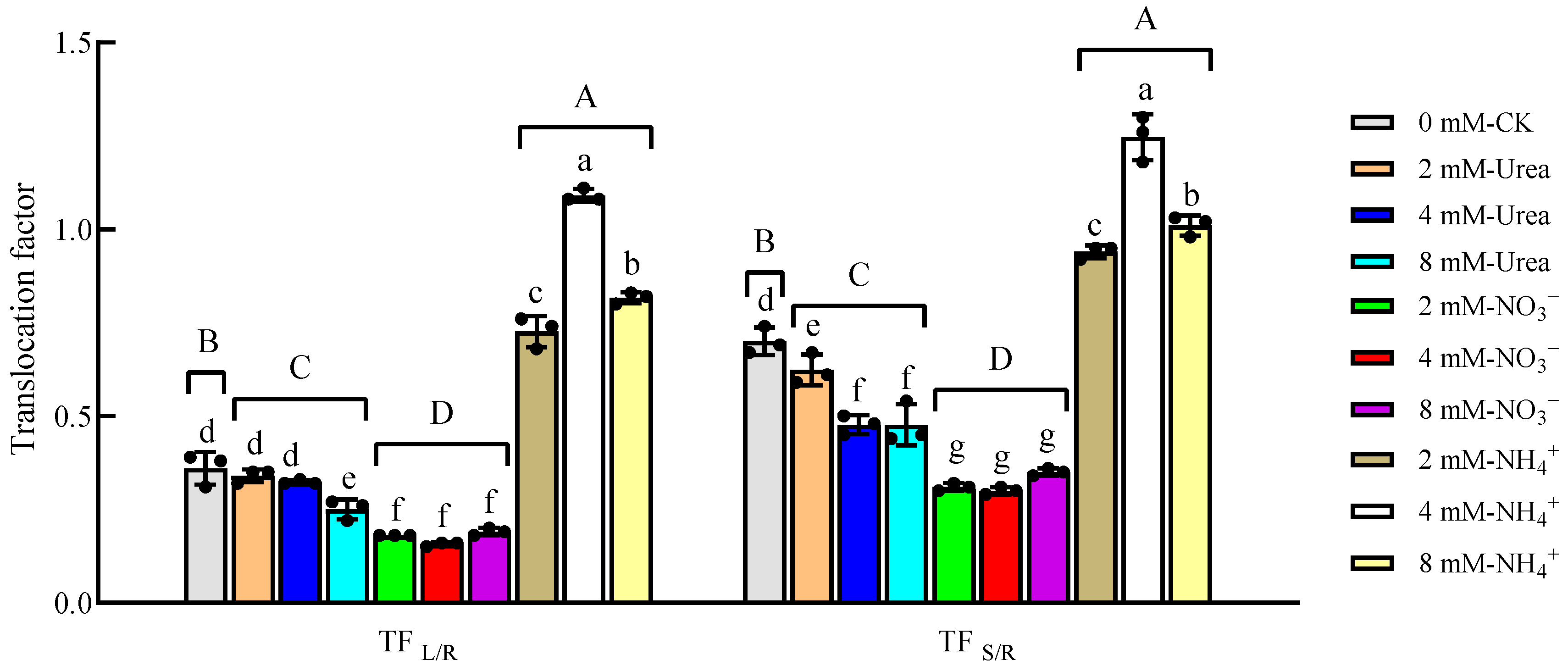
2.3.2. Translocation Factor
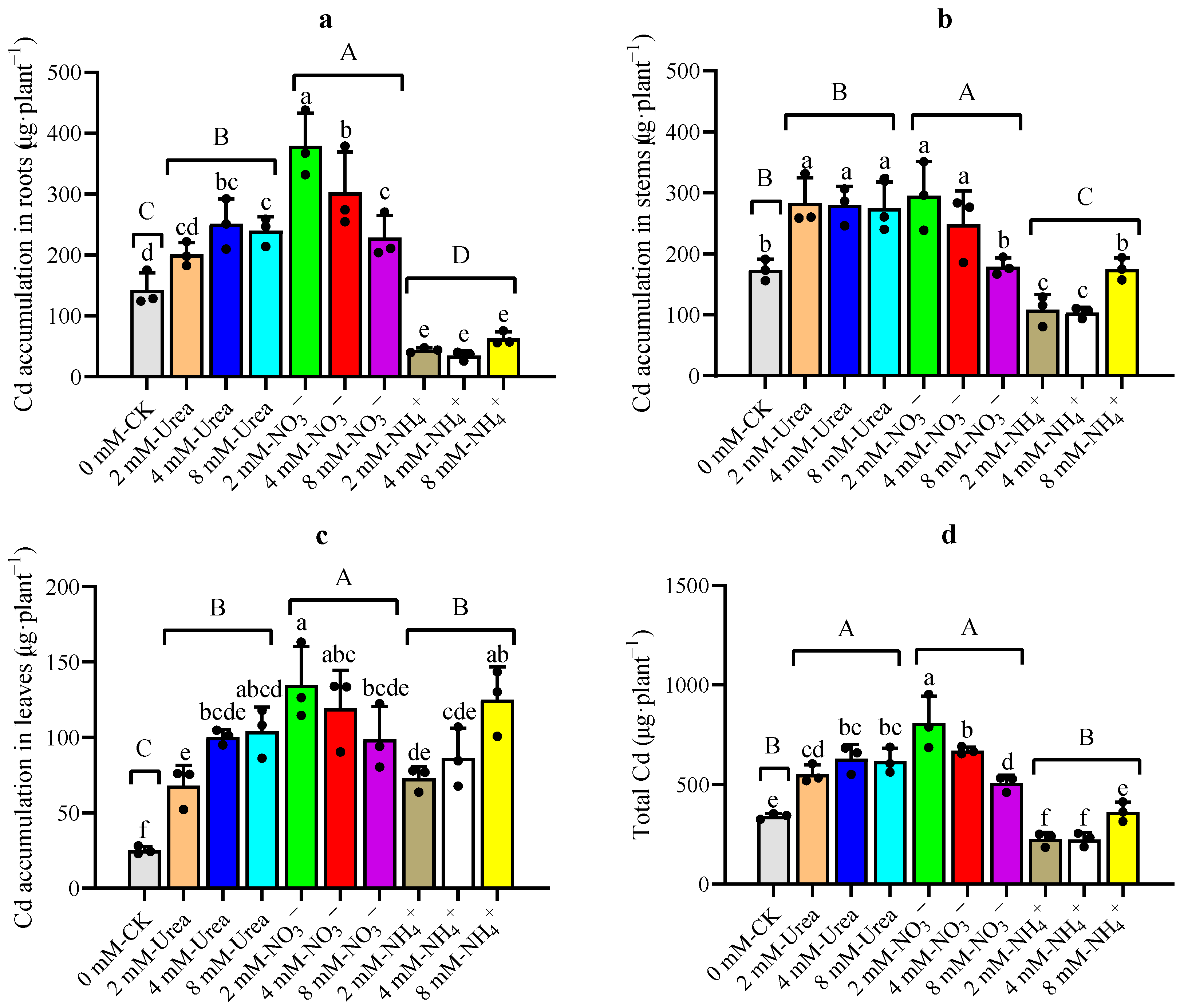
2.3.3. Cd Accumulation in Different Parts of Kenaf
2.4. Antioxidant Enzyme Activity and Proline Content
3. Discussion
3.1. Under Cd Stress, the Supply of N Promoted the Growth of the Aerial Part
3.2. NO3−–N Promotes the Absorption of Cd by Kenaf, and the Total Accumulation of Cd Was Highest under Low-Concentration Conditions
3.3. NH4+–N Promotes the Transfer of Cd in Kenaf to the Aerial Parts
3.4. Applying N Fertilizer Promotes Antioxidant Enzyme Activity of Kenaf and the NH4+–N Treatment Showed the Most Significant Enhancement
4. Materials and Methods
4.1. Plant Material and Experimental Treatments
4.2. Determination of POD, SOD, CAT and MDA Contents
4.3. Chlorophyll Contents
4.4. Growth and Biomass Analyses
4.5. Determining Cd Concentration and Translocation Factor (TF)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarwar, N.; Saifullah, M.S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.A.; Cheng, H.F.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.W.; Chen, S.; Gao, X.J. Actualities, damage and management of soil cadmium pollution in China. Anhui Agri. Sci. Bull. 2015, 21, 104–107. [Google Scholar] [CrossRef]
- Xu, J.M.; Meng, J.; Liu, X.M.; Shi, J.C.; Tang, X.J. Control of heavy metal pollution in farmland of China in terms of food security. Bull. Chin. Acad. Sci. 2018, 33, 153–159. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhu, Q.H.; Zhu, H.H.; Xu, C.; Liu, S.L. Advances and prospects of safety agro-utilization of heavy metal contaminated farmland soil. Res. Agric. Mod. 2018, 39, 1030–1043. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.B.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Tao, A.F.; Zhang, X.C.; Qi, J.M. Research progress and industrialization prospect of comprehensive utilization on kenaf. Plant Fibre Sci. China 2007, 29, 1–5. [Google Scholar]
- Li, W.L.; Jin, G.R.; Luo, X.H.; An, X.; Li, P.F.; Zhu, G.L. Comparative study on the potential of a kenaf (Hibiscus cannabinus) variety for remediating heavy metal contaminated soil. J. Agro-Environ. Sci. 2018, 37, 2150–2158. [Google Scholar] [CrossRef]
