The Effects of Irrigation Water Salinity on the Synthesis of Photosynthetic Pigments, Gas Exchange, and Photochemical Efficiency of Sour Passion Fruit Genotypes
Abstract
1. Introduction
2. Results
2.1. Analysis of Principal Components and Multivariate Variance of the Effects of Salt Stress on the Physiology of Sour Passion Fruit Genotypes
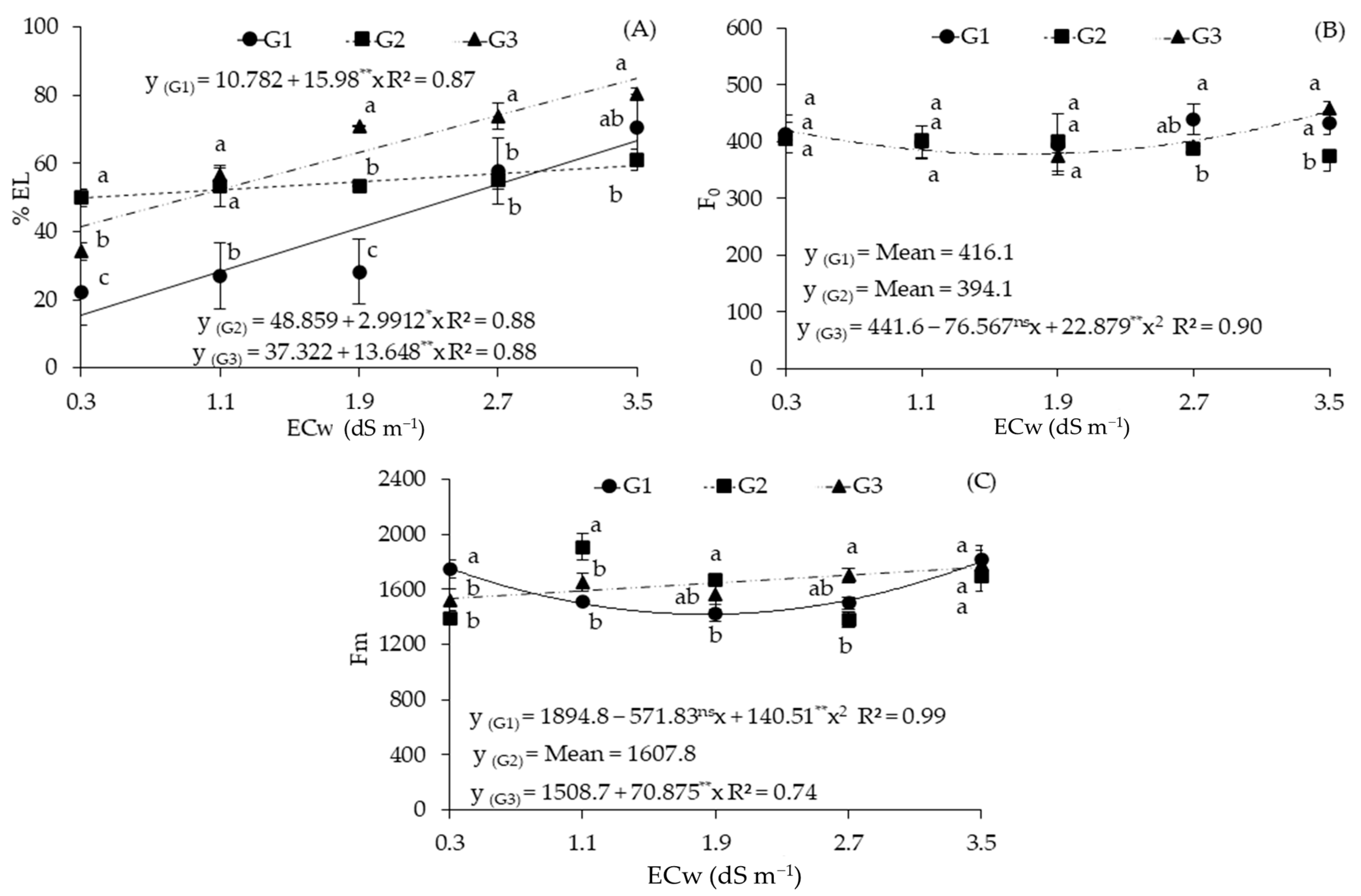
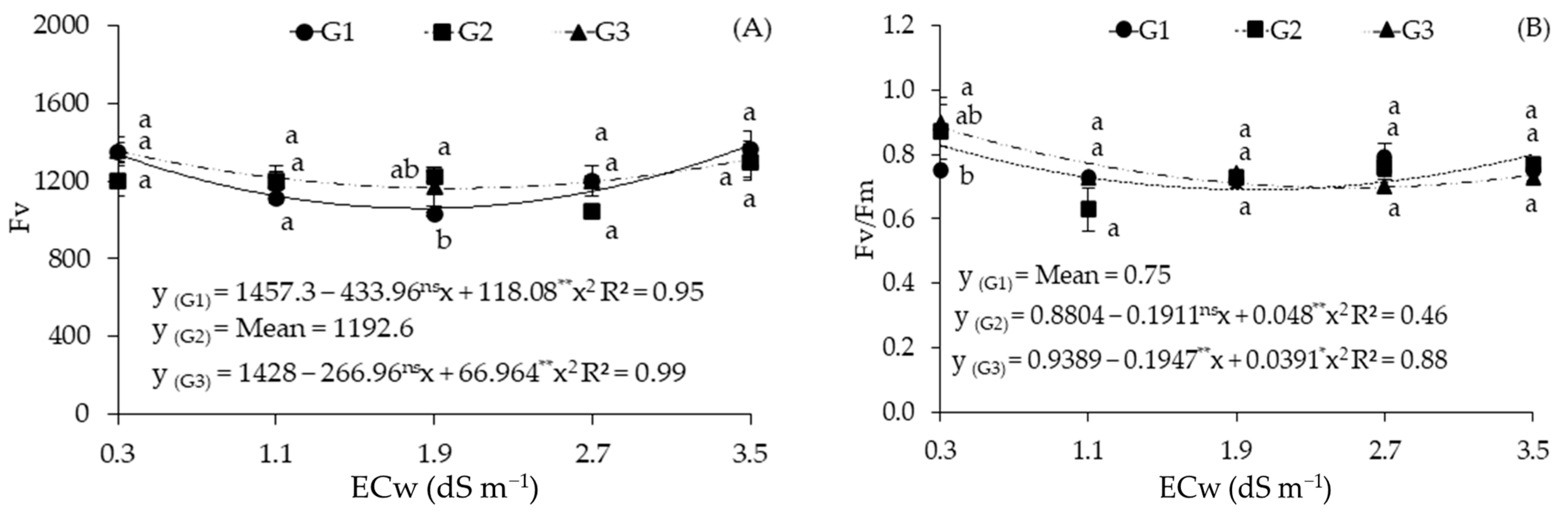
2.2. Cellular Damage and Chlorophyll a Fluorescence in Sour Passion Fruit Genotypes Irrigated with Saline Water
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca, A.M.A.; Geraldi, M.V.; Maróstica Junior, M.R.; Silvestre, A.J.D.; Rocha, S.M. Purple passion fruit (Passiflora edulis f. edulis): A comprehensive review on the nutritional value, phytochemical profile and associated health effects. Food Res. Int. 2022, 160, e111665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, L.; Li, L.; Zhu, J.; Chem, X.; Zhang, S.; Lee, L.; Yan, J.-K. Physicochemical, structural, and rheological characteristics of pectic polysaccharides from fresh passion fruit (Passiflora edulis f. flavicarpa L.) peel. Food Hydrocoll. 2023, 136, 108301. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Área Plantada e Quantidade Produzida; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2021. Available online: https://sidra.ibge.gov.br/pesquisa/pam/tabelas (accessed on 30 April 2023).
