Differential Species Richness and Ecological Success of Epiphytes and Hemiepiphytes of Neotropical Araceae and Cyclanthaceae
Abstract
:1. Introduction
2. Materials and Methods
3. Results
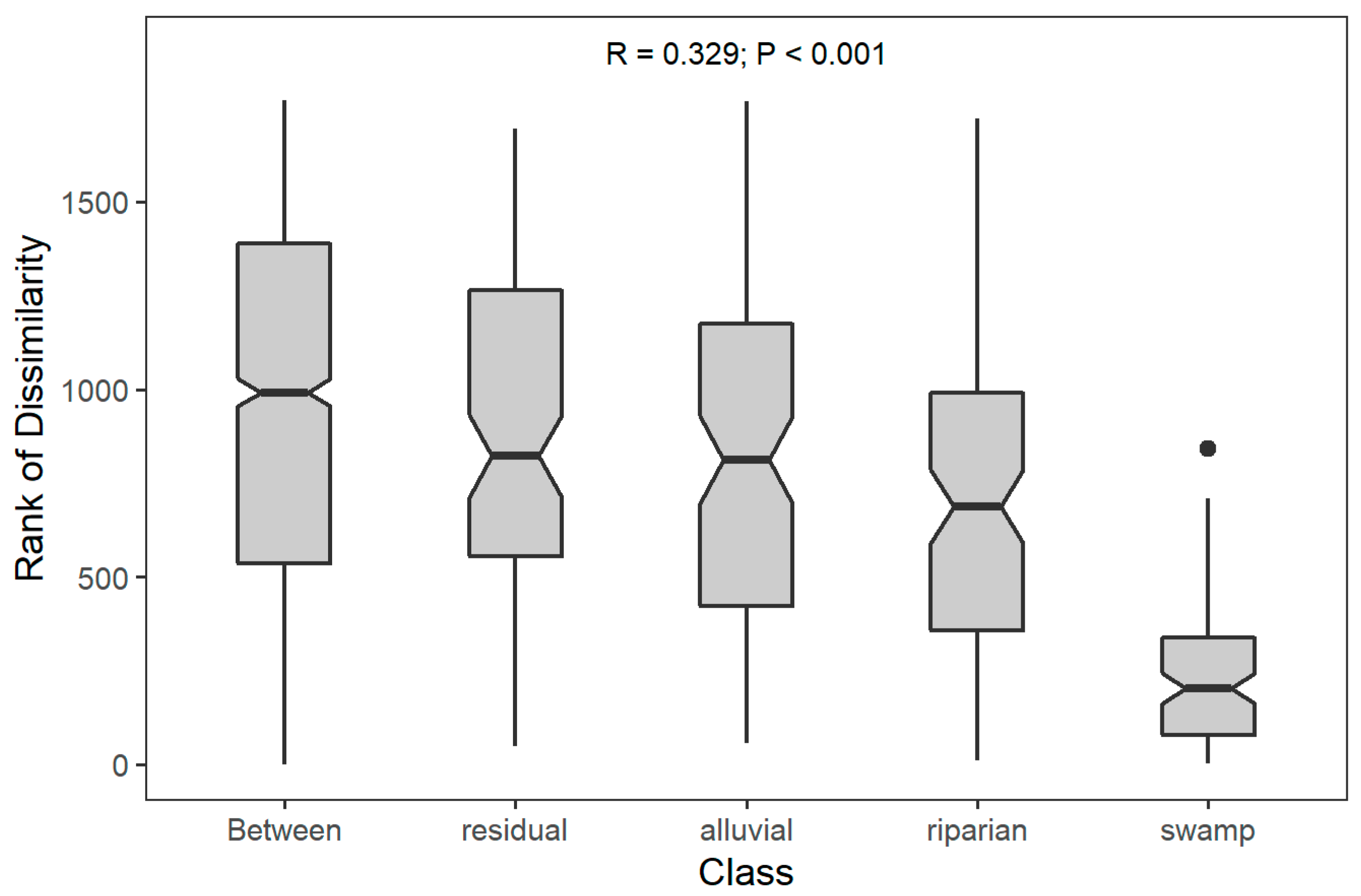
3.1. Soil Substrate
3.2. Epiphytes
3.3. Hemiepiphytes
4. Discussion
4.1. Epiphytes
4.2. Hemiepiphytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rundel, P.W.; Cooley, A.M.; Gerst, K.L.; Riordan, E.C.; Sharifi, M.R.; Sun, J.W.; Tower, J.A. Functional traits of broad-leaved monocot herbs in the understory and forest edges of a Costa Rican rainforest. PeerJ 2020, 8, e9958. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Wanek, W.; Zotz, G. Functional traits of a rainforest vascular epiphyte community: Trait covariation and indications for host specificity. Diversity 2021, 13, 97. [Google Scholar] [CrossRef]
- Zotz, G.; Weigelt, P.; Kessler, M.; Kreft, H.; Taylor, A. EpiList 1.0: A global checklist of vascular epiphytes. Ecology 2021, 102, e03326. [Google Scholar] [CrossRef]
- Males, J. Think tank: Water relations of Bromeliaceae in their evolutionary context. Bot. J. Linn. Soc. 2016, 181, 415–440. [Google Scholar] [CrossRef]
- Zotz, G. Plants on Plants-the Biology of Vascular Epiphytes; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Prats, K.A.; Brodersen, C.R. Desiccation and rehydration dynamics in the epiphytic resurrection fern Pleopeltis polypodioides. Plant Physiol. 2021, 187, 1501–1518. [Google Scholar] [CrossRef]
- Zotz, G.; Ziegler, H. The occurrence of crassulacean acid metabolism among vascular epiphytes from Central Panama. New Phytol. 1997, 137, 223–229. [Google Scholar] [CrossRef]
- Zotz, G.; Andrade, J.L.; Einzmann, H.J. CAM plants: Their importance in epiphyte communities and prospects with global change. Ann. Bot. 2023, mcac158. [Google Scholar] [CrossRef] [PubMed]
- Riordan, E.C.; Vargas Ramirez, O.; Rundel, P.W. Functional trait diversity of Cyclanthaceae and its convergent evolution with Araceae in Neotropical forests. PeerJ 2023, 11, e15557. [Google Scholar] [CrossRef]
- Strong, D.R.; Ray, T.S. Host tree location behavior of a tropical vine (Monstera gigantea) by skototropism. Science 1975, 190, 804–806. [Google Scholar] [CrossRef]
- Lopez-Portillo, J.; Ewers, F.W.; Angeles, G.; Fisher, J.B. Hydraulic architecture of Monstera acuminata: Evolutionary consequences of the hemiepiphytic growth form. New Phytol. 2000, 145, 289–299. [Google Scholar] [CrossRef]