- Chen, P.; Li, Z.; Luo, D.; Jia, R.; Huang, Z. Comparative transcriptomic analysis reveals key genes and pathways in two different cadmium tolerance kenaf (Hibiscus cannabinus L.) cultivars. Chemosphere 2021, 263, 128211. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, Q.M.; Zhao, X.L.; Wu, Z.M.; Dai, Z.G.; Zhang, M.J.; Qiu, F.Z.; Long, S.H.; Wang, Y.F. Phytoremediation with Kenaf (Hibiscus Cannabinus L.) for Cadmium-Contaminated Paddy Soil in Southern China: Translocation, Uptake and Assessment of Cultivars. Environ. Sci. Pollut. Res. Int. 2023, 30, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Lu, H.L.; Zhan, S.S. Using kenaf (Hibiscus cannabinus) to reclaim multi-metal contaminated acidic soil. Chin. J. Appl. Ecol. 2013, 24, 832–838. [Google Scholar] [CrossRef]
- Yin, M.; Tang, H.J.; Yang, D.W.; Deng, Y.; Li, D.F.; Zhao, L.N.; Huang, S.Q. Comparative repair test of different varieties of kenaf in heavily and lightly cadmium-contaminated farmland. J. Agro-Environ. Sci. 2020, 39, 2267–2276. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Zhong, X.; Yang, J.; Wang, G.; Zou, Y.; Zhang, F.; Zhu, Q.; Roland, B.; Witt, C. Challenge and opportunity in improving fertilizer-nitrogen use efficiency of irrigated rice in China. Agr. Sci. China 2002, 1, 776–785. [Google Scholar]
- He, F.; Huang, J.L.; Cui, K.H.; Wang, Q.; Tang, L.L.; Gong, W.H.; Xu, B.; Peng, S.B.; Buresh, R.J. Effect of real-time and site-specific nitrogen management on various hybrid rice. Sci. Agric. Sin. 2008, 2, 470–479. [Google Scholar]
- Zhu, Z.L.; Jin, J.Y. Fertilizer use and food security in China. Plant Nutr. Fertil. Sci. 2013, 19, 259–273. [Google Scholar]
- Yan, X.; Jin, J.Y.; Liang, Z.M. Fertilizer use efficiencies and yield-increasing rates of grain crops in China. Soils 2017, 6, 1067–1077. [Google Scholar] [CrossRef]
- Panković, D.; Plesničar, M.; Maksimoviić, I.A.; Petrović, N.; Sakač, Z.; Kastori, R. Effect of nitrogen nutrition on photosynthesis in Cd treated sunflower plants. Ann. Bot. 2000, 86, 841–847. [Google Scholar] [CrossRef]
- Yang, Y.J.; Xiong, J.; Tao, L.X.; Cao, Z.Z.; Tang, W.; Zhang, J.P.; Yu, X.Y.; Fu, G.F.; Zhang, X.F.; Lu, Y.L. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: A review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, P.; Kopittke, P.M.; Wang, A.; Sale, P.W.G.; Tang, C. Cadmium accumulation is enhanced by ammonium compared to nitrate in two hyperaccumulators, without affecting speciation. J. Exp. Bot. 2016, 67, 5041–5050. [Google Scholar] [CrossRef]
