Soybean Seed Sugars: A Role in the Mechanism of Resistance to Charcoal Rot and Potential Use as Biomarkers in Selection
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Sucrose, Raffinose, and Stachyose Analysis
2.3. Glucose and Fructose Analysis
2.4. Experimental Design and Statistical Analysis
3. Results
3.1. Analysis of Variance (ANOVA)
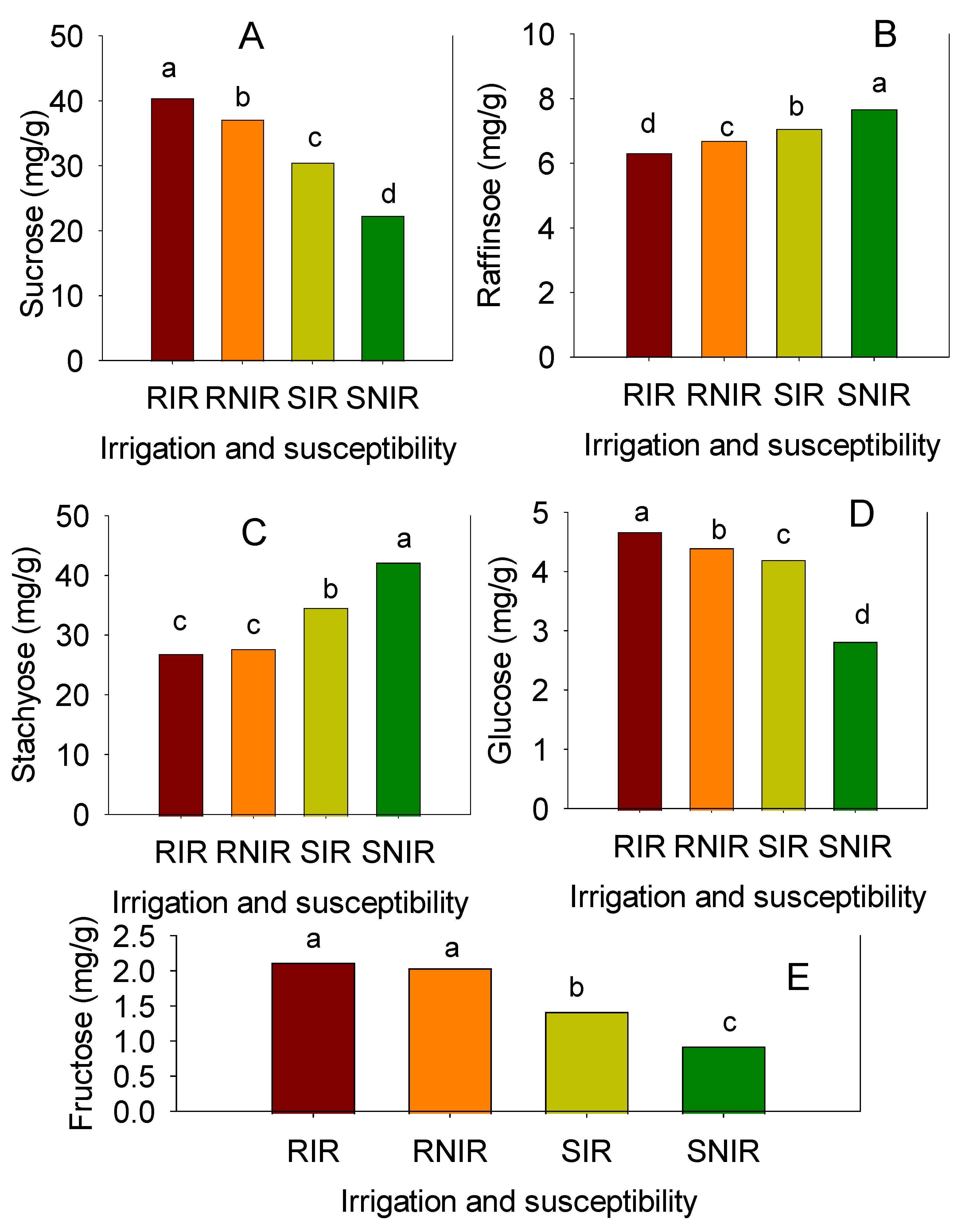
3.2. Mean Values of Sugars
3.3. Correlations between Sugars
4. Discussion
4.1. General Discussion
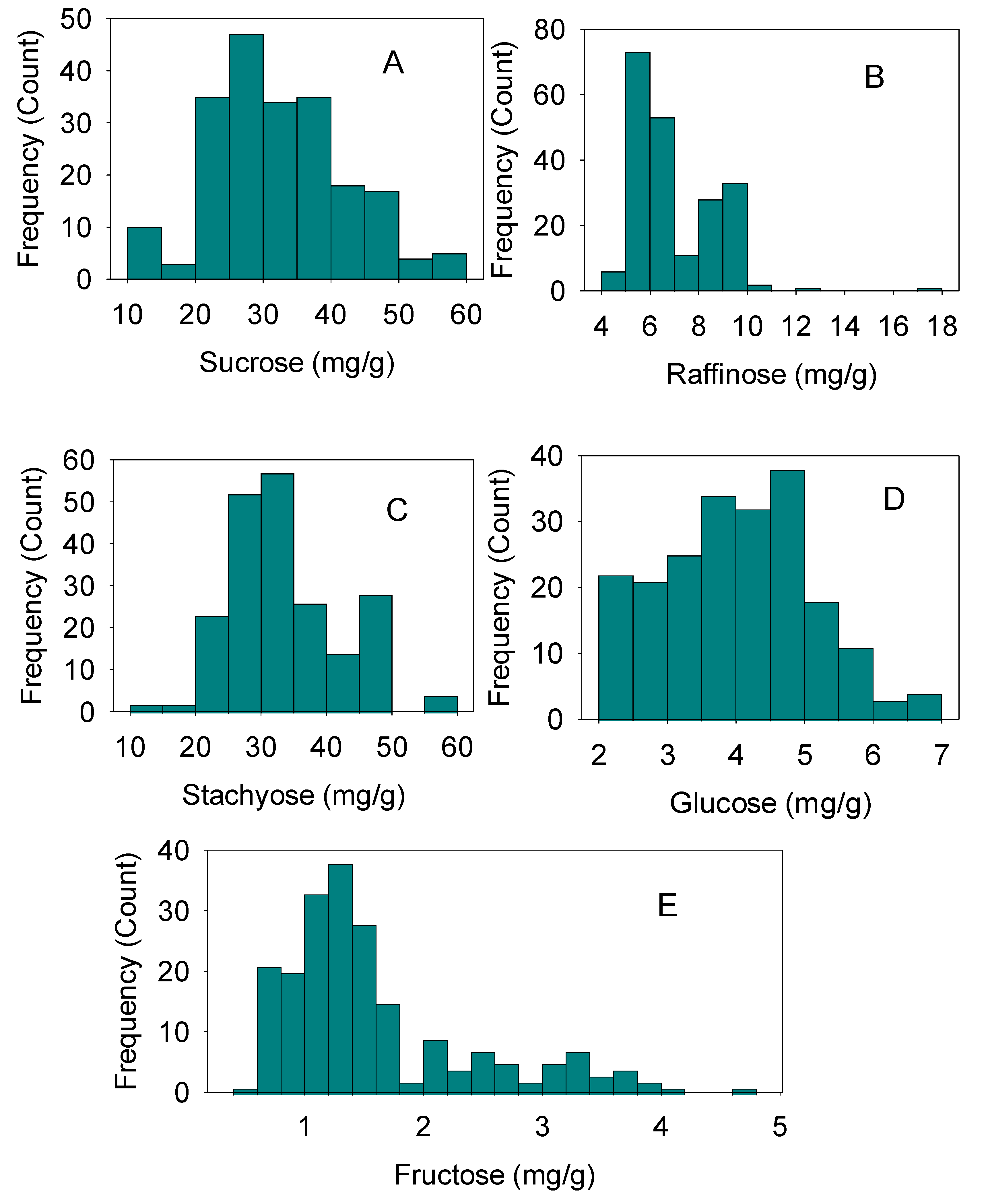
4.2. Correlations between Sugars, and Sugar Frequencies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hymowitz, T.; Collins, F.I. Variability of sugar content of seed of Glycine max (L.) Merr. and G. soja Serb. and Zucco. Agron. J. 1974, 66, 239–240. [Google Scholar] [CrossRef]
- Hou, A.; Chen, P.; Alloatti, J.; Li, D.; Mozzoni, L.; Zhang, B.; Shi, A. Genetic Variability of Seed Sugar Content in Worldwide Soybean Germplasm Collections. Crop Sci. 2009, 49, 903–912. [Google Scholar] [CrossRef]
- Bellaloui, N.; Smith, J.R.; Gillen, A.M.; Ray, J.D. Effect of Maturity on Seed Sugars as Measured on Near-Isogenic Soybean (Glycine max) Lines. Crop Sci. 2010, 50, 1978–1987. [Google Scholar] [CrossRef]
- Liu, K. Soybeans: Chemistry, Technology and Utilization; Springer US: New York, NY, USA, 1997. [Google Scholar]
- Wilson, L.A. Soy foods. In Practical Handbook of Soybean Processing and Utilization, 1st ed.; Erickson, D.R., Ed.; AOCS Press: Urbana, IL, USA, 1995; pp. 428–459. [Google Scholar]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Fisher, D.K.; Mengistu, A. Effects of Harvest-Aids on Seed Nutrition in Soybean under Midsouth USA Conditions. Plants 2020, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.L.; Chen, P.; Zhang, J.; Florez-Palacios, L.; Zeng, A.; Wang, X.; Bowen, R.A.; Miller, A.; Berry, H. Genetic analysis of sugar composition and its relationship with protein, oil, and fiber in soybean. Crop Sci. 2018, 58, 2413–2421. [Google Scholar] [CrossRef]
- Obendorf, R.L.; Horbowicz, M.; Dickerman, A.M.; Brenac, P.; Smith, M.E. Soluble Oligosaccharides and Galactosyl Cyclitols in Maturing Soybean Seeds In Planta and In Vitro. Crop Sci. 1998, 38, 78–84. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Wang, L.; Liu, X.; Liu, Y.; Phillips, J.; Deng, X. A WRKY transcription factor participates in dehydration tolerance in Boea hygrometrica by binding to the W-box elements of the galactinol synthase (BhGolS1) promoter. Planta 2009, 230, 1155–1166. [Google Scholar] [CrossRef]
