Abstract
The cytochrome P450 (CYP450) monooxygenase superfamily, which is involved in the biosynthesis pathways of many primary and secondary metabolites, plays prominent roles in plant growth and development. However, systemic information about CYP450s in Brassica napus (BnCYP450) was previously undiscovered and their biological significance are far from understood. Members of clan 86 CYP450s, such as CYP704Bs, are essential for the formation of pollen exine in plant male reproduction, and the targeted mutagenesis of CYP704B genes has been used to create new male sterile lines in many crops. In the present study, a total of 687 BnCYP450 genes were identified in Brassica napus cultivar “Zhongshuang 11” (ZS11), which has nearly 2.8-fold as many CYP450 members as in Arabidopsis thaliana. It is rationally estimated since Brassica napus is a tetraploid oil plant with a larger genome compared with Arabidopsis thaliana. The BnCYP450 genes were divided into 47 subfamilies and clustered into nine clans. Phylogenetic relationship analysis reveals that CYP86 clan consists of four subfamilies and 109 BnCYP450s. Members of CYP86 clan genes display specific expression profiles in different tissues and in response to ABA and abiotic stresses. Two BnCYP450s within the CYP704 subfamily from CYP86 clan, BnCYP704B1a and BnCYP704B1b, display high similarity to MS26 (Male Sterility 26, also known as CYP704B1). These two BnCYP704B1 genes were specifically expressed in young buds. We then simultaneously knocked-out these two BnCYP704B1 genes through a clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) genome engineering system. The edited plants displayed a pollenless, sterile phenotype in mature anthers, suggesting that we successfully reproduced genic male sterility (GMS, also known as nuclear male sterility) lines in Brassica napus. This study provides a systemic view of BnCYP450s and offers a strategy to facilitate the commercial utility of the CRISPR/Cas9 system for the rapid generation of GMS in rapeseed via knocking-out GMS controlling genes.
1. Introduction
Rapeseed (Brassica napus L., also known as canola, genome AACC, 2n = 4x = 38) is one of the most important vegetable oil crops across the world and is an allotetraploid that developed through doubling of chromosomes after the hybridization between Brassica rapa (genome AA, 2n = 20) and Brassica oleracea (genome CC, 2n = 18) [1,2]. The widespread adoption of hybrid vigor has contributed significantly to rapid and widespread increases in rapeseed production over the past few decades. Since then, the use of several pollination control systems has been proposed to promote heterosis in rapeseed, including cytoplasmic male sterility (CMS), genic male sterility (GMS), self-incompatibility, and chemical-induced male sterility (CIMS) [3]. Compared with other approaches, the major advantage of GMS is that it brings about nearly complete male sterility to a hybrid breeding program.
There are several GMS control loci used in hybrid rapeseed production, such as the S45AB, 117AB, Oro, 7365AB, 9012AB, Rs1046AB, Yi3A, and 609A systems [4,5,6,7,8]. In some of these systems, the genes controlling the male sterility have been determined. For instance, the male sterility of S45A and 117A is controlled by two duplicate genes, BnMs1 and BnMs2 [7]. The male sterile phenotypes of 7365A and 9012A are coupled with the loss of the BnMs3 gene [5,6,8]. However, the main obstacle to using GMS remains the cross-breeding of the male sterility into new rapeseed varieties and there are only a few GMS lines available for conventional genetic manipulation.
Recently, targeted mutagenesis of GMS control genes has been used to create new male sterile lines in many crops [9,10,11,12,13,14,15,16,17,18]. One of these GMS genes is ZmMS26 (also known as ZmCYP704B1), which is a cytochrome P450-like gene first identified in maize [15,16,19]. ZmMS26 encodes long-chain fatty acid omega-hydroxylase required for sporopollenin biosynthesis and essential for pollen exine formation [11,15]. Since then, protein sequences with high homology to the ZmMS26 were identified, and targeted mutagenesis was performed to create new male sterile germplasms in many plants, such as maize, rice, sorghum, and wheat [11,14,15,16,20]. However, the rapid deployment of genic male sterile lines of rapeseed at the commercial breeding level derived from artificial manipulation remains controversial.
The cytochrome P450 (CYP450) monooxygenase superfamily is one of the largest enzymatic protein families of heme-thiolate proteins [21,22]. The CYP450s catalyze a great number of NADPH- and/or O2-dependent oxygenation/hydroxylation reactions in many organisms [21,23]. The CYP450 genes are widely identified in most organisms, including bacteria, plants, animals, and humans [21,22,23,24,25,26,27,28]. In plants, the nomenclature of CYP450 genes is generally classified into two groups: A type and non-A type [21,22]. The A type CYP450s are specific to plants; the non-A type is non-plant-specific CYP450s which display high sequence similarity to animals and fungal CYP450s [22,23]. According to the current evolutionary relationship, plant CYP450 subfamilies are grouped into different clans including five single-subfamily clans (clan 51, clan 710, clan 711, clan 74 and clan 97) and four multiple-subfamily clans (clan 72, clan 85, clan 86 and clan 71) [21,22,24]. The clan 71 was categorized into A type CYP450s, and the other eight clans were categorized into non-A type CYP450s [21,22,23].
A great number of CYP450 genes have crucial roles in the biosynthesis of metabolites with distinct biological significance [21,25]. Plenty of the clan 73, clan 84, and clan 98 CYP450 genes are involved in the phenylpropanoid biosynthetic pathway, results in producing a mass of phenol related compounds, i.e., flavonoids, suberin, lignin, and polyphenols [21,25]. Members of the CYP703, CYP704, and CYP86 subfamilies are responsible for the biosynthesis of pollen exine and anther cutin, which are known as fatty acid hydroxylase [7,29,30,31,32]. The CYP701A and CYP88A genes are necessary for gibberellic acid (GA) biosynthesis, which encode ent-kaurene oxidase and ent-kaurenoic acid oxidase respectively [24,33,34]. The CYP707A is involved in abscisic acid (ABA) 8′-hydroxylation, which play an important role in ABA catabolism [35,36]. The CYP450 enzymes of the CYP74 subfamily are essential for jasmonate (JA) synthesis, which function as allene oxide synthase (AOS) [24,37]. Members of the CYP90, CYP72, CYP92, CYP85, CYP734, and CYP724 subfamilies are necessary for brassinosteroid (BR) biosynthesis [21,38]. Besides, CYP450s also act in fighting against abiotic and biotic stresses [21].
In the current study, we systematically identified and analyzed the BnCYP450 genes in the rapeseed genome. In addition, we report the introduction of GMS into rapeseed by simultaneously knocking-out two anther-specific ZmMS26 homologs through gene editing using the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 (CRISPR-associated protein 9) system.
