Decoding the Chemical Signatures and Sensory Profiles of Enshi Yulu: Insights from Diverse Tea Cultivars
Abstract
1. Introduction
2. Results
2.1. Sensory Traits of Enshi Yulu from Three Cultivars
2.2. Overall Chemical Profiles of Enshi Yulu
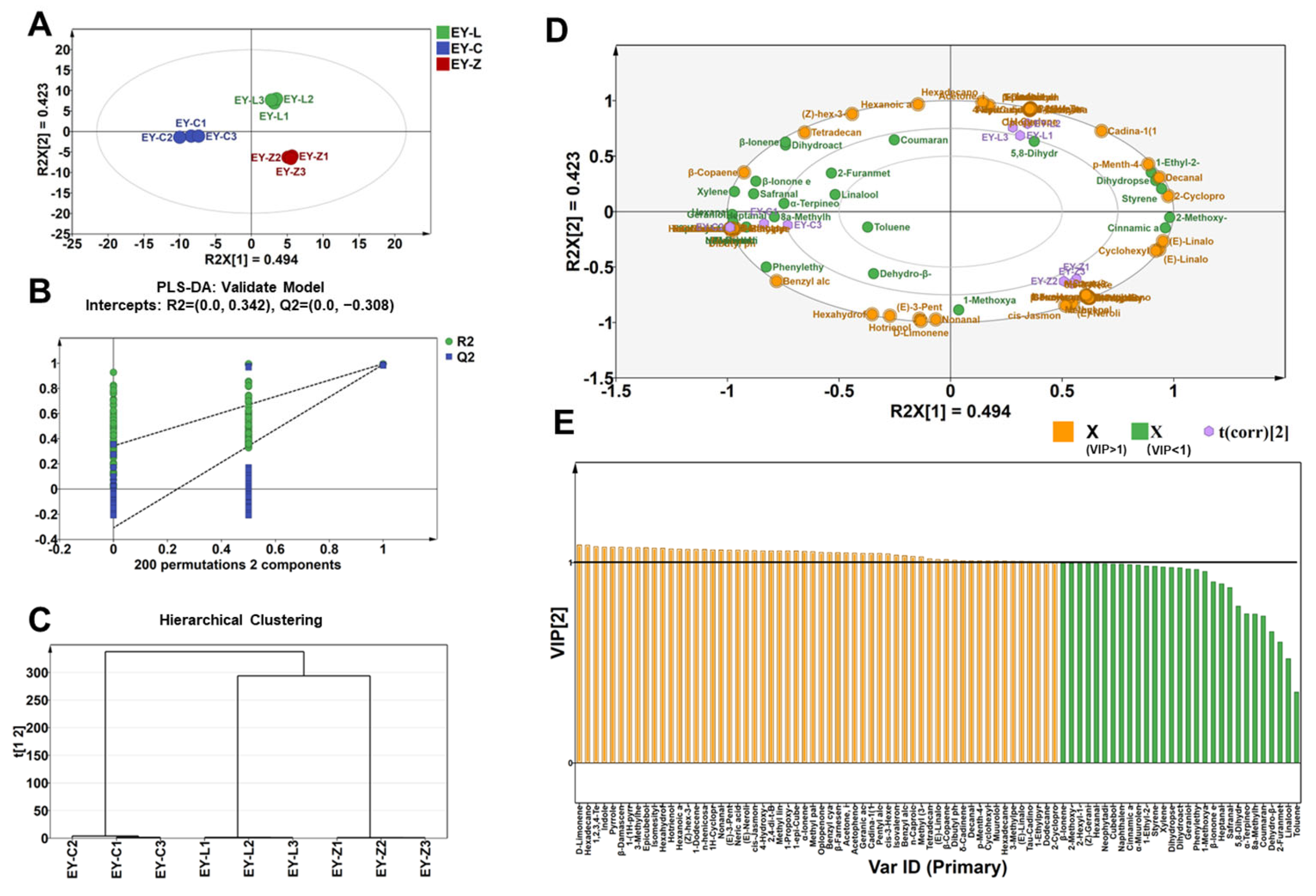
2.3. PLS-DA Analysis of Volatile Compounds for Discriminating Three Cultivars
2.4. Characteristic Volatile Composition Analysis
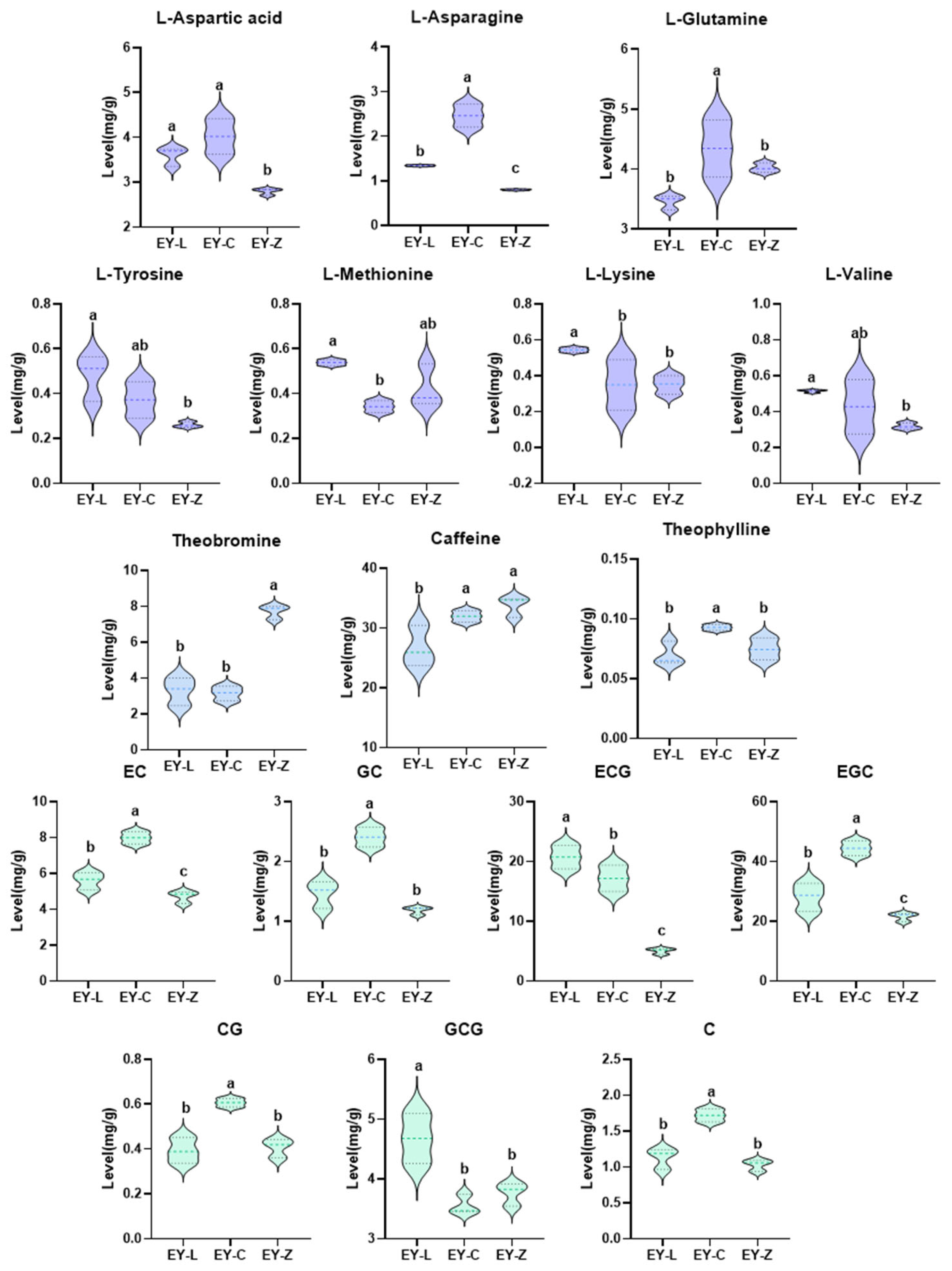
2.5. Differentiated Non-Volatile Components Analysis
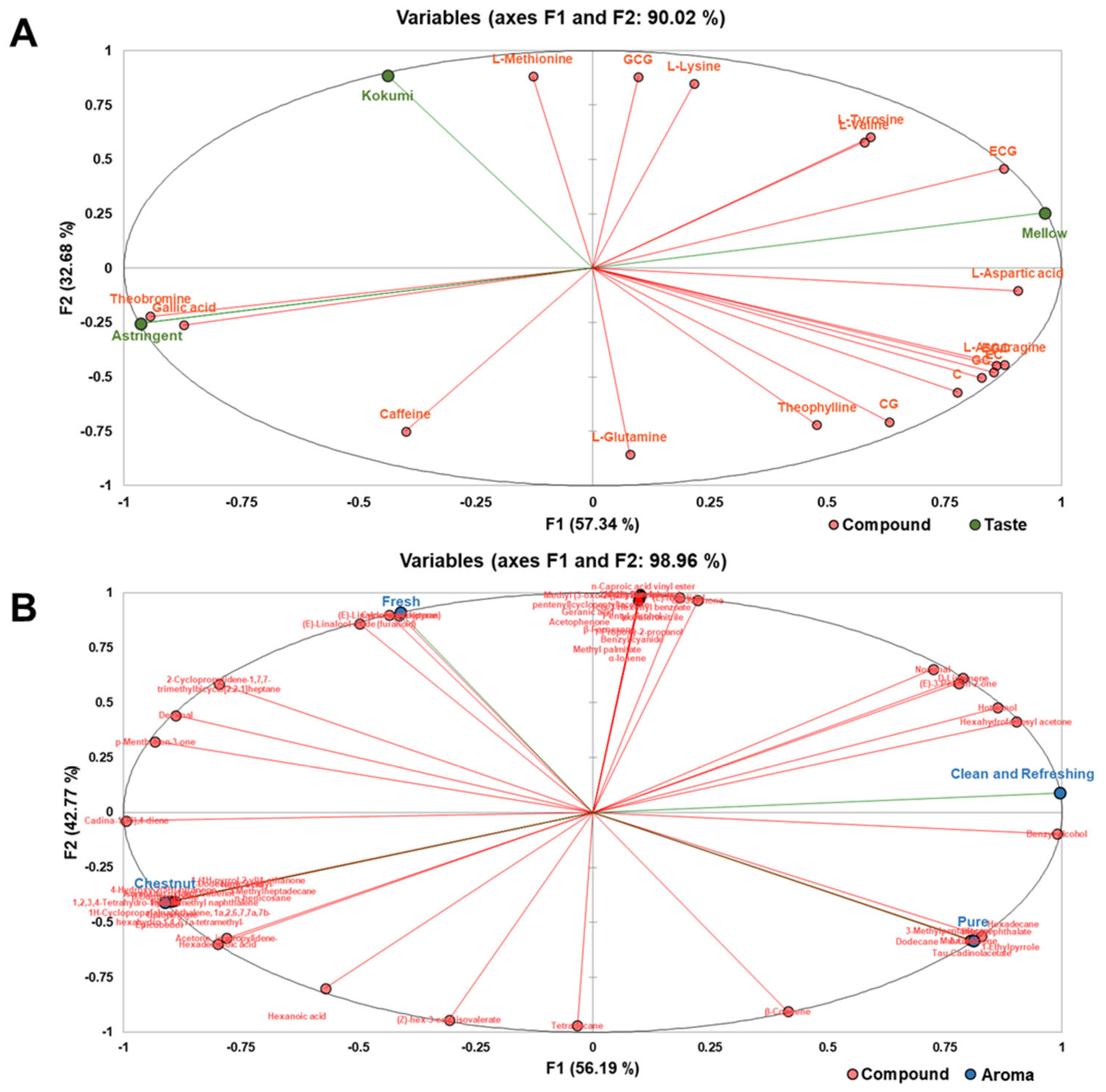
2.6. Association between Characteristic Chemical Contributors and Sensory Traits
3. Discussion
3.1. Organoleptic Quality and Chemical Profiles of Enshi Yulu
3.2. Characteristic of the Three Tea Cultivars Processing Enshi Yulu
3.3. The Relationship between Sensory Attributes and Chemical Contributors
4. Materials and Methods
4.1. Tea Sample Preparation
4.2. Reference Chemical Standards
4.3. Non-Volatile Components Determination
4.4. Extraction and Identification of Volatile Composition
4.5. Sensory Evaluation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar]
- Gui, A.; Gao, S.; Zheng, P.; Feng, Z.; Liu, P.; Ye, F.; Wang, S.; Xue, J.; Xiang, J.; Ni, D.; et al. Dynamic changes in non-volatile components during steamed green tea manufacturing based on widely targeted metabolomic analysis. Foods 2023, 12, 1551. [Google Scholar] [PubMed]
- Cui, Q.; Zhang, Q.; Liang, J.; Luo, H.; Dai, J.; Chen, Q.; Xiang, J. A study on the suitability of different tea cultivars for Enshi Yulu tea. China Tea 2021, 43, 37–42. [Google Scholar]
- Shi, Y.; Zhu, Y.; Ma, W.; Lin, Z.; Lv, H. Characterisation of the volatile compounds profile of Chinese pan-fried green tea in comparison with baked green tea, steamed green tea, and sun-dried green tea using approaches of molecular sensory science. Curr. Res. Food Sci. 2022, 5, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT 2022, 161, 113394. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Chen, J.; Wang, F.; Feng, Z.; Yin, J.; Zeng, L.; Xu, Y. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Rana, M.M.; Liu, G.; Gao, M.; Li, D.; Wu, F.; Li, X.; Wan, X.; Wei, S. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016, 212, 739–748. [Google Scholar]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 2011, 405, 620–625. [Google Scholar]
- Tan, H.R.; Lau, H.; Liu, S.Q.; Tan, L.P.; Sakumoto, S.; Lassabliere, B.; Leong, K.; Sun, J.; Yu, B. Characterisation of key odourants in Japanese green tea using gas chromatography-olfactometry and gas chromatography-mass spectrometry. LWT 2019, 108, 221–232. [Google Scholar]
- Cui, Q.; Zhang, Q.; Liang, J.; Dai, J.; Luo, H.; Chen, Q.; Xiang, J. Quality analysis of the Enshi Yulu tea. China Tea 2020, 42, 40–45. [Google Scholar]
