Molecular Characteristics of Barley Yellow Dwarf Virus—PAS—The Main Causal Agent of Barley Yellow Dwarf Disease in Poland
Abstract
:1. Introduction
2. Results
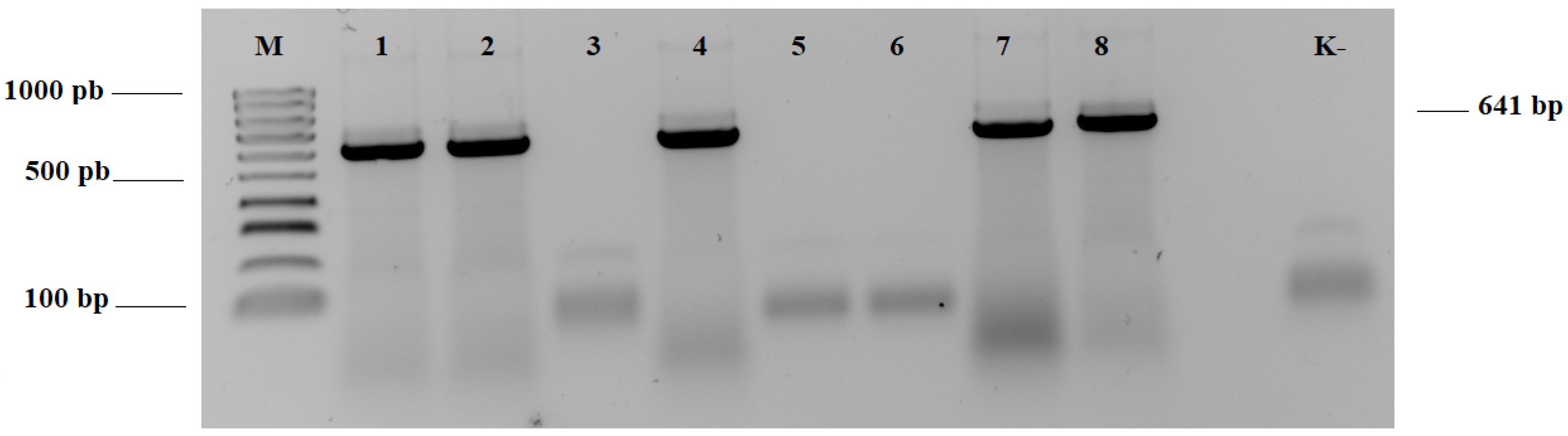
2.1. Molecular Diagnostics
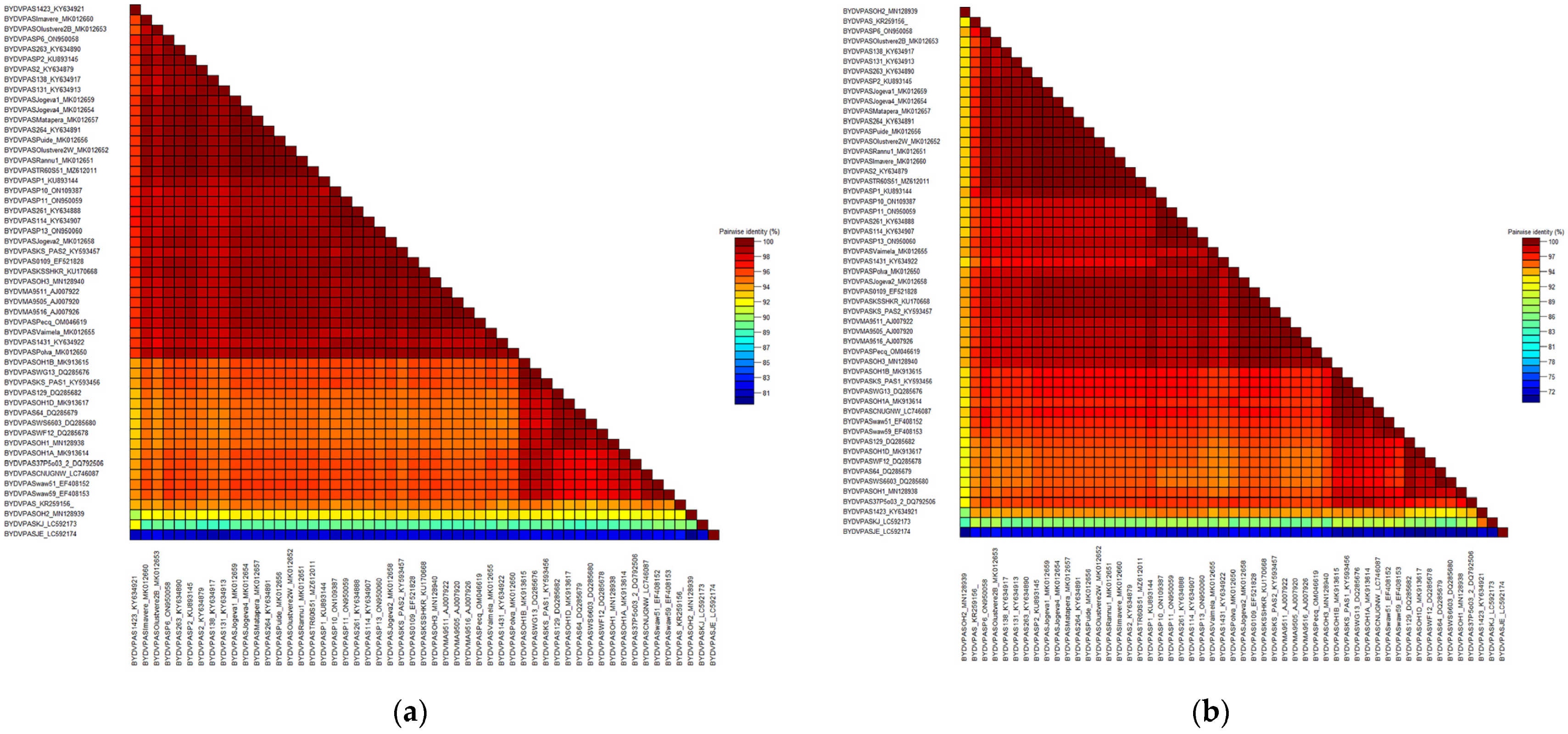
2.2. Comparative Analysis
2.3. Assessment of Variability and Recombination Analysis
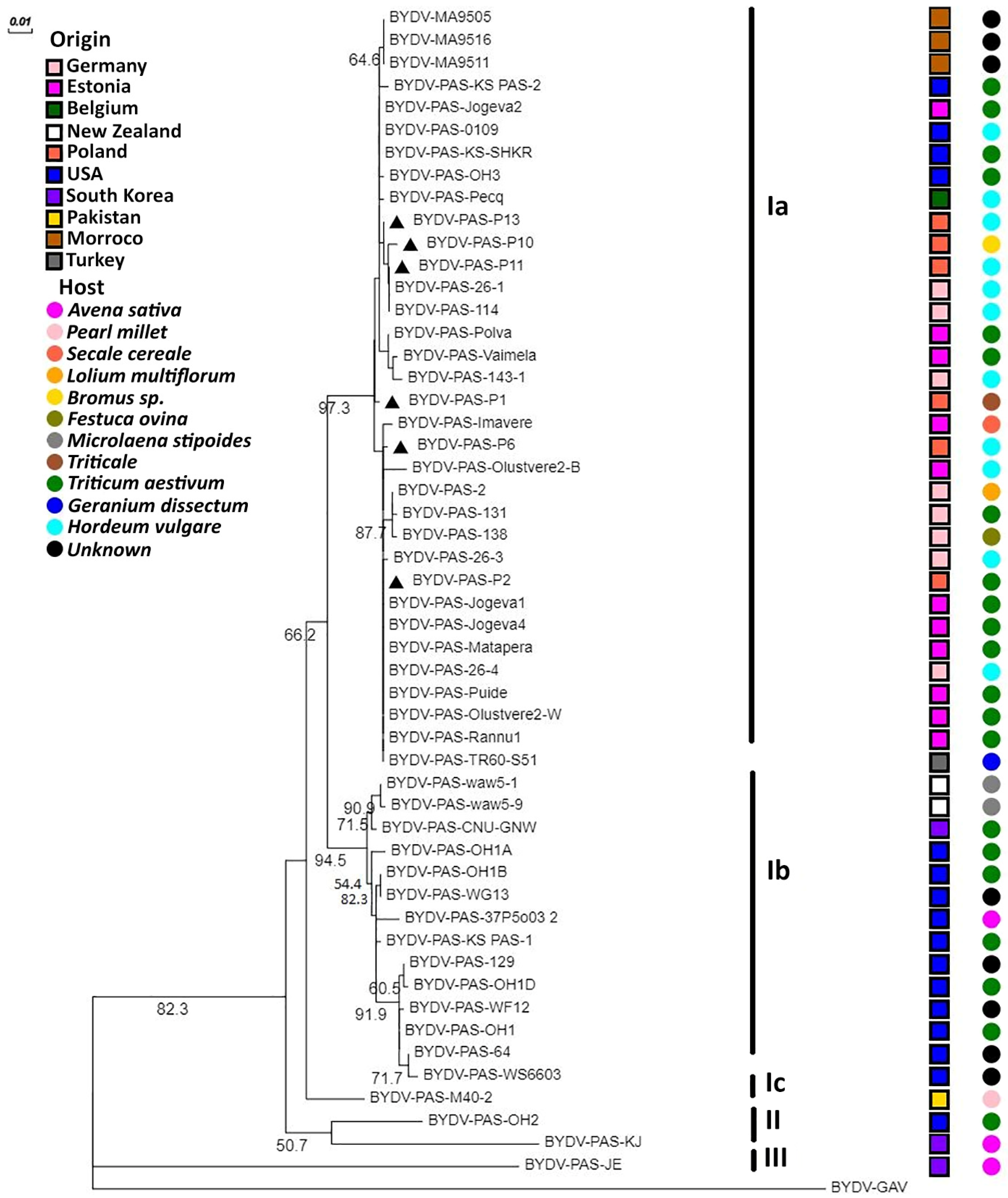
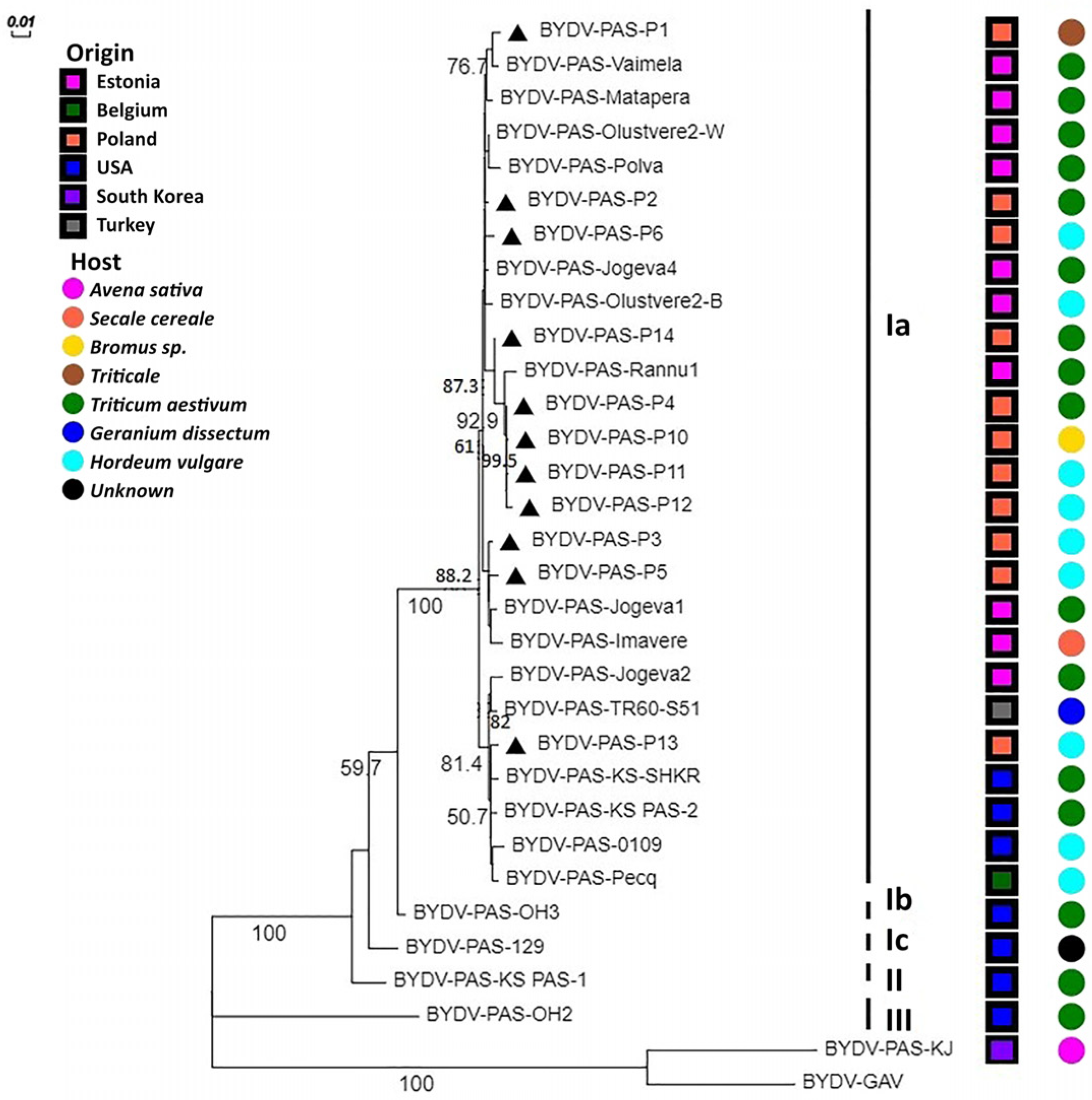
2.4. Phylogenetic Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Sources
4.2. RNA Isolation
4.3. Reverse Transcription-Polymerase Chain (RT-PCR)
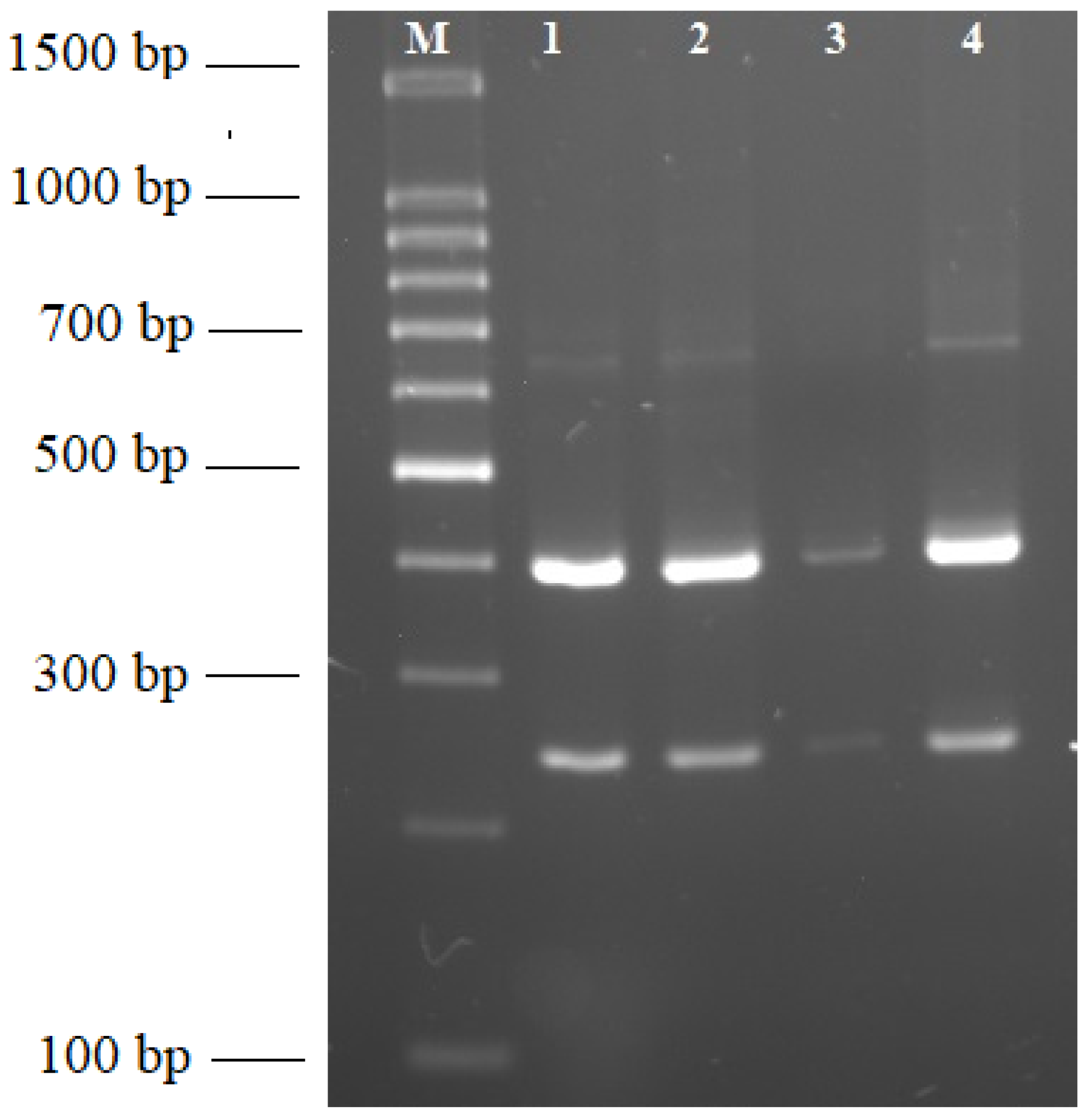
4.4. Restriction Fragment Length Polymorphism Analysis (RFLP)
4.5. Immuno-Capture-Duplex Polymerase Chain Reaction (IC-Duplex PCR)
4.6. DNA Sequencing
4.7. Estimating Sequence Similarity and Recombination Events
4.8. Phylogenetic Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Anwar, S.; Shuja, M.N.; Triparthi, R.K.; Singh, J. The genus Luteovirus from infection to disease. Eur. J. Plant Pathol. 2018, 151, 841–860. [Google Scholar] [CrossRef]
- Domier, L.L. Genome structure and function of barley yellow dwarf viruses. In Barley Yellow Dwarf 40 years of Progress, 1st ed.; D’Arcy, C.J., Burnett, P.A., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 181–201. [Google Scholar]
- Halbert, S.; Voegtlin, D. Biology and taxonomy of vectors of barley yellow dwarf viruses. In Barley Yellow Dwarf 40 Years of Progress, 1st ed.; D’Arcy, C.J., Burnett, P.A., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 217–258. [Google Scholar]
- Rochow, W.F. Biological properties of four isolates of Barley yellow dwarf virus. Phytopathology 1969, 59, 1580–1588. [Google Scholar] [PubMed]
- Rochow, W.F.; Muller, I. Fifth variant of Barley yellow dwarf virus in New-York. Plant Dis. Rep. 1971, 55, 874–879. [Google Scholar]