- Filartiga, A.L.; Mantuano, D.; Vieira, R.C.; Gama De Toni, K.L.; Vasques, G.M.; André Mantovan, A. Root morphophysiology changes during the habitat transition from soil to canopy of the aroid vine Rhodospatha oblongata. Ann. Bot. 2021, 127, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Filartiga, A.L.; Vieira, R.; Mantovani, A. Aerial root hydraulic conductivity increases with plant size for the aroid vine Rhodospatha oblongata (Araceae). J. Plant Hydraul. 2017, 4, e-006. [Google Scholar] [CrossRef]
- Bautista-Bello, A.P.; Krömer, T.; Acebey, A.R.; Weichgrebe, L.; Zotz, G. Variación biológica en las aráceas trepadoras. Acta Bot. Mex. 2021, 128, e1819. [Google Scholar] [CrossRef]
- Balcázar-Vargas, M.P.; Van Andel, T.R.; Westers, P.; Zuidema, P.A. What drives the vital rates of secondary hemiepiphytes? A first assessment for three species of Heteropsis (Araceae) in the Colombian Amazon. J. Trop. Ecol. 2015, 31, 251–265. [Google Scholar] [CrossRef]
- Croat, T.B.; Ortiz, O.O. Distribution of Araceae and the diversity of life forms. Acta Soc. Bot. Pol. 2020, 89, 8939. [Google Scholar] [CrossRef]
- Went, F.W. Über Haft- und Nährwurzeln bei Kletterpflanzen und Epiphyten. Ann. Jard. Bot. Buitenzor 1895, 12, 1–72. [Google Scholar]
- Schimper, A.F.W. Planzengeographie auf Physiologischer Grundlage; Clarendon Press: Oxford, UK, 1898. [Google Scholar]
- Williams-Linera, G.; Lawton, R.O. Ecology of hemiepiphytes in forest canopies. In Forest Canopies; Lowman, M.D., Nadkarni, N.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 255–283. [Google Scholar]
- Lawton, R.O.; Williams-Linera, G. Hemiepiphyte-host relationships: Research problems and prospects. Selbyana 1995, 17, 71–74. [Google Scholar]
- Holbrook, N.M.; Putz, F.E. Physiology of tropical vines and hemiepiphytes: Plants that climb up and plants that climb down. In Tropical Plant Ecophysiology; Mulkey, S., Chazdon, P.L., Smith, A.P., Eds.; Springer: Boston, MA, USA, 1996; pp. 363–394. [Google Scholar]
- Leimbeck, R.M.; Balslev, H. Species richness and abundance of epiphytic Araceae on adjacent floodplain and upland forest in Amazonian Ecuador. Biodiv. Conserv. 2001, 10, 1579–1593. [Google Scholar] [CrossRef]
- Jácome, J.; Galeano, G.; Amaya, M.; Mora, M.M. Vertical Distribution of epiphytic and hemiepiphytic Araceae in a tropical rain forest in Chocó, Colombia. Selbyana 2004, 25, 118–125. [Google Scholar]