2. Results
2.1. Genome-Wide Identification of BnCYP450s
There are 687 putative BnCYP450s with conserved P450 domains isolated from the genome of Brassica napus cultivar “Zhongshuang 11” (ZS11), which is around 2.8 folds of AtCYP450s. This is not surprising since Brassica napus is tetraploid and Arabidopsis is diploid with a very small genome size. We found 318 and 367 BnCYP450 genes in the A and C subgenome respectively (Table 1). The BnCYP450s were renamed in terms of their sequences’ similarity to AtCYP450s (Table S1). The protein length of BnCYP450s was between 80 and 1971 amino acids (aa) with predicted molecular weight (Mw) in the range of 8.67–224 kDa. The theoretical isoelectric points (pI) of BnCYP450s ranged from 4.1 to 11.52 (Table S1). The pI of 547 BnCYP450s (79.6%) showed values greater than 7. Other characteristics of BnCYP450s, such as gene position and accession number, are presented in Table S1.

Table 1.
Enumeration of AtCYP450 and BnCYP450 gene superfamily.
2.2. Classification and Phylogenetic Analyses of BnCYP450 Proteins
We divided BnCYP450 proteins into 46 subfamilies according to the P450 nomenclature (Table S1). To explore the evolutionary relationships between AtCYP450s and BnCYP450s, an unrooted NJ tree with the 251 AtCYP450s and 687 BnCYP450s was constructed (Figure S1). Based on it, BnCYP450s were also categorized into nine clans. However, there were no members identified in CYP716 subfamily. The BnCYP450s were also classified into non-A type and A-type, which is consistent to previous evolutionary analysis [23]. The non-A type BnCYP450s consisted of eight clans with 27 subfamilies containing 275 members. The A-type BnCYP450s were all grouped into CYP71 clan with 19 subfamilies comprising 412 members (Table 1). The CYP721, CYP734, CYP718, CYP720, CYP701, CYP75, and CYP93 subfamilies all had only two members, while the largest subfamily CYP71 harbored 138 members.
2.3. Gene Duplication and Collinearity Analysis of the BnCYP450 Genes
To study the putative tandem and segmental duplication events, we determined the chromosomal distribution of BnCYP450 family members. In total, the isolated 687 BnCYP450 genes, except BnCYP81F4a and BnCYP94B2a, were widely distributed on 19 chromosomes (Table S1). BnCYP81F4a and BnCYP94B2a were anchored on HiCscaffold2150 and HiCscaffold2213 respectively. The distributing density of BnCYP450s on chromosomes was extremely uneven. A relatively high density was observed on chromosomes A09, C03 and C07 (more than 50 genes) while a relatively low density on chromosomes A07, A10 and C06 (fewer than 20 genes). We identified a total of 32 tandem duplication events and 608 segmental duplications in the Brassica napus genome according to the BLAST and MCScanX results (Figure 1 and Tables S2 and S3). Moreover, only nine segmental duplication events were observed within the same chromosome and five of them were observed on chromosomes C04, whereas 599 segmental duplications were detected across chromosomes (Table S3). Further analysis revealed that there are 88 duplication events located on the AA subgenome and 91 events on the CC subgenome, whereas there are 429 events occurred between AA and CC subgenomes (Tables S4 and S5). In terms of the U triangle, allopolyploidization may have key roles in the expansion of the BnCYP450s in Brassica napus. To ascertain the evolution of the BnCYP450 genes, we determined the synteny between Brassica napus and Arabidopsis thaliana at the whole genome level. A total of 458 collinear gene pairs were observed between the two genomes (Figure S2 and Table S6). Most (113 out of 142) AtCYP450s genes have multiple orthologous genes in Brassica napus. For instance, AtCYP706A4 has 11 collinear BnCYP450 genes. However, there are 29 AtCYP450 genes having only one collinear BnCYP450 gene (Table S6).
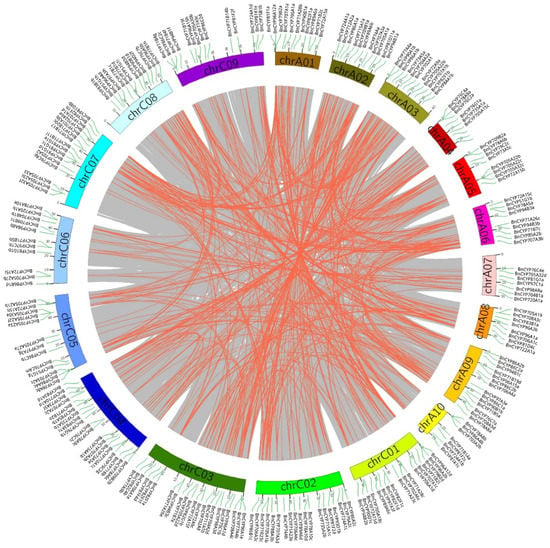
Figure 1.
The synteny analysis of BnCYP450 genes in Brassica napus. The gray lines display all synteny blocks, and the red lines indicate the segmental duplicate gene pairs in Brassica napus Cultivar ZS11 genome respectively.
2.4. Gene Structure Analyses of the Clan 86 BnCYP450 Genes
As above mentioned, members of clan 86 CYP450s, such as CYP704Bs, are essential for the formation of pollen exine in plant male reproduction and targeted mutagenesis of CYP704B genes has been used to create new male sterile lines in many crops [11,14,16,30]. Therefore, we are focused on analysis of clan 86 CYP450s. The overall exon/intron profile is an index to understand the phylogenetic relationships within a particular gene family [39]. So, we first determined the intron and exon organization of the clan 86 BnCYP450 genes. The majority of CYP96 subfamily genes (29 out of 52) contains no intron. Most members of the CYP86 (12 out of 29) and CYP94 (9 out of 20) subfamilies also have intronless genes (Figure 2). All members of the CYP704 subfamily have at least three introns. There are two genes from CYP94 subfamily (BnCYP94B1a and BnCYP94B1f) and one gene from CYP96 subfamily (BnCYP96A15c) having at most 21 introns (Figure 2). In short, these gene architectures within a subfamily may also be attributed to the high quality of ‘ZS11’ genome assembly after comparing with the ‘Darmor-bzh’ genome assembly as previously described [1].
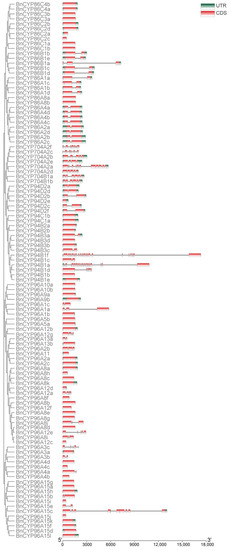
Figure 2.
Schematic exon/intron structures of the clan 86 BnCYP450 genes. The red boxes represent exons and black lines represent introns. The UTR region of are indicated in green boxes. The length of CDS can be estimated by the scale at the bottom.
2.5. Tissue-Specific Transcript Accumulation Patterns of the Clan 86 BnCYP450 Genes
In order to determine the transcript abundance of the clan 86 BnCYP450 genes, we investigated their expression profiles in 12 different tissues (stem, sepal, pistil, stamen, ovule, pericarp, blossomy pistil, wilting pistil, root, flower, leaf and silique) using public available RNA-seq data of ZS11 as above mentioned [40,41]. Eighty-seven genes were observed to be expressed in at least one tissue, while 22 genes were not observed to be expressed in any of the analyzed tissues (Figure 3 and Table S7). Moreover, three genes were specifically expressed in one tissue, whereas 24 genes were expressed in all of the analyzed tissues (Figure 3 and Table S7). There are 13 genes highly expressed in flower. These results suggest that some members of the clan 86 BnCYP450s may have distinct roles in cell differentiation and tissue development, and the flower expressed members may play conserved roles in control of fertility.
