The Postharvest Application of Carvone, Abscisic Acid, Gibberellin, and Variable Temperature for Regulating the Dormancy Release and Sprouting Commencement of Mini-Tuber Potato Seeds Produced under Aeroponics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
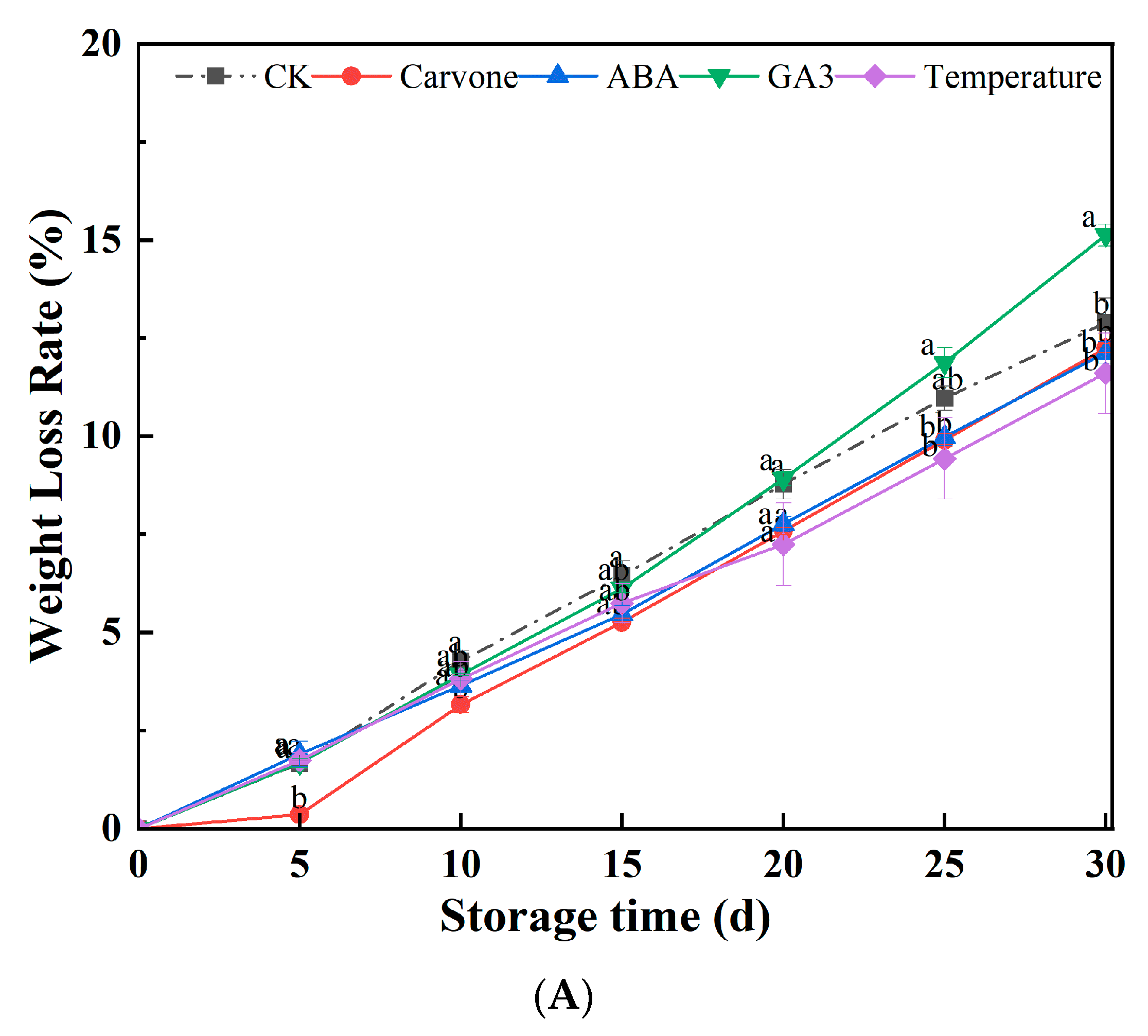
2.2. Weight Loss Rate
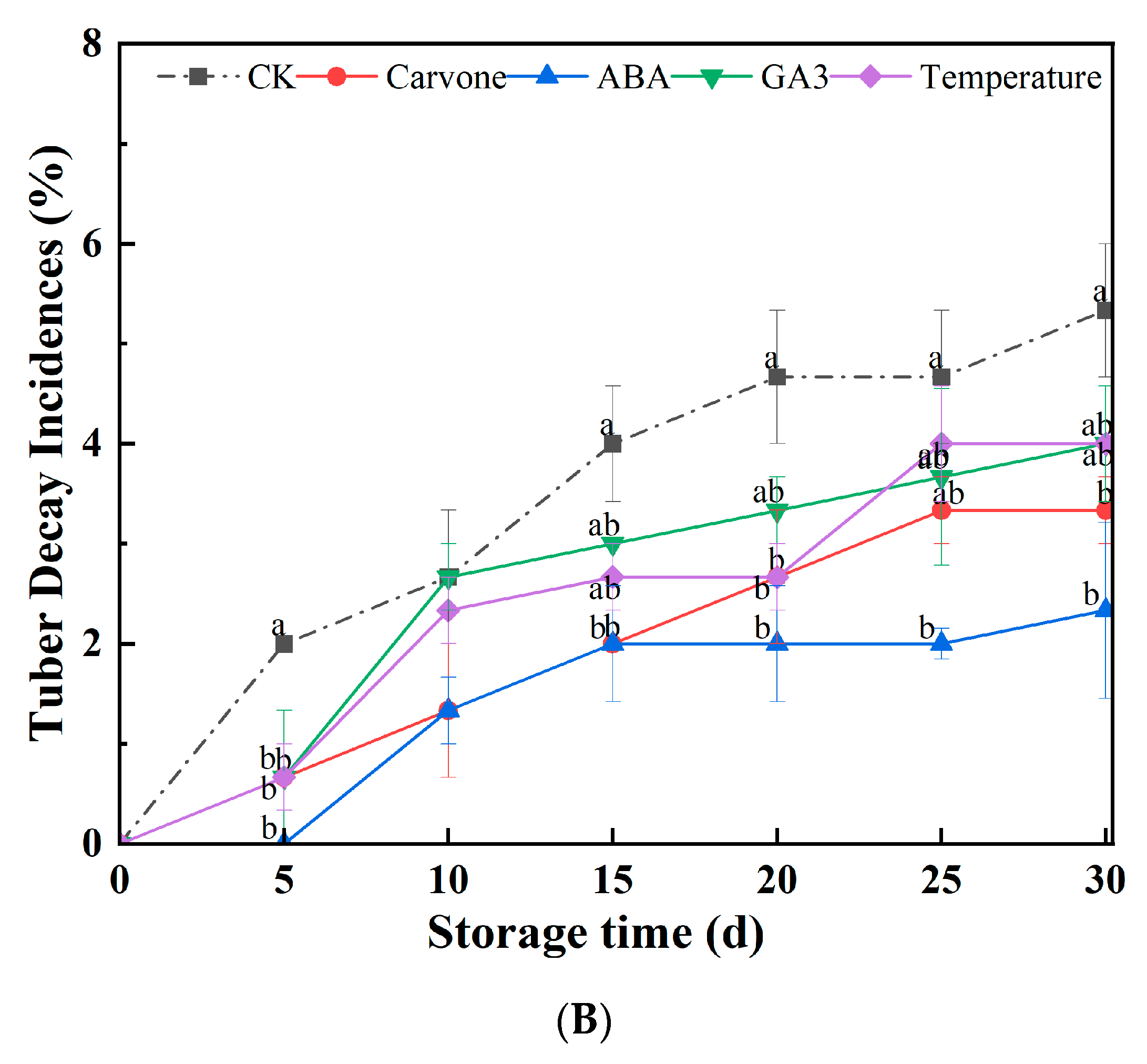
2.3. Tuber Decay Incidences
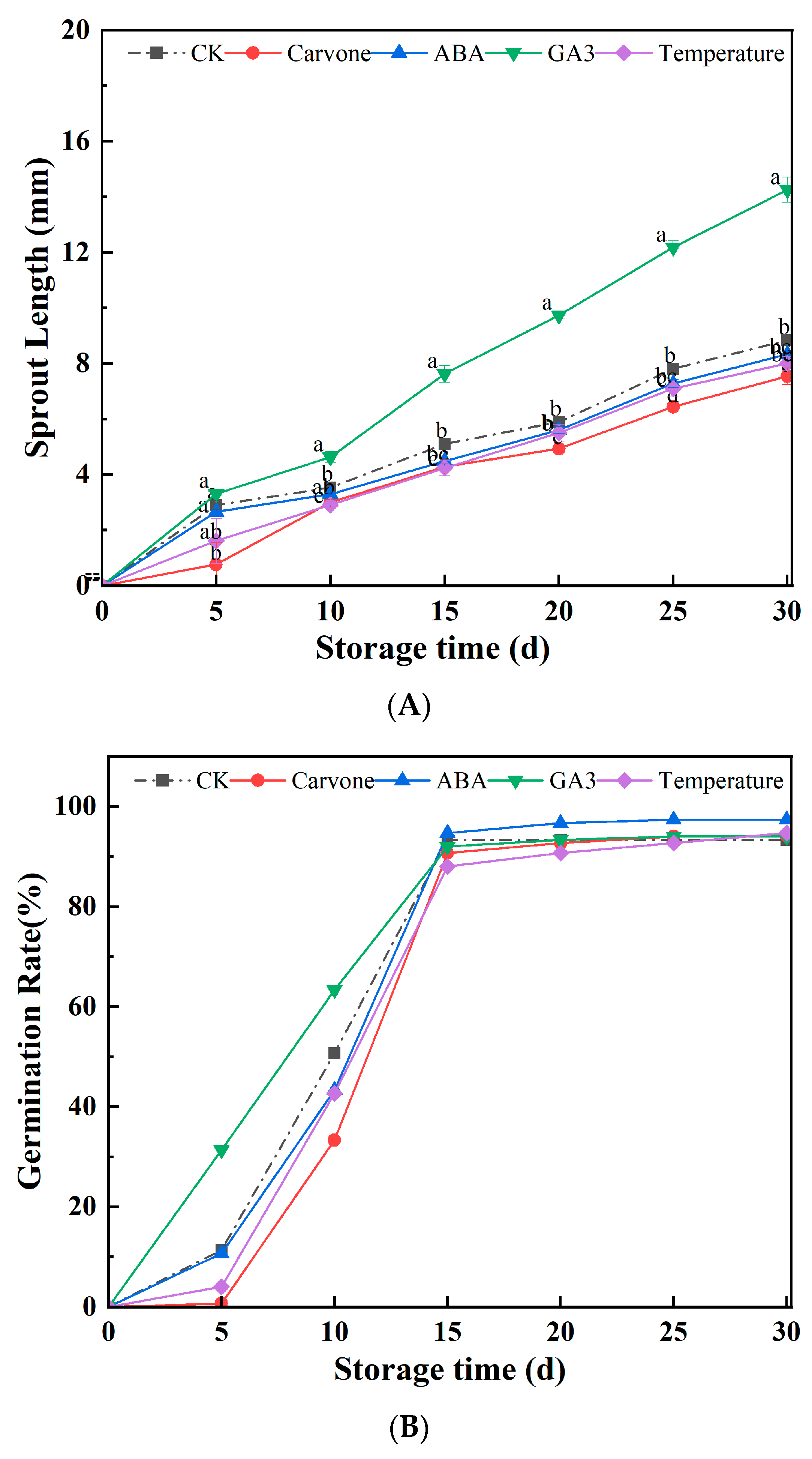
2.4. Sprout Length and Germination Rate
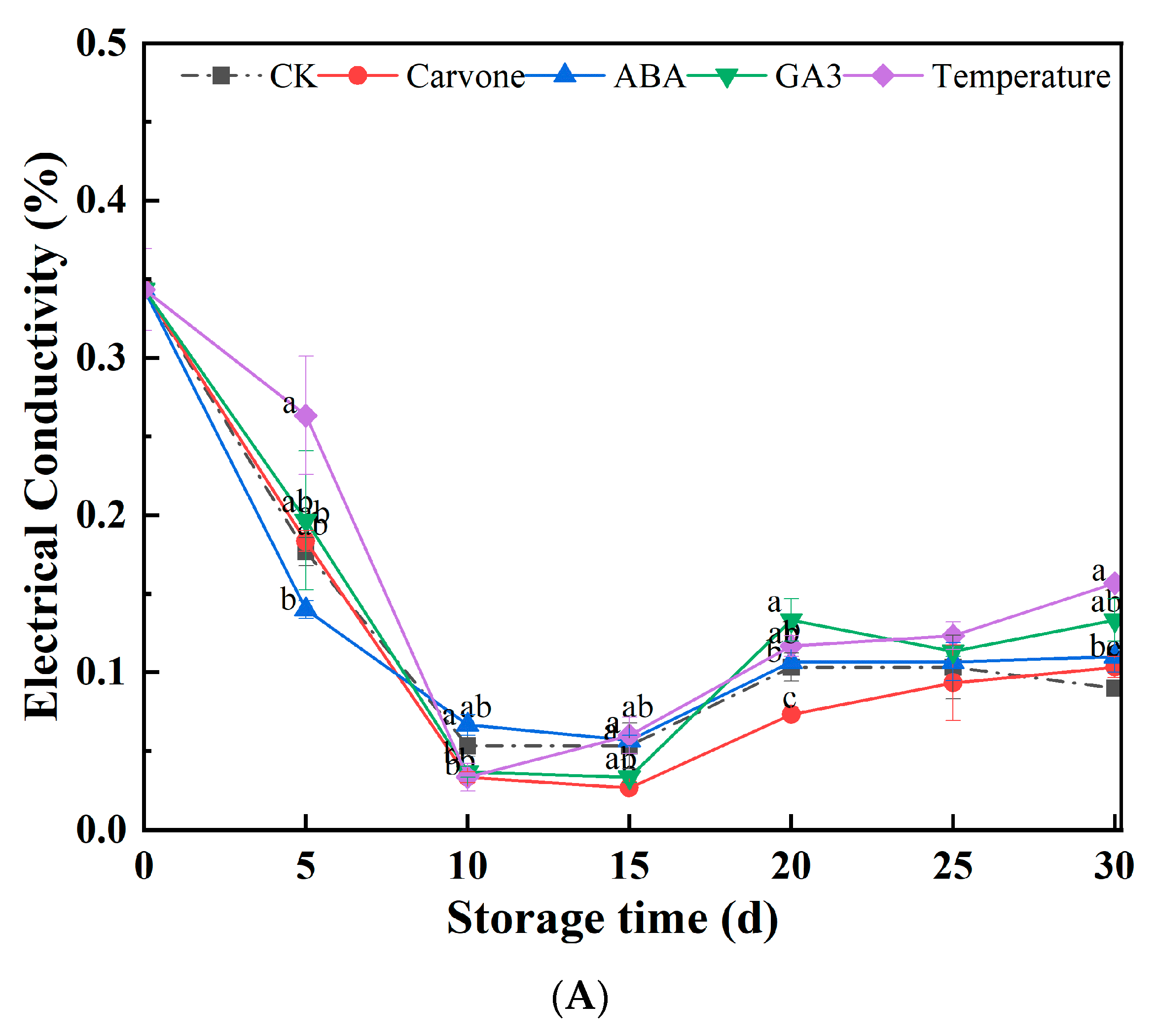
2.5. Relative Conductivity and MDA Content
2.6. Extraction and Assay of Enzyme Activities
2.7. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss Rate and Tuber Decay Incidences
3.2. Sprout Length and Germination Rate
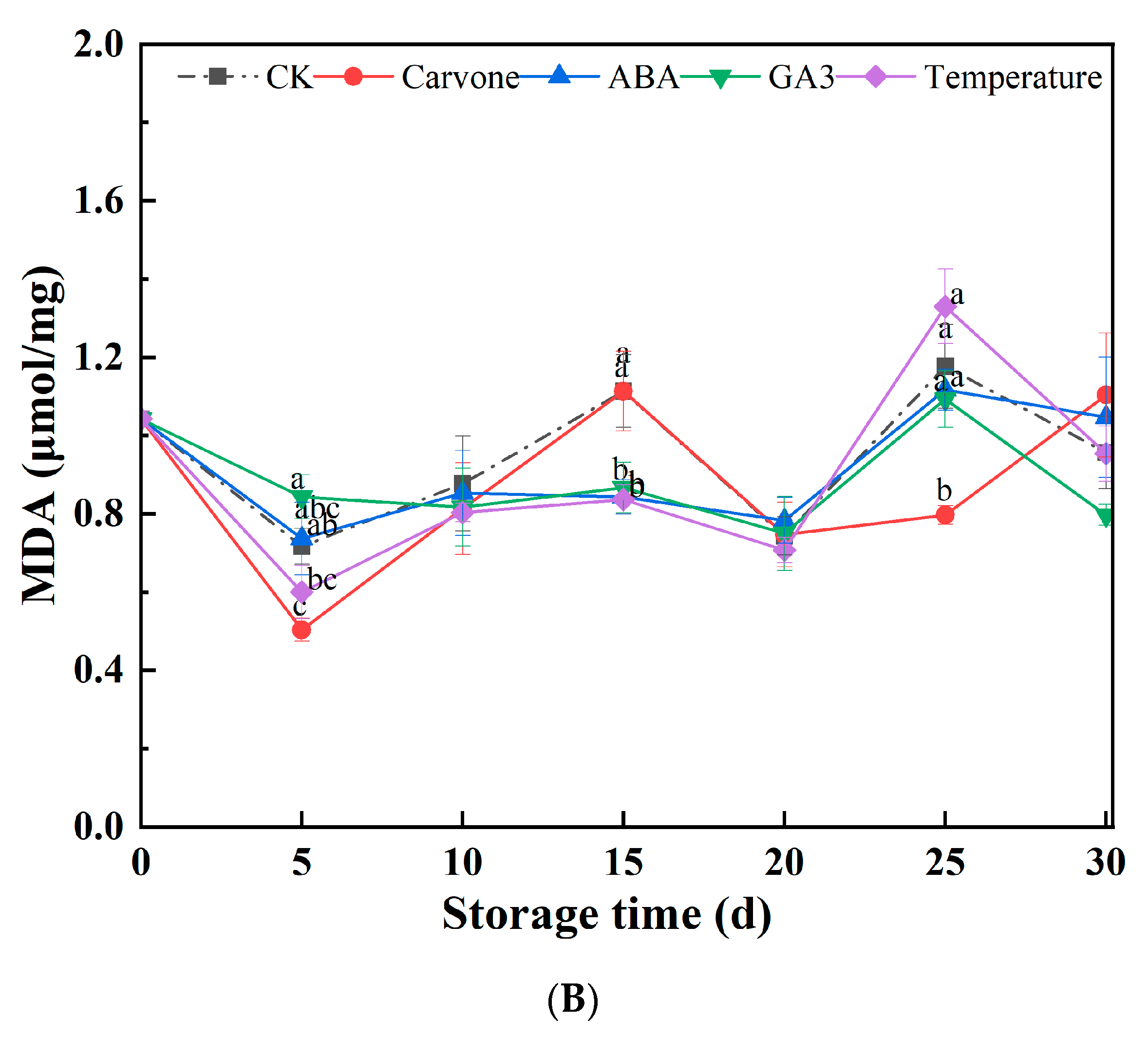
3.3. Electrical Conductivity and Malondialdehyde Content
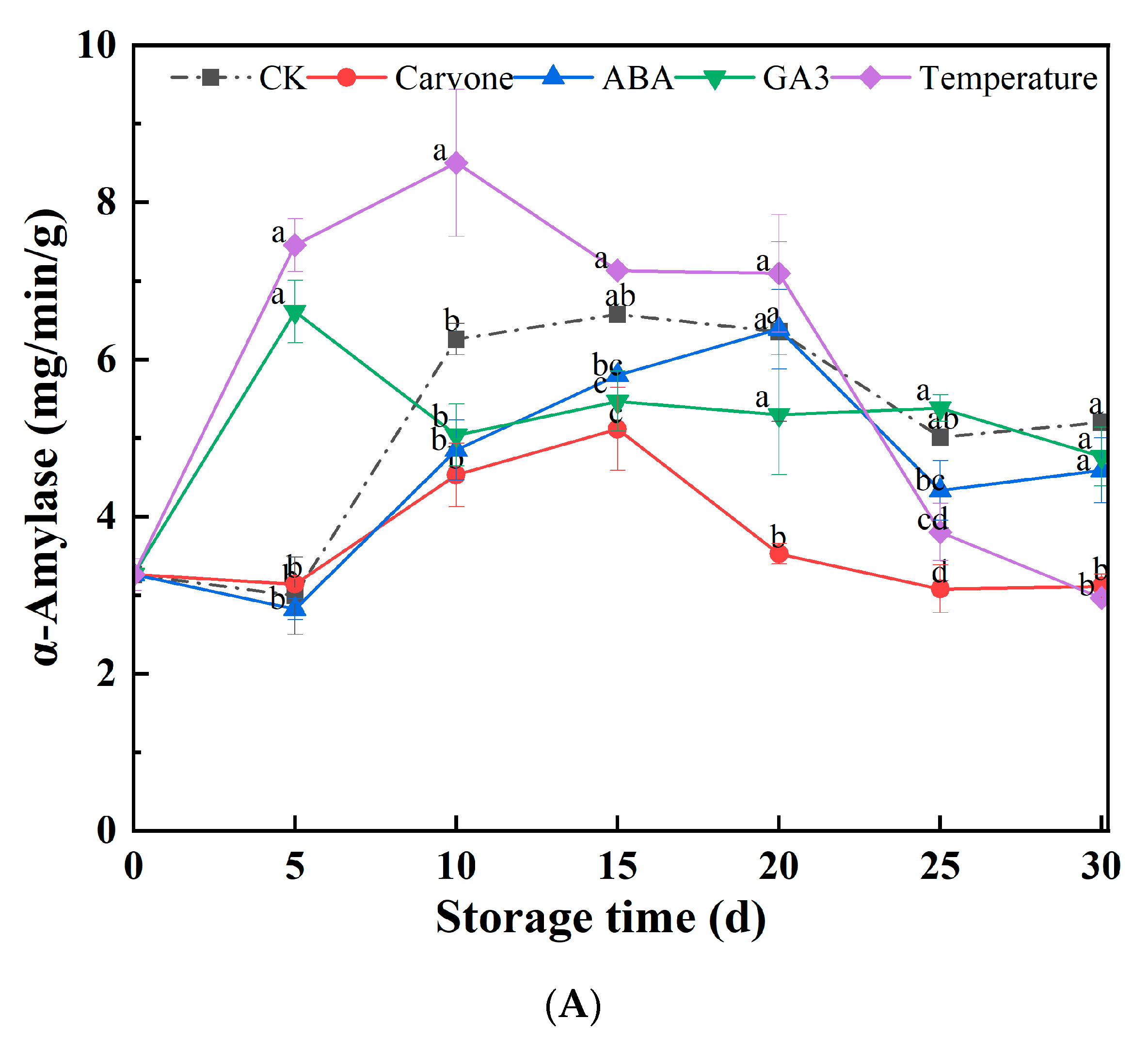
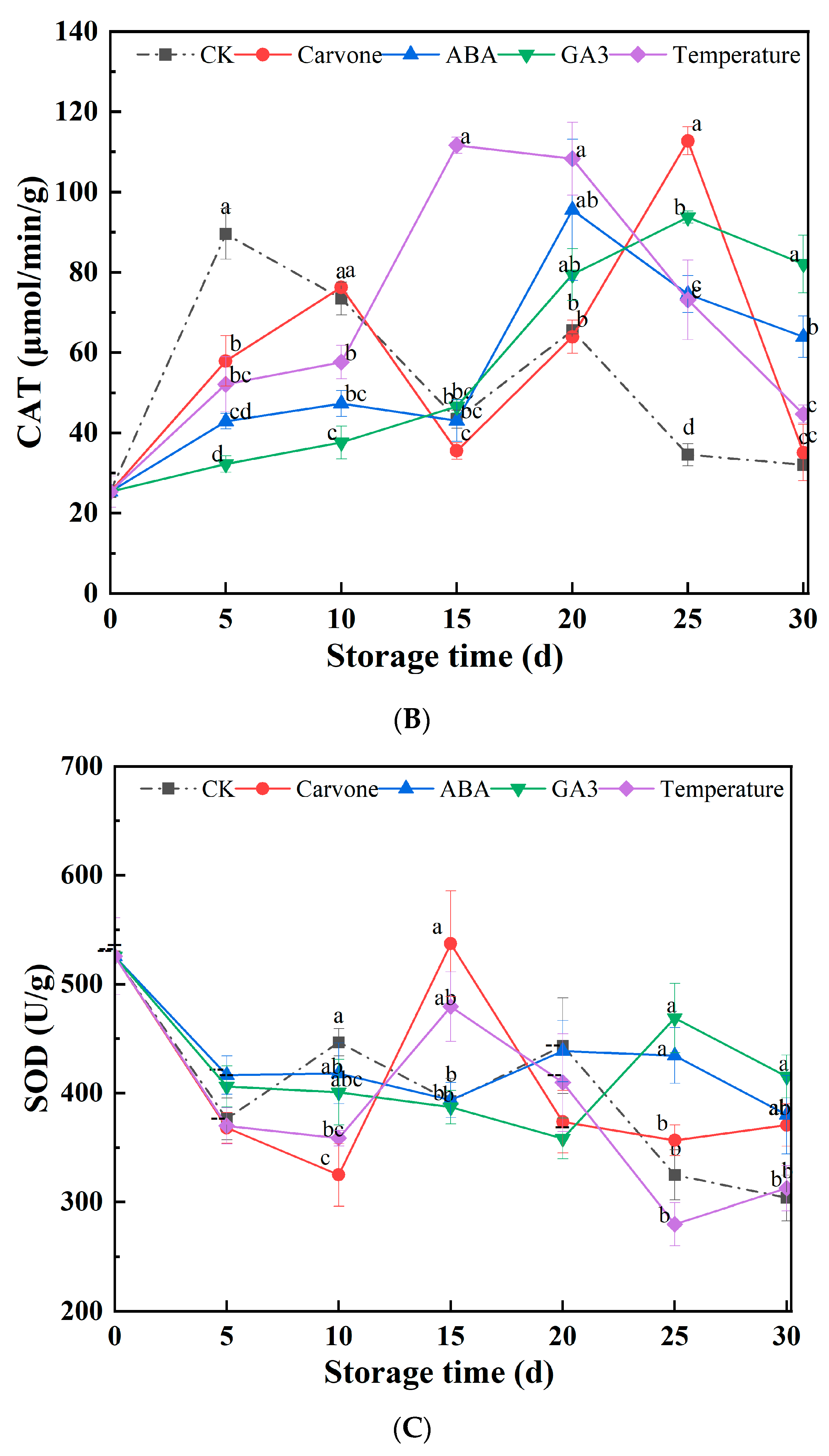
3.4. α-Amylase, CAT, and SOD Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Rasheed, H.; Ahmad, D.; Bao, J.S. Genetic diversity and health properties of polyphenols in potato. Antioxidants 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global food security, contributions from sustainable potato agri-food systems. In Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–35. ISBN 978-3-030-28683-5. [Google Scholar]