- Rochow, W.F.; Carmichael, L.E. Specificity among barley yellow dwarf viruses in enzyme immunosorbent assays. Virology 1979, 95, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.M.; He, X.Y.; Wu, M.S.; Zhou, G.H. Nucleotide sequence of coat protein gene for GPV isolate of Barley yellow dwarf virus and construction of expression plasmid for plant. Sci. China Ser. C 1996, 39, 534–543. [Google Scholar]
- Wang, X.; Chang, S.; Jin, Z.; Li, L.; Zhou, G. Nucleotide sequences of the coat protein and readthrough protein genes of the Chinese GAV isolate of Barley yellow dwarf virus. Acta Virol. 2001, 45, 249–252. [Google Scholar]
- Mayo, M.A. ICTV at the Paris ICV: Results of the plenary session and the binomial ballot. Arch. Virol. 2002, 147, 2254–2260. [Google Scholar] [CrossRef]
- Domier, L.L. Family Luteoviridae. In Virus taxonomy classification and nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, 1st ed.; King, A.M.Q., Adams, M.L., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2012; pp. 1045–1053. [Google Scholar]
- Miller, W.A.; Liu, S.; Beckett, R. Barley yellow dwarf virus: Luteoviridae or Tombusviridae? Mol Plant Pathol 2002, 3, 177–183. [Google Scholar] [CrossRef]
- Brakke, M.K. Virus disease in wheat. In Wheat and Wheat Improvement, 2nd ed.; Heyne, E.G., Ed.; American Social of Agronomy: Madison, WI, USA, 1987; pp. 585–603. [Google Scholar]
- Perry, K.L.; Kolb, F.L.; Samsons, B.; Lawson, C.; Cisar, G.; Ohm, H. Yield effects of barley yellow dwarf virus in soft red winter wheat. Phytopathology 2000, 90, 1043–1048. [Google Scholar] [CrossRef]
- Jones, R.; McKirdy, S.; Shivas, R. Occurrence of barley yellow dwarf viruses in over-summering grasses and cereal crops in Western Australia. Australas. Plant Pathol. 1990, 19, 90–96. [Google Scholar] [CrossRef]