- Zuther, E.; Büchel, K.; Hundertmark, M.; Stitt, M.; Hincha, D.K.; Heyer, A.G. The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett. 2004, 576, 169–173. [Google Scholar] [CrossRef]
- Koster, K.; Leopold, A.C. Sugars and Desiccation Tolerance in Seeds. Plant Physiol. 1988, 88, 829–832. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Elsayed, A.I.; Rafudeen, M.S.; Golldack, D. Physiological aspects of raffinose family oligosaccharides in plants: Protection against abiotic stress. Plant Biol. 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, T.D.; Scott, D.H. Charcoal rot of soybean: Current status. In Soybean diseases of the North Central Region; Amer Phytopathological Society: St. Paul, MN, USA, 1988; pp. 106–113. [Google Scholar]
- Mengistu, A.; Ray, J.D.; Smith, J.R.; Arelli, P.R.; Bellaloui, N.; Chen, P.; Shannon, G.; Boykin, D. Effect of charcoal rot on selected putative drought tolerant soybean genotypes and yield. Crop Prot. 2018, 105, 90–101. [Google Scholar] [CrossRef]
- Coser, S.M.; Reddy, R.V.C.; Zhang, J.; Mueller, D.S.; Mengistu, A.; Wise, K.A.; Allen, T.W.; Singh, A.; Singh, A.K. Genetic Architecture of Charcoal Rot (Macrophomina phaseolina) Resistance in Soybean Revealed Using a Diverse Panel. Front. Plant Sci. 2017, 8, 1626. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Dhillon, G.S.; Brar, S.K.; Vallad, G.E.; Chand, R.; Chauhan, V.B. Emerging phytopathogen Macrophomina phaseolina: Biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 2012, 38, 136–151. [Google Scholar] [CrossRef]
- Mengistu, A.; Wrather, A.; Rupe, J.C. Charcoal rot. In Compendium of Soybean Diseases and Pests; Hartman, G.L., Rupe, J.C., Sikora, E.F., Domier, L.L., Davis, J.A., Steffey, K.L., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2015; pp. 67–69. [Google Scholar]
- Bellaloui, N.; Mengistu, A.; Smith, J.R.; Abbas, H.K.; Accinelli, C.; Shier, W.T. Effects of Charcoal Rot on Soybean Seed Composition in Soybean Genotypes That Differ in Charcoal Rot Resistance under Irrigated and Non-Irrigated Conditions. Plants 2021, 10, 1801. [Google Scholar] [CrossRef] [PubMed]
- Radwan, O.; Rouhana, L.V.; Hartman, G.L.; Korban, S.S. Genetic Mechanisms of Host–Pathogen Interactions for Charcoal Rot in Soybean. Plant Mol. Biol. Rep. 2014, 32, 617–629. [Google Scholar] [CrossRef]
- Khaledi, N.; Taheri, P. Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina phaseolina. J. Plant Prot. Res. 2016, 56, 21–31. [Google Scholar] [CrossRef]
- Guo, J. Evaluations of Soybean Genotypes for Drought Tolerance and Charcoal Rot Resistance. Ph.D. Thesis, University of Illinois Urbana-Champaign, Champaign, IL, USA, 2018. [Google Scholar]