- Lima, G.S.; Fernandes, C.G.J.; dos Soares, L.A.; Gheyi, H.R.; Fernandes, P.D. Gas exchange, chloroplast pigments and growth of passion fruit cultivated with saline water and potassium fertilization. Rev. Caatinga 2020, 33, 184–194. [Google Scholar] [CrossRef]
- Oliveira, F.F.M.; Morais, M.B.; Silva, M.E.S.; Saraiva, Y.K.F.; Arruda, M.V.M.; Silva, J.N.C.; Albuquerque, C.C. Ecophysiological response of Lippia gracilis (Verbanaceae) to duration of salt stress. Ecotoxicol. Environ. Saf. 2019, 178, 202–210. [Google Scholar] [CrossRef]
- Da Lima, A.F.; da Luz, L.N.; dos Santos, M.F.; da Silva Filho, F.V.; de Gouveia, F.A.L.; de Casemiro, J.A.O. Eficiência fisiológica e desempenho do amendoim sob estresse salino e inoculado com Bradyrhizobium. Water Resour. Irrigat. Manag. 2022, 11, 22–35. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Vangelisti, A.; Zambrano, L.S.; Caruso, G.; Macheda, D.; Bernardi, R.; Usai, G.; Giordani, F.M.T.; Gucci, R.; Cavallini, A.; Natali, L. How an ancient, salt-tolerant fruit crop, Ficus carica L., copes with salinity: A transcriptome analysis. Sci. Rep. 2019, 9, 109037. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Mota, K.N.A.B.; Silva, P.C.C.; Cova, A.M.W.; Ribas, R.F.; Gheyi, H.R. Selection of sunflower genotypes for salt stress and mechanisms of salt tolerance in contrasting genotypes. Ciênc. Agrotec. 2020, 44, e020120. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- De Lima, G.S.; de Lacerda, C.N.; dos Soares, L.A.; Gheyi, H.R.; Rocha, R.H.C. Production characteristics of sesame genotypes under different strategies of saline water application. Rev. Caatinga 2020, 33, 490–499. [Google Scholar] [CrossRef]
- Veloso, L.L.S.A.; Azevedo, C.A.V.; Nobre, R.G.; de Lima, G.S.; Silva, I.J.; Lacerda, C.N. Hydrogen peroxide in the acclimation of colored-fiber cotton genotypes to salt stress. Rev. Caatinga 2023, 36, 414–423. [Google Scholar] [CrossRef]
- Araújo Neto, A.C.; Nunes, R.T.C.; de Costa, R.Q.; Moreira, G.L.P.; de Silva, R.A.; São José, A.R. Germinação e crescimento inicial de Vigna unguiculata (L). sob estresse salino. Rev. Ciências Agrárias 2020, 43, 283–292. [Google Scholar] [CrossRef]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Da Prazeres, S.S.; de Lacerda, C.F.; Barbosa, F.E.L.; Amorim, A.V.; Araújo, I.C.S.; Cavalcante, L.F. Crescimento e trocas gasosas de plantas de feijão-caupi sob irrigação salina e doses de potássio. Rev. Agro@Mbiente 2015, 9, 111–118. [Google Scholar] [CrossRef][Green Version]
- Silva, E.M.; Lima, G.S.; Gheyi, H.R.; Nobre, R.G.; da Sá, F.V.S.; de Souza, L.P. Growth and gas exchanges in soursop under irrigation with saline water and nitrogen sources. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 776–781. [Google Scholar] [CrossRef]
- De Lima, G.S.; Pinheiro, F.W.A.; de Souza, W.B.B.; dos Soares, L.A.A.; Gheyi, H.R.; Nobre, R.G.; de Queiroga, R.C.F.; Fernandes, P.D. Physiological indices of sour passion fruit under brackish water irrigation strategies and potassium fertilization. Rev. Bras. Eng. Agríc. Ambient. 2023, 27, 383–392. [Google Scholar] [CrossRef]
- De Souto, A.D.L.; Cavalcante, L.F.; de Melo, E.N.; Cavalcante, Í.H.L.; da Oliveira, R.Í.L.S.; de Mesquita, E.F.; Mendonça, R.M.N. Gas exchange and yield of grafted yellow passion fruit under salt stress and plastic mulching. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 823–830. [Google Scholar] [CrossRef]
- Alvarenga, C.F.S.; Silva, E.M.; Nobre, R.G.; Gheyi, H.R.; de Lima, G.S.; de Silva, L.A. Morfofisiologia de aceroleira irrigada com águas salinas sob combinações de doses de nitrogênio e potássio. Rev. Ciências Agrárias 2019, 42, 194–205. [Google Scholar] [CrossRef]