- Zaccheo, P.; Laura, C.; Valeria, D.M.P. Ammonium nutrition as a strategy for cadmium mobolisation in the rhizosphere of sunflower. Plant Soil. 2006, 283, 43–56. [Google Scholar] [CrossRef]
- Hassan, M.J.; Zhang, G.; Zhu, Z. Influence of cadmium toxicity on plant growth and nitrogen uptake in rice as affected by nitrogen form. J. Plant Nutr. 2008, 31, 251–262. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.B.; Li, Z.; Wu, L.H.; Luo, Y.M. Effects of Nitrogen Forms on Growth and Zn/Cd Uptake of Sedum Plumbizincicola. J. Agro-Environ. Sci. 2014, 33, 2118–2124. [Google Scholar]
- Wángstrand, H.; Eriksson, J.; Öborn, I. Cadmium concentration in winter wheat as affected by nitrogen fertilization. Eur. J. Agron. 2007, 26, 209–214. [Google Scholar] [CrossRef]
- Yang, Y.J.; Xiong, J.; Chen, R.J.; Fu, G.F.; Chen, T.T.; Tao, L.X. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ. Exp. Bot. 2016, 122, 141–149. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, W.L.; Li, Q.S.; Wang, L.L.; He, T.; Wang, F.P.; Xu, Z.M. Nitrogen fertilizer management affects remobilization of the immobilized cadmium in soil and its accumulation in crop tissues. Environ. Sci. Pollut. Res. Int. 2021, 28, 31640–31652. [Google Scholar] [CrossRef]
- Schwartz, C.; Echevarria, G.; Morel, J.L. Phytoextraction of cadmium with Thlaspi caerulescens. Plant Soil. 2003, 249, 27–35. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, X.; Zhong, Y. Nitrogen as an important detoxification factor to cadmium stress in poplar plants. J. Plant Interact. 2014, 9, 249–258. [Google Scholar] [CrossRef]
- Sun, L.; Hao, X.Z.; Zhou, D.M.; Zhang, J. Influence of urea on the uptake of Cu, Zn and Cd of Corn at different stages of growth. J. Soil. Water Conserv. 2014, 28, 227–231. [Google Scholar] [CrossRef]
- Li, Z.X.; Xiang, Y.C.; Li, H.D.; Chen, Z. Effects of nitrogen application levels on Cd, Pb uptake and accumulation by maize. J. Soil. Water Conserv. 2014, 28, 143–147+166. [Google Scholar] [CrossRef]
- Bloom, A.J.; Frensch, J.; Taylor, A. Influence of inorganic nitrogen and pH on the elongation of maize seminal roots. Ann. Bot. 2006, 97, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, M.A.; Chen, J.H.; Zhang, G.P. Effect of Nitrogen Fertilizer Forms on Growth, Photosynthesis, and Yield of Rice Under Cadmium Stress. J. Plant Nutr. 2009, 32, 1081–1085. [Google Scholar] [CrossRef]