- Paris, R.L.; Mengistu, A.; Tyler, J.M.; Smith, J.R. Registration of Soybean Germplasm Line DT97–4290 with Moderate Resistance to Charcoal Rot. Crop Sci. 2006, 46, 2324. [Google Scholar] [CrossRef]
- Mengistu, A.; Arelli, P.; Bond, J.; Nelson, R.; Rupe, J.; Shannon, G.; Wrather, A. Identification of Soybean Accessions Resistant to Macrophomina phaseolina by Field Screening and Laboratory Validation. Plant Health Prog. 2013, 14, 1. [Google Scholar] [CrossRef]
- Pawlowski, M.L.; Hill, C.B.; Hartman, G.L. Resistance to Charcoal Rot Identified in Ancestral Soybean Germplasm. Crop Sci. 2015, 55, 1230–1235. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Spec. Rep. 80; Iowa State University of Science and Technology: Ames, IA, USA, 1977; p. 11. [Google Scholar]
- Bellaloui, N.; Smith, J.R.; Mengistu, A. Seed nutrition and quality, seed coat boron and lignin are influenced by delayed harvest in exotically-derived soybean breeding lines under high heat. Front. Plant Sci. 2017, 8, 1563. [Google Scholar] [CrossRef]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. Statistical Analysis Systems (SAS); SAS Institute: Cary, NC, USA, 2002–2012. [Google Scholar]
- Romero Luna, M.P.; Mueller, D.; Mengistu, A.; Singh, A.K.; Hartman, G.L.; Wise, K.A. Advancing Our Understanding of Charcoal Rot in Soybeans. J. Integr. Pest Manag. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- da Silva, M.P. Genetic and Phytopathological Studies on Charcoal Rot Resistance in Soybean [Glycine max (L) Merr.]. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2018. Available online: https://scholarworks.uark.edu/etd/2654 (accessed on 16 August 2022).
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 1995; pp. 379–396. [Google Scholar]
- Graham, N.; Graham, M.Y. Gyceollinelicitors induce major but distinctly different shifts in isoflavonoid metabolism in proximal and distal soybean cell populations. Mol. Plant. Microbe Interact. 1991, 4, 60–68. [Google Scholar] [CrossRef]
- Spann, T.M.; Schumann, A.W. Mineral Nutrition Contributes to Plant Disease and Pest Resistance; HS1181, One of a Series of the Horticultural Sciences Department; University of Florida/IFAS Extension: Gainesville, FL, USA, 2013. [Google Scholar]
- Bellaloui, N.; Mengistu, A.; Zobiole, L.H.S.; Shier, W.T. Resistance to toxin-mediated fungal infection: Role of lignins, isoflavones, other seed phenolics, sugars, and boron in the mechanism of resistance to charcoal rot disease in soybean. Toxin Rev. 2012, 31, 16–26. [Google Scholar] [CrossRef]