- Wei, K.; Wang, L.; Zhou, J.; He, W.; Zeng, J.; Jiang, Y.; Cheng, H. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012, 130, 720–724. [Google Scholar] [CrossRef]
- Liang, J.; Cui, Q.; Zhang, Q. Comparative analysis of sensory evaluation of Enshi Yulu tea samples. Beijing Agric. 2014, 36, 350–351. [Google Scholar]
- Ai, S.R.; Wu, R.M.; Yan, L.Y.; Wu, Y.H. Measurement of the ratio of tea polyphenols to amino acids in green tea infusion based on near infrared spectroscopy. Adv. Mater. Res. 2011, 301–303, 1093–1097. [Google Scholar] [CrossRef]
- Li, P.; Dai, W.; Lu, M.; Xie, D.; Tan, J.; Yang, C.; Zhu, Y.; Lv, H.; Peng, Q.; Zhang, Y.; et al. Metabolomic analysis reveals the composition differences in 13 Chinese tea cultivars of different manufacturing suitabilities. J. Sci. Food Agric. 2018, 98, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, C.; Yu, X.; Zhou, J.; Ran, W.; Chen, Y.; Ni, D. Effects of red-light withering on the taste of black tea as revealed by non-targeted metabolomics and transcriptomics analysis. LWT 2021, 147, 111620. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar]
- Wang, L.; Wang, Y.; Cao, H.; Hao, X.; Zeng, J.; Yang, Y.; Wang, X. Transcriptome analysis of an anthracnose-resistant tea plant cultivar reveals genes associated with resistance to Colletotrichum camelliae. PLoS ONE 2016, 11, e148535. [Google Scholar]
- Pang, X.; Chen, F.; Liu, G.; Zhang, Q.; Ye, J.; Lei, W.; Jia, X.; He, H. Comparative analysis on the quality of Wuyi Rougui (Camellia sinensis) tea with different grades. Food Sci. Technol. 2022, 42, e115321. [Google Scholar]
- Shi, Y.; Zhu, Y.; Ma, W.; Shi, J.; Peng, Q.; Lin, Z.; Lv, H. Comprehensive investigation on non-volatile and volatile metabolites in four types of green teas obtained from the same tea cultivar of Longjing 43 (Camellia sinensis var. sinensis) using the widely targeted metabolomics. Food Chem. 2022, 394, 133501. [Google Scholar]
- Du, L.; Wang, C.; Zhang, C.; Ma, L.; Xu, Y.; Xiao, D. Characterization of the volatile and sensory profile of instant Pu-erh tea using GC × GC-TOFMS and descriptive sensory analysis. Microchem. J. 2019, 146, 986–996. [Google Scholar] [CrossRef]
- Zhu, D.; Yue, P.; Yuan, D. Effects of different fixation methods on the quality of green tea. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2009, 25, 275–279. [Google Scholar]
- Wu, Y.; Xie, G.; Yu, S.; Tong, Z.; Liu, C.; Li, H. Influence of different killing methods on the quality of green tea in Wuyuan. Newsl. Seric. Tea 2018, 1, 18–21. [Google Scholar]
- Hu, Y.; Huang, J.; Sh, I.Z. The effect of different fixation methods on quality of green tea. J. Tea 2008, 34, 24–28. [Google Scholar]