- Tang, D.; Jia, Y.X.; Zhang, J.Z.; Li, H.B.; Cheng, L.; Wang, P.; Bao, Z.G.; Liu, Z.H.; Feng, S.S.; Zhu, X.J.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 609, E14. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liu, H.; Zeng, F.K.; Yang, Y.C.; Xu, D.; Zhao, Y.C.; Liu, X.F.; Kaur, L.; Liu, G.; Singh, J. Potato processing industry in China: Current scenario, future trends and global impact. Potato Res. 2023, 66, 543–562. [Google Scholar] [CrossRef]
- Fernie, A.R.; Willmitzer, L. Molecular and biochemical triggers of potato tuber development. Plant Physiol. 2001, 127, 1459–1465. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, G.B.; Park, Y.E.; Jin, Y.I.; Choi, J.G.; Seo, J.H.; Cheon, C.G.; Chang, D.C.; Cho, J.H.; Kang, J.H. Effects of seed tuber size on dormancy and growth characteristics in potato double cropping. Hortic. Environ. Biotechnol. 2023, 64, 167–178. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.M.; Shaikh, S.A.; Lakhiar, I.A.; Qureshi, W.A.; Solangi, K.A.; Chandio, F.A. Potato production in aeroponics: An emerging food growing system in sustainable agriculture for food security. Chil. J. Agric. Res. 2020, 80, 118–132. [Google Scholar] [CrossRef]
- Tierno, R.; Carrasco, A.; Ritter, E.; de Galarreta, J.I.R. Differential growth response and minituber production of three potato cultivars under aeroponics and greenhouse bed culture. Am. J. Potato Res. 2014, 91, 346–353. [Google Scholar] [CrossRef]
- Brocic, Z.; Oljaca, J.; Pantelic, D.; Rudic, J.; Momcilovic, I. Potato aeroponics: Effects of cultivar and plant origin on minituber production. Horticulturae 2022, 8, 915. [Google Scholar] [CrossRef]