- Islam, S.; Haque, S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.M.; Hossain, Z.; Ahmed, B.; Rahim, S.; Rahman, S.; et al. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Heiler, S.; Mendgen, K.; Deising, H. Cellulolytic enzymes of the obligately biotrophic rust fungus Uromyces viciae-fabae are regulated differentiation-specifically. Mycol. Res. 1993, 97, 77–85. [Google Scholar] [CrossRef]
- Amadioha, A.C. The production and activity of extracellular amylase by Rhizoctonia bataticola. Arch. Phytopathol. Plant Prot. 2000, 33, 1–9. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Siddiqui, K.A.; Ali, E. Phytotoxic metabolites of Macrophomina phaseolina. Indian J. Mycol. Plant Pathol. 1992, 22, 54–57. [Google Scholar]
- Shier, W.T.; Abbas, H.K.; Baird, R.E.; Sciumbato, G.L. (-)-Botryodiplodin, a unique ribose-analog toxin. Toxin Reviews. 2007, 26, 343–386. [Google Scholar] [CrossRef]
- Abbas, H.K.; Bellaloui, N.; Butler, A.M.; Nelson, J.L.; Abou-Karam, M.; Shier, W.T. Phytotoxic Responses of Soybean (Glycine max L.) to Botryodiplodin, a Toxin Produced by the Charcoal Rot Disease Fungus, Macrophomina phaseolina. Toxins 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Bellaloui, N.; Accinelli, C.; Smith, J.R.; Shier, W.T. Toxin production in soybean (Glycine max L.) plants with charcoal rot disease and by Macrophomina phaseolina, the fungus that causes the disease. Toxins 2019, 11, 645. [Google Scholar] [CrossRef]
- Ospina-Giraldo, M.D.; Griffith, J.G.; Laird, E.W.; Mingora, C. The CAZyome of Phytophthora spp.: A comprehensive analysis of the gene complement coding for carbohydrate-active enzymes in species of the genus Phytophthora. BMC Genom. 2010, 11, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.A.I.; Gupta, A.K.; Paul, A.K.; Banerjee, A.K. Purification and properties of a heat-resistant exotoxin produced by Macrophomina phaseolina (Tassi) Goid in culture. Cell Mol. Life Sci. 1979, 35, 1222–1223. [Google Scholar] [CrossRef] [PubMed]
- Dhar, T.K.; Siddiqui, K.A.I.; Ali, E. Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Lett. 1982, 23, 5459–5462. [Google Scholar]
- Mahato, S.B.; Siddiqui, K.A.I.; Bhattacharya, G.; Ghosal, T.; Miyahara, K.; Sholichin, M.; Kawasaki, T. Structure and Stereochemistry of Phaseolinic Acid: A New Acid from Macrophomina phaseolina. J. Nat. Prod. 1987, 50, 245–247. [Google Scholar] [CrossRef]
- Ramezani, M.; Shier, W.T.; Abbas, H.K.; Tonos, J.L.; Baird, R.E.; Sciumbato, G.L. Soybean Charcoal Rot Disease Fungus Macrophomina phaseolina in Mississippi Produces the Phytotoxin (−)-Botryodiplodin but No Detectable Phaseolinone. J. Nat. Prod. 2007, 70, 128–129. [Google Scholar] [CrossRef]
