Morphological Characteristics and Expression Patterns of CmCYC2c of Different Flower Shapes in Chrysanthemum morifolium
Abstract
:1. Introduction
2. Results
2.1. Structural Aspects of Ray Floret and Disc Floret Development
2.2. Morphological Observations of Different Inflorescence Forms of the Chrysanthemum
2.3. Comparison of the Early Development of Different Capitulum Types
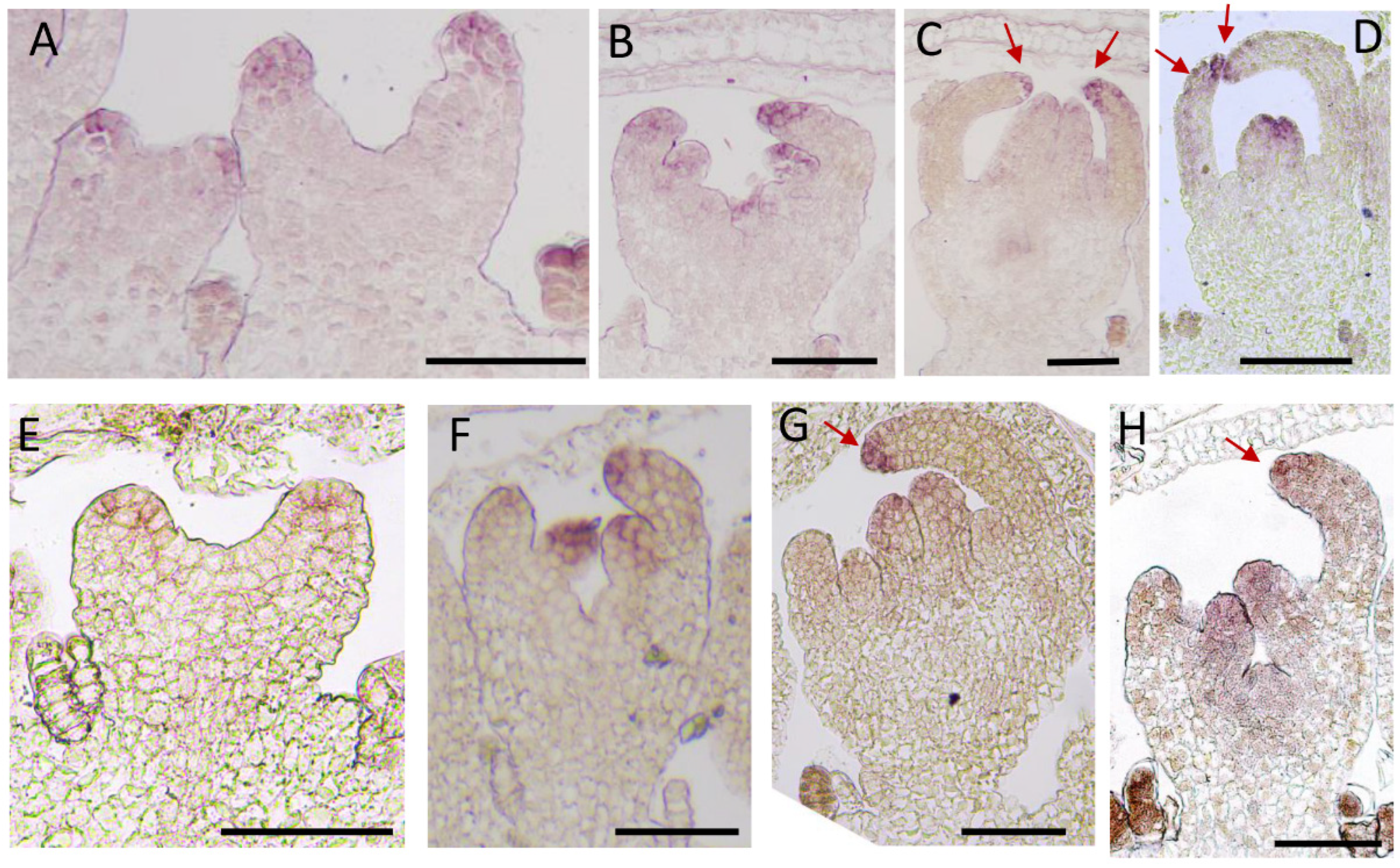
2.4. Expression Pattern Analysis of CmCYC2c in Different Inflorescence Types of Chrysanthemum
3. Discussion
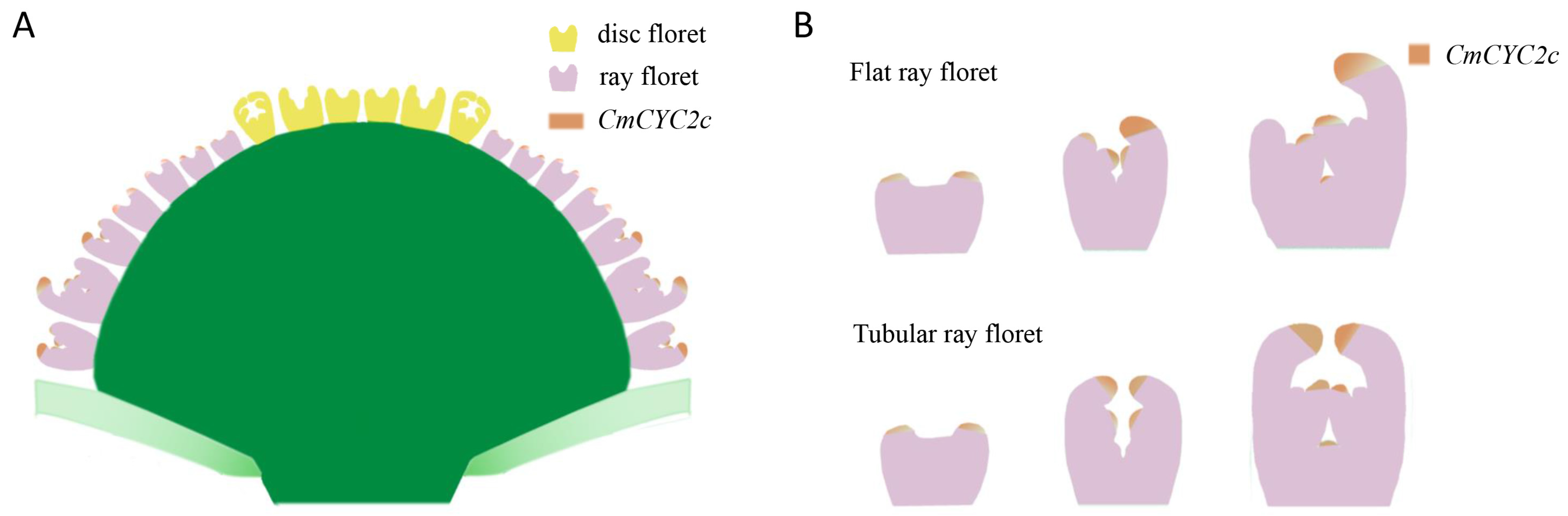
3.1. Whether the Dorsal Domain of the Ray Florets Elongates during Early Flower Development Is the Main Factor for Flower Shape Diversity in the Chrysanthemum
3.2. CmCYC2c Is Responsible for the Morphogenesis of Flat, Spoon, and Tubular Ray Petals
4. Materials and Methods
4.1. Plant Materials
4.2. Morphological Observations and Electron Microscopy Scanning
4.3. Paraffin Sections
4.4. RNA In Situ Hybridization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teixeira da Silva, J.A. Chrysanthemum: Advances in tissue culture, cryopreservation, postharvest technology, genetics and transgenic biotechnology. Biotechnol. Adv. 2003, 21, 715–766. [Google Scholar] [CrossRef]
- Anderson, N.O. Flower Breeding and Genetics; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Liu, H.; Luo, C.; Chen, D.; Wang, Y.; Guo, S.; Chen, X.; Bai, J.; Li, M.; Huang, X.; Cheng, X.; et al. Whole-transcriptome analysis of differentially expressed genes in the mutant and normal capitula of Chrysanthemum morifolium. BMC Genom. Data 2021, 22, 2. [Google Scholar] [CrossRef]
- Song, X.; Xu, Y.; Gao, K.; Fan, G.; Zhang, F.; Deng, C.; Dai, S.; Huang, H.; Xin, H.; Li, Y. High-density genetic map construction and identification of loci controlling flower-type traits in Chrysanthemum (Chrysanthemum × morifolium Ramat.). Hortic. Res. 2020, 7, 108. [Google Scholar] [CrossRef]
- Lim, J.H.; Shim, M.S.; Sim, S.-C.; Oh, K.H.; Seo, J.Y. Genetic variation of flower characteristics in a population derived from a cross between the Chrysanthemum cultivars ‘Falcao’ and ‘Frill Green’. Hortic. Environ. Biotechnol. 2014, 55, 322–328. [Google Scholar] [CrossRef]