- Salimi, F.; Shekari, F.M.R.; Azimi, E.Z. Role of methyl jasmonate on improving salt resistance through some physiological characters in German chamomile (Matricaria chamomilla L.). Iran. J. Med. Aromat. Plants. 2012, 27, 700–711. [Google Scholar] [CrossRef]
- Khaliq, A.; Haq, M.Z.U.; Aslam, F.; Matloob, A.; Saddam, H. Salinity tolerance in wheat cultivars is related to enhanced activities of enzymatic antioxidants and reduced lipid peroxidation. Clean Soil Air Water 2015, 43, 248–1258. [Google Scholar] [CrossRef]
- Do Sacramento, B.L.; Cruz, T.S.; Silva, L.L.; Mota, K.N.A.B.; de Azevedo Neto, A.D. Pigmentos e teores de solutos orgânicos em plantas de aguapé sob estresse salino. Encic. Biosf. 2014, 10, 33–54. [Google Scholar]
- Da Paiva, F.J.S.; de Lima, G.S.; de Lima, V.L.A.; Ramos, J.G.; Gheyi, H.R.; de Farias, M.S.S.; Fernandes, P.D.; de Azevedo, C.A.V. Growth, photosynthetic pigments, and photochemical efficiency of sour passion fruit as a function of the cationic nature of water. Semina Ciênc. Agrár. 2021, 42, 583–598. [Google Scholar] [CrossRef]
- Habermann, G.; Machado, E.C.; Rodrigues, J.D.; Medina, C.L. CO2 assimilation, photosynthetic light response curves, and water relations of ‘Pêra’ sweet orange plants infected with Xylella fastidious. Braz. J. Plant Physiol. 2003, 15, 79–87. [Google Scholar] [CrossRef]
- Aycan, M.; Baslam, M.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Development of new high-salt tolerant bread wheat (Triticum aestivum L.) genotypes and insight into the tolerance mechanisms. Plant Physiol. Biochem. 2021, 166, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E.; Myller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal; ARTMED: Porto Alegre, Brazil, 2017; 858p. [Google Scholar]
- Silva, E.N.; Ribeiro, R.V.; Silva-Ferreira, S.L.; Vieira, S.A.; Ponte, L.F.A.; Silveira, A.G. Coordinate changes in photosynthesis, sugar accumulation and anti-oxidative enzymes improve the performance of Jatropha curcas plants under drought stress. Biomass Bioenergy 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Pompeiano, A.; Landi, M.; Meloni, G.; Vita, F.; Guglielminetti, L.; Guidi, L. Allocation pattern, ion partitioning, and chlorophyll a fluorescence in Arundo donax L. in responses to salinity stress. Plant Biosyst. 2016, 151, 613–622. [Google Scholar] [CrossRef]
- De Lima, G.S.; de Souza, W.B.B.; dos Soares, L.A.A.; Pinheiro, F.W.A.; Gheyi, H.R.; Oliveira, V.K.N. Dano celular e pigmentos fotossintéticos do maracujazeiro-azedo em função da natureza catiônica da água. Irriga 2020, 25, 663–669. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Híbrido de Maracujazeiro-Azedo de Alta Produtividade: BRS Gigante Amarelo; Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA: Brasília, Brazil, 2014; 2p. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Híbrido de Maracujazeiro-Azedo para mesa e Indústria: BRS Sol do Cerrado; Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA: Brasília, Brazil, 2014; 2p. [Google Scholar]
- Petry, H.B.; Bruna, E.D.; Moreto, A.L.; Brancher, A.; Sônego, M. ‘SCS437 Catarina’: Maracujá-azedo de alta qualidade para o mercado de mesa. Agropec. Catarin. 2019, 32, 49–52. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; 573p. [Google Scholar]
- De Costa, A.F.S.; Costa, A.N.; Ventura, J.A.; Fanton, C.J.; de Lima, I.M.; Caetano, L.C.S.; de Santana, E.N. Recomendações Técnicas para o Cultivo do Maracujazeiro; (Incaper. Documentos, 162); Incaper: Vitória, Brazil, 2008; 56p. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Department of Agriculture: Washington, DC, USA, 1954; 160p.