- Rogers, L.A.; Campbell, M.M. The genetic control of lignin deposition during plant growth and development. New Phytol. 2004, 164, 17–30. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and Biotic Stresses and Changes in the Lignin Content and Composition in Plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Sederoff, R.R. Variation in Lignin Content and Composition (Mechanisms of Control and Implications for the Genetic Improvement of Plants). Plant Physiol. 1996, 110, 3–13. [Google Scholar] [CrossRef]
- Capeleti, I.; Bonini, E.A.; Ferrarese, M.D.L.L.; Teixeira, A.C.N.; Krzyzanowski, F.C.; Ferrarese-Filho, O. Lignin content and peroxidase activity in soybean seed coat susceptible and resistant to mechanical damage. Acta Physiol. Plant. 2005, 27, 103–108. [Google Scholar] [CrossRef]
- Dixon, R.A.; Steele, C.L. Flavonoids and isoflavonoids–A gold mine for metabolic engineering. Trends Plant Sci. 1999, 4, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.K.; Ohlrogge, J.B.; Shachar-Hill, Y. The role of light in soybean seed filling metabolism. Plant J. 2009, 58, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.; A Aznar-Moreno, J.; Bailey, S.R.; Arp, J.J.; Chu, K.L.; Bilyeu, K.D.; Durrett, T.P.; Allen, D.K. Temporal changes in metabolism late in seed development affect biomass composition. Plant Physiol. 2021, 186, 874–890. [Google Scholar] [CrossRef] [PubMed]
- Bellaloui, N.; Smith, J.R.; Ray, J.D.; Mengistu, A.; Gillen, A.M.; Fisher, D.K.; Singh, G. Responses of seed yield, quality, and composition to the harvest-aid paraquat in soybean grown in Mississippi. Agrosystems Geosci. Environ. 2022, 5, e20262. [Google Scholar] [CrossRef]
- Collakova, E.; Aghamirzaie, D.; Fang, Y.; Klumas, C.; Tabataba, F.; Kakumanu, A.; Myers, E.; Heath, L.S.; Grene, R. Metabolic and Transcriptional Reprogramming in Developing Soybean (Glycine max) Embryos. Metabolites 2013, 3, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hur, M.; Lee, J.Y.; Zhou, W.; Song, Z.; Ransom, N.; Demirkale, C.Y.; Nettleton, D.; Westgate, M.; Arendsee, Z.; et al. A systems biology approach toward understanding seed composition in soybean. BMC Genom. 2015, 16, S9. [Google Scholar] [CrossRef]

| Genotype | Maturity Group | Resistance/Susceptibility to Charcoal Rot |
|---|---|---|
| DS-880 | MG V | Moderately Resistant |
| DT97-4290 | MG IV | Moderately Resistant |
| R07-7232 | MG V | Moderately Resistant |
| USG 75Z38 | MG V | Moderately Resistant |
| USG Allen | MG V | Moderately Resistant |
| Osage | MG V | Moderately Resistant |
| Dyna-Gro 36C44 | MG IV | Susceptible |
| Progeny 4408 | MG IV | Susceptible |