- Fan, J.; Huang, J.; Pu, Y.; Niu, Y.; Zhang, M.; Dai, S.; Huang, H. Transcriptomic analysis reveals the formation mechanism of anemone-type flower in chrysanthemum. BMC Genom. 2022, 23, 846. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, H.; Wen, X.; Lu, C.; Zhang, B.; Gu, X.; Qi, S.; Fan, G.; Wang, W.; Dai, S. Comprehensive transcriptomic analysis provides new insights into the mechanism of ray floret morphogenesis in chrysanthemum. BMC Genom. 2020, 21, 728. [Google Scholar] [CrossRef]
- Song, X.; Gao, K.; Fan, G.; Zhao, X.; Liu, Z.; Dai, S. Quantitative Classification of the Morphological Traits of Ray Florets in Large-flowered Chrysanthemum. HortSci. Horts 2018, 53, 1258–1265. [Google Scholar] [CrossRef]
- Dejong, J.; Drennan, D.L. Genetic analysis in Chrysanthemum morifolium. II. Flower doubleness and ray floret corolla splitting. Euphytica 1984, 33, 465–470. [Google Scholar] [CrossRef]
- Pu, Y.; Liao, M.; Li, J.; Tian, Y.; Wang, Z.; Song, X.; Dai, S. Floral Development Stage-Specific Transcriptomic Analysis Reveals the Formation Mechanism of Different Shapes of Ray Florets in Chrysanthemum. Genes 2023, 14, 766. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, S.; Hong, Y.; Song, X. Application of genomic SSR locus polymorphisms on the identification and classification of Chrysanthemum cultivars in China. PLoS ONE 2014, 9, e104856. [Google Scholar] [CrossRef]
- National Chrysanthemum Society. Line Drawings Chrysanthemum Classes; National Chrysanthemum Society: Burke, Virginia, 2014. [Google Scholar]
- Yang, Z.; Hai, T. Discussion on the classification of ornamental chrysanthemums. Xiandai Horticult. 2016, 81–82. [Google Scholar] [CrossRef]
- Wen, X.; Qi, S.; Yang, L.; Hong, Y.; Dai, S. Expression pattern of candidate genes in early capitulum morphogenesis of Chrysanthemum lavandulifolium. Sci. Hortic. 2019, 252, 332–341. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, K.; Zhang, X.; Song, A.; Su, J.; Hu, Y.; Zhao, W.; Jiang, J.; Chen, F. Comprehensive characterization of a floral mutant reveals the mechanism of hooked petal morphogenesis in Chrysanthemum morifolium. Plant Biotechnol. J. 2019, 17, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Song, A.; Zhang, X.; Li, S.; Su, J.; Xia, W.; Zhao, K.; Zhao, W.; Guan, Y.; Fang, W.; et al. The core regulatory networks and hub genes regulating flower development in Chrysanthemum morifolium. Plant Mol. Biol. 2020, 103, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Howarth, D.G.; Donoghue, M.J. Phylogenetic analysis of the “ECE” (CYC/TB1) clade reveals duplications predating the core eudicots. Proc. Natl. Acad. Sci. USA 2006, 103, 9101–9106. [Google Scholar] [CrossRef]