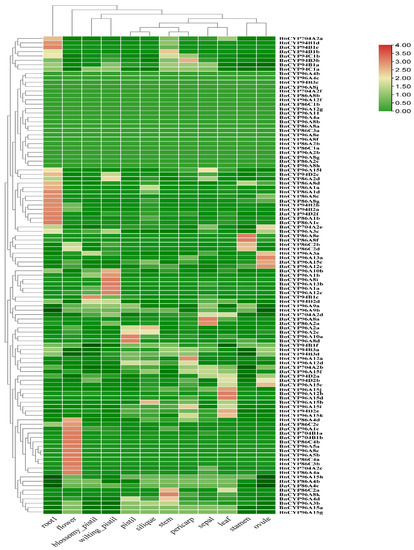
Figure 3.
Expression profiles of the clan 86 BnCYP450 genes in different tissues. The expression level is equal to the mean values and transforms log2 values for normalization. The color scale represents relative expression levels from low (green colored) to high (red colored).
2.6. Expression Profiles of the Clan 86 BnCYP450 Genes in Response to Abiotic Stresses
To examine the transcript abundance of the clan 86 BnCYP450 genes in response to abiotic stresses, we investigated their expression profiles under dehydration, NaCl, ABA, and cold conditions with publicly available RNA-seq data of ZS11 as above mentioned [40,41]. Sixty-seven genes were observed to be induced in response to at least one analyzed treatment, while nine genes were observed to be repressed in response to at least one analyzed treatment (Figure 4 and Table S8). There were 33 genes that could not be detected in response to any of the analyzed treatments. Moreover, there are some genes significantly induced by certain treatments, such as BnCYP94B3a and BnCYP96A15l by dehydration and cold or BnCYP94B1d by ABA. Consistent with a previous study [30], the expression of some BnCYP704 genes, such as BnCYP704B1b, BnCYP704A2e, BnCYP704A2b, BnCYP704A2d, BnCYP704A2a, and BnCYP704B1a, are affected by abiotic stresses (Figure 4 and Table S8).
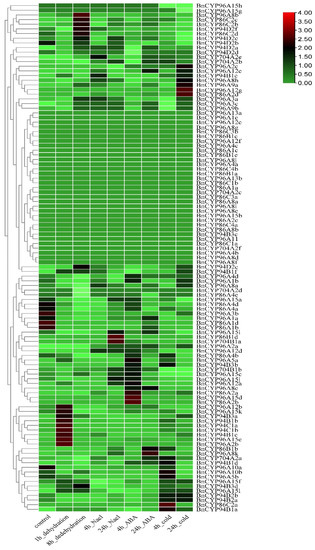
Figure 4.
Expression profiles of the clan 86 BnCYP450 genes under different stress conditions. The expression level is equal to the mean values and transforms log2 values for normalization. The color scale represents relative expression levels from low (green colored) to high (red colored).
2.7. The Brassica Napus Genome Encodes Two Putative ZmMS26-Like Genes
As displayed in Figure 5a and Table S1, there are two orthologous sequences for AtMS26 found in the rapeseed genome, BnCYP704B1a (ZS11A07G034230) and BnCYP704B1b (ZS11C06G043970), both of which contained six exons and five introns (Figure 5b). To remain consistent with previous MS26 nomenclature, we renamed BnCYP704B1a as BnMS26a and BnCYP704B1b as BnMS26b, respectively. Both BnCYP704B1a and BnCYP704B1b encode predicted proteins with 519 amino acids, estimated molecular masses of 59.8 kDa, and predicted pI of 8.58. Comparison of the predicted amino acid sequences and coding sequences of BnCYP704B1a and BnCYP704B1b revealed that they are highly similar, displaying about 98% amino acid identity (Figure S3) and 97% nucleotide identity (Figure S4). All of these indicate that BnCYP704B1a and BnCYP704B1b are segmental duplicating genes in rapeseed, and they originated from different progenitor species according to the U triangle [2]. In other words, BnCYP704B1a originated from Brassica rapa and BnCYP704B1b originated from Brassica oleracea. Phylogenetic analysis shows that ZmMS26 homologs in monocots (rice, maize, sorghum, Brachypodium, tomato and wheat) and dicot (the Brassicaceae species Arabidopsis, cabbage and rapeseed) are divided into two subfamilies (Figure 5a).
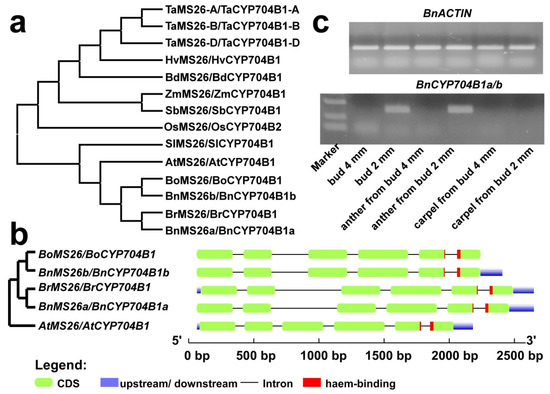
Figure 5.
Phylogenetic analysis of MS26 orthologs and anther-specific expression of BnCYP704B1a and BnCYP704B1b. (a) Phylogenetic analysis of MS26 orthologs. The protein sequences of MS26 orthologs were aligned by the MUSCLE tool; the maximum likelihood tree was generated using MEGA 6.0. (b) Genetic architecture of BoMS26, BnCYP704B1b, BrMS26, BnCYP704B1a and AtMS26. (c) RT-PCR analysis of BnCYP704B1a and BnCYP704B1b in buds, anther and carpel released from buds using BnACTIN as control. The exon/intron distribution of selected MS26 genes was determined using the online Gene Structure Display Server 2.0. The CDS (corresponding to exons) is represented by green boxes. The introns are indicated as black lines. The haem-binding motif region on the genomic sequence is denoted by red box.
2.8. BnCYP704B1a and BnCYP704B1b Are Mainly Expressed in Young Anther
The expression of the ZmMS26 gene is limited to developing anthers [11,15,16], which is consistent with its role in pollen development. Moreover, the ZmMS26 orthologs in rice, sorghum, and wheat also confer male sterility when targeted by mutagenesis, strongly supporting the conserved function in anthers [11,15,16]. To examine the expression patterns of the BnCYP704B1a and BnCYP704B1b genes, we first compared their expression levels in different tissues by analyzing available RNA-seq data from rapeseed [42]. The expression of BnCYP704B1a and BnCYP704B1b was only significantly detected in young buds (around 2 mm in diameter, Figure S4). To gain an insight into whether they also have a conserved anther-expressed pattern, we determined the expression levels of BnCYP704B1a and BnCYP704B1b in different flower tissues using RT-PCR. Concerning their high sequence identity, we used degenerate primers to detect their combined expression. Consistent with the transcriptome data, the expression of BnCYP704B1a and BnCYP704B1b could be only detected in young buds (Figure 5c). In flower parts isolated from young buds, the expression of BnCYP704B1a and BnCYP704B1b was only detected in anther, but not in carpel (Figure 5b and Figure S5), suggesting that they may also have a role at the very early stage of pollen development.