- Hu, Q.N.; Tang, C.T.; Zhou, X.Y.; Yang, X.Z.; Luo, Z.S.; Wang, L.; Yang, M.Y.; Li, D.; Li, L. Potatoes dormancy release and sprouting commencement: A review on current and future prospects. Food Front. 2023, 4, 1001–1018. [Google Scholar] [CrossRef]
- Destefano-Beltrán, L.; Knauber, D.; Huckle, L.; Suttle, J. Chemically forced dormancy termination mimics natural dormancy progression in potato tuber meristems by reducing ABA content and modifying expression of genes involved in regulating ABA synthesis and metabolism. J. Exp. Bot. 2006, 57, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.W.; Ayyub, C.M.; Malik, A.U.; Ahmad, R. Plant growth regulators and electric current break tuber dormancy by modulating antioxidant activities of potato. Pak. J. Agric. Sci. 2019, 56, 867–877. [Google Scholar]
- Ge, X.; Xu, R.; Li, M.; Tian, J.C.; Li, S.Q.; Cheng, J.X.; Tian, S.L. Regulation mechanism of carvone on seed potato sprouting. Sci. Agric. Sin. 2020, 53, 4929–4939. [Google Scholar]
- Xie, Y.J.; Onik, J.C.; Hu, X.J.; Duan, Y.Q.; Lin, Q. Effects of (S)-carvone and gibberellin on sugar accumulation in potatoes during low temperature storage. Molecules 2018, 23, 3118. [Google Scholar] [CrossRef]