- Arnon, D.I. Copper enzymes in isolated cloroplasts: Polyphenoloxidases in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I- The field easurements of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Scotti-Campos, P.; Pham-Thi, A.-T.; Semedo, J.N.; Pais, I.P.; Ramalho, J.C.; Matos, M.C. Physiological responses and membrane integrity in three Vigna genotypes with contrasting drought tolerance. Emir. J. Food Agric. 2013, 25, 1002–1013. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Lichter, K.; Dendooven, L.; Deckers, J. Influence of permanent raised bed planting and residue management on physical and chemical soil quality in rain fed maize/wheat systems. Plant Soil 2007, 291, 39–54. [Google Scholar] [CrossRef]
- Hotelling, H.; Eisenhart, C.; Hastay, M.W.; Wallis, W.A. Multivariate Quality Control; Techniques of statistical analysis; John Wiley & Sons: New York, NY, USA, 1947; 73p. [Google Scholar]
- Hair, F.J.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados, 6th ed; Tradução Adonai Schlup Sant’Anna; Bookman: Porto Alegre, Brazil, 2009; 688p. [Google Scholar]
- Statsoft, I.N.C. Programa Computacional Statistica 7.0; The European Association of Urology: Arnhem, The Netherlands, 2004. [Google Scholar]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
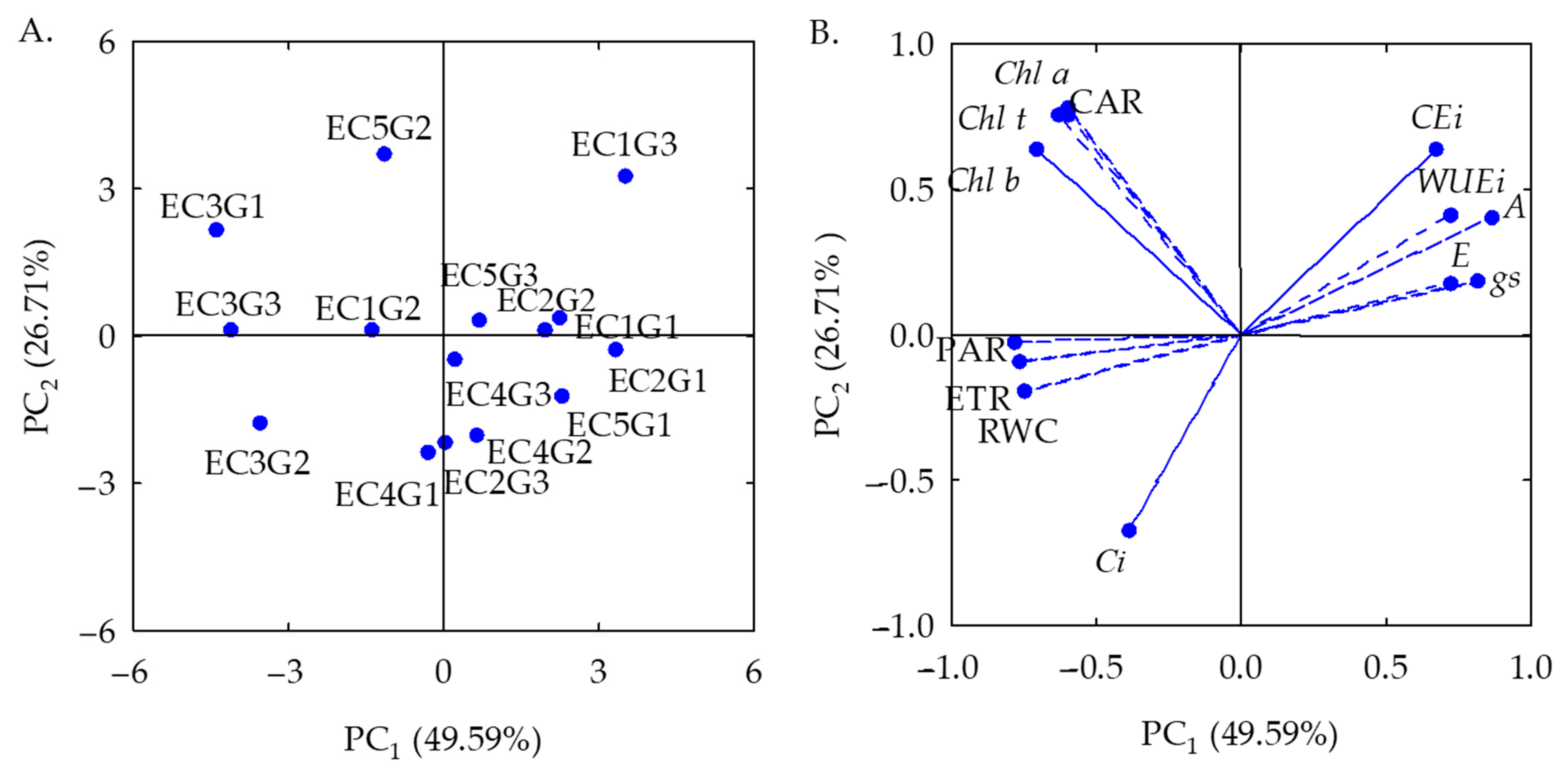


| Principal Components (PCs) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | ||||||||||||
| Eigenvalues (λ) | 6.44 | 3.47 | |||||||||||
| Percentage of total variance (S2%) | 49.59 | 26.71 | |||||||||||
| Hotelling test (T2) for electrical conductivity (ECw) | 0.01 | 0.01 | |||||||||||
| Hotelling test (T2) for genotypes (GEN) | 0.01 | 0.01 | |||||||||||
| Hotelling test (T2) for ECw × GEN interaction | 0.01 | 0.01 | |||||||||||
| PCs | Correlation coefficient | ||||||||||||
| RWC | Chl a | Chl b | Chl t | Car | Ci | E | gs | A | WUEi | CEi | ETR | PAR | |
| PC1 | −0.75 | −0.60 | −0.70 | −0.62 | −0.60 | −0.39 | 0.72 | 0.81 | 0.87 | 0.72 | 0.67 | −0.76 | −0.78 |
| PC2 | −0.19 | 0.78 | 0.63 | 0.76 | 0.76 | −0.66 | 0.17 | 0.18 | 0.40 | 0.41 | 0.63 | −0.08 | −0.02 |
| TREAT | Mean values | ||||||||||||