| R01-581F | MG V | Susceptible |
| R02-1325 | MG V | Susceptible |
| Trisoy 4788 | MG IV | Susceptible |
| LS98-0358 | MG IV | Susceptible |
| Pharaoh | MG IV | Susceptible |
| 2012 | 2013 | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Irrigated | Non-Irrigated | Irrigated | Non-Irrigated | ||||
| DS-880 | 1572 | cd * | 722 | gf | 1409 | e | 3175 | dc |
| DT97-4290 | 212 | e | 590 | g | 1350 | e | 1278 | d |
| R07-7232 (R07) | 1364 | cd | 1818 | egf | 1976 | e | 5731 | c |
| USG 75Z38 (USG75) | 1158 | cde | 2459 | edf | 1643 | e | 3062 | dc |
| USG Allen (USGAl) | 567 | ed | 986 | gf | 2257 | ed | 3826 | c |
| Osage | 2249 | bcd | 4787 | edc | 1953 | e | 3833 | c |
| Dyna-Gro 36C44 (Dayna) | 11,427 | ab | 39,704 | ba | 7555 | bc | 43,326 | a |
| Progeny 4408 (P4408) | 1171 | cde | 10,770 | bc | 6206 | bcd | 24,428 | ba |
| R01-581F (R01) | 5195 | abc | 10,431 | bc | 25,879 | a | 30,004 | ba |
| R02-1325 (R02) | 3634 | abc | 12,694 | bac | 3491 | ecd | 39,340 | a |
| Trisoy 4788 (T4) | 3104 | bcd | 9438 | dc | 3730 | ecd | 15,142 | b |
| LS98-0358 (LS98) | 6635 | abc | 37,097 | ba | 15,528 | ba | 42,328 | a |
| Pharaoh | 18,905 | a | 43,547 | a | 17,633 | ba | 32,183 | ba |
| Sucrose | Raffinose | Stachyose | Glucose | Fructose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | DF | F | p | F | p | F | p | F | p | F | p |
| Year (Y) | 1 | 44.17 | *** | 72.34 | *** | 0.48 | ns | 0.06 | ns | 190.57 | *** |
| Genotype (G) | 12 | 12.59 | ** | 2.97 | ns | 16.43 | *** | 6.32 | *** | 23.93 | *** |
| Y*G | 12 | 4.18 | ns | 2.18 | ns | 1.38 | 0.18 | 5.27 | ** | 12.30 | *** |
| Irrigation (IR) | 1 | 32.70 | *** | 4.49 | ns | 28.94 | *** | 42.45 | *** | 26.78 | *** |
| Y*IR | 1 | 0.46 | ns | 1.46 | ns | 6.02 | * | 9.50 | ** | 2.26 | ns |
| G*IR | 12 | 0.79 | ns | 0.31 | ns | 1.53 | ns | 2.31 | ns | 1.27 | ns |
| Y*G*IR | 12 | 0.16 | ns | 0.27 | ns | 0.99 | ns | 0.59 | ns | 0.72 | ns |
| Residuals | 28.51 | 0.18 | 35.94 | 0.38 | 0.15 |
| IR | 2012 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | DS- 880 | DT97-4290 | Dyna | Osage | R07 | USG75 | USGAl | MR Mean | LS98 | Pharaoh | P4408 | R01 | R02 | T4 | S Mean | LSD * |
| Resistance | MR | MR | MR | MR | MR | MR | MR | S | S | S | S | S | S | |||
| Sugars | ||||||||||||||||
| Suc | 37.85 | 30.38 | 31.50 | 30.70 | 39.83 | 29.23 | 37.73 | 33.89 | 30.58 | 24.85 | 32.78 | 35.38 | 27.68 | 27.30 | 29.76 | 1.90 |
| Raff | 5.27 | 5.25 | 6.29 | 5.48 | 5.55 | 5.50 | 5.55 | 5.56 | 6.88 | 6.13 | 6.13 | 5.75 | 6.28 | 6.55 | 6.29 | 0.31 |
| Stac | 25.38 | 25.98 | 42.10 | 26.53 | 28.80 | 25.28 | 27.75 | 28.83 | 40.88 | 37.15 | 31.90 | 33.75 | 37.20 | 31.88 | 35.46 | 2.50 |