- Sorce, C.; Lorenzi, R.; Ranalli, P. The effects of (S)-(+)-carvone treatments on seed potato tuber dormancy and sprouting. Potato Res. 1997, 40, 155–161. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Murigi, W.W.; Nyankanga, R.O.; Shibairo, S.I. Effect of storage temperature and postharvest tuber treatment with chemical and biorational inhibitors on suppression of sprouts during potato storage. J. Hort. Res. 2021, 29, 83–94. [Google Scholar] [CrossRef]
- Alamar, M.C.; Tosetti, R.; Landahl, S.; Bermejo, A.; Terry, L.A. Assuring potato tuber quality during storage: A future perspective. Front. Plant Sci. 2017, 8, 2034. [Google Scholar] [CrossRef]
- Gong, H.L.; Dusengemungu, L.; Igiraneza, C.; Rukundo, P. Molecular regulation of potato tuber dormancy and sprouting: A mini-review. Plant Biotechnol. Rep. 2021, 15, 417–434. [Google Scholar] [CrossRef]
- Emragi, E.; Kalita, D.; Jayanty, S.S. Effect of edible coating on physical and chemical properties of potato tubers under different storage conditions. LWT-Food Sci. Technol. 2022, 153, 112580. [Google Scholar] [CrossRef]
- Nyankanga, R.O.; Murigi, W.W.; Shibairo, S.I. Effect of packaging material on shelf life and quality of ware potato tubers stored at ambient tropical temperatures. Potato Res. 2018, 61, 283–296. [Google Scholar] [CrossRef]
- Mahto, R.; Das, M. Effect of gamma irradiation on the physico-mechanical and chemical properties of potato (Solanum tuberosum L.), cv. ‘Kufri Sindhuri’, in non-refrigerated storage conditions. Postharvest Biol. Technol. 2014, 92, 37–45. [Google Scholar] [CrossRef]