- Achon, M.A.; Serrano, L.; Ratti, C.; Rubies-Autonell, C. First detection of Wheat dwarf virus in barley in Spain associated with an outbreak of barley yellow dwarf. Plant Dis 2006, 90, 970. [Google Scholar] [CrossRef]
- Jeżewska, M.; Cajza, M.; Buchowska-Ruszkowska, M. Monitoring and molecular diagnostics of cereal viruses. In Reducing Losses in Crop Yields with Food Safety, 1st ed.; Sosnowska, D., Ed.; Instytut Ochrony Roślin–PIB: Poznań, Poland, 2010; pp. 157–180. [Google Scholar]
- Jeżewska, M.; Trzmiel, K. Outbreak of barley yellow dwarf in winter cereals in Poland in the season 2014/2015. Prog. Plant Prot. 2016, 56, 296–301. [Google Scholar]
- Trzmiel, K. Identification of barley yellow dwarf viruses in Poland. J Plant Pathol 2017, 99, 493–497. [Google Scholar]
- Trzmiel, K. Occurrence of Wheat dwarf virus and Barley yellow dwarf virus species in Poland in the spring of 2019. J. Plant Prot. Res. 2020, 60, 345–350. [Google Scholar]
- Trzmiel, K.; Klejdysz, T. Detection of barley- and wheat-specific forms of Wheat dwarf virus in their vector Psammotettix alienus by duplex PCR assay. J. Plant Prot. Res. 2018, 58, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Liu, Y.; Xie, J.; Gray, S.M.; Zhou, G.; Gao, B. A Chinese isolate of barley yellow dwarf virus-PAV represents a third distinct species within the PAV serotype. Arch. Virol. 2007, 152, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, R.; Van Leeuwen, T.; Haesaert, G. Identifying drivers of spatio-temporal dynamics in barley yellow dwarf virus epidemiology as a critical factor in disease control. Pest. Manag. Sci. 2020, 76, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, Y.C. Identyfication and annual variation of variants of barley yellow dwarf virus in Ontario and Quebec. J. Plant Pathol. 1982, 4, 59–64. [Google Scholar]