| Glu | 3.92 | 4.21 | 4.28 | 4.73 | 4.50 | 4.09 | 4.81 | 4.36 | 4.70 | 4.48 | 5.62 | 4.82 | 5.00 | 4.71 | 4.89 | 0.35 |
| Fru | 1.64 | 1.48 | 1.44 | 1.56 | 1.56 | 1.47 | 1.55 | 1.53 | 1.35 | 1.26 | 1.21 | 1.15 | 1.28 | 1.40 | 1.28 | 0.07 |
| NIR | 2012 | |||||||||||||||
| Genotype | DS-880 | DT97-4290 | Dyna | Osage | R07 | USG75 | USGAl | MR mean | LS98 | Pharaoh | P4408 | R01 | R02 | T4 | S mean | LSD * |
| Resistance | MR | MR | MR | MR | MR | MR | MR | S | S | S | S | S | S | |||
| Sugars | ||||||||||||||||
| Suc | 31.85 | 27.25 | 19.95 | 28.00 | 36.38 | 24.60 | 34.73 | 28.97 | 20.80 | 17.53 | 24.05 | 19.60 | 21.30 | 22.30 | 20.93 | 2.87 |
| Raff | 5.50 | 5.57 | 6.44 | 5.75 | 5.85 | 5.73 | 5.07 | 5.70 | 6.15 | 6.30 | 6.42 | 6.61 | 6.65 | 7.33 | 6.58 | 0.44 |
| Stac | 29.08 | 28.73 | 45.43 | 27.70 | 29.70 | 27.30 | 26.35 | 30.61 | 45.85 | 40.50 | 33.00 | 34.85 | 42.65 | 35.05 | 38.65 | 2.67 |
| Glu | 3.43 | 3.86 | 2.53 | 3.92 | 3.92 | 3.55 | 4.48 | 3.67 | 2.90 | 2.61 | 2.82 | 2.73 | 2.46 | 4.88 | 3.07 | 0.30 |
| Fru | 1.35 | 1.21 | 1.03 | 1.25 | 1.24 | 1.13 | 1.16 | 1.20 | 0.84 | 0.93 | 0.73 | 0.78 | 0.93 | 0.79 | 0.83 | 0.08 |
| IR | 2013 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | DS-880 | DT97-4290 | Dyna | Osage | R07 | USG75 | USGAl | MR Mean | LS98 | Pharaoh | P4408 | R01 | R02 | T4 | S Mean | LSD * |
| Resistance | MR | MR | MR | MR | MR | MR | MR | S | S | S | S | S | S | |||
| Sugars | ||||||||||||||||
| Suc | 34.73 | 50.55 | 35.23 | 47.55 | 39.70 | 45.30 | 46.70 | 42.82 | 27.75 | 28.90 | 29.85 | 31.65 | 31.35 | 31.63 | 30.19 | 2.91 |
| Raff | 5.07 | 8.02 | 9.07 | 7.42 | 6.99 | 6.59 | 6.30 | 7.07 | 9.37 | 8.82 | 9.29 | 6.38 | 6.05 | 5.85 | 7.63 | 0.52 |
| Stac | 26.35 | 26.53 | 36.95 | 28.00 | 28.20 | 27.45 | 28.33 | 28.83 | 32.65 | 32.13 | 31.25 | 33.18 | 30.70 | 34.25 | 32.36 | 3.32 |
| Glu | 4.48 | 4.58 | 3.62 | 4.83 | 4.38 | 6.32 | 4.64 | 4.69 | 3.93 | 3.73 | 3.93 | 3.76 | 3.22 | 3.12 | 3.62 | 0.35 |
| Fru | 1.16 | 2.75 | 1.27 | 3.42 | 2.59 | 2.21 | 2.26 | 2.24 | 1.37 | 1.46 | 1.15 | 1.89 | 1.69 | 2.22 | 1.63 | 0.30 |
| NIR | 2013 | |||||||||||||||
| Genotype | DS-880 | DT97-4290 | Dyna | Osage | R07 | USG75 | USGAl | MR mean | LS98 | Pharaoh | P4408 | R01 | R02 | T4 | S mean | LSD * |
| Resistance | MR | MR | MR | MR | MR | MR | MR | S | S | S | S | S | S | |||
| Sugars | ||||||||||||||||
| Suc | 47.98 | 46.43 | 27.30 | 46.28 | 37.95 | 42.05 | 41.53 | 41.36 | 23.40 | 22.00 | 24.60 | 20.13 | 22.18 | 27.63 | 23.32 | 2.66 |