- Zhang, W.; Kramer, E.M.; Davis, C.C. Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proc. Natl. Acad. Sci. USA 2010, 107, 6388–6393. [Google Scholar] [CrossRef]
- Luo, D.; Carpenter, R.; Vincent, C.; Copsey, L.; Coen, E. Origin of floral asymmetry in Antirrhinum. Nature 1996, 383, 794–799. [Google Scholar] [CrossRef]
- Tähtiharju, S.; Rijpkema, A.S.; Vetterli, A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Evolution and Diversification of the CYC/TB1 Gene Family in Asteraceae—A Comparative Study in Gerbera (Mutisieae) and Sunflower (Heliantheae). Mol. Biol. Evol. 2011, 29, 1155–1166. [Google Scholar] [CrossRef]
- Chapman, M.A.; Tang, S.; Draeger, D.; Nambeesan, S.; Shaffer, H.; Barb, J.G.; Knapp, S.J.; Burke, J.M. Genetic analysis of floral symmetry in Van Gogh’s sunflowers reveals independent recruitment of CYCLOIDEA genes in the Asteraceae. PLoS Genet. 2012, 8, e1002628. [Google Scholar] [CrossRef]
- Broholm, S.K.; Tahtiharju, S.; Laitinen, R.A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc. Natl. Acad. Sci. USA 2008, 105, 9117–9122. [Google Scholar] [CrossRef]
- Juntheikki-Palovaara, I.; Tähtiharju, S.; Lan, T.; Broholm, S.K.; Rijpkema, A.S.; Ruonala, R.; Kale, L.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Functional diversification of duplicated CYC2 clade genes in regulation of inflorescence development in Gerbera hybrida (Asteraceae). Plant J. 2014, 79, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cui, M.L.; Cubas, P.; Gillies, A.; Lee, K.; Chapman, M.A.; Abbott, R.J.; Coen, E. Regulatory genes control a key morphological and ecological trait transferred between species. Science 2008, 322, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Garcês, H.M.; Spencer, V.M.; Kim, M. Control of Floret Symmetry by RAY3, SvDIV1B, and SvRAD in the Capitulum of Senecio vulgaris. Plant Physiol. 2016, 171, 2055–2068. [Google Scholar] [CrossRef]