- D’Arcy, C.J.; Domier, L.L. Family Luteoviridae. In Virus Taxonomy: VIIIth Report of International Committee on Taxonomy of Viruses, 1st ed.; Faequet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 891–900. [Google Scholar]
- Robertson, N.L.; French, R. Genetic structure in natural populations of barley/cereal yellow dwarf virus isolates from Alaska. Arch. Virol. 2007, 152, 891–902. [Google Scholar] [CrossRef]
- Delmiglio, C. The Incidence and Phylogenetic Analysis of Viruses Infecting New Zealand’s Native Grasses. Ph.D. Thesis, University of Aukland, Auckland, New Zealand, 2008. [Google Scholar]
- Kim, N.K.; Lee, H.J.; Kim, S.M.; Jeong, R.D. Identification of viruses infecting oats in Korea by metatranscriptomics. Plants 2022, 11, 256. [Google Scholar] [CrossRef]
- Bencharki, B.; Muttere, J.; El Yamani, M.; Ziegler-Graff, V.; Zaoui, D.; Jonard, G. Severity of infection of Moroccan barley yellow dwarf virus PAV isolates correlates with variability in their coat protein sequences. Ann. Appl. Biol. 1999, 13, 843–853. [Google Scholar] [CrossRef]
- Mastari, J.; Lapierre, H.; Dessens, J.T. Asymetrical distribution of barley yellow dwarf virus PAV variants between host plant species. Phytopathology 1998, 88, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K. First report of barley yellow dwarf virus-PAS in wheat and barley grown in the Czech Republic. Plant Dis. 2008, 92, 1587. [Google Scholar] [CrossRef] [PubMed]
- Sõmera, M.; Massart, S.; Tamisier, L.; Sooväli, P.; Sathees, K.; Kvarnheden, A. A survey using high-throughput sequencing suggests that the diversity of cereal and barley yellow dwarf viruses is underestimated. Front. Microbiol. 2021, 12, 673218. [Google Scholar]