- Benavides, A.M.; Duque, A.J.M.; Duivenvoorden, J.F.; Vasco, G.A.; Callejas, R. A first quantitative census of vascular epiphytes in rain forests of Colombian Amazonia. Biodiv. Conserv. 2005, 14, 739–758. [Google Scholar] [CrossRef]
- Pos, E.T.; Sleegers, A.D.M. Vertical distribution and ecology of vascular epiphytes in a lowland tropical rain forest of Brazil. Bol. Do Mus. Para. Emílio Goeldi-Ciências Nat. 2010, 5, 335–344. [Google Scholar] [CrossRef]
- Irume, M.V.; Morais, M.d.L.d.C.S.; Zartman, C.E.; do Amaral, I.L. Floristic composition and community structure of epiphytic angiosperms in a terra firme forest in central Amazonia. Acta Bot. Brasílica 2013, 27, 378–393. [Google Scholar] [CrossRef]
- Hammel, B.E.; Grayum, M.H.; Herrera, C.; Zamora, N. Manual de Plantas de Costa Rica; Missouri Botanical Garden Press: St. Louis, MO, USA, 2010; Volume 2. [Google Scholar]
- Sanford, R.L.P.; Sancho, F.M.; Mata, R.C.; Sanford, R.L. Climate, geomorphology, and aquatic systems. In La Selva: Ecology and Natural History of a Neotropical Rain Forest; McDade, L., Hartshorn, G.S., Eds.; University Chicago Press: Chicago, IL, USA, 1994; pp. 19–33. [Google Scholar]
- Sollins, P.; Paaby, P.; Luvall, J.C.; Phillips, E. The La Selva ecosystem: Climate geomorphology, the aquatic systems. In La Selva: Ecology and Natural History of a Neotropical Rain Forest; McDade, L., Hartshorn, G.S., Eds.; University Chicago Press: Chicago, IL, USA, 1994; pp. 34–53. [Google Scholar]
- Hartshorn, G.; Hammel, B.E. La Selva vegetation types and floristic patterns. In La Selva: Ecology and Natural History of a Neotropical Rain Forest; McDade, L., Hartshorn, G.S., Eds.; University Chicago Press: Chicago, IL, USA, 1994; pp. 73–89. [Google Scholar]
- Moffett, M.W. What’s “up”? A critical look at the basic terms of canopy biology. Biotropica 2000, 32, 569–596. [Google Scholar] [CrossRef]
- Zotz, G.; Almeda, F.; Arias, S.; Hammel, B.; Pansarin, G. Do secondary hemiepiphytes exist? J. Trop. Ecol. 2021, 37, 286–290. [Google Scholar] [CrossRef]
- Zotz, G.; Almeda, F.; Bautista-Bello, A.P.; Eskov, A.K.; Giraldo-Cañas, D.; Hammel, B.E.; Harrison, R.D.; Köster, N.; Krömer, T.; Lowry II, P.P.; et al. Hemiepiphytes revisited. Perspect. Plant Ecol. Evol. Syst. 2021, 51, 125620. [Google Scholar] [CrossRef]