| RWC | Chl a | Chl b | Chl t | Car | Ci | E | gs | A | WUEi | CEi | ETR | PAR | |
| EC1G1 | 83.98 ± 0.59 | 537.66 ± 77.61 | 106.76 ± 2.03 | 644.43 ± 79.64 | 106.01 ± 6.11 | 232.00 ± 9.24 | 3.60 ± 0.16 | 0.40 ± 0.01 | 31.71 ± 0.56 | 8.87 ± 0.53 | 0.14 ± 0.01 | 25.83 ± 2.93 | 95.00 ± 8.08 |
| EC1G2 | 89.49 ± 2.67 | 672.01 ± 61.11 | 225.95 ± 19.64 | 897.97 ± 54.03 | 221.51 ± 5.79 | 228.00 ± 2.89 | 2.43 ± 2.89 | 0.32 ± 0.05 | 15.64 ± 2.25 | 6.40 ± 0.76 | 0.07 ± 0.01 | 37.40 ± 2.48 | 153.33 ± 5.49 |
| EC1G3 | 79.02 ± 4.12 | 723.31 ± 18.04 | 154.94 ± 5.19 | 878.25 ± 23.96 | 192.45 ± 14.29 | 140.00 ± 23.09 | 3.42 ± 23.09 | 0.34 ± 0.01 | 38.38 ± 7.03 | 11.44 ± 2.54 | 0.31 ± 0.11 | 20.30 ± 1.96 | 92.00 ± 1.15 |
| EC2G1 | 76.84 ± 3.27 | 289.36 ± 44.45 | 31.85 ± 3.95 | 321.21 ± 42.61 | 52.4 ± 8.04 | 209.50 ± 2.60 | 3.77 ± 0.14 | 0.45 ± 0.04 | 30.94 ± 2.25 | 8.29 ± 0.91 | 0.15 ± 0.01 | 32.25 ± 1.01 | 113.33 ± 6.64 |
| EC2G2 | 81.68 ± 1.18 | 299.27 ± 10.94 | 88.37 ± 1.45 | 387.64 ± 9.48 | 185.08 ± 46.99 | 239.00 ± 5.51 | 2.94 ± 0.20 | 0.52 ± 0.03 | 29.21 ± 2.28 | 9.98 ± 0.78 | 0.12 ± 0.01 | 39.40 ± 2.37 | 174.00 ± 8.08 |
| EC2G3 | 85.47 ± 2.83 | 201.6 ± 1.51 | 43.9 ± 0.21 | 245.49 ± 1.29 | 59.6 ± 1.80 | 230.00 ± 4.62 | 2.93 ± 0.39 | 0.17 ± 0.02 | 15.79 ± 0.12 | 5.61 ± 0.86 | 0.07 ± 0.01 | 39.35 ± 4.24 | 155.33 ± 7.80 |
| EC3G1 | 88.16 ± 3.37 | 1386.47 ± 137.18 | 295.34 ± 52.70 | 1681.81 ± 189.88 | 337.28 ± 22.75 | 206.00 ± 3.46 | 2.81 ± 0.31 | 0.12 ± 0.04 | 17.87 ± 0.83 | 6.48 ± 0.58 | 0.09 ± 0.01 | 77.55 ± 6.90 | 477.00 ± 58.31 |
| EC3G2 | 88.74 ± 4.14 | 593.98 ± 22.22 | 215.94 ± 8.632 | 809.92 ± 30.85 | 188.32 ± 24.68 | 345.50 ± 10.68 | 1.66 ± 0.04 | 0.10 ± 0.01 | 9.51 ± 0.14 | 5.75 ± 0.12 | 0.03 ± 0.01 | 43.90 ± 1.91 | 321.00 ± 15.01 |
| EC3G3 | 85.96 ± 2.91 | 1155.18 ± 103.71 | 275.06 ± 35.36 | 1430.23 ± 139.07 | 280.77 ± 33.62 | 292.00 ± 11.26 | 2.38 ± 0.12 | 0.12 ± 0.01 | 9.11 ± 0.46 | 3.74 ± 0.33 | 0.04 ± 0.01 | 55.50 ± 3.70 | 260.33 ± 26.85 |
| EC4G1 | 90.28 ± 0.61 | 159.14 ± 12.50 | 31.13 ± 1.07 | 190.27 ± 10.79 | 46.63 ± 3.83 | 209.00 ± 12.12 | 3.62 ± 0.25 | 0.24 ± 0.03 | 15.08 ± 1.05 | 4.18 ± 0.27 | 0.07 ± 0.01 | 58.43 ± 5.82 | 191.00 ± 42.15 |