2.9. BnCYP704B1a and BnCYP704B1b Simultaneous Knockout Mutants Are Male Sterile
To determine whether the BnCYP704B1a and BnCYP704B1b could be edited by CRISPR/Cas9 system, we designed six sgRNA (Target-1~Target-6) to simultaneously target the first or the second exon (Figure 6a, Table S9). The six sgRNAs were divided into three groups and inserted into pHSE401 expression vectors as two gRNA expression cassettes [43]. The resulting three recombinant plasmids were transformed into Agrobacterium tumefaciens and co-cultured with the hypocotyls of rapeseed. As previously reported, CRISPR/Cas9-induced editing events could take place in the callus cells or in the regenerated shoots derivate from transformed hypocotyl before regeneration in rapeseed [44]. As expected, homozygous mutants with gRNA-directed mutation were acquired in the regenerated shoots (Figure S6). The editing efficiency of each sgRNA expression cassette was measured in the regenerated shoots. The sgRNA expression cassette harboring Target-1 and Target-2 was more likely to edit at both target sites (Figure S6). The sgRNA expression cassette harboring Target-5 and Target-6 was prone to edit at target site 5 (Figure S6). No editing event was observed at target sites 3 and 4 (Figure S6). Moreover, editing events with deletions were frequently observed in BnCYP704B1a and BnCYP704B1b in all of the regenerated shoots (Figure S6). We obtained two T2 plants harboring mutations in both BnCYP704B1a and BnCYP704B1b from the edited shoots (Figure 6b). All of these lines exhibited a pollenless, sterile phenotype in mature anthers (Figure 6). Sequencing results confirmed that all of these lines harbored the editing events at the targeted sites observed in the regenerated shoots (Figure 6b). To investigate whether the deletions generated by the CRISPR/Cas9 system could be transmitted to the next generation, they were cross-pollinated with the recipient line K407. The fertility of the corresponding F1 and F2 progenies were determined. All F1 progenies developed normal pollen grain in the mature anthers and produced seeds via self-fertilization. Around a quarter of the F2 progeny plants displayed the pollenless, sterile phenotype, indicating that BnCYP704B1a and BnCYP704B1b are two duplicate, recessive genes. Sequencing results also confirmed that the observed editing events were able to be transmitted to the next generations. Furthermore, the sterile edited BnCYP704B1a and BnCYP704B1b plants were able to set seed via artificial pollination with pollen from the recipient line K407, indicating normal female organ development and fertilization potential.

Figure 6.
Male sterility phenotypes of CRISPR/Cas9-edited GMS lines. (a) Gene structures of BnCYP704B1a and BnCYP704B1b and the corresponding CRISPR/Cas9 guide RNA target sites, marked by black arrows. (b) Mutated genotypes of BnCYP704B1a and BnCYP704B1b after CRISPR/Cas9 editing. The target sites sequences are marked in BnCYP704B1a and BnCYP704B1b sequences, with DEFINE (PAM) highlighted in green. (c–e) inflorescence; (f–h) flower; (i–k) flower without petal; (l–q) pollen fertility in CK (represents the recipient line K407) and CRISPR/Cas9-edited plants; (o–q) the pollen was stained with I2—KI solution. No detectable pollen was observed from CRISPR/Cas9-edited GMS plants. (c–k), scale bars, 1 cm; (l–q), scale bars, 50 μm.
To examine whether any off-target event occurs, we determined the potential off-target sites for BnCYP704B1a and BnCYP704B1b, respectively. After sequencing the amplified fragments of those potential off-target sites, no genome editing was observed in any examined samples (Table S10). These results indicated that the two sgRNA expression cassettes harboring Target-1 and Target-2 or Target-5 and Target-6 had the highest editing efficiency and an undetectable off-target effect.
3. Discussion
In the present study, a total of 687 BnCYP450 genes were identified from Brassica napus cultivar ZS11, which is assigned into 22 subfamilies and clustered into nine clans (Table 1). The number of BnCYP450s was greater than those of Arabidopsis (251), Brassica rapa (354), Brassica oleracea (343), rice (355), apple (348), tomato (457), cotton (461), model legume (346), grape (236), and fewer than in wheat (1700) [22,23,24,25,27,28]. Some subfamilies and clans showed deep phylogenetic relationships. For example, the CYP720 subfamily was inside the CYP90 subfamily on the CYP85 clan branch. The CYP704 subfamily was separated into two clades by the CYP94 and CYP86 subfamily on the CYP86 clan branch. The CYP89 subfamily was clustered inside the CYP77 subfamily on the CYP71 clan branch. The CYP76 subfamily from the CYP71 clan was separated into two clades by the CYP72 clan on the phylogenetic tree (Figure S1). Based on the above analysis, we speculated that the CYP450 superfamily has a significant degree of evolutionary extension in Brassica napus. Moreover, the large range of Mw and pI of BnCYP450s may determine their functional diversity in distinct biosynthetic pathways.
It is conceivable that gene duplication represents a major force to gene expansion [21,40,45]. Compared with those of AtCYP450s (251), BrCYP450s (354), and BoCYP450s (343), the number of BnCYP450s (687) was significantly expanded in Brassica napus, which may be attributed to the genome polyploidy event in terms of the U’ triangle [1,2,46]. Indeed, there were 32 tandem duplication events and 608 segmental duplications observed across AA and CC subgenomes (Table S3). Moreover, there were 88 and 91 duplication events observed within AA or CC subgenomes, respectively (Tables S4 and S5). So, tetraploid, segmental, and tandem duplication are the main types of gene duplication events taking place in the BnCYP450s superfamily. Under evolutionary pressures, duplication events have extended the gene family members and mutations in the upstream regions can alter the expression patterns of new members [47,48].
As mentioned above, some members of the CYP86 clan (CYP704, CYP86 and CYP94 subfamilies) are recognized as fatty acid hydroxylases responsible for the biosynthesis of pollen exine and anther cutin [7,21,29,30,31,32]. It is tempting to speculate that gene expression patterns are clues to their biological significance [21,39,49]. The expression patterns of the CYP86 clan BnCYP450s genes from 12 tissues and four types of abiotic stresses were determined. There were only a few of the CYP86 clan BnCYP450s genes displayed tissue-specific expression (Figure 3 and Table S7), such as BnCYP96A8c, BnCYP96A5b and BnCYP96A5a. It is interesting that most of the CYP86 clan BnCYP450s genes are induced by at least one analyzed treatment (Figure 4 and Table S8). Further RT-PCR analysis revealed that BnCYP704B1a and BnCYP704B1b are specifically expressed in young anther (Figure 5b), neither of which could be detected in any of the analyzed tissue or treatment (Figure 3 and Figure 4 and Tables S7 and S8). Concerning the importance of BnCYP450 superfamily members, the expression patterns of the CYP86 clan BnCYP450s examined in the present study may provide an important way to uncover the biological functions of other BnCYP450s in future.