- Qin, Z.; Pang, X.; Chen, D.; Cheng, H.; Hu, X.; Wu, J. Evaluation of Chinese tea by the electronic nose and gas chromatography–mass spectrometry: Correlation with sensory properties and classification according to grade level. Food Res. Int. 2013, 53, 864–874. [Google Scholar] [CrossRef]
- Sun, M.; Guo, G.; Zhang, J. Analysis of aroma quality of steamed green tea and roasted Xinyang Maojian tea by GC-MS. Food Sci. 2014, 35, 151–155. [Google Scholar]
- Li, Y.; Ran, W.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chem. X 2022, 14, 100289. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Masuda, H.; Henze, A.; Hofmann, T. Molecular and sensory studies on the umami taste of Japanese green tea. J. Agric. Food Chem. 2006, 54, 2688–2694. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Gao, L.; Shi, X.; Zhao, X.; Ma, X.; Xia, T.; Wang, Y. Molecular evidence for catechin synthesis and accumulation in tea buds (Camellia sinensis). J. Agric. Food Chem. 2018, 66, 63–69. [Google Scholar] [CrossRef]
- Shimoda, M.; Shigematsu, H.; Shiratsuchi, H.; Osajima, Y. Comparison of volatile compounds among different grades of green tea and their relations to odor attributes. J. Agric. Food Chem. 1995, 43, 1621–1625. [Google Scholar] [CrossRef]
- Khairallah, R.; Reynolds, A.G.; Bowen, A.J. Harvest date effects on aroma compounds in aged Riesling icewines. J. Sci. Food Agric. 2016, 96, 4398–4409. [Google Scholar] [CrossRef]
- Noge, K.; Tamogami, S. Isovaleronitrile co-induced with its precursor, l-leucine, by herbivory in the common evening primrose stimulates foraging behavior of the predatory blue shield bug. Biosci. Biotechnol. Biochem. 2018, 82, 395–406. [Google Scholar] [CrossRef]
- Xiao, M.; Zheng, F.; Xiao, M.; Qi, A.; Wang, H.; Dai, Q. Contribution of aroma-active compounds to the aroma of Lu’an Guapian tea. Flavour Fragr. J. 2022, 37, 83–95. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics combined with proteomics provides a novel interpretation of the compound differences among Chinese tea cultivars (Camellia sinensis var. sinensis) with different manufacturing suitabilities. Food Chem. 2022, 377, 131976. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Y.; Pan, Y.; Mei, S.; Zhang, G.; Chu, Q.; Chen, P. Sensory-guided identification and characterization of kokumi-tasting compounds in green tea (Camellia sinensis L.). Molecules 2022, 27, 5677. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah Lotfabadi, S.; Mortazavi, S.A.; Yeganehzad, S. Study on the release and sensory perception of encapsulated d-limonene flavor in crystal rock candy using the time–intensity analysis and HS-GC/MS spectrometry. Food Sci. Nutr. 2020, 8, 933–941. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, Y.; Qu, F.; Liu, L.; Wang, B.; Wang, P.; Zhang, X. HS–SPME/GC–MS reveals the season effects on volatile compounds of green tea in high–latitude region. Foods 2022, 11, 3016. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, K.; Ni, H.; Li, T.; Li, L.J.; Li, Q.B.; Chen, F. Aroma enhancement of instant green tea infusion using β-glucosidase and β-xylosidase. Food Chem. 2020, 315, 126287. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.; Kim, M.K. Volatile and non-volatile compounds in green tea affected in harvesting time and their correlation to consumer preference. J. Food Sci. Technol. 2016, 53, 3735–3743. [Google Scholar] [CrossRef]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of key odorants in Xinyang Maojian green tea and their changes during the manufacturing process. J. Agric. Food Chem. 2022, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.; Moo-Huchin, V.; Sosa-Moguel, O.; Sauri-Duch, E.; Cuevas-Glory, L. Characterization of aroma-active compounds in choch (Lucuma hypoglauca Standley) fruit. Int. J. Food Prop. 2017, 20 (Suppl. S1), S444–S448. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Guo, X.; Yang, Y.; Xie, Y.; Ju, F.; Guo, W. Characterization of the aromatic profile in “zijuan” and “pu-erh” green teas by headspace solid-phase microextraction coupled with GC-O and GC-MS. Anal. Methods 2016, 8, 4727–4735. [Google Scholar] [CrossRef]
- Yamanishi, T.; Kosuge, M.; Tokitomo, Y.; Maeda, R. Flavor constituents of pouchong tea and a comparison of the aroma pattern with jasmine tea. Agric. Biol. Chem. 1980, 44, 2139–2142. [Google Scholar]
- Xiaofen, L.; Yuqing, L.; Hua, F.; Xiaozhen, Z.; Liubo, Y.; Xiangpei, W. Analysis of Meitan Cuiya Tea Aroma Components by GC-MS 2018/02, 2018; Atlantis Press: Dordrecht, The Netherlands, 2018; pp. 559–563. [Google Scholar]
- Wang, G.; Wang, Y.; Li, J.; Zhou, Z.; Yang, S.; Wang, L.; Peng, J.; Jiao, H.; Sun, X. Analysis of floral differences in terpenoids between two genotypes of Jerusalem artichoke (Helianthus tuberosus L.) by HS-SPME-GC/MS-based on the metabolome and transcriptome. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100403. [Google Scholar] [CrossRef]
- Hazrati, S.; Lotfi, K.; Govahi, M.; Ebadi, M.T. A comparative study: Influence of various drying methods on essential oil components and biological properties of Stachys lavandulifolia. Food Sci. Nutr. 2021, 9, 2612–2619. [Google Scholar] [CrossRef]
- Schulz, H.; Özkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Daferera, D.; Polissiou, M.; Sokmen, A. Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J. Food Eng. 2005, 66, 447–454. [Google Scholar] [CrossRef]
- Chen, S.; Rui, R.; Wang, S.; He, X. Comparative analysis of the floral fragrance compounds of Panax notoginseng flowers under the Panax notoginseng-pinus agroforestry system using SPME-GC-MS. Molecules 2022, 27, 3565. [Google Scholar] [CrossRef]