- Xu, Y.J.; Wang, D.; Zhao, W.T.; Zheng, Y.Y.; Wang, Y.B.; Wang, P.; Ma, Y.; Zhao, X.Y. Low frequency ultrasound treatment enhances antibrowning effect of ascorbic acid in fresh-cut potato slices. Food Chem. 2022, 380, 132190. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.W.; Nafeesa, M.; Amina, M.; Asadb, H.U.; Ahmad, I. Physiology of tuber dormancy and its mechanism of release in potato. J. Hortic. Sci. Technol. 2021, 4, 13–21. [Google Scholar] [CrossRef]
- Haider, M.W.; Nafees, M.; Ahmad, I.; Ali, B.; Maryam; Iqbal, R.; Vodnar, D.C.; Marc, R.A.; Kamran, M.; Saleem, M.H.; et al. Postharvest dormancy-related changes of endogenous hormones in relation to different dormancy-breaking methods of potato (Solanum tuberosum L.) tubers. Front. Plant Sci. 2022, 13, 945256. [Google Scholar] [CrossRef]
- Ge, X.; Huang, Z.; Tian, J.; Xu, R.; Wu, X.; Tian, S. S-(+)-carvone/HPβCD inclusion complex: Preparation, characterization and its application as a new sprout suppressant during potato storage. Food Chem. Adv. 2022, 1, 100100. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, X. Advancements in the development of field precooling of fruits and vegetables with/without phase change materials. J. Energy Storage 2023, 73, 109007. [Google Scholar] [CrossRef]
- Thoma, J.; Zheljazkov, V.D. Sprout suppressants in potato storage: Conventional options and promising essential oils-A review. Sustainability 2022, 14, 6382. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Bashyal, B.M.; Shanmugam, V.; Lal, M.K.; Kumar, R.; Sharma, S.; Vinod; Gaikwad, K.; Singh, B.; Aggarwal, R. Impact of Fusarium dry rot on physicochemical attributes of potato tubers during postharvest storage. Postharvest Biol. Technol. 2021, 181, 111638. [Google Scholar] [CrossRef]
- Sanli, A.; Karadogan, T. Carvone containing essential oils as sprout suppressants in potato (Solanum tuberosum L.) tubers at different storage temperatures. Potato Res. 2019, 62, 345–360. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Hartmans, K.J.; Huizing, H.J. Inhibition of potato (Solanum tuberosum) sprout growth by the monoterpene S-carvone: Reduction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity without effect on its mRNA level. J. Plant Physiol. 1993, 141, 463–469. [Google Scholar] [CrossRef]
- Suttle, J.C. Postharvest changes in endogenous ABA levels and ABA metabolism in relation to dormancy in potato-tubers. Physiol. Plant. 1995, 95, 233–240. [Google Scholar] [CrossRef]
- Wang, K.T.; Zhang, N.; Fu, X.; Zhang, H.H.; Liu, S.Y.; Pu, X.; Wang, X.; Si, H.J. StTCP15 regulates potato tuber sprouting by modulating the dynamic balance between abscisic acid and gibberellic acid. Front. Plant Sci. 2022, 13, 1009552. [Google Scholar] [CrossRef]
- Tosetti, R.; Waters, A.; Chope, G.A.; Cools, K.; Alamar, M.C.; McWilliam, S.; Thompson, A.J.; Terry, L.A. New insights into the effects of ethylene on ABA catabolism, sweetening and dormancy in stored potato tubers. Postharvest Biol. Technol. 2021, 173, 111420. [Google Scholar] [CrossRef] [PubMed]