Gene editing by the CRISPR/Cas9 system provides a rapid and specific way to manipulate the genetic material used in crop breeding. Since its first application in plants in 2013, targeted mutagenesis of interested genes has been used to create new germplasms in many crops [11,12,14,17]. One of these strategies is to target the mutagenesis of male-sterile control genes to create new male sterile lines in crops [9,11,12,13,14,18,20]. One of such male-sterile control genes is ZmMS26 [7,11,16,20,29,31,32]. Targeted mutagenesis of orthologues of MS26 have created male sterile lines in maize, rice, wheat, sorghum, and bread wheat [11,16,20]. In the current work, gene editing directed the knock-out of two orthologues of MS26 in Brassica napus is capable of generating a pure male sterile line (Figure 6). Moreover, editing events with deletions were frequently observed upon BnCYP704B1a and BnCYP704B1b in the obtained male sterile plants. This may be mainly attributed to the error-prone character of the two sgRNA expression cassettes, in which sgRNA directed deletion is observed to occur at high frequencies [43,50]. Target sequences with high editing and low off-target efficiencies will improve the breeding efficiency and reduce the breeding cost associated with the development of commercially applied genic male sterility lines.
We have also established male sterile lines in tomato via CRISPR/Cas9 directed knock-out of tomato CYP704B1 homolog [14]. As previously described, the conserved haem-binding loop in the C-terminal is essential for catalytic activity of haem-thiolate cytochrome P450s [11,16,20]. Targeted mutagenesis of the conserved haem domain of ZmMS26 (mainly located in exon 5 or exon 6) orthologs confers male sterility in monocots (such as maize, rice, sorghum, wheat) [11,16,20]. As mentioned above, our designed gRNAs were mainly located in exon 1 and exon 2 and conferred male sterility when simultaneously edited (Figure 6). Taken together, these results indicated that CRISPR/Cas9 directed simultaneously knock-out of ZmMS26 orthologs could be used as a universal strategy to establish male sterile lines in allotetraploid plants (such as rapeseed and wheat).
It is well-known that propagating a pure male sterile line is essential for commercial hybrid seed production [10]. The created male sterile line in the present study can serve as the foundation for applications in the two-line hybrid breeding of rapeseed. Taken together, our findings suggest that practical pure male sterile lines can be developed by the simultaneous, targeted knock-out of BnCYP704B1a and BnCYP704B1b using the CRISPR/Cas9 system, which is an important step towards capturing heterosis in commercial hybrid rapeseed production. Isolation of the inherited mutations in Cas9-free (also known as transgene clean) plants can ensure the stable transmission of the identified mutations to next generations. Moreover, the screening process for the male sterile line is time-consuming, laborious, and inefficient (50% fertile plants need to be removed before fertilizing). A long period of plant regeneration could be avoided with the selection of pure male sterile plants in advance, which are almost indistinguishable from classical male sterile lines during the molecular breeding period. Recently, a fluorescence-based seed sorting strategy has been adopted in the streamlined identification of male sterile lines in rice [10]. A convenient and efficient DsRed-based visual screening method has also been established in Brassica napus [51]. To further simplify the screening procedure for pure male sterile plants, a similar strategy also needs to be developed in hybrid rapeseed production in future.
4. Materials and Methods
4.1. Identification of the Cytochrome P450 Gene Superfamily in Brassica napus
Protein sequences of the rapeseed cultivar ZS11 were obtained from National Genomics Data Center (NGDC, accession number PRJCA002883) [1]. The cytochrome P450 genes of Brassica napus (BnCYP450s) were isolated from ZS11 through the HMM profile corresponding to the Pfam cytochrome P450 family PF00067 by running the HUMMER3.1 software of Linux version, with the threshold was e-value < e−10. A total of 251 AtCYP450 protein sequences download from the cytochrome P450 homepage (https://drnelson.uthsc.edu/, accessed on 11 November 2022) [22] were used as queries to perform a BLASTP search in the local protein database of ZS11. Then, the putative BnCYP450s were obtained via taking the intersection of the HUMMER and BLASTP methods. Finally, these proteins were submitted to the NCBI-CDD server (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 11 November 2022) and the SMART (Simple Modular Architecture Research Tool, http://smart.embl-heidelberg.de/, accessed on 11 November 2022) database to perform the cytochrome P450 domain predictions as described [40]. The theoretical molecular weight and isoelectric points of BnCYP450s were calculated by DNAstar as previously described [49].
4.2. Gene Duplication and Genomic Synteny of BnCYP450s
Gene duplication and genomic synteny of BnCYP450s were determined as previously described [40]. All of the BnCYP450 protein sequences were aligned using BLASTP with the threshold was e-value of e−100. Then, the duplication pattern of BnCYP450 genes was determined through the MCScanX software with default parameters and divided into tandem and segmental duplication as previously described [40,52]. Similarly, all of the BnCYP450 and AtCYP450 protein sequences were aligned using BLASTP with the threshold e-value of e−100, and all putative syntenic blocks were mapped with JCVI software as described [40,46].
4.3. Phylogenetic and Gene Structure Analysis
Protein sequences of the clan 86 BnCYP450 members were aligned with the FFT-NS-I method of the MAFFT software at first. The phylogenetic tree was constructed via FastTree software with the maximum likelihood method as previously described [40,53]. Then, the phylogenetic tree was visualized by Figtree software [39]. The overall intron/exon organization of the clan 86 BnCYP450 genes were displayed based on GFF annotation files by TBtools software as previously described [40,54]. Several MS26 orthologs, namely AtMS26 (Arabidopsis thaliana MS26, At1g69500) [29], BoMS26 (Brassica oleracea MS26, Bo6g108940) [31], BrMS26 (Brassica rapa MS26, Bra004386), BnMS26a (Brassica napus MS26a), BnMS26b (Brassica napus MS26b), SlMS26 (Solanum lycopersicum MS26, XM_004228326.4) [14], OsMS26 (Oryza sativa MS26, XP_015629295.1), HvMS26 (Hordeum vulgare MS26, BAK08270.1), ZmMS26 (Zea mays MS26, NP_001130648.10) [11,15,20], SbMS26 (Sorghum bicolor MS26, XP_002465796.1), BdMS26 (Brachypodium distachyon MS26, XP_003558727.1), TaMS26-A (Triticum aestivum MS26-A, TraesCS4A03G0032700.1), TaMS26-B (Triticum aestivum MS26-B, TraesCS4B03G0752700.1), and TaMS26-D (Triticum aestivum MS26-D, TraesCS4D03G0673200.1) [16], were aligned using the MUSCLE tool, and the maximum likelihood trees were generated using MEGA 6.0. The online Gene Structure Display Server (GSDS2.0, http://gsds.cbi.pku.edu.cn/ accessed on 30 November 2022) was used to decipher the intron-exon distribution of AtMS26, BoMS26, BrMS26, BnMS26a, and BnMS26b genes as previously described [31].