- Chay, C.A.; Smith, D.M.; Vaughan, R.; Gray, S.M. Diversity among isolates within the PAV serotype of barley yellow dwarf virus. Phytopathology 1996, 86, 370–377. [Google Scholar] [CrossRef]
- Hodge, B.A.; Paul, P.A.; Stewart, L.R. Occurrence and High-Throughput Sequencing of Viruses in Ohio wheat. Plant Dis. 2020, 104, 1789–1800. [Google Scholar] [CrossRef]
- Kundu, J.K.; Jarošova, J.; Gadiou, S.; Červena, G. Discrimination of three BYDV species by one-step RT-PCR-RFLP and sequence based methods in cereal plants from the Czech Republic. Cereal Res. Commun. 2009, 37, 541–550. [Google Scholar] [CrossRef]
- Jarošova, J.; Chrpova, J.; Šip, V.; Kundu, J.K. A comparative study of the Barley yellow dwarf virus species PAV and PAS: Distribution, accumulation and host resistance. Plant Pathol. 2013, 62, 436–443. [Google Scholar] [CrossRef]
- Singh, K.; Jarošova, J.; Fousek, J.; Chen, H.; Kundu, J.K. Virome identification in wheat in the Czech Republic using small RNA deep sequencing. J. Integr. Agric. 2020, 19, 1825–1833. [Google Scholar] [CrossRef]
- Strażyński, P.; Roik, K. Registration of the flight dynamics of economically important aphid species using the Jonson’s suction trap and its importance in the integrated protection of agricultural crops. Prog. Plant Prot. 2021, 61, 246–252. [Google Scholar]
- Hall, G. Selective constraint and genetic differentiation in geographically distant barley yellow dwarf virus populations. J. Gen. Virol. 2006, 87, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Blanchard-Letort, A.; Liu, Y.; Zhou, G.; Wang, X.; Elena, S.F. Dynamics of Molecular Evolution and Phylogeography of Barley yellow dwarf virus-PAV. PLoS ONE 2011, 6, e16896. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Trzmiel, K.; Szydło, W.; Hasiów-Jaroszewska, B. Biological and molecular characterisation of the two Polish Wheat streak mosaic virus isolates and their transmission by wheat curl mites. Plant Prot. Sci. 2021, 57, 171–178. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choise. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res 2019, 47, W270–W275. [Google Scholar] [CrossRef]