- Gerst, K.L.; Sharifi, M.R.; Prigge, B.; Rundel, P.W. Distribution and photosynthetic assimilation of rosulate aroid epiphytes in a Costa Rican lowland rainforest. Flora 2021, 279, 151830. [Google Scholar] [CrossRef]
- Croat, T.B. A revision of the genus Anthurium section Pachyneurium (Araceae). Ann. Miss. Bot. Gard. 1991, 78, 539–585. [Google Scholar] [CrossRef]
- Meyer, C.F.J.; Zotz, G. Do growth and survival of aerial roots limit the vertical distribution of hemiepiphytic aroids? Biotropica 2004, 366, 483–491. [Google Scholar]
- Zotz, G.; Wilhelm, K.; Becker, A. Heteroblasty—A review. Bot. Rev. 2011, 77, 109–151. [Google Scholar] [CrossRef]
- Lee, D.W.; Richards, J.H. Heteroblastic development in vines. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 205–243. [Google Scholar]
- Ray, T.S. Growth correlations within the segment in the Araceae. Am. J. Bot. 1986, 73, 993–1001. [Google Scholar] [CrossRef]
- Ray, T.S. Cyclic heterophylly in Syngonium (Araceae). Am. J. Bot. 1987, 74, 16–26. [Google Scholar] [CrossRef]
- Ray, T.S. Leaf types in the Araceae. Am. J. Bot. 1987, 74, 1359–1372. [Google Scholar] [CrossRef]
- Moodley, D.; Procheş, Ş.; Wilson, J.R.U. Assessing and managing the threat posed by Epipremnum aureum in South Africa. S. Afr. J. Bot. 2017, 109, 178–188. [Google Scholar] [CrossRef]
- Moodley, D.; Procheş, Ş.; Wilson, J.R. A global assessment of a large monocot family highlights the need for group-specific analyses of invasiveness. AoB Plants 2016, 8, plw009. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. Leaf morpho-physiology and distribution of epiphytic aroids along a vertical gradient in a Brazilian rain forest. Selbyana 1999, 20, 241–249. [Google Scholar]
- Mantovani, A. Leaf orientation in epiphytic aroids: Effect on water and temperature balances of the leaves. Leandra 2000, 15, 91–103. [Google Scholar]
- Mantovani, A.; Pereira, T.E.; Mantuano, D. Allomorphic growth of Epipremnum aureum (Araceae) as characterized by changes in leaf morphophysiology during the transition from ground to canopy. Braz. J. Bot. 2017, 40, 177–191. [Google Scholar] [CrossRef]


| Residual | Alluvial | Riparian | Swamp | |
|---|---|---|---|---|
| Epiphytes | ||||
| Araceae | ||||
| Species | 15 | 14 | 13 | 14 |
| Summed frequencies | 35.89 | 50.28 | 33.25 | 67.75 |
| Cyclanthaceae | ||||
| Species | 2 | 2 | 3 | 2 |
| Summed frequencies | 0.6 | 0.79 | 0.52 | 3.31 |
| Ratios Araceae to Cyclanthaceae | ||||