4.4. Expression Profiles of the Clan 86 BnCYP450 Genes
RNA-seq data from different tissues and under abiotic stresses of ZS11 were downloaded from the NGDC (accession numbers PRJNA394926 and CA001775) [2]. All of these RNA-seq data were mapped to the reference genome of ZS11 with HISAT2 software [55]. Transcript abundance of the clan 86 BnCYP450 genes was calculated by the TPM (Transcripts Per Million) values with FeatureCounts R package and a histogram was generated via TBtools software as previously described [54].
4.5. Determination of the Expression Profiles of BnCYP704B1a and BnCYP704B1b
Brassica napus RNA-seq data derived from different tissues (http://yanglab.hzau.edu.cn/BnTIR, accessed on 11 November 2022) [42] was used to profile the expression levels of BnCYP704B1a and BnCYP704B1b. To examine the anther-specific expression of BnCYP704B1a and BnCYP704B1b, semi-quantitative RT-PCR was used. Briefly, a total RNA was extracted from young buds (around 2 mm or 4 mm in diameter), anther (isolated from young buds), and carpel (isolated from young buds) with TRIzol reagent (TaKaRa). The complementary DNA (cDNA) was obtained after reverse transcription with M-MLV Reverse transcriptase (Promega). Semi-quantitative RT-PCR was performed to assess the expression levels of BnCYP704B1a and BnCYP704B1b and was normalized to ZS11C02G003910, which is an actin-like gene in Brassica napus.
4.6. Guide RNA Design and CRISPR/Cas9 Vector Construction
Guide RNAs targeting BnCYP704B1a and BnCYP704B1b were designed by CRISPR RGEN Tools (http://www.rgenome.net/cas-designer/, accessed on 11 November 2022), which provides both bulge-allowed RNA-guided Endonuclease (RGEN) targets via Cas-Designer [56] and potential off-targets within a 2-nt mismatch and optional 3-nt bulges via Cas-OFFinder [57]. It is advised to design at least two guide RNAs when intending to knock-out a single gene [43,56,57]. Since we were targeting two homologous genes with high sequence similarity, guide RNAs targeting either BnCYP704B1a or BnCYP704B1b were chosen to co-edit them. The CRISPR/Cas9 toolkit was set to design two guide RNA expression cassettes for genome editing in rapeseed as described previously [44]. Briefly, two guide RNAs were incorporated into two forward or two reverse primers, respectively, with the two forward or two reverse primers partially overlapping. The expression cassettes were amplified from pCBC-DT1T2 with these guide RNA-incorporated primers. Purified expression cassettes were digested with BsaI and inserted into pHSE401 via T4 DNA ligase (TaKaRa) reactions. The oligos used to construct the CRISPR/Cas9 vector for BnCYP704B1a and BnCYP704B1b (pHSE401-BnCYP704B1) are listed in Table S9.
4.7. Plant Material and Plant Transformation
Seeds of the winter rapeseed selfing line K407 were obtained from Hybrid Rapeseed Research Centre of Shaanxi Province, China. K407 shows high transformation efficiency (from our preliminary data, not shown). Rapeseed hypocotyl transformation followed the protocol as previously described [44,51], with modifications to Agrobacterium tumefaciens depletion and hygromycin selection. Briefly, seeds were surface sterilized and germinated in the dark. After 5–6 days, the etiolated hypocotyls were cut into 0.8–1-cm segments and co-cultivated with Agrobacterium tumefaciens harboring the pHSE401-BnCYP704B1 construct for 2 days in a solid M1 medium. Following co-cultivation, the hypocotyl explants were placed on M2 medium with 300 μg/mL Timentin and 10 μg/mL hygromycin to remove Agrobacterium tumefaciens and select transgenic plants respectively. Further sub-culturing of the explants was conducted on M3 shooting medium to induce callus and shoot regeneration at intervals of 2–3 weeks. After 6–8 weeks of selection and when normal shoots appeared, explants were transferred to M4 rooting medium and kept at 4 °C for 2–4 weeks for vernalization. The resulting plants were placed into soil after the formation of roots.
4.8. Determination of CRISPR/Cas9-Mediated Editing Events
Genomic DNA was extracted from regenerated shoots or plants with DNA easy extraction solution [40]. Fragments covering the sgRNA target region were amplified using genotyping primers (Table S9). The PCR products from regenerated shoots or plants were sent out for sequencing (Invitrogen). Moreover, PCR products from T2 plants were cloned into a pMD19 T-easy cloning vector (TaKaRa). Bacterial colony PCR was conducted, and positive single colonies were chosen for sequencing. To identify editing events for the target site, the generated sequences were aligned with BnCYP704B1a and BnCYP704B1b gene sequences. The editing events were verified via checking the corresponding peaks incorporated into the sequencing reports.
4.9. Phenotypic Analysis
The anthers of the edited plants were collected just before anthesis. Pollen grains were released by breaking anthers and suspending them in a 1% iodine/potassium iodide solution (KI/I2) to assess pollen viability. The released and stained pollen was examined and photographed under a microscope (BX53, Olympus Corporation, Tokyo, Japan) as previously described [12,14].
5. Conclusions
The cytochrome P450 (CYP450) monooxygenase superfamily plays essential roles in plant growth and development via producing many primary and secondary metabolites. This study isolated a total of 687 BnCYP450 genes in Brassica napus cultivar ZS11. The BnCYP450 genes were categorized into 47 subfamilies, clustered into nine clans, and grouped into non-A-type and A-type. Gene duplication and syntenic analysis provided a clear collinear relationship between BnCYP450s and AtCYP450s. Phylogeny, gene architecture, expression profiles of the CYP86 clan genes were determined in detail. Two ZmMS26 orthologous genes, BnCYP704B1a and BnCYP704B1b, were observed to be specifically expressed in young anthers. Simultaneously targeted knocked-out of these two genes through CRISPR/Cas9 brought about a male-sterile line Brassica napus. Taken together, a global investigation of the BnCYP450s and targeted editing of two anther-expressed ZmMS26 homologous genes were conducted in the current study, which could provide valuable solutions to investigate the biological significance of other BnCYP450s in future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12020365/s1. Table S1. Details of the identified BnCYP450 genes in Brassica napus; Table S2. List of tandem duplicated gene pairs of BnCYP450s; Table S3. List of segmental duplication of the BnCYP450 genes across AA/CC subgenome in Brassica napus; Table S4. List of segmental duplication of the BnCYP450 genes in AA subgenome; Table S5. List of segmental duplication of the BnCYP450 genes in CC subgenome; Table S6. One-to-one orthologous relationships of the BnCYP450 genes between Brassica napus and Arabidopsis thaliana; Table S7. Expression (TPM) of BnCYP450 genes in different tissues; Table S8. Expression (TPM) of BnCYP450 genes under different treatments; Table S9. Primers used in this study; Table S10. Potential off-target sites of Target-5 and Target-6 detected in GMS plants; Figure S1. Phylogenetic analysis of BnCYP450s; Figure S2. Synteny analysis of AtCYP450 and BnCYP450 genes; Figure S3. Alignment the protein sequences of BnCYP704B1a and BnCYP704B1b; Figure S4. Alignment the CDS sequences of BnCYP704B1a and BnCYP704B1b; Figure S5. The expression profiles of BnCYP704B1a and BnCYP704B1b; Figure S6. The sgRNA target sites and induced mutations upon BnCYP704B1a and BnCYP704B1b in regenerated shoots.