- Hattori, S.; Takagaki, H.; Fujimori, T. Evaluation of Japanese green tea extract using GC/O with original aroma simultaneously input to the sniffing port method (OASIS). Food Sci. Technol. Res. 2003, 9, 350–352. [Google Scholar] [CrossRef][Green Version]
- Yang, P.; Song, H.; Lin, Y.; Guo, T.; Wang, L.; Granvogl, M.; Xu, Y. Differences of characteristic aroma compounds in Rougui tea leaves with different roasting temperatures analyzed by switchable GC-O-MS and GC × GC-O-MS and sensory evaluation. Food Funct. 2021, 12, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC × GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Liu, C.; Ma, L.; Li, J. Effects of glycosidase on glycoside-bound aroma compounds in grape and cherry juice. J. Food Sci. Technol. 2023, 60, 761–771. [Google Scholar] [CrossRef]
- Xu, W.; Tong, Y.; Tong, Q.; Liu, Y.; Wang, Z. Effects of different re-fermentation methods on the quality characteristics of kombucha beverages. J. Food Sci. Technol. 2023, 60, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Fang, S.; Wang, J.; Zhuo, C.; Luo, Y.; Yu, Y.; Li, L.; Wang, Y.; Deng, W.; Ning, J. Sensomics analysis of the effect of the withering method on the aroma components of Keemun black tea. Food Chem. 2022, 395, 133549. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.; Schwab, W.; Wan, X. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chem. 2021, 363, 130328. [Google Scholar] [CrossRef]
- Lv, H.; Zhong, Q.; Lin, Z.; Wang, L.; Tan, J.; Guo, L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC–olfactometry. Food Chem. 2012, 130, 1074–1081. [Google Scholar] [CrossRef]
- Matich, A.J.; Bunn, B.J.; Hunt, M.B. The enantiomeric composition of linalool and linalool oxide in the flowers of kiwifruit (Actinidia) species. Chirality 2010, 22, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.; Shao, C.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.; Tan, J.; Peng, Q.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Y.; Yang, J.; Wang, H.; Ding, Y.; Lei, P. Comprehensive profiling of volatile components in Taiping Houkui green tea. LWT 2022, 163, 113523. [Google Scholar] [CrossRef]
- Laurentin, A.; Edwards, C.A. A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal. Biochem. 2003, 315, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Huafu, W.; Xiaoqing, Y. Free and glycosidically bound monoterpene alcohols in Qimen black tea. Food Chem. 1996, 56, 395–398. [Google Scholar] [CrossRef]


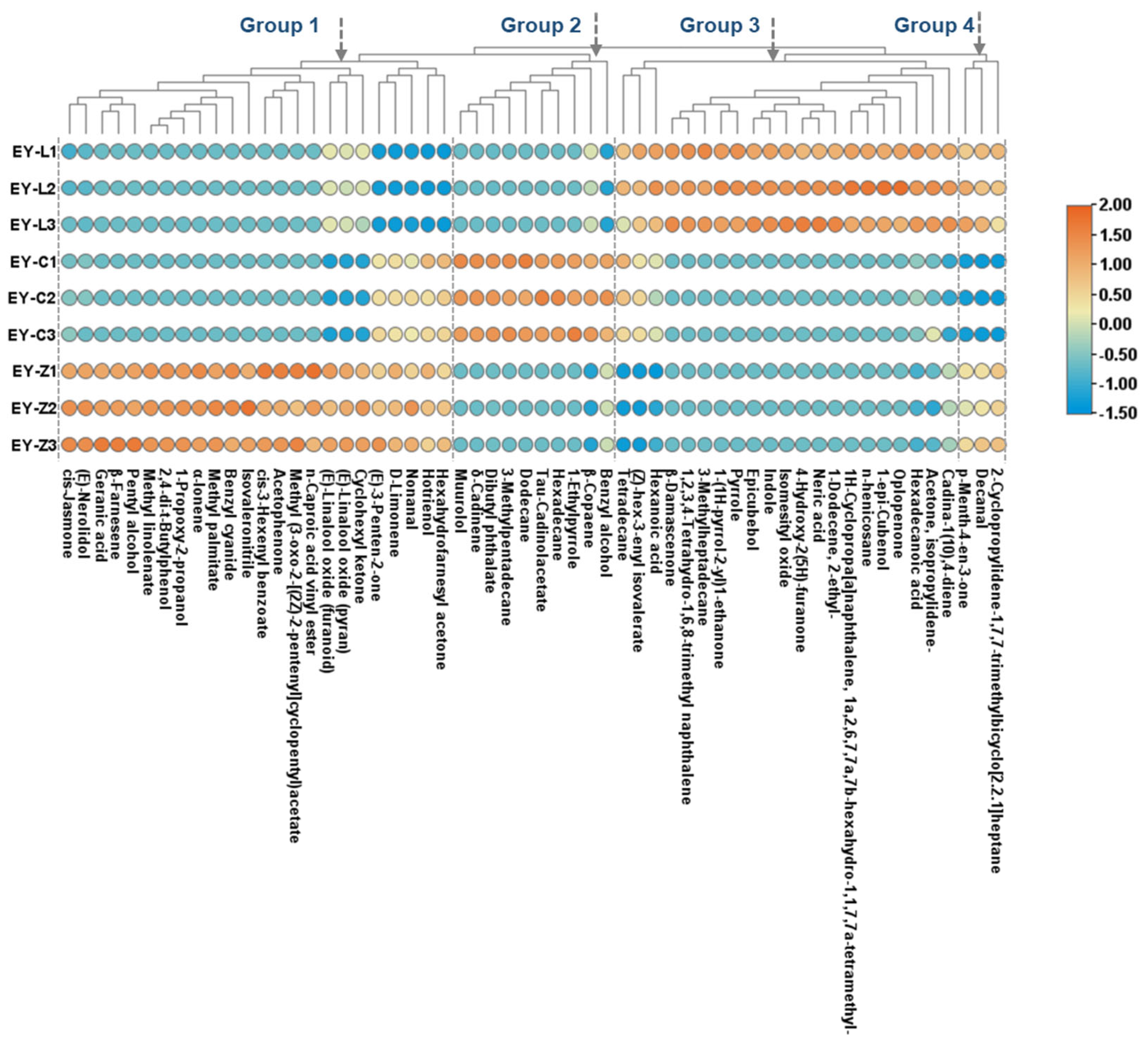
| Compound | Odor | Categories | EY-L1 | EY-L2 | EY-L3 | EY-C1 | EY-C2 | EY-C3 | EY-Z1 | EY-Z2 | EY-Z3 | CAS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Content | ||||||||||||||
| Clean and refreshing | D-Limonene | Citrus | Alkenes | / | / | / | 0.162 | 0.163 | 0.154 | 0.219 | 0.204 | 0.211 | 5989-27-5 | |
| Hexahydrofarnesyl acetone | Floral | Ketones | / | / | / | 0.025 | 0.022 | 0.021 | 0.020 | 0.023 | 0.024 | 502-69-2 | ||