| EC4G2 | 86.53 ± 2.15 | 138.27 ± 13.18 | 32.35 ± 4.12 | 170.62 ± 17.29 | 45.55 ± 3.21 | 258.50 ± 4.33 | 3.10 ± 0.03 | 0.34 ± 0.02 | 22.69 ± 0.14 | 7.33 ± 0.02 | 0.09 ± 0.01 | 48.50 ± 1.56 | 250.33 ± 11.26 |
| EC4G3 | 87.7 ± 1.35 | 487.55 ± 0.36 | 48.54 ± 0.09 | 536.09 ± 0.44 | 126.45 ± 1.93 | 214.00 ± 2.02 | 3.21 ± 0.04 | 0.31 ± 0.03 | 25.18 ± 0.85 | 7.86 ± 0.25 | 0.12 ± 0.01 | 51.80 ± 3.15 | 287.67 ± 25.12 |
| EC5G1 | 77.76 ± 5.33 | 218.43 ± 29.02 | 49.47 ± 5.88 | 267.9 ± 35.49 | 86.51 ± 6.34 | 274.50 ± 1.44 | 3.66 ± 0.24 | 0.39 ± 0.01 | 25.81 ± 2.38 | 7.03 ± 0.18 | 0.09 ± 0.01 | 24.40 ± 2.31 | 146.00 ± 6.35 |
| EC5G2 | 86.52 ± 2.52 | 1439.23 ± 76.14 | 290.96 ± 25.16 | 1730.19 ± 101.30 | 365.84 ± 20.52 | 150.50 ± 7.79 | 3.48 ± 0.22 | 0.38 ± 0.02 | 22.13 ± 0.38 | 6.40 ± 0.43 | 0.15 ± 0.01 | 40.30 ± 0.98 | 167.00 ± 6.35 |
| EC5G3 | 82.33 ± 1.91 | 642.39 ± 47.18 | 138.9 ± 6.57 | 781.28 ± 53.75 | 168.52 ± 12,59 | 231.50 ± 10.10 | 3.69 ± 0.34 | 0.37 ± 0.01 | 24.26 ± 0.08 | 6.70 ± 0.65 | 0.11 ± 0.01 | 40.20 ± 0.75 | 166.33 ± 7.22 |
| Sources of Variation | DF | Mean Squares | ||||
|---|---|---|---|---|---|---|
| %EL | F0 | Fm | Fv | Fv/Fm | ||
| Electrical conductivity (ECw) | 4 | 1716.72 ** | 1312.57 ns | 96,165.81 ** | 65,375.66 ** | 0.02 ** |
| Linear Regression | 1 | 6809.31 ** | 664.22 ns | 62,357.34 * | 2230.04 ns | 0.01 ns |
| Quadratic Regression | 1 | 3.56 ns | 4165.87 * | 58,933.53 * | 24,9600.50 ** | 0.04 ** |
| Genotypes (GEN) | 2 | 1860.60 ** | 1857.05 ns | 8633.95 ns | 13,440.15 ns | 0.00 ns |
| Interaction (ECw × GEN) | 8 | 371.57 ** | 1713.15 * | 87,023.17 ** | 17,940.35 * | 0.00 ns |
| Blocks | 2 | 7.24 | 21,774.15 | 85,552.15 | 102,639.62 | 0.00 |
| Residual | 28 | 25.69 | 581.50 | 11,812.66 | 6728.00 | 0.00 |
| CV (%) | 9.57 | 5.94 | 6.72 | 6.76 | 4.33 | |
| Sources of Variation | DF | Mean Squares | |||
|---|---|---|---|---|---|
| Fs | Fms | Y | LTemp | ||
| Electrical Conductivity (ECw) | 4 | 2357.88 ** | 21,234.08 ** | 0.03 ** | 1.31 ns |
| Linear Regression | 1 | 3074.17 ** | 67,240.00 ** | 0.03 ** | 0.18 ns |
| Quadratic Regression | 1 | 528.28 ns | 12,520.12 ** | 0.10 ** | 0.58 ns |