Author Contributions
C.X., J.M. and Y.Z. conceived the project and designed the experiment plans; Z.W., Y.Z., M.S., X.T., S.H., B.L., P.J., W.G., F.L., L.X. and R.A. conducted the experiments and performed bioinformatics analyses; C.X. and J.M. analyzed the data, prepared Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 and wrote the article; X.Z., W.H. and Y.Z. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Innovation Capability Support Program of Shaanxi Province (2020TD-051); by the State Basic Research Project from the Department of Science and Technology of Shaanxi Province (2021JM-086); by the Key Research and Development Program of Yangling Seed Industry Innovation Center (Ylzy-yc-2021-02); by the Scientific Research and Sharing Platform Construction project of Shaanxi Province (2021PT-036); by the State Key Research and Development Project from the Department of Science and Technology of Shaanxi Province (2018NY-097); by the National Key Research and Development Project from the Ministry of Science and Technology of China (2016YFD0101900).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Chen Qijun (China Agricultural University) for providing the CRISPR/Cas9 system and Wang Qinhu (Northwest A&F University) for help with bioinformatics analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, X.; Tong, C.; Zhang, X.; Song, A.; Hu, M.; Dong, W.; Chen, F.; Wang, Y.; Tu, J.; Liu, S.; et al. A high-quality Brassica napus genome reveals expansion of transposable elements, subgenome evolution and disease resistance. Plant Biotechnol. J. 2021, 19, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. Cell Mol. Biol. 2017, 92, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, L.; Hu, P.; Liu, Q.; Zhu, X.; Qian, Q.; Wang, K.; Wang, Y. Developing disease-resistant thermosensitive male sterile rice by multiplex gene editing. J. Integr. Plant Biol. 2019, 61, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Li, X.; Wang, Z.; Jiang, Y.; Wan, L.; Dong, F.; Chen, F.; Hong, D.; Yang, G. Map-based cloning reveals the complex organization of the BnRf locus and leads to the identification of BnRf(b), a male sterility gene, in Brassica napus. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2016, 129, 53–64. [Google Scholar] [CrossRef]
- Dun, X.; Zhou, Z.; Xia, S.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Tu, J.; Fu, T. BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J. Cell Mol. Biol. 2011, 68, 532–545. [Google Scholar] [CrossRef]
- Li, J.; Hong, D.; He, J.; Ma, L.; Wan, L.; Liu, P.; Yang, G. Map-based cloning of a recessive genic male sterility locus in Brassica napus L. and development of its functional marker. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2012, 125, 223–234. [Google Scholar] [CrossRef]
- Yi, B.; Zeng, F.; Lei, S.; Chen, Y.; Yao, X.; Zhu, Y.; Wen, J.; Shen, J.; Ma, C.; Tu, J.; et al. Two duplicate CYP704B1-homologous genes BnMs1 and BnMs2 are required for pollen exine formation and tapetal development in Brassica napus. Plant J. Cell Mol. Biol. 2010, 63, 925–938. [Google Scholar] [CrossRef]
- Zhou, Z.; Dun, X.; Xia, S.; Shi, D.; Qin, M.; Yi, B.; Wen, J.; Shen, J.; Ma, C.; Tu, J.; et al. BnMs3 is required for tapetal differentiation and degradation, microspore separation, and pollen-wall biosynthesis in Brassica napus. J. Exp. Bot. 2012, 63, 2041–2058. [Google Scholar] [CrossRef]
- Barman, H.N.; Sheng, Z.; Fiaz, S.; Zhong, M.; Wu, Y.; Cai, Y.; Wang, W.; Jiao, G.; Tang, S.; Wei, X.; et al. Generation of a new thermo-sensitive genic male sterile rice line by targeted mutagenesis of TMS5 gene through CRISPR/Cas9 system. BMC Plant Biol. 2019, 19, 109. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Z.; Wang, N.; Xie, G.; Lu, J.; Yan, W.; Zhou, J.; Tang, X.; Deng, X.W. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 2016, 113, 14145–14150. [Google Scholar] [CrossRef]
- Djukanovic, V.; Smith, J.; Lowe, K.; Yang, M.; Gao, H.; Jones, S.; Nicholson, M.G.; West, A.; Lape, J.; Bidney, D.; et al. Male-sterile maize plants produced by targeted mutagenesis of the cytochrome P450-like gene (MS26) using a re-designed I-CreI homing endonuclease. Plant J. Cell Mol. Biol. 2013, 76, 888–899. [Google Scholar]
- Jiang, B.; Chen, L.; Yang, C.; Wu, T.; Yuan, S.; Wu, C.; Zhang, M.; Gai, J.; Han, T.; Hou, W.; et al. The cloning and CRISPR/Cas9-mediated mutagenesis of a male sterility gene MS1 of soybean. Plant Biotechnol. J. 2021, 19, 1098–1100. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Si, X.; Tian, Y.; Chen, K.; Liu, J.; Chen, H.; Gao, C. Generation of thermosensitive male-sterile maize by targeted knockout of the ZmTMS5 gene. J. Genet. Genom. Yi Chuan Xue Bao 2017, 44, 465–468. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Wang, H.; Luo, B.; Cai, Y.; Li, X.; Zhang, Y.; Wang, X. Rapid generation of tomato male-sterile lines with a marker use for hybrid seed production by CRISPR/Cas9 system. Mol. Breed. 2021, 41, 25. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, C.; Zhu, J.; Liu, C.; Huang, C.; Li, X.; Xie, C. Genome editing enables next-generation hybrid seed production technology. Mol. Plant 2020, 13, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, M.; Thilges, K.; Cho, M.J.; Cigan, A.M. MS26/CYP704B is required for anther and pollen wall development in bread wheat (Triticum aestivum L.) and combining mutations in all three homeologs causes male sterility. PLoS ONE 2017, 12, e0177632. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, Y.; Zhu, J.K.; Botella, J.R. Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 2021, 63, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, M.; Li, J.; Chen, L.; Huang, Z.; Zheng, S.; Zhu, L.; Ni, E.; Jiang, D.; Zhao, B.; et al. Development of commercial thermo-sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9-mediated TMS5 editing system. Sci. Rep. 2016, 6, 37395. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, R.; Zanella, C.; Trimnell, M.R.; Fox, T.W.; Albertsen, M.C.; Bedinger, P. Two male-sterile mutants of Zea mays (Poaceae) with an extra cell division in the anther wall. Am. J. Bot. 2000, 87, 1193–1201. [Google Scholar] [CrossRef]
- Cigan, A.M.; Singh, M.; Benn, G.; Feigenbutz, L.; Kumar, M.; Cho, M.J.; Svitashev, S.; Young, J. Targeted mutagenesis of a conserved anther-expressed P450 gene confers male sterility in monocots. Plant Biotechnol. J. 2017, 15, 379–389. [Google Scholar] [CrossRef]
- Mizutani, M.; Ohta, D. Diversification of P450 genes during land plant evolution. Annu. Rev. Plant Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef]