| Virus Isolate | Host | Origin | Acc. No. of RNA [CP * and RdRP #] |
|---|---|---|---|
| BYDV-PAS-P1 1 | Triticale | Poland, 2015 | KU893144 *; ON950062 # |
| BYDV-PAS-P2 | Triticum aestivum | Poland, 2015 | KU893145 *; ON950063 # |
| BYDV-PAS-P3 | Hordeum vulgare | Poland, 2015 | ON950056 *; ON950064 # |
| BYDV-PAS-P4 | Triticum aestivum | Poland, 2015 | OM685192 *; ON950065 # |
| BYDV-PAS-P5 | Hordeum vulgare | Poland, 2018 | ON950057 *; ON950066 # |
| BYDV-PAS-P6 | Hordeum vulgare | Poland, 2018 | ON950058 *; ON950067 # |
| BYDV-PAS -IDA-P7 | Hordeum vulgare | Poland, 2021 | OM685192 *; ON950065 # |
| BYDV-PAS-P8 | Hordeum vulgare | Poland, 2021 | OM685192 *; ON950065 # |
| BYDV-PAS-P9 | Hordeum vulgare | Poland, 2021 | OM685192 *; ON950065 # |
| BYDV-PAS-Br-P10 | Bromus sp. | Poland, 2021 | ON109387 *; ON950068 # |
| BYDV-PAS-P11 | Hordeum vulgare | Poland, 2021 | ON950059 *; ON950069 # |
| BYDV-PAS-P12 | Hordeum vulgare | Poland, 2021 | OM685192 *; ON950070 # |
| BYDV-PAS-P13 | Hordeum vulgare | Poland, 2021 | ON950060 *; ON950071 # |
| BYDV-PAS-P14 | Triticum aestivum | Poland, 2021 | ON950061 *; ON950072 # |
| BYDV-PAS-Jogeva1 2 | Triticum aestivum | Estonia, 2015 | MK012659 |
| BYDV-PAS-Jogeva2 | Triticum aestivum | Estonia, 2015 | MK012658 |
| BYDV-PAS-Jogeva4 | Triticum aestivum | Estonia, 2015 | MK012654 |
| BYDV-PAS-Matapera | Triticum aestivum | Estonia, 2015 | MK012657 |
| BYDV-PAS-Vaimela | Triticum aestivum | Estonia, 2014 | MK012655 |
| BYDV-PAS-Puide | Triticum aestivum | Estonia, 2015 | MK012656 |