- Jiaka, L.T.; Yu, H.; Feng, W.Q.; Qin, Y.S.; Zhao, J.; Liao, M.L.; Wang, C.Q.; Tu, S.H. Effect of nitrogen fertilizer type and application rate on cadmium uptake and grain yield of paddy rice. Chin. J. Eco-Agric. 2010, 18, 281–285. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chao, Y.Y.; Huang, W.D.; Kao, C.H. Effect of nitrogen deficiency on antioxidant status and Cd toxicity in rice seedlings. Plant Growth Regul. 2011, 64, 263–273. [Google Scholar] [CrossRef]
- Xie, H.L.; Jiang, R.F.; Zhang, F.S.; McGrath, S.P.; Zhao, F.J. Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator Thlaspi caerulescens. Plant Soil 2009, 318, 205–215. [Google Scholar] [CrossRef]
- Yang, Y.J. Effects of Nitrogen Fertilizer Form and Dosage on Cadmium Accumulation and Toxicity and Its Regulatory Mechanism in Rice. Ph.D. Thesis, Chinese Academy of Agricultural Sciences Dissertation, Hangzhou, China, 2016. [Google Scholar]
- Chang, Y.S.; Chang, Y.J.; Lin, C.T.; Lee, M.C.; Wu, C.W.; Lai, Y.H. Nitrogen fertilization promotes the phytoremediation of cadmium in Pentas lanceolata. Int. Biodeter. Biodegr. 2013, 85, 709–714. [Google Scholar] [CrossRef]
- Kong, X.S.; Zhao, Y.X.; Tian, K.; He, X.B.; Jia, Y.Y.; He, Z.H.; Wang, W.W.; Xiang, C.G.; Tian, X.J. Insight into nitrogen and phosphorus enrichment on cadmium phytoextraction of hydroponically grown Salix matsudana Koidz cuttings. Environ. Sci. Pollut. Res. Int. 2020, 27, 8406–8417. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.E.H.; Asp, H. Influence of nitrogen supply on cadmium accumulation in potato tubers. J. Plant Nutr. 2011, 34, 345–360. [Google Scholar] [CrossRef]
- Luo, B.F.; Du, S.T.; Lu, K.X.; Liu, W.J.; Lin, X.Y.; Jin, C.W. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2012, 63, 3127–3136. [Google Scholar] [CrossRef]
- Mao, Q.; Guan, M.; Lu, K.; Du, S.; Fan, S.; Ye, Y.; Lin, X.; Jin, C. Inhibition of nitrate transporter 1.1-controlled nitrate uptake reduces cadmium uptake in Arabidopsis. Plant Physiol. 2014, 166, 934–944. [Google Scholar] [CrossRef]