- Nelson, D.R. The cytochrome p450 homepage. Hum. Genom. 2009, 4, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tehrim, S.; Wang, L.; Dossa, K.; Zhang, X.; Ke, T.; Liao, B. Evolutionary history and functional divergence of the cytochrome P450 gene superfamily between Arabidopsis thaliana and Brassica species uncover effects of whole genome and tandem duplications. BMC Genom. 2017, 18, 733. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; He, T.; Wang, Y.; Liu, S.; Gao, Y.; Yan, H.; Xiang, Y. Genome and transcriptome analysis to understand the role diversification of cytochrome P450 gene under excess nitrogen treatment. BMC Plant Biol. 2021, 21, 447. [Google Scholar] [CrossRef]
- Jiu, S.; Xu, Y.; Wang, J.; Wang, L.; Liu, X.; Sun, W.; Sabir, I.A.; Ma, C.; Xu, W.; Wang, S.; et al. The cytochrome P450 monooxygenase inventory of grapevine (Vitis vinifera L.): Genome-wide identification, evolutionary characterization and expression analysis. Front. Genet. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, L.; Li, Q.; Zhang, Q.; Sun, C.; Liu, X.; Yang, N. Global investigation of cytochrome P450 genes in the chicken genome. Genes 2019, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Fang, H.; Chen, Y.; Zhuang, Z.; Chen, Q.; Shan, T.; Khan, M.K.R.; Zhang, J.; Wang, B. Genome-wide analysis of the cytochrome P450 gene family involved in salt tolerance in Gossypium hirsutum. Front. Plant Sci. 2021, 12, 685054. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, J.; Ma, L.; Yan, S.; Pang, Y. Genome-wide identification and analyses of drought/salt-responsive cytochrome P450 genes in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9957. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Moller, B.L.; Preuss, D. CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Shi, J.X.; Cui, M.H.; Yang, L.; Kim, Y.J.; Zhang, D.B. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Ji, J.L.; Yang, L.M.; Fang, Z.Y.; Zhuang, M.; Zhang, Y.Y.; Lv, H.H.; Liu, Y.M.; Li, Z.S. Recessive male sterility in cabbage (Brassica oleracea var. capitata) caused by loss of function of BoCYP704B1 due to the insertion of a LTR-retrotransposon. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2017, 130, 1441–1451. [Google Scholar] [CrossRef]
- Li, H.; Pinot, F.; Sauveplane, V.; Werck-Reichhart, D.; Diehl, P.; Schreiber, L.; Franke, R.; Zhang, P.; Chen, L.; Gao, Y.; et al. Cytochrome P450 family member CYP704B2 catalyzes the {omega}-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 2010, 22, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Morrone, D.; Chen, X.; Coates, R.M.; Peters, R.J. Characterization of the kaurene oxidase CYP701A3, a multifunctional cytochrome P450 from gibberellin biosynthesis. Biochem. J. 2010, 431, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.; Daviere, J.M.; Heintz, D.; Lange, T.; Achard, P. The gibberellin biosynthetic genes AtKAO1 and AtKAO2 have overlapping roles throughout Arabidopsis development. Plant J. Cell Mol. Biol. 2014, 80, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J. Regulation of brassinosteroid biosynthesis and inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, W.; Hu, X.; Sun, C.; Li, Y.; Wang, D.; Wang, Q.; Pei, G.; Zhang, Y.; Guo, A.; et al. Genome-wide analysis of the bZIP gene family identifies two ABI5-Like bZIP transcription factors, BrABI5a and BrABI5b, as positive modulators of ABA signalling in Chinese cabbage. PLoS ONE 2016, 11, e0158966. [Google Scholar] [CrossRef]
- Song, M.; Linghu, B.; Huang, S.; Li, F.; An, R.; Xie, C.; Zhu, Y.; Hu, S.; Mu, J.; Zhang, Y. Genome-wide survey of leucine-rich repeat receptor-like protein kinase genes and CRISPR/Cas9-targeted mutagenesis BnBRI1 in Brassica napus. Front. Plant Sci. 2022, 13, 865132. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, Y.; Chen, Y.; Wu, D.; Jiang, L. Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in Brassica napus. BMC Plant Biol. 2020, 20, 543. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, L.; Wei, L.; Yu, P.; Wang, J.; Zhao, H.; Zhang, Y.; Zhang, S.; Yang, Z.; Chen, G.; et al. BnTIR: An online transcriptome platform for exploring RNA-seq libraries for oil crop Brassica napus. Plant Biotechnol. J. 2021, 19, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.L.; Dong, L.; Wang, Z.P.; Zhang, H.Y.; Han, C.Y.; Liu, B.; Wang, X.C.; Chen, Q.J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Braatz, J.; Harloff, H.J.; Mascher, M.; Stein, N.; Himmelbach, A.; Jung, C. CRISPR-Cas9 targeted mutagenesis leads to simultaneous modification of different homoeologous gene copies in polyploid oilseed rape (Brassica napus L.). Plant Physiol. 2017, 174, 935–942. [Google Scholar] [CrossRef]
- Freeling, M. Bias in plant gene content following different sorts of duplication: Tandem, whole-genome, segmental, or by transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef]
- Tang, H.; Bowers, J.E.; Wang, X.; Ming, R.; Alam, M.; Paterson, A.H. Synteny and collinearity in plant genomes. Science 2008, 320, 486–488. [Google Scholar] [CrossRef]
- Heidari, P.; Abdullah, S.F.; Poczai, P. Magnesium transporter gene family: Genome-wide identification and characterization in Theobroma cacao, Corchorus capsularis, and Gossypium hirsutum of Family Malvaceae. Agronomy 2021, 11, 1651. [Google Scholar] [CrossRef]
- Heidari, P.; Puresmaeli, F.; Mora-Poblete, F. Genome-wide identification and molecular evolution of the magnesium transporter (MGT) gene family in Citrullus lanatus and Cucumis sativus. Agronomy 2022, 12, 2253. [Google Scholar] [CrossRef]
- Li, G.; Hu, X.; Hou, L.; Cao, L.; Wang, Q.; Wang, D.; Mu, X.; Zhang, Y.; Zhou, X.; Zhao, Y.; et al. Molecular identification of BrHAB2a, one of the two AtHAB2-like proteins in Brassica rapa, is an important component of ABA signaling. Biochem. Biophys. Res. Commun. 2018, 503, 495–500. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, X.; Guo, R.; Huang, J.; Wang, W.; Tang, J.; Tan, L.; Zhu, J.K.; Chu, C.; Qian, Y. Genome-wide targeted mutagenesis in rice using the CRISPR/Cas9 system. Mol. Plant 2017, 10, 1242–1245. [Google Scholar] [CrossRef]
- Zhang, K.; He, J.; Liu, L.; Xie, R.; Qiu, L.; Li, X.; Yuan, W.; Chen, K.; Yin, Y.; Kyaw, M.M.M.; et al. A convenient, rapid and efficient method for establishing transgenic lines of Brassica napus. Plant Methods 2020, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bae, S.; Kim, J.S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, J.; Kim, J.S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).