| Genotypes (GEN) | 2 | 1912.08 ** | 9881.31 ** | 0.05 ** | 0.20 ns |
| Interaction (ECw × GEN) | 8 | 2146.42 ** | 4205.16 ** | 0.00 * | 0.08 ns |
| Blocks | 2 | 401.42 | 6175.31 | 0.02 | 5.22 |
| Residual | 28 | 150.18 | 914.99 | 0.00 | 0.16 |
| CV (%) | 10.03 | 10.55 | 10.05 | 1.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, F.J.d.S.; Lima, G.S.d.; Lima, V.L.A.d.; Souza, W.B.B.d.; Soares, L.A.d.A.; Torres, R.A.F.; Gheyi, H.R.; Silva, L.d.A.; Sá, F.V.d.S.; Sá, V.K.N.O.d.; et al. The Effects of Irrigation Water Salinity on the Synthesis of Photosynthetic Pigments, Gas Exchange, and Photochemical Efficiency of Sour Passion Fruit Genotypes. Plants 2023, 12, 3894. https://doi.org/10.3390/plants12223894
Paiva FJdS, Lima GSd, Lima VLAd, Souza WBBd, Soares LAdA, Torres RAF, Gheyi HR, Silva LdA, Sá FVdS, Sá VKNOd, et al. The Effects of Irrigation Water Salinity on the Synthesis of Photosynthetic Pigments, Gas Exchange, and Photochemical Efficiency of Sour Passion Fruit Genotypes. Plants. 2023; 12(22):3894. https://doi.org/10.3390/plants12223894
Chicago/Turabian StylePaiva, Francisco Jean da Silva, Geovani Soares de Lima, Vera Lúcia Antunes de Lima, Weslley Bruno Belo de Souza, Lauriane Almeida dos Anjos Soares, Rafaela Aparecida Frazão Torres, Hans Raj Gheyi, Luderlândio de Andrade Silva, Francisco Vanies da Silva Sá, Valeska Karolini Nunes Oliveira de Sá, and et al. 2023. "The Effects of Irrigation Water Salinity on the Synthesis of Photosynthetic Pigments, Gas Exchange, and Photochemical Efficiency of Sour Passion Fruit Genotypes" Plants 12, no. 22: 3894. https://doi.org/10.3390/plants12223894
APA StylePaiva, F. J. d. S., Lima, G. S. d., Lima, V. L. A. d., Souza, W. B. B. d., Soares, L. A. d. A., Torres, R. A. F., Gheyi, H. R., Silva, L. d. A., Sá, F. V. d. S., Sá, V. K. N. O. d., Silva, S. T. d. A., Fátima, R. T. d., Fernandes, P. D., & Almeida, A. K. C. d. (2023). The Effects of Irrigation Water Salinity on the Synthesis of Photosynthetic Pigments, Gas Exchange, and Photochemical Efficiency of Sour Passion Fruit Genotypes. Plants, 12(22), 3894. https://doi.org/10.3390/plants12223894