- Li, F.T.; Qi, J.M.; Zhang, G.Y.; Lin, L.H.; Fang, P.P.; Tao, A.F.; Xu, J.T. Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. J. Integrat. Agric. 2017, 12, 610–620. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Li, Z.Q.; Jia, R.X.; Luo, D.J.; Tang, M.Q.; Lu, H.; Hu, Y.L.; Yue, J.; Huang, Z. Transcriptome analysis revealed key genes and pathways related to cadmium-stress tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970. [Google Scholar] [CrossRef]
- Zeng, Q. Influences of Different Types and Amounts of Nitrogen Fertilizer on Yield and Cadmium Accumulation in Rice. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2018. [Google Scholar]
- Xiong, J.; Fu, G.; Tao, L.X.; Zhu, C. Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch. Biochem. Biophys. 2010, 497, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.W.; Du, S.T.; Zhang, Y.S.; Lin, X.Y.; Tang, C.X. Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum). Ann. Bot. 2009, 104, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, W.; Xu, S.; Shi, H.; Wen, D.; Huang, Y.; Peng, L.; Deng, T.; Du, R.; Li, F.; et al. Increasing ammonium nutrition as a strategy for inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environ. Exp. Bot. 2018, 155, 734–741. [Google Scholar] [CrossRef]
- Chen, C.; Lv, X.; Li, J.; Yi, H.; Gong, J. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Zhang, S.S.; Gou, X.; Zhang, L.Y. Effects of different nitrogen fertilizers on Cd enrichment in amaranthus hypochondriacus L. Sci. Technol. Eng. 2020, 20, 6286–6291. [Google Scholar]
- Chai, M.; Li, R.; Shen, X.; Tam, N.; Zan, Q.; Li, R. Does ammonium nitrogen affect accumulation, subcellular distribution and chemical forms of cadmium in Kandelia obovata? Ecotox. Environ. Saf. 2018, 162, 430–437. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Jobe, T.O.; Hauser, F.; Schroeder, J.I. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 2011, 14, 554–562. [Google Scholar] [CrossRef]
- Leite, S.D.T.; Monteiro, A.F. Nitrogen form regulates cadmium uptake and accumulation in Tanzania guinea grass used for phytoextraction. Chemosphere 2019, 236, 124324. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, D.; Huang, Y.; Huang, S. Physiological response to cadmium stress in kenaf (Hibiscus cannabinus L.) seedlings. Ind. Crop. Prod. 2017, 107, 453–457. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L.) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar] [CrossRef]