- Destefano-Beltrán, L.; Knauber, D.; Huckle, L.; Suttle, J.C. Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Mol. Biol. 2006, 61, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Gumbo, N.; Magwaza, L.S.; Ngobese, N.Z. Evaluating ecologically acceptable sprout suppressants for enhancing dormancy and potato storability: A review. Plants 2021, 10, 2307. [Google Scholar] [CrossRef]
- Lamont, B.B.; Pausas, J.G. Seed dormancy revisited: Dormancy-release pathways and environmental interactions. Funct. Ecol. 2023, 37, 1106–1125. [Google Scholar] [CrossRef]
- Boivin, M.; Bourdeau, N.; Barnabé, S.; Desgagné-Penix, I. Sprout suppressive molecules effective on potato (Solanum tuberosum) tubers during storage: A review. Am. J. Potato Res. 2020, 97, 451–463. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cao, Y.H.; Tang, J.; He, X.J.; Li, M.; Li, C.; Ren, X.L.; Ding, Y.D. Physiology and application of gibberellins in postharvest horticultural crops. Horticulturae 2023, 9, 625. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H. The seed and the metabolism regulation. Biology 2022, 11, 168. [Google Scholar] [CrossRef]
- Haider, M.W.; Nafees, M.; Iqbal, R.; Asad, H.U.; Azeem, F.; Ali, B.; Shaheen, G.; Iqbal, J.; Vyas, S.; Arslan, M.; et al. Postharvest starch and sugars adjustment in potato tubers of wide-ranging dormancy genotypes subjected to various sprout forcing techniques. Sci. Rep. 2023, 13, 14845. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Pei, H.; Li, Z.; Zhang, M.; Chen, C.; Li, S. The Postharvest Application of Carvone, Abscisic Acid, Gibberellin, and Variable Temperature for Regulating the Dormancy Release and Sprouting Commencement of Mini-Tuber Potato Seeds Produced under Aeroponics. Plants 2023, 12, 3952. https://doi.org/10.3390/plants12233952
Zhu T, Pei H, Li Z, Zhang M, Chen C, Li S. The Postharvest Application of Carvone, Abscisic Acid, Gibberellin, and Variable Temperature for Regulating the Dormancy Release and Sprouting Commencement of Mini-Tuber Potato Seeds Produced under Aeroponics. Plants. 2023; 12(23):3952. https://doi.org/10.3390/plants12233952
Chicago/Turabian StyleZhu, Tiandi, Huaidi Pei, Zhongwang Li, Minmin Zhang, Chen Chen, and Shouqiang Li. 2023. "The Postharvest Application of Carvone, Abscisic Acid, Gibberellin, and Variable Temperature for Regulating the Dormancy Release and Sprouting Commencement of Mini-Tuber Potato Seeds Produced under Aeroponics" Plants 12, no. 23: 3952. https://doi.org/10.3390/plants12233952
APA StyleZhu, T., Pei, H., Li, Z., Zhang, M., Chen, C., & Li, S. (2023). The Postharvest Application of Carvone, Abscisic Acid, Gibberellin, and Variable Temperature for Regulating the Dormancy Release and Sprouting Commencement of Mini-Tuber Potato Seeds Produced under Aeroponics. Plants, 12(23), 3952. https://doi.org/10.3390/plants12233952