| Hotrienol | Tropical | Alcohols | / | / | / | 0.037 | 0.028 | 0.030 | 0.038 | 0.035 | 0.031 | 29957-43-5 | ||
| Nonanal | Aldehydic | Aldehydes | / | / | / | 0.091 | 0.111 | 0.096 | 0.116 | 0.171 | 0.147 | 124-19-6 | ||
| (E)-3-Penten-2-one | / | Ketones | / | / | / | 0.011 | 0.012 | 0.013 | 0.013 | 0.015 | 0.019 | 3102-33-8 | ||
| Pure | Benzyl alcohol | Floral | Alcohols | / | / | / | 0.201 | 0.223 | 0.183 | 0.104 | 0.101 | 0.099 | 100-51-6 | |
| Dibutyl phthalate | Faint | Esters | 0.007 | 0.006 | 0.005 | 0.383 | 0.375 | 0.380 | 0.016 | 0.020 | 0.018 | 84-74-2 | ||
| δ-Cadinene | / | Alkenes | / | / | / | 0.043 | 0.042 | 0.039 | / | / | / | 483-76-1 | ||
| Muurolol | Herbal | Alcohols | / | / | / | 0.015 | 0.014 | 0.013 | / | / | / | 19435-97-3 | ||
| Hexadecane | / | Alkanes | / | / | / | 0.013 | 0.014 | 0.012 | / | / | / | 544-76-3 | ||
| 3-Methylpentadecane | / | Alkanes | / | / | / | 0.011 | 0.009 | 0.011 | / | / | / | 2882-96-4 | ||
| Tau-Cadinolacetate | / | Esters | / | / | / | 0.013 | 0.015 | 0.012 | / | / | / | 149197-48-8 | ||
| 1-Ethylpyrrole | Burnt | Aromatic heterocycles | / | / | / | 0.019 | 0.019 | 0.023 | / | / | / | 617-92-5 | ||
| Dodecane | / | Alkanes | / | / | / | 0.029 | 0.022 | 0.025 | / | / | / | 112-40-3 | ||
| β-Copaene | / | Alkenes | 0.009 | 0.007 | 0.008 | 0.015 | 0.017 | 0.017 | / | / | / | 18252-44-3 | ||
| Chestnut | Hexadecanoic acid | Waxy | Acids | 0.377 | 0.373 | 0.373 | 0.082 | 0.105 | 0.070 | / | / | / | 57-10-3 | |
| 1,2,3,4-Tetrahydro-1,6,8-trimethyl naphthalene | / | Arenes | 0.005 | 0.005 | 0.005 | / | / | / | / | / | / | 30316-36-0 | ||
| Indole | Animal | Aromatic heterocycles | 0.019 | 0.021 | 0.021 | / | / | / | / | / | / | 120-72-9 | ||
| Pyrrole | Nutty | Aromatic heterocycles | 0.011 | 0.011 | 0.010 | / | / | / | / | / | / | 109-97-7 | ||
| β-Damascenone | Floral | Ketones | 0.005 | 0.005 | 0.006 | / | / | / | / | / | / | 23726-93-4 | ||
| 1-(1H-pyrrol-2-yl)1-ethanone | Musty | Ketones | 0.120 | 0.138 | 0.116 | / | / | / | / | / | / | 1072-83-9 | ||
| 3-Methylheptadecane | / | Alkanes | 0.006 | 0.005 | 0.005 | / | / | / | / | / | / | 6418-44-6 | ||
| Epicubebol | / | Alcohols | 0.008 | 0.009 | 0.009 | / | / | / | / | / | / | 38230-60-3 | ||
| Isomesityl oxide | Vegetable | Ketones | 0.004 | 0.005 | 0.005 | / | / | / | / | / | / | 673542 | ||
| (4E)-4-Hexenyl hexanoate | / | Esters | 0.023 | 0.025 | 0.021 | 0.016 | 0.015 | 0.016 | 0.007 | 0.009 | 0.010 | / | ||
| 1-Dodecene, 2-ethyl- | / | Alkenes | 0.004 | 0.005 | 0.005 | / | / | / | / | / | / | 19780-34-8 | ||
| 1H-Cyclopropa[a]naphthalene, 1a,2,6,7,7a,7b-hexahydro-1,1,7,7a-tetramethyl- | / | Alkenes | 0.009 | 0.012 | 0.008 | / | / | / | / | / | / | 154098-14-3 | ||