| BYDV-PAS-Olustvere2-W | Triticum aestivum | Estonia, 2014 | MK012652 |
| BYDV-PAS-Olustvere2-B | Hordeum vulgare | Estonia, 2014 | MK012653 |
| BYDV-PAS-Rannu1 | Triticum aestivum | Estonia, 2014 | MK012651 |
| BYDV-PAS-Imavere | Secale cereale | Estonia, 2015 | MK012660 |
| BYDV-PAS-Polva | Triticum aestivum | Estonia, 2013 | MK012650 |
| BYDV-PAS-26-1 | Hordeum vulgare | Germany, 2015 | KY634888 * |
| BYDV-PAS-26-3 | Hordeum vulgare | Germany, 2015 | KY634890 * |
| BYDV-PAS-26-4 | Hordeum vulgare | Germany, 2015 | KY634891 * |
| BYDV-PAS-2 | Lolium multiflorum | Germany, 2008 | KY634879 * |
| BYDV-PAS-131 | Triticum aestivum | Germany, 2015 | KY634913 * |
| BYDV-PAS-138 | Festuca ovina | Germany, 2008 | KY634917 * |
| BYDV-PAS-114 | Hordeum vulgare | Germany, 2015 | KY634907 * |
| BYDV-PAS-143-1 | Hordeum vulgare | Germany, 2016 | KY634922 * |
| BYDV-PAS-142-3 | Hordeum vulgare | Germany, 2016 | KY634921 * |
| BYDV-PAS-Pecq | Hordeum vulgare | Belgium, 2013 | OM046619 * |
| BYDV-PAS-TR60-S51 | Geranium dissectum | Turkey, 2018 | MZ612011 |
| BYDV-MA9505 | - | Morocco, 1998 | AJ007920 * |
| BYDV-MA9511 | - | Morocco, 1998 | AJ007922 * |
| BYDV-MA9516 | - | Morocco, 1998 | AJ007926 * |
| BYDV-PAS-0109 | Hordeum vulgare | USA: Iowa, 2007 | EF521828 |
| BYDV-PAS-OH1 | Triticum aestivum | USA: Ohio, 2016 | MN128938 * |
| BYDV-PAS-OH1A | Triticum aestivum | USA: Ohio, 2016 | MK913614 * |
| BYDV-PAS-OH1B | Triticum aestivum | USA: Ohio, 2016 | MK913615 * |
| BYDV-PAS-OH1D | Triticum aestivum | USA: Ohio, 2016 | MK913617 * |
| BYDV-PAS-OH2 | Triticum aestivum | USA: Ohio, 2016 | MN128939 |
| BYDV-PAS-OH3 | Triticum aestivum | USA: Ohio, 2016 | MN128940 |
| BYDV-PAS-KS PAS-1 | Triticum aestivum | USA: Kansas, 2012 | KY593456 |
| BYDV-PAS-KS PAS-2 | Triticum aestivum | USA: Kansas, 2012 | KY593457 |