- Sun, P.; Bao, Y.; Zhu, Y.J.; Huang, N.; Wang, X.R.; Wu, Z.Y. Possible role of the CYC2c gene in the cornflower-like ray floret phenotype of Gaillardia cultivars. J. Plant Res. 2022, 135, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, X.; Sun, M.; Zhang, T.; Pan, H.; Cheng, T.; Wang, J.; Zhang, Q. Identification and Characterization of CYC-Like Genes in Regulation of Ray Floret Development in Chrysanthemum morifolium. Front. Plant Sci. 2016, 7, 1633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cieslak, M.; Owens, A.; Wang, F.; Broholm, S.K.; Teeri, T.H.; Elomaa, P.; Prusinkiewicz, P. Phyllotactic patterning of gerbera flower heads. Proc. Natl. Acad. Sci. USA 2021, 118, e2016304118. [Google Scholar] [CrossRef]
- Liu, H.; Sun, M.; Pan, H.; Cheng, T.; Wang, J.; Zhang, Q. Two Cyc2CL transcripts (Cyc2CL-1 and Cyc2CL-2) may play key roles in the petal and stamen development of ray florets in chrysanthemum. BMC Plant Biol. 2021, 21, 105. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Broholm, S.K.; Tähtiharju, S.; Mouhu, K.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Evolutionary Co-Option of Floral Meristem Identity Genes for Patterning of the Flower-Like Asteraceae Inflorescence. Plant Physiol. 2016, 172, 284–296. [Google Scholar] [CrossRef]
- Yin, X.; Tsukaya, H. Fibonacci spirals may not need the Golden Angle. Quant. Plant Biol. 2022, 3, e13. [Google Scholar] [CrossRef]
- Stuessy, T.F.; Urtubey, E. Phylogenetic implications of corolla morphology in subfamily Barnadesioideae (Asteraceae). Flora 2006, 201, 340–352. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Fan, G.; Gao, K.; Dai, S.; Zhang, M.; Ma, C.; Wu, X. Genetic analysis of the corolla tube merged degree and the relative number of ray florets in chrysanthemum (Chrysanthemum × morifolium Ramat.). Sci. Hortic. 2018, 242, 214–224. [Google Scholar] [CrossRef]
- Luo, D.; Carpenter, R.; Copsey, L.; Vincent, C.; Clark, J.; Coen, E. Control of organ asymmetry in flowers of Antirrhinum. Cell 1999, 99, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Horn, S.; Mühlhausen, A.; Mummenhoff, K.; Zachgo, S. Corolla monosymmetry: Evolution of a morphological novelty in the Brassicaceae family. Mol. Biol. Evol. 2012, 29, 1241–1254. [Google Scholar] [CrossRef]
- Cubas, P.; Coen, E.; Zapater, J.M. Ancient asymmetries in the evolution of flowers. Curr. Biol. 2001, 11, 1050–1052. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.Z.; Guo, Y.P.; Rao, G.Y. Patterning the Asteraceae Capitulum: Duplications and Differential Expression of the Flower Symmetry CYC2-Like Genes. Front. Plant Sci. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Fox, S.; Hanna, A.I.; Baxter, C.; Coen, E. Evolution of regulatory interactions controlling floral asymmetry. Development 2005, 132, 5093–5101. [Google Scholar] [CrossRef]