- Rehman, M.; Maqbool, Z.; Peng, D.; Liu, L. Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ. Sci. Pollut. Res. Int. 2019, 26, 5851–5861. [Google Scholar] [CrossRef]
| Treatment | N Concentration (mM) | Plant Height (cm) | Stem Diameter (mm) | Root Dry Weight (g·Plant−1) | Stem Dry Weight (g·Plant−1) | Leaf Dry Weight (g·Plant−1) | Maximum Root Length (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 0 | 34.08 ± 3.15 ef | B | 3.06 ± 0.23 cd | B | 0.62 ± 0.09 abcd | AB | 1.09 ± 0.02 c | B | 0.31 ± 0.04 e | C | 29.83 ± 3.74 a | A |
| Urea | 2 | 40.54 ± 1.45 cd | A | 3.52 ± 0.26 ab | A | 0.77 ± 0.10 abc | A | 1.57 ± 0.25 ab | A | 0.77 ± 0.16 d | B | 21.25 ± 3.03 bc | B |
| 4 | 46.92 ± 0.80 ab | 3.81 ± 0.12 a | 0.81 ± 0.12 a | 1.90 ± 0.22 a | 1.00 ± 0.07 cd | 23.83 ± 1.44 b | |||||||
| 8 | 45.42 ± 0.29 abc | 3.57 ± 0.21 ab | 0.66 ± 0.11 abcd | 1.75 ± 0.31 ab | 1.14 ± 0.23 bcd | 25.08 ± 3.17 b | |||||||
| NO3− | 2 | 49.92 ± 6.39 a | A | 3.68 ± 0.34 a | A | 0.80 ± 0.12 ab | AB | 1.99 ± 0.36 a | A | 1.56 ± 0.27 a | A | 24.92 ± 1.04 b | B |
| 4 | 43.58 ± 3.39 bc | 3.65 ± 0.39 a | 0.60 ± 0.15 bcd | 1.63 ± 0.35 ab | 1.50 ± 0.29 ab | 25.42 ± 3.30 b | |||||||
| 8 | 38.08 ± 3.09 de | 2.70 ± 0.08 d | 0.49 ± 0.06 d | 1.10 ± 0.08 c | 1.13 ± 0.24 bcd | 22.42 ± 2.31 b | |||||||
| NH4+ | 2 | 30.83 ± 1.91 f | B | 3.09 ± 0.24 cd | A | 0.58 ± 0.03 cd | B | 1.33 ± 0.09 bc | A | 1.33 ± 0.16 abc | A | 16.83 ± 1.01 d | C |
| 4 | 32.25 ± 2.84 f | 3.21 ± 0.07 bc | 0.55 ± 0.12 d | 1.53 ± 0.32 abc | 1.26 ± 0.26 abc | 17.42 ± 0.76 cd | |||||||
| 8 | 42.33 ± 1.18 bcd | 3.84 ± 0.22 a | 0.65 ± 0.10 abcd | 1.81 ± 0.20 a | 1.59 ± 0.29 a | 24.25 ± 1.00 b | |||||||
| Variable | N Form | Concentration | N Form × Concentration |
|---|---|---|---|
| Plant height | 27.39 ** | 0.61 | 14.33 ** |
| Stem diameter | 3.99 * | 1.45 | 12.34 ** |
| Root dry weight | 4.96 * | 2.8 | 3.1 * |
| Stem dry weight | 1.57 | 2.52 | 5.71 ** |
| Leaf dry weight | 11.33 ** | 0.22 | 3.63 * |
| Maximum root length | 10.44 ** | 3.5 | 4.61 ** |
| Chlorophyll a | 7.67 ** | 2.68 | 11.65 ** |
| Chlorophyll b | 42.08 ** | 5.96 ** | 20.49 ** |
| Cd content in leaf | 38.9 ** | 5.21 ** | 4.53 ** |
| Cd content in stem | 441 ** | 25.8 ** | 3.42 * |
| Cd content in root | 1292.62 ** | 12.63 ** | 13.25 ** |
| Translocation factor leaf/root | 2359.46 ** | 63.25 ** | 75.72 ** |
| Translocation factor stem/root | 1137.41 ** | 8.34 ** | 38.91 ** |