| Neric acid | Green | Acids | 0.014 | 0.018 | 0.020 | / | / | / | / | / | / | 4613-38-1 | ||
| 4-Hydroxy-2(5H)-furanone | / | Lactones | 0.027 | 0.036 | 0.040 | / | / | / | / | / | / | 541-57-1 | ||
| 1-epi-Cubenol | / | Alcohols | 0.009 | 0.013 | 0.009 | / | / | / | / | / | / | 19912-67-5 | ||
| Oplopenone | / | Ketones | 0.007 | 0.009 | 0.006 | / | / | / | / | / | / | 28305-60-4 | ||
| Isobutenyl methyl ketone | Vegetable | Ketones | 0.014 | 0.015 | 0.014 | 0.010 | 0.010 | 0.012 | 0.010 | 0.009 | 0.009 | 141-79-7 | ||
| Cadina-1(10),4-diene | / | Alkenes | 0.021 | 0.024 | 0.025 | / | / | / | 0.010 | 0.009 | 0.008 | 16729-01-4 | ||
| p-Menth-4-en-3-one | / | Ketones | 0.014 | 0.018 | 0.019 | / | / | / | 0.012 | 0.010 | 0.013 | 5113-66-6 | ||
| Fresh | (E)-Nerolidol | Floral | Alcohols | 0.007 | 0.007 | 0.008 | 0.015 | 0.016 | 0.010 | 0.068 | 0.077 | 0.077 | 40716-66-3 | |
| cis-Jasmone | Floral | Ketones | 0.027 | 0.029 | 0.031 | 0.032 | 0.034 | 0.035 | 0.058 | 0.062 | 0.063 | 488-10-8 | ||
| 2,4-di-t-Butylphenol | / | Phenols | / | / | / | / | / | / | 0.006 | 0.006 | 0.006 | 96-76-4 | ||
| Methyl linolenate | Oily | Esters | / | / | / | / | / | / | 0.387 | 0.383 | 0.383 | 301-00-8 | ||
| 1-Propoxy-2-propanol | Mild Ether | Alcohols | / | / | / | / | / | / | 0.039 | 0.041 | 0.041 | 1569-01-3 | ||
| Ionene | / | Arenes | / | / | / | / | / | / | 0.005 | 0.005 | 0.004 | 475-03-6 | ||
| Methyl palmitate | Waxy | Esters | / | / | / | / | / | / | 0.014 | 0.017 | 0.016 | 112-39-0 | ||
| Benzyl cyanide | Aromatic | Nitriles | / | / | / | / | / | / | 0.022 | 0.022 | 0.017 | 140-29-4 | ||
| β-Farnesene | Woody | Alkenes | / | / | / | / | / | / | 0.006 | 0.006 | 0.008 | 18794-84-8 | ||
| Acetophenone | Floral | Ketones | / | / | / | / | / | / | 0.022 | 0.016 | 0.019 | 98-86-2 | ||
| Geranic acid | Green | Acids | / | / | / | / | / | / | 0.019 | 0.021 | 0.025 | 459-80-3 | ||
| Pentyl alcohol | Fermented | Alcohols | / | / | / | / | / | / | 0.035 | 0.034 | 0.045 | 71-41-0 | ||
| cis-3-Hexenyl benzoate | Green | Esters | / | / | / | / | / | / | 0.008 | 0.006 | 0.007 | 25152-85-6 | ||
| Isovaleronitrile | / | Nitriles | / | / | / | / | / | / | 0.017 | 0.025 | 0.019 | 625-28-5 | ||
| n-Caproic acid vinyl ester | / | Esters | / | / | / | / | / | / | 0.008 | 0.006 | 0.005 | 3050-69-9 | ||
| Methyl (3-oxo-2-[(2Z)-2-pentenyl]cyclopentyl)acetate | Floral | Esters | / | / | / | / | / | / | 0.011 | 0.007 | 0.010 | 42536-97-0 | ||
| (E)-Linalool oxide (pyran) | / | Alcohols | 0.062 | 0.057 | 0.060 | / | / | / | 0.112 | 0.109 | 0.120 | 39028-58-5 | ||
| Decanal | Aldehydic | Aldehydes | 0.006 | 0.005 | 0.006 | / | / | / | 0.004 | 0.004 | 0.005 | 112-31-2 | ||
| Cyclohexyl ketone | Minty | Ketones | 0.010 | 0.010 | 0.008 | / | / | / | 0.016 | 0.020 | 0.019 | 119-60-8 | ||