| Raff | 8.54 | 7.95 | 11.33 | 8.26 | 8.03 | 6.99 | 7.09 | 8.31 | 9.85 | 8.92 | 9.41 | 6.25 | 8.66 | 7.07 | 8.36 | 0.85 |
| Stac | 25.28 | 25.23 | 45.53 | 28.70 | 29.50 | 26.20 | 30.13 | 30.08 | 42.40 | 46.98 | 43.70 | 44.00 | 45.30 | 47.28 | 44.94 | 2.98 |
| Glu | 4.53 | 5.15 | 2.83 | 4.63 | 4.51 | 6.06 | 4.84 | 4.65 | 2.91 | 2.36 | 2.84 | 2.48 | 2.84 | 2.46 | 2.65 | 0.23 |
| Fru | 2.79 | 3.33 | 0.92 | 3.51 | 2.80 | 2.61 | 2.41 | 2.62 | 0.89 | 1.16 | 0.84 | 1.14 | 0.99 | 1.38 | 1.07 | 0.25 |
| 2012 | IR | 2012 | NIR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Suc | Raff | Stac | Glu | Suc | Raff | Stac | Glu | ||
| Raff | R = −0.27 | Raff | R = −0.33 | ||||||
| p = * | p = ** | ||||||||
| Stac | R = ns | 0.40 | Stac | R = −0.39 | 0.31 | ||||
| p = ns | *** | p = *** | * | ||||||
| Glu | R = ns | ns | ns | Glu | R = 0.40 | ns | −0.36 | ||
| p = ns | ns | ns | p = *** | ns | ** | ||||
| Fru | R = ns | ns | −0.41 | −0.27 | Fru | R = 0.42 | −0.42 | −0.41 | ns |
| p = ns | ns | *** | * | p = *** | *** | ** | ns | ||
| 2013 | IR | 2013 | NIR | ||||||
| Suc | Raff | Stac | Glu | Suc | Raff | Stac | Glu | ||
| Raff | R = ns | Raff | ns | ||||||
| p = ns | ns | ||||||||
| Stac | R = ns | ns | Stac | −0.76 | ns | ||||
| p = ns | ns | *** | ns | ||||||
| Glu | R = 0.56 | ns | ns | Glu | 0.74 | −0.29 | −0.78662 | ||
| p = *** | ns | ns | *** | * | *** | ||||
| Fru | R = 0.52 | −0.30 | −0.41 | 0.41 | Fru | 0.79 | ns | −0.72 | 0.73 |
| p = *** | * | ** | ** | *** | ns | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellaloui, N.; Mengistu, A.; Smith, J.R.; Abbas, H.K.; Accinelli, C.; Shier, W.T. Soybean Seed Sugars: A Role in the Mechanism of Resistance to Charcoal Rot and Potential Use as Biomarkers in Selection. Plants 2023, 12, 392. https://doi.org/10.3390/plants12020392
Bellaloui N, Mengistu A, Smith JR, Abbas HK, Accinelli C, Shier WT. Soybean Seed Sugars: A Role in the Mechanism of Resistance to Charcoal Rot and Potential Use as Biomarkers in Selection. Plants. 2023; 12(2):392. https://doi.org/10.3390/plants12020392
Chicago/Turabian StyleBellaloui, Nacer, Alemu Mengistu, James R. Smith, Hamed K. Abbas, Cesare Accinelli, and W. Thomas Shier. 2023. "Soybean Seed Sugars: A Role in the Mechanism of Resistance to Charcoal Rot and Potential Use as Biomarkers in Selection" Plants 12, no. 2: 392. https://doi.org/10.3390/plants12020392
APA StyleBellaloui, N., Mengistu, A., Smith, J. R., Abbas, H. K., Accinelli, C., & Shier, W. T. (2023). Soybean Seed Sugars: A Role in the Mechanism of Resistance to Charcoal Rot and Potential Use as Biomarkers in Selection. Plants, 12(2), 392. https://doi.org/10.3390/plants12020392