- Yang, X.; Pang, H.B.; Liu, B.L.; Qiu, Z.J.; Gao, Q.; Wei, L.; Dong, Y.; Wang, Y.Z. Evolution of double positive autoregulatory feedback loops in CYCLOIDEA2 clade genes is associated with the origin of floral zygomorphy. Plant Cell 2012, 24, 1834–1847. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Huang, D.; Yang, Y.; Sun, M.; Cheng, T.; Wang, J.; Pan, H.; Zhang, Q. CmCYC2-like transcription factors may interact with each other or bind to the promoter to regulate floral symmetry development in Chrysanthemum morifolium. Plant Mol. Biol. 2020, 103, 159–171. [Google Scholar] [CrossRef]
- Zhao, Y.; Broholm, S.K.; Wang, F.; Rijpkema, A.S.; Lan, T.; Albert, V.A.; Teeri, T.H.; Elomaa, P. TCP and MADS-Box Transcription Factor Networks Regulate Heteromorphic Flower Type Identity in Gerbera hybrida. Plant Physiol. 2020, 184, 1455–1468. [Google Scholar] [CrossRef]
- Ding, L.; Yan, S.; Jiang, L.; Liu, M.; Zhang, J.; Zhao, J.; Zhao, W.; Han, Y.Y.; Wang, Q.; Zhang, X. HANABA TARANU regulates the shoot apical meristem and leaf development in cucumber (Cucumis sativus L.). J. Exp. Bot. 2015, 66, 7075–7087. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Li, S.; Zhao, K.; Jia, D.; Chen, F.; Ding, L. Morphological Characteristics and Expression Patterns of CmCYC2c of Different Flower Shapes in Chrysanthemum morifolium. Plants 2023, 12, 3728. https://doi.org/10.3390/plants12213728
Qiu T, Li S, Zhao K, Jia D, Chen F, Ding L. Morphological Characteristics and Expression Patterns of CmCYC2c of Different Flower Shapes in Chrysanthemum morifolium. Plants. 2023; 12(21):3728. https://doi.org/10.3390/plants12213728
Chicago/Turabian StyleQiu, Taijia, Song Li, Kunkun Zhao, Diwen Jia, Fadi Chen, and Lian Ding. 2023. "Morphological Characteristics and Expression Patterns of CmCYC2c of Different Flower Shapes in Chrysanthemum morifolium" Plants 12, no. 21: 3728. https://doi.org/10.3390/plants12213728
APA StyleQiu, T., Li, S., Zhao, K., Jia, D., Chen, F., & Ding, L. (2023). Morphological Characteristics and Expression Patterns of CmCYC2c of Different Flower Shapes in Chrysanthemum morifolium. Plants, 12(21), 3728. https://doi.org/10.3390/plants12213728