| Species | 7.5 | 6.5 | 6 | 6.5 |
| Summed frequencies | 59.8 | 63.6 | 63.9 | 20.5 |
| Hemiepiphytes | ||||
| Araceae | ||||
| Species | 31 | 32 | 32 | 34 |
| Summed frequencies | 101.19 | 130.97 | 140.85 | 212.52 |
| Cyclanthaceae | ||||
| Species | 4 | 4 | 4 | 4 |
| Summed frequencies | 8.26 | 18.04 | 15.6 | 17.13 |
| Ratios Araceae to Cyclanthaceae | ||||
| Species | 7.8 | 8 | 8 | 8.5 |
| Summed frequencies | 12.3 | 7.3 | 9.0 | 12.4 |
| Residual | Alluvial | Riparian | Swamp | |
|---|---|---|---|---|
| Araceae—Epiphytes | ||||
| Anthurium acutangulum | 0.34 | 1.01 | 0.26 | 0.12 |
| A. austin-smithii | 0.86 | 0.34 | 0.77 | 0.24 |
| A. bakeri | 6.02 | 10.15 | 10.18 | 17.85 |
| A. bradeanum | 0.09 | 0.34 | 0.26 | 0.24 |
| A. clavigerum | 0.95 | 3.83 | 0.77 | 4.49 |
| A. consobrinum | 19.62 | 20.52 | 11.34 | 22.46 |
| A. cuspidatum | 0.43 | 1.92 | 3.74 | 4.61 |
| A. friedrichsthalii | 0.52 | 0.11 | 0 | 0.24 |
| A. gracile | 0.09 | 0 | 0 | 0 |
| A. llanense | 0.77 | 0.56 | 0.13 | 0 |
| A. obtusum | 0 | 0 | 0 | 0.24 |
| A. ramonense | 2.32 | 4.74 | 2.19 | 5.67 |
| A. spathiphyllum | 0.95 | 0.68 | 0.52 | 1.18 |
| A. upalaense | 1.03 | 1.69 | 0.77 | 3.55 |
| Philodendron radiatum | 1.38 | 3.04 | 1.29 | 3.43 |
| P. wendlandii | 0.52 | 1.35 | 1.03 | 3.43 |
| Total | 35.89 | 50.28 | 33.25 | 67.75 |
| Cyclanthaceae—Epiphytes | ||||
| Chorigyne pendula | 0.43 | 0.56 | 0.26 | 2.25 |
| Ludovia integrifolia | 0.17 | 0.23 | 0.13 | 1.06 |
| Sphaeradenia acutitepala | 0 | 0 | 0.13 | 0 |
| Total | 0.6 | 0.79 | 0.52 | 3.31 |
| Residual | Alluvial | Riparian | Swamp | |
|---|---|---|---|---|
| Araceae—Hemipiphytes | ||||
| Anthurium flexile | 0 | 0.11 | 0 | 0 |
| A. formosum | 1.03 | 0.79 | 2.58 | 6.26 |
| A. interruptum | 0.86 | 2.59 | 1.03 | 2.6 |
| A. pentaphyllum | 2.67 | 1.92 | 1.55 | 0.35 |
| A. subsignatum | 0.43 | 1.01 | 1.03 | 4.26 |
| A. trisectum | 0.86 | 0.11 | 6.83 | 3.55 |
| Philodendron alliodorum | 26.25 | 32.81 | 38.14 | 55.79 |
| P. aromaticum | 2.75 | 3.27 | 2.58 | 4.26 |
| P. cretosum | 0 | 2.14 | 1.29 | 5.67 |
| P. brevispathum | 0 | 0 | 0 | 0.12 |
| P. brunneicaule | 0 | 0.11 | 0.9 | 0.47 |
| P. cretosum | 0.77 | 1.47 | 3.22 | 1.54 |
| P. davidsonii | 0.34 | 0.11 | 0.39 | 1.18 |
| P. fragrantissimum | 10.41 | 0 | 0 | 0 |
| P. hederaceum | 0.09 | 0.34 | 0.26 | 0.59 |
| P. herbaceum | 1.72 | 6.99 | 5.15 | 5.44 |
| P. inaequilaterum | 4.48 | 9.13 | 3.22 | 5.08 |