| (E)-Linalool oxide (furanoid) | Floral | Alcohols | 0.017 | 0.016 | 0.016 | / | / | / | 0.030 | 0.024 | 0.029 | 34995-77-2 | ||
| 2-Cyclopropylidene-1,7,7-trimethylbicyclo[2.2.1]heptane | / | Alkenes | 0.008 | 0.007 | 0.006 | / | / | / | 0.007 | 0.006 | 0.007 | / | ||
| Compound | Categories | EY-L1 | EY-L2 | EY-L3 | EY-C1 | EY-C2 | EY-C3 | EY-Z1 | EY-Z2 | EY-Z3 | CAS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/g) | ||||||||||||
| Mellow | L-Aspartic acid | Amino acids | 3.355 | 3.737 | 3.700 | 4.022 | 3.622 | 4.423 | 2.835 | 2.707 | 2.833 | 56-84-8 |
| L-Asparagine | Amino acids | 1.342 | 1.359 | 1.325 | 2.461 | 2.202 | 2.719 | 0.814 | 0.784 | 0.800 | 70-47-3 | |
| L-Tyrosine | Amino acids | 0.364 | 0.512 | 0.564 | 0.291 | 0.454 | 0.372 | 0.276 | 0.250 | 0.257 | 60-18-4 | |
| L-Valine | Amino acids | 0.517 | 0.519 | 0.505 | 0.276 | 0.579 | 0.427 | 0.338 | 0.302 | 0.314 | 72-18-4 | |
| EGC | Catechins | 32.634 | 23.338 | 28.698 | 44.391 | 46.860 | 41.922 | 22.465 | 22.162 | 19.816 | 970-74-1 | |
| EC | Catechins | 5.672 | 5.072 | 6.045 | 7.989 | 8.335 | 7.644 | 4.951 | 4.846 | 4.312 | 490-46-0 | |
| ECG | Catechins | 20.731 | 18.752 | 22.710 | 14.956 | 19.367 | 17.162 | 5.400 | 5.204 | 4.455 | 1257-08-5 | |
| Astringent | Gallic acid | Acids | 0.854 | 1.054 | 1.247 | 0.864 | 1.215 | 1.188 | 1.855 | 1.859 | 1.690 | 149-91-7 |
| Theobromine | Alkaloids | 2.454 | 3.406 | 4.016 | 2.720 | 3.540 | 3.175 | 7.887 | 7.990 | 7.266 | 83-67-0 | |
| Kokumi | L-Methionine | Amino acids | 0.552 | 0.539 | 0.526 | 0.316 | 0.369 | 0.342 | 0.533 | 0.355 | 0.381 | 63-68-3 |
| L-Lysine | Amino acids | 0.544 | 0.557 | 0.531 | 0.209 | 0.490 | 0.349 | 0.400 | 0.297 | 0.354 | 56-87-1 | |
| GCG | Catechins | 4.676 | 4.259 | 5.094 | 3.451 | 3.746 | 3.467 | 3.827 | 3.913 | 3.543 | 4233-96-9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Shen, Y.; Hu, B.; Ye, H.; Guo, H.; Chu, Q.; Chen, P. Decoding the Chemical Signatures and Sensory Profiles of Enshi Yulu: Insights from Diverse Tea Cultivars. Plants 2023, 12, 3707. https://doi.org/10.3390/plants12213707
Guo Y, Shen Y, Hu B, Ye H, Guo H, Chu Q, Chen P. Decoding the Chemical Signatures and Sensory Profiles of Enshi Yulu: Insights from Diverse Tea Cultivars. Plants. 2023; 12(21):3707. https://doi.org/10.3390/plants12213707
Chicago/Turabian StyleGuo, Yating, Yili Shen, Boya Hu, Huichun Ye, Haowei Guo, Qiang Chu, and Ping Chen. 2023. "Decoding the Chemical Signatures and Sensory Profiles of Enshi Yulu: Insights from Diverse Tea Cultivars" Plants 12, no. 21: 3707. https://doi.org/10.3390/plants12213707
APA StyleGuo, Y., Shen, Y., Hu, B., Ye, H., Guo, H., Chu, Q., & Chen, P. (2023). Decoding the Chemical Signatures and Sensory Profiles of Enshi Yulu: Insights from Diverse Tea Cultivars. Plants, 12(21), 3707. https://doi.org/10.3390/plants12213707