| Total Cd in root | 127.25 ** | 1.77 | 7.11 ** |
| Total Cd in stem | 44.64 ** | 0.87 | 5.72 ** |
| Total Cd in leaf | 6.04 ** | 2.2 | 5.79 ** |
| Total Cd in plant | 109.37 ** | 0.68 | 12.47 ** |
| Treatment | N Concentration (mM) | SOD (U·g−1 FW) | POD (U·g−1 FW) | CAT (nmol·g−1 FW) | MDA (nmol·g−1 FW) |
|---|---|---|---|---|---|
| CK | 0 | 339.36 ± 9.34 h | 33.96 ± 1.33 h | 9.74 ± 0.50 h | 4.54 ± 0.29 e |
| Urea | 2 | 382.15 ± 13.28 g | 63.95 ± 1.07 d | 21.82 ± 0.14 g | 5.13 ± 0.30 bc |
| 4 | 561.36 ± 36.19 e | 58.12 ± 0.92 e | 25.41 ± 0.82 f | 4.74 ± 0.12 cde | |
| 8 | 637.94 ± 32.95 d | 63.54 ± 1.37 d | 47.97 ± 0.82 a | 6.34 ± 0.36 a | |
| NO3− | 2 | 413.44 ± 3.40 fg | 43.89 ± 0.74 g | 29.53 ± 1.03 e | 4.67 ± 0.18 de |
| 4 | 549.41 ± 5.42 e | 49.47 ± 0.35 f | 20.24 ± 0.43 g | 5.20 ± 0.17 b | |
| 8 | 425.75 ± 9.84 g | 62.39 ± 1.01 d | 30.44 ± 1.03 e | 5.22 ± 0.14 b | |
| NH4+ | 2 | 941.21 ± 21.69 a | 95.16 ± 0.34 a | 41.76 ± 1.10 c | 5.24 ± 0.27 b |
| 4 | 805.66 ± 13.22 b | 84.62 ± 0.43 c | 46.23 ± 1.43 b | 4.93 ± 0.19 bcde | |
| 8 | 721.17 ± 25.43 c | 87.18 ± 2.14 b | 33.13 ± 0.44 d | 4.98 ± 0.17 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, C.; Deng, Y.; Luo, X.; Liu, T.; An, X.; Zou, L.; Luan, M.; Li, D. Influence of Nitrogen Supply on Growth, Antioxidant Capacity and Cadmium Absorption of Kenaf (Hibiscus cannabinus L.) Seedlings. Plants 2023, 12, 4067. https://doi.org/10.3390/plants12234067
Li W, Chen C, Deng Y, Luo X, Liu T, An X, Zou L, Luan M, Li D. Influence of Nitrogen Supply on Growth, Antioxidant Capacity and Cadmium Absorption of Kenaf (Hibiscus cannabinus L.) Seedlings. Plants. 2023; 12(23):4067. https://doi.org/10.3390/plants12234067
Chicago/Turabian StyleLi, Wenlue, Changli Chen, Yong Deng, Xiahong Luo, Tingting Liu, Xia An, Lina Zou, Mingbao Luan, and Defang Li. 2023. "Influence of Nitrogen Supply on Growth, Antioxidant Capacity and Cadmium Absorption of Kenaf (Hibiscus cannabinus L.) Seedlings" Plants 12, no. 23: 4067. https://doi.org/10.3390/plants12234067
APA StyleLi, W., Chen, C., Deng, Y., Luo, X., Liu, T., An, X., Zou, L., Luan, M., & Li, D. (2023). Influence of Nitrogen Supply on Growth, Antioxidant Capacity and Cadmium Absorption of Kenaf (Hibiscus cannabinus L.) Seedlings. Plants, 12(23), 4067. https://doi.org/10.3390/plants12234067