| BYDV-PAS-KS-SHKR | Triticum aestivum | USA: Kansas, 2011 | KU170668 |
| BYDV-PAS-WG13 | - | USA, 2005 | DQ285676 * |
| BYDV-PAS-64 | - | USA, 2005 | DQ285679 * |
| BYDV-PAS-WF12 | - | USA, 2005 | DQ285678 * |
| BYDV-PAS-WS6603 | - | USA, 2005 | DQ285680 * |
| BYDV-PAS-129 | - | USA, 2005 | AF218798 |
| BYDV-PAS37P5o03-2 | Avena sativa | USA, 2003 | DQ792506 * |
| BYDV-PAS-M40-2 | Pearl millet | Pakistan, 2011 | KR259156 * |
| BYDV-PAS-waw5-1 | Microlaena stipoides | New Zealand | EF408152 * |
| BYDV-PAS-waw5-9 | Microlaena stipoides | New Zealand | EF408153 * |
| BYDV-PAS-KJ | Avena sativa | Republic of Korea, 2020 | LC592173 |
| BYDV-PAS-JE | Avena sativa | Republic of Korea, 2020 | LC592174 |
| BYDV-PAS-CNU-GNW | Triticum aestivum | Republic of Korea, 2021 | LC746087 * |
| BYDV-GAV | - | China | AY220739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzmiel, K.; Hasiów-Jaroszewska, B. Molecular Characteristics of Barley Yellow Dwarf Virus—PAS—The Main Causal Agent of Barley Yellow Dwarf Disease in Poland. Plants 2023, 12, 3488. https://doi.org/10.3390/plants12193488
Trzmiel K, Hasiów-Jaroszewska B. Molecular Characteristics of Barley Yellow Dwarf Virus—PAS—The Main Causal Agent of Barley Yellow Dwarf Disease in Poland. Plants. 2023; 12(19):3488. https://doi.org/10.3390/plants12193488
Chicago/Turabian StyleTrzmiel, Katarzyna, and Beata Hasiów-Jaroszewska. 2023. "Molecular Characteristics of Barley Yellow Dwarf Virus—PAS—The Main Causal Agent of Barley Yellow Dwarf Disease in Poland" Plants 12, no. 19: 3488. https://doi.org/10.3390/plants12193488
APA StyleTrzmiel, K., & Hasiów-Jaroszewska, B. (2023). Molecular Characteristics of Barley Yellow Dwarf Virus—PAS—The Main Causal Agent of Barley Yellow Dwarf Disease in Poland. Plants, 12(19), 3488. https://doi.org/10.3390/plants12193488