| P. jodavisianum | 7.57 | 7.33 | 4.12 | 8.27 |
| P. ligulatum | 0.17 | 0 | 0.26 | 0.47 |
| P. opacum | 0.17 | 0 | 1.16 | 1.89 |
| P. platypetiolatum | 4.04 | 7.89 | 11.34 | 15.37 |
| P. rhodoaxis | 0.52 | 5.07 | 5.28 | 10.17 |
| P. rigidifolium | 10.07 | 2.03 | 2.19 | 1.42 |
| P. rothschuhianum | 0.26 | 0.45 | 0 | 0 |
| P. sagittifolium | 0.86 | 0.79 | 0.64 | 1.18 |
| P. schottii | 5.85 | 3.49 | 4.25 | 2.96 |
| P. sulcatum | 0.34 | 0.9 | 0.52 | 3.55 |
| P. tenue | 0 | 0.45 | 0.13 | 1.18 |
| P. tripartitum | 2.75 | 5.52 | 4.64 | 6.26 |
| Rhodospatha pellucida | 0.17 | 0.11 | 0.52 | 4.14 |
| R. wendlandii | 9.72 | 18.94 | 22.16 | 32.03 |
| Syngonium hoffmannii | 0.52 | 1.01 | 0.26 | 1.42 |
| S. macrophyllum | 1.64 | 3.27 | 3.22 | 2.36 |
| S. peliocladum | 0 | 1.35 | 0.13 | 1.18 |
| S. rayi | 0.52 | 0 | 0 | 0.12 |
| S. schottianum | 0.69 | 1.24 | 1.29 | 4.37 |
| S. triphyllum | 2.67 | 8.23 | 10.57 | 17.02 |
| Total | 101.19 | 130.97 | 140.85 | 212.52 |
| Cyclanthaceae—Epiphytes | ||||
| Asplundia euryspatha | 0 | 0.23 | 1.29 | 0.12 |
| A. ferruginea | 2.84 | 1.24 | 2.84 | 0.83 |
| A. multistaminata | 0.77 | 0.79 | 1.29 | 0.35 |
| A. utilis | 2.67 | 8.79 | 4.51 | 6.26 |
| Evodianthus funifer | 1.98 | 6.99 | 5.67 | 9.57 |
| Total | 8.26 | 18.04 | 15.6 | 17.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riordan, E.C.; Gerst, K.L.; Vargas Ramirez, O.; Rundel, P.W. Differential Species Richness and Ecological Success of Epiphytes and Hemiepiphytes of Neotropical Araceae and Cyclanthaceae. Plants 2023, 12, 4004. https://doi.org/10.3390/plants12234004
Riordan EC, Gerst KL, Vargas Ramirez O, Rundel PW. Differential Species Richness and Ecological Success of Epiphytes and Hemiepiphytes of Neotropical Araceae and Cyclanthaceae. Plants. 2023; 12(23):4004. https://doi.org/10.3390/plants12234004
Chicago/Turabian StyleRiordan, Erin C., Katharine L. Gerst, Orlando Vargas Ramirez, and Philip W. Rundel. 2023. "Differential Species Richness and Ecological Success of Epiphytes and Hemiepiphytes of Neotropical Araceae and Cyclanthaceae" Plants 12, no. 23: 4004. https://doi.org/10.3390/plants12234004
APA StyleRiordan, E. C., Gerst, K. L., Vargas Ramirez, O., & Rundel, P. W. (2023). Differential Species Richness and Ecological Success of Epiphytes and Hemiepiphytes of Neotropical Araceae and Cyclanthaceae. Plants, 12(23), 4004. https://doi.org/10.3390/plants12234004




